Potato Spindle Tuber Viroid RNA-Templated Transcription: Factors and Regulation
Abstract
1. Introduction
2. The Discovery and Biogenesis of TFIIIA in Plants
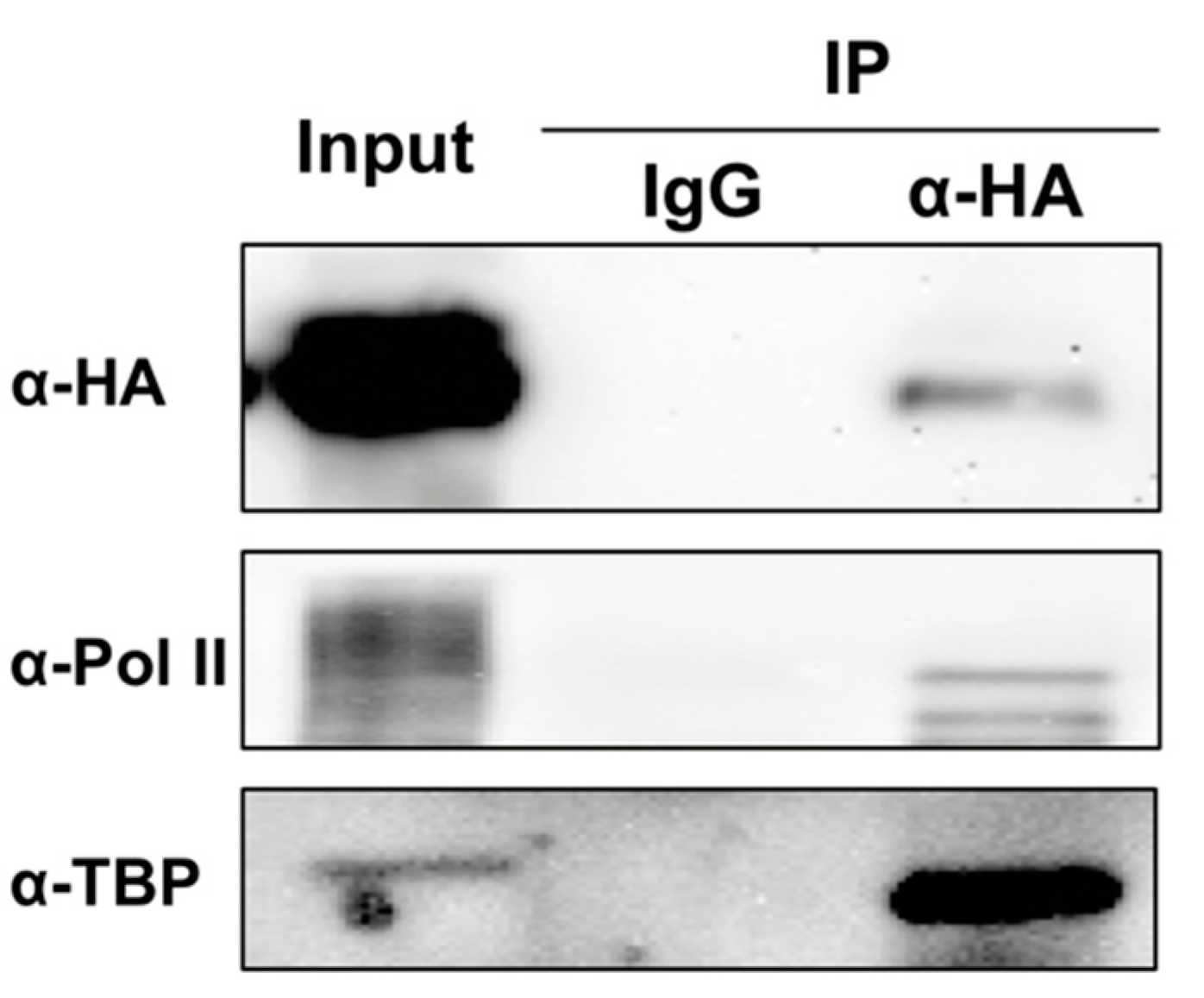
3. TFIIIA-7ZF Is Required for PSTVd-Templated Transcription by Pol II
4. Other Factors Involved in PSTVd-Templated Transcription Catalyzed by Pol II
5. RPL5 as a Regulator of TFIIIA Splicing and PSTVd Replication
6. Possible PSTVd RNA Conformations during Transcription
7. Transcription on (−)-Strand PSTVd
8. RNA-Templated Transcription of PSTVd and HDV: A Brief Comparison
9. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ding, B. The biology of viroid-host interactions. Annu. Rev. Phytopathol. 2009, 47, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Gago-Zachert, S.; Serra, P.; Sanjuan, R.; Elena, S.F. Viroids: Survivors from the RNA world? Annu. Rev. Microbiol. 2014, 68, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Li, S.F.; Matoušek, J.; Owens, R.A.; Pallás, V.; Randles, J.W.; Sano, T.; Verhoeven, J.T.J.; Vidalakis, G.; Flores, R. ICTV Report Consortium. ICTV Virus Taxonomy Profile: Avsunviroidae. J. Gen. Virol. 2018, 99, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Pelchat, M.; Grenier, C.; Perreault, J.P. Characterization of a viroid-derived RNA promoter for the DNA-dependent RNA polymerase from Escherichia coli. Biochemistry 2002, 41, 6561–6571. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.A.; Vera, A.; Flores, R. A chloroplastic RNA polymerase resistant to tagetitoxin is involved in replication of avocado sunblotch viroid. Virology 2000, 68, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Dezelee, S.; Sentenac, A.; Fromageot, P. Role of deoxyribonucleic acid-ribonucleic acid hybrids in eukaryotes. Synthetic ribo- and deoxyribopolynucleotides as template for yeast ribonucleic acid polymerase B. (or II). J. Biol. Chem. 1974, 249, 5978–5983. [Google Scholar] [PubMed]
- Rackwitz, H.R.; Rohde, W.; Sanger, H.L. DNA-dependent RNA polymerase II of plant origin transcribes viroid RNA into full-length copies. Nature 1981, 291, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Boege, F.; Rohde, W.; Sanger, H.L. In vitro transcription of viroid RNA into full-length copies by RNA-dependent RNA polymerase from healthy tomato leaf tissue. Biosci. Rep. 1982, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Fels, A.; Hu, K.; Riesner, D. Transcription of potato spindle tuber viroid by RNA polymerase II starts predominantly at two specific sites. Nucleic Acids Res. 2001, 29, 4589–4597. [Google Scholar] [CrossRef] [PubMed]
- Flores, R. Synthesis of RNAs Specific to Citrus Exocortis Viroid by a Fraction Rich in Nuclei from Infected Gynura aurantiaca: Examination of the Nature of the Products and Solubilization of the Polymerase-Template Complex. J. Gen. Virol. 1989, 70, 2695–2706. [Google Scholar] [CrossRef]
- Flores, R.; Semancik, J.S. Properties of a cell-free system for synthesis of citrus exocortis viroid. Proc. Natl. Acad. Sci. USA 1982, 79, 6285–6288. [Google Scholar] [CrossRef] [PubMed]
- Kolonko, N.; Bannach, O.; Aschermann, K.; Hu, K.H.; Moors, M.; Schmitz, M.; Steger, G.; Riesner, D. Transcription of potato spindle tuber viroid by RNA polymerase II starts in the left terminal loop. Virology 2006, 347, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Goodman, T.C.; Nagel, L.; Rappold, W.; Klotz, G.; Riesner, D. Viroid replication: Equilibrium association constant and comparative activity measurements for the viroid-polymerase interaction. Nucleic Acids Res. 1984, 12, 6231–6246. [Google Scholar] [CrossRef] [PubMed]
- Spiesmacher, E.; Muhlbach, H.P.; Tabler, M.; Sanger, H.L. Synthesis of (+) and (−) RNA molecules of potato spindle tuber viroid (PSTV) in isolated nuclei and its impairment by transcription inhibitors. Biosci. Rep. 1985, 5, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Muhlbach, H.P.; Sanger, H.L. Viroid replication is inhibited by alpha-amanitin. Nature 1979, 278, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Bustamante, R.F.; Semancik, J.S. Properties of a Viroid-replicating Complex Solubilized from Nuclei. J. Gen. Virol. 1989, 70, 2707–2716. [Google Scholar] [CrossRef]
- Semancik, J.S.; Harper, K.L. Optimal conditions for cell-free synthesis of citrus exocortis viroid and the question of specificity of RNA polymerase activity. Proc. Natl. Acad. Sci. USA 1984, 81, 4429–4433. [Google Scholar] [CrossRef] [PubMed]
- Schindler, I.M.; Mühlbach, H.P. Involvement of nuclear DNA-dependent RNA polymerases in potato spindle tuber viroid replication: A. reevaluation. Plant Sci. 1992, 84, 221–229. [Google Scholar] [CrossRef]
- Warrilow, D.; Symons, R.H. Citrus exocortis viroid RNA is associated with the largest subunit of RNA polymerase II in tomato in vivo. Arch. Virol. 1999, 144, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Takahashi, T. Inhibition of hop stunt viroid replication by α-amanitin. Z. Pflanzenkr. Pflanzenschutz. 1986, 93, 62–71. [Google Scholar]
- Wang, Y.; Qu, J.; Ji, S.; Wallace, A.J.; Wu, J.; Li, Y.; Gopalan, V.; Ding, B. A land plant-specific transcription factor directly enhances transcription of a pathogenic noncoding RNA template by DNA-dependent RNA polymerase II. Plant Cell 2016, 28, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.X.; Smith, E.R.; Shilatifard, A. Born to run: Control of transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 2018, 19, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Hantsche, M.; Cramer, P. Conserved RNA polymerase II initiation complex structure. Curr. Opin. Struct. Biol. 2017, 47, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; di Serio, F.; Hernández, C. Viroids: The noncoding genomes. Semin. Virol. 1997, 8, 65–73. [Google Scholar] [CrossRef]
- Layat, E.; Probst, A.V.; Tourmente, S. Structure, function and regulation of transcription factor IIIA: From xenopus to Arabidopsis. Biochim. Biophys. Acta 2013, 1829, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.; Barciszewska, M.Z.; Erdmann, V.A.; Barciszewski, J. 5S rRNA: Structure and interactions. Biochem. J. 2003, 371, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Hanas, J.S.; Gaskins, C.J.; Smith, J.F.; Ogilvie, M.K. Structure, function, evolution of transcription factor IIIA. Prog. Nucleic Acid Res. Mol. Biol. 1992, 43, 205–239. [Google Scholar] [PubMed]
- Mathieu, O.; Yukawa, Y.; Prieto, J.L.; Vaillant, I.; Sugiura, M.; Tourmente, S. Identification and characterization of transcription factor IIIA and ribosomal protein l5 from Arabidopsis thaliana. Nucleic Acids Res. 2003, 31, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Bannach, O.; Chen, H.; Teune, J.H.; Schmitz, A.; Steger, G.; Xiong, L.; Barbazuk, W.B. Alternative splicing of anciently exonized 5s rRNA regulates plant transcription factor TFIIIA. Genome Res. 2009, 19, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.C.; Wachter, A.; Breaker, R.R. A plant 5S ribosomal RNA mimic regulates alternative splicing of transcription factor IIIA pre-mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Layat, E.; Cotterell, S.; Vaillant, I.; Yukawa, Y.; Tutois, S.; Tourmente, S. Transcript levels, alternative splicing and proteolytic cleavage of TFIIIA control 5S rRNA accumulation during Arabidopsis thaliana development. Plant J. 2012, 71, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Garcia, S.; Voinnet, O. Nonsense-mediated decay serves as a general viral restriction mechanism in plants. Cell Host Microbe 2014, 16, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Si, X.; Ji, X.; Fan, R.; Liu, J.; Chen, K.; Wang, D.; Gao, C. Genome editing of upstream open reading frames enables translational control in plants. Nat. Biotechnol. 2018, 36, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, Y.; Makita, Y.; Kawashima, M.; Fujita, T.; Iwasaki, S.; Matsui, M. Transcripts from downstream alternative transcription start sites evade uorf-mediated inhibition of gene expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 7831–7836. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; T Wardell, S.J.; Kleffmann, T.; Brown, C.M. The exon-intron gene structure upstream of the initiation codon predicts translation efficiency. Nucleic Acids Res. 2018, 46, 4575–4591. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yuan, M.; Ai, C.; Liu, L.; Zhuang, E.; Karapetyan, S.; Wang, S.; Dong, X. Uorf-mediated translation allows engineered plant disease resistance without fitness costs. Nature 2017, 545, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Ribone, P.A.; Capella, M.; Arce, A.L.; Chan, R.L. A uorf represses the transcription factor athb1 in aerial tissues to avoid a deleterious phenotype. Plant Physiol. 2017, 175, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Smith, H.N.; Ren, D.; Dissanayaka, S.M.; Dawe, A.L.; Wang, L.; Wang, Y. Potato spindle tuber viroid modulates its replication through a direct interaction with a splicing regulator. J. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Eiras, M.; Nohales, M.A.; Kitajima, E.W.; Flores, R.; Daros, J.A. Ribosomal protein l5 and transcription factor IIIA from Arabidopsis thaliana bind in vitro specifically potato spindle tuber viroid RNA. Arch. Virol. 2011, 156, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Archual, A.J.; Amin, A.A.; Ding, B. A genomic map of viroid RNA motifs critical for replication and systemic trafficking. Plant Cell 2008, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Bojic, T.; Beeharry, Y.; Zhang, D.J.; Pelchat, M. Tomato RNA polymerase II interacts with the rod-like conformation of the left terminal domain of the potato spindle tuber viroid positive RNA genome. J. Gen. Virol. 2012, 93, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Maniataki, E.; Martinez de Alba, A.E.; Sagesser, R.; Tabler, M.; Tsagris, M. Viroid RNA systemic spread may depend on the interaction of a 71-nucleotide bulged hairpin with the host protein VirP1. RNA 2003, 9, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Gozmanova, M.; Denti, M.A.; Minkov, I.N.; Tsagris, M.; Tabler, M. Characterization of the RNA motif responsible for the specific interaction of potato spindle tuber viroid RNA (PSTVd) and the tomato protein VirP1. Nucleic Acids Res. 2003, 31, 5534–5543. [Google Scholar] [CrossRef] [PubMed]
- Kalantidis, K.; Denti, M.A.; Tzortzakaki, S.; Marinou, E.; Tabler, M.; Tsagris, M. VirP1 is a host protein with a major role in potato spindle tuber viroid infection in nicotiana plants. J. Virol. 2007, 81, 12872–12880. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Kalantidis, K.; Rao, A.L. A bromodomain-containing host protein mediates the nuclear importation of a satellite RNA of cucumber mosaic virus. J. Virol. 2014, 88, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, E.; Brueckner, F.; Cramer, P. Molecular basis of RNA-dependent RNA polymerase II activity. Nature 2007, 450, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Abrahem, A.; Pelchat, M. Formation of an RNA polymerase II preinitiation complex on an RNA promoter derived from the hepatitis delta virus RNA genome. Nucleic Acids Res. 2008, 36, 5201–5211. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Filipovska, J.; Yano, K.; Furuya, A.; Inukai, N.; Narita, T.; Wada, T.; Sugimoto, S.; Konarska, M.M.; Handa, H. Stimulation of RNA polymerase II elongation by hepatitis delta antigen. Science 2001, 293, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Nolte, R.T.; Conlin, R.M.; Harrison, S.C.; Brown, R.S. Differing roles for zinc fingers in DNA recognition: Structure of a six-finger transcription factor IIIA complex. Proc. Natl. Acad. Sci. USA 1998, 95, 2938–2943. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; McLachlan, A.D.; Klug, A. Repetitive zinc-binding domains in the protein transcription factor IIIA from xenopus oocytes. EMBO J. 1985, 4, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Churchill, M.E.; Tullius, T.D.; Klug, A. Mode of interaction of the zinc finger protein TFIIIA with a 5S RNA gene of xenopus. Proc. Natl. Acad. Sci. USA 1990, 87, 5528–5532. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.B.; Clemens, K.R.; Tennant, L.; Wright, P.E.; Gottesfeld, J.M. Specific interaction of the first three zinc fingers of TFIIIA with the internal control region of the xenopus 5S RNA gene. J. Mol. Biol. 1992, 223, 857–871. [Google Scholar] [CrossRef]
- Keese, P.; Symons, R.H. Domains in viroids: Evidence of intermolecular rna rearrangements and their contribution to viroid evolution. Proc. Natl. Acad. Sci. USA 1985, 82, 4582–4586. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrasco, A.; Flores, R. Dissecting the secondary structure of the circular RNA of a nuclear viroid in vivo: A “naked” rod-like conformation similar but not identical to that observed in vitro. RNA Biol. 2017, 14, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Gast, F.U.; Kempe, D.; Spieker, R.L.; Sanger, H.L. Secondary structure probing of potato spindle tuber viroid (PSTVd) and sequence comparison with other small pathogenic RNA replicons provides evidence for central non-canonical base-pairs, large a-rich loops, and a terminal branch. J. Mol. Biol. 1996, 262, 652–670. [Google Scholar] [CrossRef] [PubMed]
- Giguere, T.; Adkar-Purushothama, C.R.; Perreault, J.P. Comprehensive secondary structure elucidation of four genera of the family Pospiviroidae. PLoS ONE 2014, 9, e98655. [Google Scholar] [CrossRef] [PubMed]
- Riesner, D.; Henco, K.; Rokohl, U.; Klotz, G.; Kleinschmidt, A.K.; Domdey, H.; Jank, P.; Gross, H.J.; Sanger, H.L. Structure and structure formation of viroids. J. Mol. Biol. 1979, 133, 85–115. [Google Scholar] [CrossRef]
- Dingley, A.J.; Steger, G.; Esters, B.; Riesner, D.; Grzesiek, S. Structural characterization of the 69 nucleotide potato spindle tuber viroid left-terminal domain by NMR and thermodynamic analysis. J. Mol. Biol. 2003, 334, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, B. Viroids: Small probes for exploring the vast universe of RNA trafficking in plants. J. Integr. Plant Biol. 2010, 52, 28–39. [Google Scholar] [CrossRef] [PubMed]
- McBryant, S.J.; Veldhoen, N.; Gedulin, B.; Leresche, A.; Foster, M.P.; Wright, P.E.; Romaniuk, P.J.; Gottesfeld, J.M. Interaction of the RNA binding fingers of xenopus transcription factor IIIA with specific regions of 5S ribosomal RNA. J. Mol. Biol. 1995, 248, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Neely, L.S.; Lee, B.M.; Xu, J.; Wright, P.E.; Gottesfeld, J.M. Identification of a minimal domain of 5S ribosomal RNA sufficient for high affinity interactions with the RNA-specific zinc fingers of transcription factor IIIA. J. Mol. Biol. 1999, 291, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Scripture, J.B.; Huber, P.W. Analysis of the binding of xenopus ribosomal protein l5 to oocyte 5S rRNA. The major determinants of recognition are located in helix III-loop C. J. Biol. Chem. 1995, 270, 27358–27365. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Leontis, N.; Qian, S.; Itaya, A.; Qi, Y.; Boris-Lawrie, K.; Ding, B. Tertiary structural and functional analyses of a viroid RNA motif by isostericity matrix and mutagenesis reveal its essential role in replication. J. Virol. 2006, 80, 8566–8581. [Google Scholar] [CrossRef] [PubMed]
- Branch, A.D.; Benenfeld, B.J.; Robertson, H.D. Ultraviolet light-induced crosslinking reveals a unique region of local tertiary structure in potato spindle tuber viroid and hela 5S RNA. Proc. Natl. Acad. Sci. USA 1985, 82, 6590–6594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zirbel, C.L.; Leontis, N.B.; Ding, B. RNA 3-dimentional structural motifs as a critical constraint of viroid RNA evolution. PLoS Pathog. 2018, 14, e1006801. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Nie, X.; Chang, H.E.; Han, Z.; Taylor, J. Transcription of hepatitis delta virus RNA by RNA polymerase II. J. Virol. 2008, 82, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.L.; Chen, P.J.; Tu, S.J.; Wang, C.J.; Chen, D.S. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc. Natl. Acad. Sci. USA 1991, 88, 8490–8494. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.Y.; Chao, M.; Taylor, J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: Role of delta antigen. J. Virol. 1989, 63, 1945–1950. [Google Scholar] [PubMed]
- Polson, A.G.; Ley, H.L., 3rd; Bass, B.L.; Casey, J.L. Hepatitis delta virus RNA editing is highly specific for the amber/w site and is suppressed by hepatitis delta antigen. Mol. Cell Biol. 1998, 18, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.L. Hepatitis delta virus RNA editing. In Madame Curie Bioscience Database; Landes BioScience: Austin, TX, USA, 2013; pp. 52–65. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dissanayaka Mudiyanselage, S.D.; Qu, J.; Tian, N.; Jiang, J.; Wang, Y. Potato Spindle Tuber Viroid RNA-Templated Transcription: Factors and Regulation. Viruses 2018, 10, 503. https://doi.org/10.3390/v10090503
Dissanayaka Mudiyanselage SD, Qu J, Tian N, Jiang J, Wang Y. Potato Spindle Tuber Viroid RNA-Templated Transcription: Factors and Regulation. Viruses. 2018; 10(9):503. https://doi.org/10.3390/v10090503
Chicago/Turabian StyleDissanayaka Mudiyanselage, Shachinthaka D., Jie Qu, Nancy Tian, Jian Jiang, and Ying Wang. 2018. "Potato Spindle Tuber Viroid RNA-Templated Transcription: Factors and Regulation" Viruses 10, no. 9: 503. https://doi.org/10.3390/v10090503
APA StyleDissanayaka Mudiyanselage, S. D., Qu, J., Tian, N., Jiang, J., & Wang, Y. (2018). Potato Spindle Tuber Viroid RNA-Templated Transcription: Factors and Regulation. Viruses, 10(9), 503. https://doi.org/10.3390/v10090503






