Pospiviroid Infection of Tomato Regulates the Expression of Genes Involved in Flower and Fruit Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Infection
2.2. Total RNA and miRNA Isolation and Processing
2.3. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Sequencing and Quantitative RT-PCR (qRT-PCR) for Viroid Titer
2.4. Gene Expression Analysis
2.4.1. cDNA Synthesis
2.4.2. Quantitative PCR (qPCR)
2.4.3. Stem-Loop Quantitative RT-PCR
2.4.4. Analysis of qPCR Data
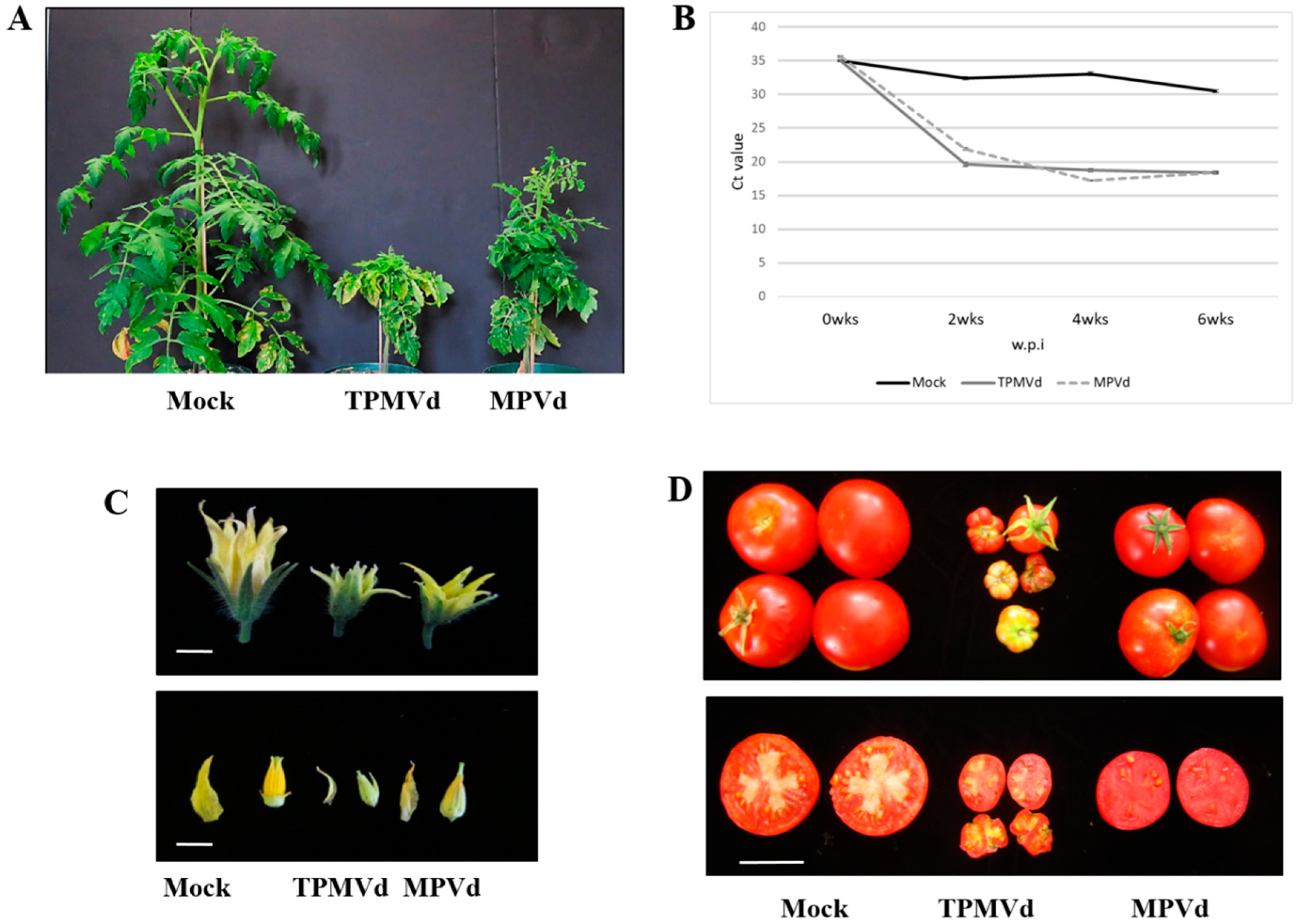
3. Results and Discussion
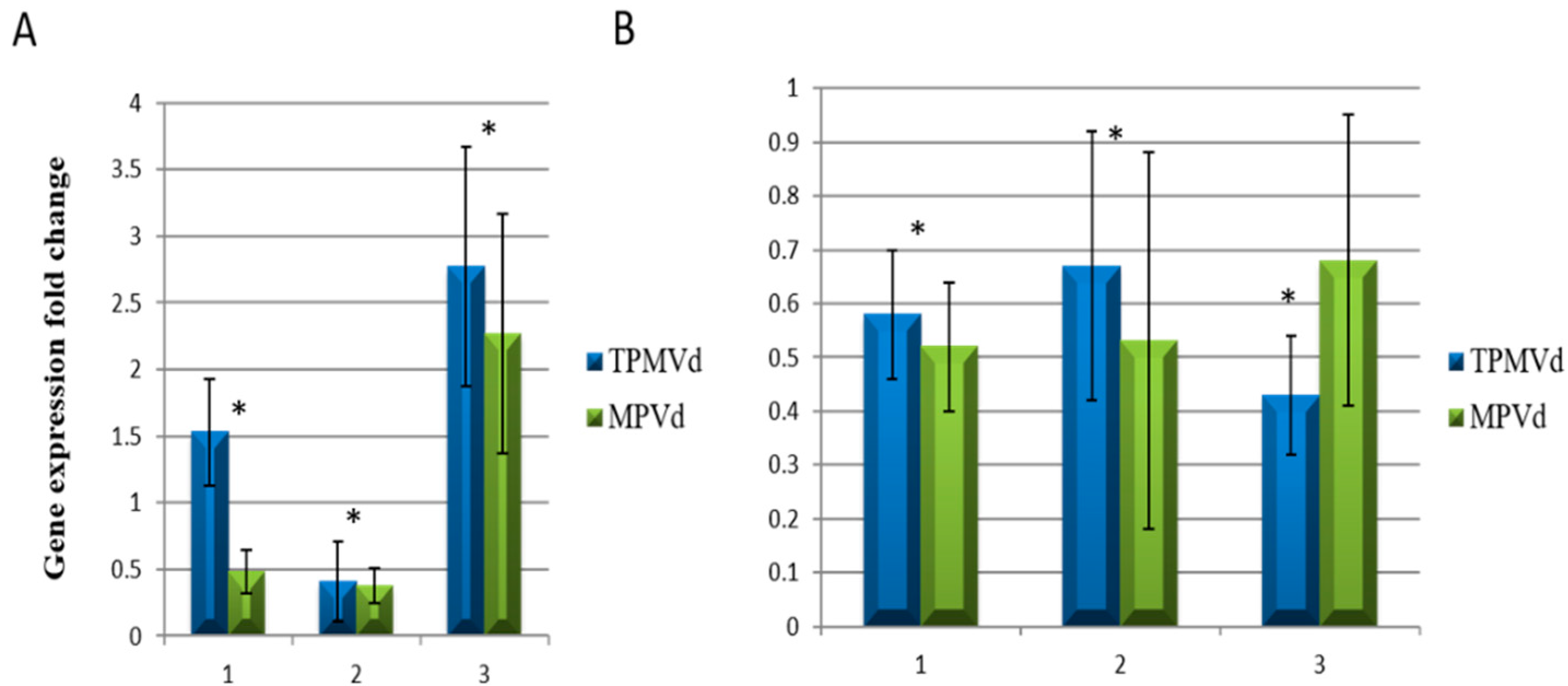
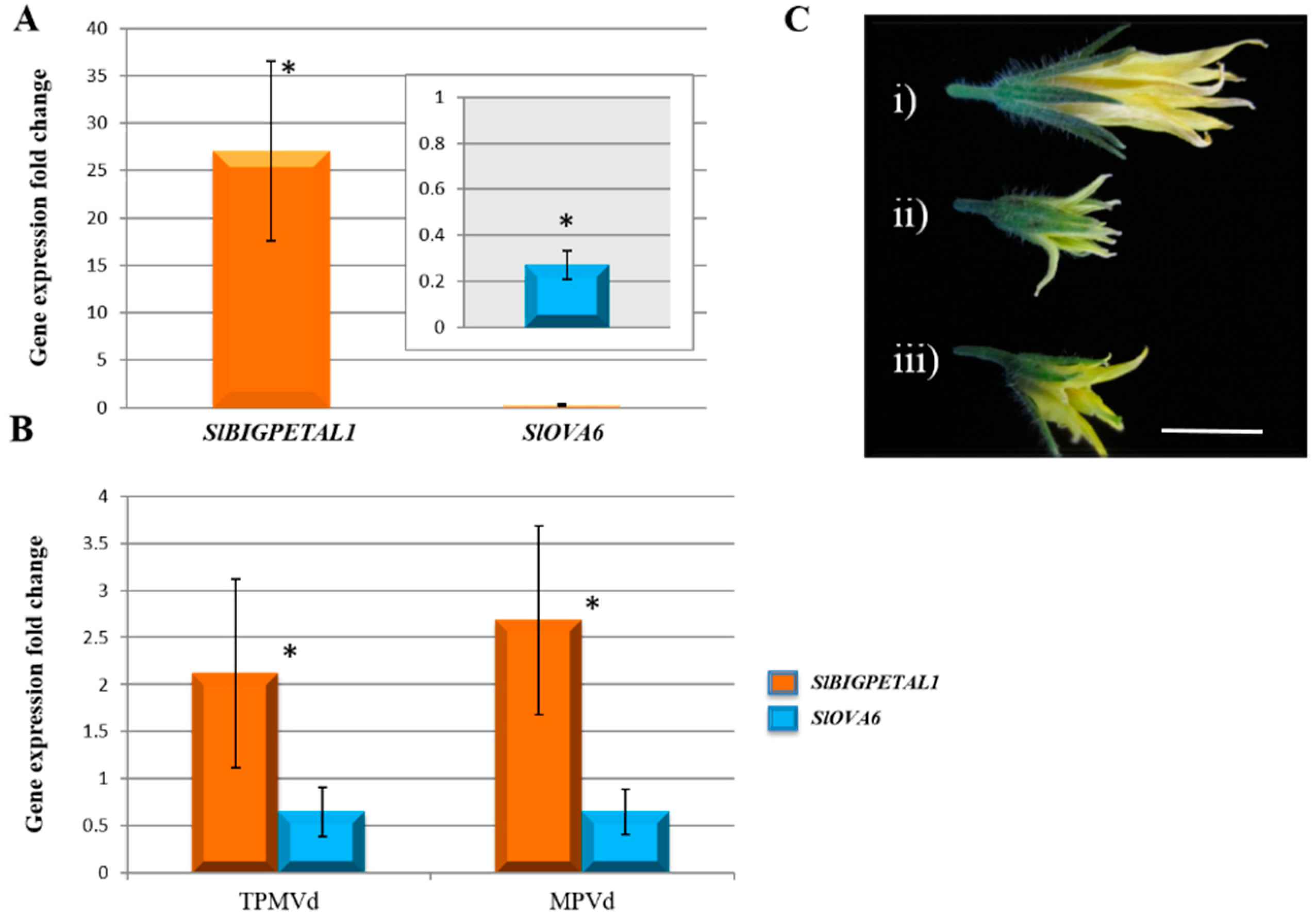
3.1. Gene Expression Changes Associated with Floral Phenotypes
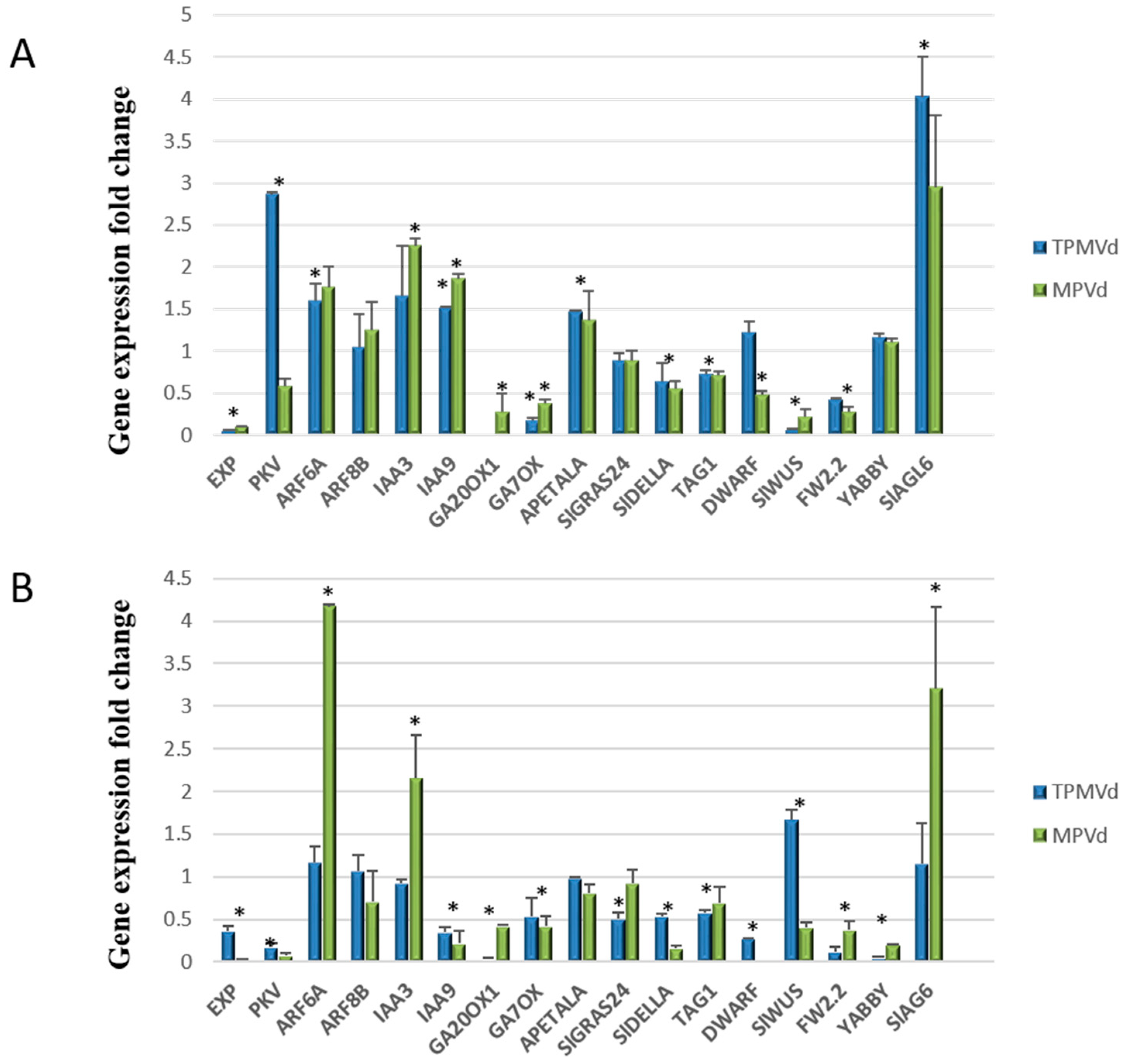
3.2. Relative Expression of Additional Tomato Genes That Regulate Plant Growth and Development
3.2.1. Gibberellin Biosynthesis and Signaling
3.2.2. Auxin Signaling Pathways
3.2.3. Coordinated Signaling among Hormone Pathways
3.2.4. Transcriptional Control of MADS-Box Transcription Factors and Floral/Fruit Development
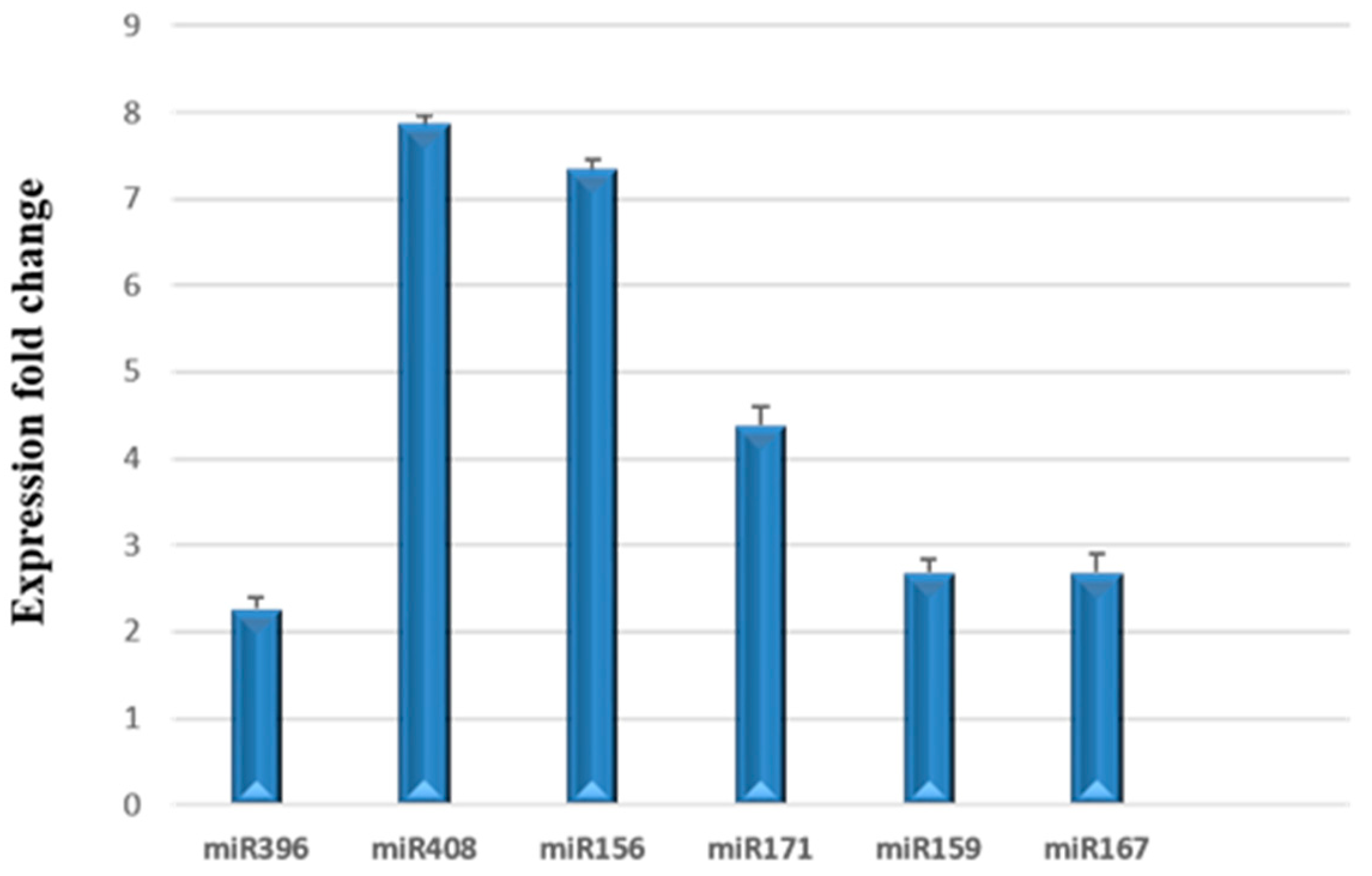
3.3. Relative Expression of miRNAs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diener, T.O. The Viroids; Plenum Press: New York, NY, USA, 1987; 344p, ISBN 0-306-42523-8. [Google Scholar]
- Hadidi, A.; Flores, R.; Randles, J.W.; Palukaitis, P. Viroids and Satellites, 1st ed.; Academic Press (Elsevier): San Diego, CA, USA, 2017; 753p, ISBN 978-0-12-801498-1. [Google Scholar]
- Avina-Padilla, K.; Martinez de la Vega, O.; Rivera-Bustamante, R.; Martinez-Soriano, J.P.; Owens, R.A.; Hammond, R.W.; Vielle-Calzada, J.-P. In silico prediction and validation of potential gene targets for pospiviroid-derived small RNAs during tomato infection. Gene 2015, 564, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, S.A.; Hamzehzarghani, H.; Izadpanah, K.; Djavaheri, M. Effects of potato spindle tuber viroid infection on tomato metabolic profile. J. Plant Physiol. 2016, 201, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Itaya, A. Viroid: A useful model for studying the basic principles of infection and RNA biology. Mol. Plant-Microbe Interact. 2007, 20, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Hernandez, C.; Martinez de Alba, A.E.; Daròs, J.A.; Di Serio, F. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 2005, 43, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Navarro, B.; Gisel, A.; Rodio, M.E.; Delgado, S.; Flores, R.; Di Serio, F. Small RNAs containing the pathogenic determinant of a chloroplast-replicating viroid guide the degradation of a host mRNA as predicted by RNA silencing. Plant J. 2012, 6, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Tabler, M.; Tsagris, M. Viroids: Petite RNA pathogens with distinguished talents. Trends Plant Sci. 2004, 9, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Y.; Ding, B.; Fei, Z. Comprehensive transcriptome analyses reveal that potato spindle tuber viroid triggers genome-wide changes in alternative splicing, inducible trans-acting activity of phasiRNAs and immune responses. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Bitters, W.P.; Duran-Vila, I.; Semancik, J.S. Effect of citrus exocortis viroid on flower and fruit structure and development in Etrog citron. Plant Dis. 1987, 71, 397–399. [Google Scholar] [CrossRef]
- Ding, B. The biology of viroid-host interactions. Annu. Rev. Phytopathol. 2009, 47, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Horst, R.K.; Langhans, R.W.; Smith, S.H. Effects of chrysanthemum stunt, chlorotic mottle, aspermy and mosaic on flowering and rooting of chrysanthemums. Phytopathology 1977, 67, 9–14. [Google Scholar] [CrossRef]
- Martin, R.; Arenas, C.; Daròs, J.A.; Covarrubias, A.; Reyes, J.L.; Chua, N.H. Characterization of small RNAs derived from citrus exocortis viroid (CEVd) in infected tomato plants. Virology 2007, 367, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.T.J.; Jansen, C.C.; Roenhorst, J.W.; Flores, R.; de la Peña, M. Pepper chat fruit viroid: Biological and molecular properties of a proposed new species of the genus Pospiviroid. Virus. Res. 2009, 144, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.W. Analysis of the virulence modulating region of potato spindle tuber viroid (PSTVd) by site-directed mutagenesis. Virology 1992, 187, 654–662. [Google Scholar] [CrossRef]
- Li, R.; Padmanabhan, C.; Ling, K.-S. A single base pair in the right terminal domain of tomato planta macho viroid is a virulence determinant factor on tomato. Virology 2017, 500, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Schnölzer, M.; Haas, B.; Ramm, K.; Hofmann, H.; Sänger, H.L. Correlation between structure and pathogenicity of potato spindle tuber viroid (PSTV). EMBO J. 1985, 4, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Galindo, A.J.; Smith, D.R.; Diener, T.O. Etiology of Plant Macho, a viroid disease of tomato. Phytopathology 1982, 72, 49–54. [Google Scholar] [CrossRef]
- Kiefer, M.C.; Owens, R.A.; Diener, T.O. Structural similarities between viroids and transposable genetic elements. Proc. Natl. Acad. Sci. USA 1988, 80, 6234–6238. [Google Scholar] [CrossRef]
- Martínez-Soriano, J.P.; Galindo-Alonso, J.; Maroon, C.J.M.; Yucel, I.; Smith, D.R.; Diener, T.O. Mexican papita viroid: Putative ancestor of crop viroids. Proc. Natl. Acad. Sci. USA 1996, 93, 9397–9401. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Flores, R.; Verhoeven, J.T.J.; Li, S.-F.; Pallas, V.; Randles, J.W.; Sano, T.; Vidalakis, G.; Owens, R.A. Current status of viroid taxonomy. Arch. Virol. 2014, 159, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.T.J.; Roenhorst, J.W.; Owens, R.A. Mexican papita viroid and tomato planta macho viroid belong to a single species in the genus Pospiviroid. Arch. Virol. 2011, 156, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Conejero, V.; Vera, P. Genes encoding acidic and basic class III β-1,3-glucanases are expressed in tomato plants upon viroid infection. Plant Mol. Biol. 1994, 24, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Gadea, J.; Mayda, M.E.; Conejero, V.; Vera, P. Characterization of defense-related genes ectopically expressed in viroid-infected tomato plants. Mol. Plant-Microbe Interact. 1996, 9, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Itaya, A.; Matsuda, Y.; Gonzales, R.A.; Nelson, R.S.; Ding, B. Potato spindle tuber viroid strains of different pathogenicity induces and suppresses expression of common and unique genes in infected tomato. Mol. Plant-Microbe Interact. 2002, 10, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.A.; Tech, K.B.; Shao, J.Y.; Sano, T.; Baker, C.J. Global analysis of tomato gene expression during potato spindle tuber viroid infection reveals a complex array of changes affecting hormone signaling. Mol. Plant-Microbe Interact. 2012, 25, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Tornero, P.; Conejero, V.; Vera, P. Primary structure and expression of a pathogen-induced protease (PR-P69) in tomato plants: Similarity of functional domains to subtilisin-like endoproteases. Proc. Natl. Acad. Sci. USA 1996, 93, 6332–6337. [Google Scholar] [CrossRef] [PubMed]
- Vera, P.; Hernandez-Yago, J.; Conejero, V. “Pathogenesis-related” P1 (p14) protein. Vacuolar and apoplastic localization in leaf tissue from tomato plants infected with citrus exocortis viroid: In vitro synthesis and processing. J. Gen. Virol. 1989, 70, 1933–1942. [Google Scholar] [CrossRef]
- Vera, P.; Tornero, P.; Conejero, V. Cloning and expression of a viroid-induced peroxidase from tomato plants. Mol. Plant-Microbe Interact. 1993, 6, 790–794. [Google Scholar] [PubMed]
- Więsyk, A.; Iwanicka-Nowicka, R.; Fogtman, A.; Zagórski-Ostoja, W.; Góra-Sochacka, A. Time-course microarray analysis reveals differences between transcriptional changes in tomato leaves triggered by mild and severe variants of potato spindle tuber viroid. Viruses 2018, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.L.; García-Martínez, J.L.; Flores, R. The relationship between plant growth substances content and infection of Gynura aurantiaca DC by citrus exocortis viroid. Physiol. Plant Pathol. 1978, 13, 355–363. [Google Scholar] [CrossRef]
- Vidal, A.M.; Ben-Cheikh, W.; Talón, M.; García-Martínez, J.L. Regulation of gibberellin 20-oxidase gene expression and gibberellin content in citrus by temperature and citrus exocortis viroid. Planta 2003, 217, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Diermann, N.; Matousek, J.; Junge, M.; Riesner, D.; Steger, G. Characterization of plant miRNAs and small RNAs derived from potato spindle tuber viroid (PSTVd) in infected tomato. Biol. Chem. 2010, 391, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.; Martinez, G.; Pallas, V. Interplay between viroid-induced pathogenesis and RNA silencing pathways. Trends Plant Sci. 2009, 14, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Itaya, A.; Zhong, X.; Bundschuh, R.; Qi, Y.; Wang, Y.; Takeda, R.; Harris, A.R.; Molina, C.; Nelson, R.C.; Ding, B. A structured viroid RNA serves as a substrate for dicer-like cleavage to produce biologically active small RNAs but is resistant to RNA-induced silencing complex-mediated degradation. J. Virol. 2007, 81, 2980–2994. [Google Scholar] [CrossRef] [PubMed]
- Adkar-Purushothama, C.R.; Iyer, P.S.; Perreault, J.-P. Potato spindle tuber viroid infection triggers degradation of chloride channel protein CLC-b-like and ribosomal protein S3a-like mRNAs in tomato plants. Sci. Rep. 2017, 7, 8341. [Google Scholar] [CrossRef] [PubMed]
- Machida, S.; Yamahata, N.; Watanuki, H.; Owens, R.A.; Sano, T. Successive accumulation of two size classes of viroid-specific small RNAs in potato spindle tuber viroid-infected plants. J. Gen. Virol. 2007, 88, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Kovalskaya, N.; Hammond, R.W. Molecular biology of viroid-host interactions and disease control strategies. Plant Sci. 2014, 228, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Papaefthimiou, I.; Hamilton, A.J.; Denti, M.A.; Baulcombe, D.C.; Tsagris, M.; Tabler, M. Replicating potato spindle tuber viroid RNA is accompanied by short RNA fragments that are characteristic of posttranscriptional gene silencing. Nucleic Acids Res. 2001, 29, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.B.; Bian, X.Y.; Wu, L.M.; Liu, L.X.; Smith, N.A.; Isenegger, D.; Wu, R.M.; Masuta, C.; Vance, V.B.; Watson, J.M.; et al. On the role of RNA silencing in the pathogenicity and evolution of viroids and viral satellites. Proc. Natl. Acad. Sci. USA 2004, 101, 3275–3280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shibuya, M.; Taneda, A.; Kurauchi, T.; Senda, M.; Owens, R.A.; Sano, T. Accumulation of Potato spindle tuber viroid-specific small RNAs is accompanied by specific changes in gene expression on two tomato cultivars. Virology 2011, 413, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Adkar-Purushothama, C.R.; Sano, T.; Perreault, J.P. Viroid derived small RNA induces early flowering in tomato plants by RNA silencing. Mol. Plant Pathol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.A.; Jakoby, M.; Werber, M.; Martin, C.; Weisshaar, B.; Bailey, P.C. The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 2003, 20, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ortiz, G.; Huq, E.; Quail, P.H. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 2003, 15, 1749–1770. [Google Scholar] [CrossRef]
- Szécsi, J.; Joly, C.; Bordji, K.; Varaud, E.; Cock, J.M.; Dumas, C.; Bendahmane, M. BIGPETALp, a bHLH transcription factor is involved in the control of Arabidopsis petal size. EMBO J. 2006, 25, 3912–3920. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Rogers, R.; Muralla, R.; Meinke, D. Requirement of aminoacyl-tRNA synthetases for gametogenesis and embryo development in Arabidopsis. Plant J. 2005, 44, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, P.; Masiero, S.; Eubel, H.; Braun, H.P.; Bhushan, S.; Glaser, E.; Salamini, F.; Leister, D. Nuclear photosynthetic gene expression is synergistically modulated by rates of protein synthesis in chloroplasts and mitochondria. Plant Cell 2006, 4, 970–991. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.T.J.; Jansen, C.; Willemen, T.M.; Kox, L.F.F.; Owens, R.A.; Roenhorst, J.W. Natural infections of tomato by Citrus exocortis viroid, Columnea latent viroid, Potato spindle tuber viroid and Tomato chlorotic dwarf viroid. Eur. J. Plant Pathol. 2004, 110, 823–831. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem–loop RT–PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, P.; Wrzeksińska, B.; Obrępalska-Stęplowska, A. Assessment of reference gene stability influenced by extremely divergent disease symptoms in Solanum lycopersicum L. J. Virol. Methods 2013, 194, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions. R Package version 1.14.4. 2013. Available online: http://www.R-project.org/ (accessed on 21 May 2018).
- Martínez de Alba, A.E.; Flores, R.; Hernández, C. Two chloroplastic viroid induce the accumulation of small RNAs associated with posttranscriptional gene silencing. J. Virol. 2002, 76, 13094–13096. [Google Scholar] [CrossRef] [PubMed]
- Da Grça, J.V.; Mason, T.E. Detection of avocado sunblotch viroid in flower buds by polyacrylamide gel electrophoresis. J. Phytopathol. 1983, 108, 262–266. [Google Scholar] [CrossRef]
- Matsushita, Y.; Usugi, T.; Tsuda, S. Distribution of tomato chlorotic dwarf viroid in floral organs of tomato. Eur. J. Plant Pathol. 2011, 130, 441–447. [Google Scholar] [CrossRef]
- Krizek, B.; Anderson, J.T. Control of flower size. J. Exp. Bot. 2013, 64, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.H.; Ellis, C.M.; Ploense, S.E.; Wu, M.-F.; Yadav, V.; Tholl, D.; Chetelat, A.; Haupt, I.; Kennerley, B.J.; Hodgens, C.; et al. A regulatory network for coordinated flower maturation. PLoS Genet. 2012, 8, e1002506. [Google Scholar] [CrossRef] [PubMed]
- Tanksley, S.D. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16, S181–S189. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.W.; Zhao, Y. Characterization of a tomato protein kinase gene induced by infection by Potato spindle tuber viroid. Mol. Plant-Microbe Interact. 2000, 13, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.W.; Zhao, Y. Modification of tobacco plant development by sense and antisense expression of the tomato viroid-induced AGC VIIIa protein kinase PKV suggests involvement in gibberellin signaling. BMC Plant Biol. 2009, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Hu, Y.; Zhao, Y.; Zhou, D.X. Regulatory networks involving YABBY genes in rice shoot development. Plant Signal. Behav. 2007, 2, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Barrero, L.S.; Tanksley, S.D. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 2008, 40, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Varaud, E.; Brioudes, F.; Szécsi, J.; Leroux, J.; Brown, S.; Perrot-Rechenmann, C.; Bendahmane, M. AUXIN RESPONSE FACTOR8 regulates Arabidopsis petal growth by interacting with the bHLH transcription factor BIGPETALp. Plant Cell 2011, 23, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Hooper, L.C.; Johnson, S.D.; Rodrigues, J.C.M.; Vivian-Smith, A.; Koltunow, A.M. Expression of aberrant forms of Auxin Response Factor8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol. 2007, 145, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wu, S.; Van Houten, J.; Wang, Y.; Ding, B.; Fei, Z.; Clarke, T.H.; Reed, J.W.; van der Knapp, E. Down-regulation of AUXIN RESPONSE FACTORS 6 and 8 by microRNA167 leads to floral development defects and female sterility in tomato. J. Exp. Bot. 2014, 65, 2507–2520. [Google Scholar] [CrossRef] [PubMed]
- Chaabouni, S.; Jones, B.; Delalande, C.; Wang, H.; Li, Z.; Mila, I.; Frasse, P.; Latché, A.; Pech, J.-C.; Bouzayen, M. Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signaling involved in differential growth. J. Exp. Bot. 2009, 60, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jones, B.; Li, A.; Frasse, P.; Delalande, C.; Regad, F.; Chaabouni, S.; Latché, A.; Pech, J.-C.; Bouzayan, M. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 2005, 17, 2676–2692. [Google Scholar] [CrossRef] [PubMed]
- Sandalio, L.M.; Rodríiguez-Serrano, M.; Romero-Puertas, M.C. Leaf epinasty and auxin: A biochemical and molecular overview. Plant Sci. 2016, 253, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, K.; Wu, Y.; Zhang, R.; Bonar, N.; Morris, J.; Hedley, P.E.; Bryan, G.J.; Kalantidis, K.; Hornyik, C. Insight on genes affecting tuber development in potato upon potato spindle tuber viroid (PSTVd) infection. PLoS ONE 2016, 11, e0150711. [Google Scholar] [CrossRef] [PubMed]
- Klahre, U.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Yokota, T.; Nomura, T.; Yoshida, S.; Chua, N.H. The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell 1998, 10, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xian, Z.; Kang, X.; Tang, N.; Li, Z. Genome-wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Peng, S.; Xian, Z.; Lin, D.; Hu, G.; Yang, L.; Ren, M.; Li, Z. Overexpression of a tomato miR171 target gene SlGRAS24 impacts multiple agronomical traits via regulating gibberellins and auxin homeostasis. Plant Biotechnol. J. 2017, 15, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Frary, A.; Nesbitt, T.C.; Grandillo, S.; Knapp, E.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; Tanksley, S.D. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ding, B. Inhibition of cell growth and shoot development by a specific nucleotide sequence in a noncoding viroid RNA. Plant Cell 2003, 15, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Saedler, H.; Becker, A.; Winter, K.U.; Kirchner, C.; Theissen, G. MADS-box genes are involved in floral development and evolution. Acta Biochim. Pol. 2001, 48, 351–358. [Google Scholar] [PubMed]
- Gimenez, E.; Castaneda, L.; Pineda, B.; Pan, I.L.; Moreno, V.; Angosto, T.; Lozano, R. TOMATO AGAMOUS1 and ARLEQUIN/TOMATO AGAMOUS-LIKE1 MADS-box genes have redundant and divergent functions required for tomato reproductive development. Plant Mol. Biol. 2016, 91, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Klap, C.; Yeshayahou, E.; Bolger, A.M.; Arazi, T.; Gupta, S.K.; Shabtai, S.; Usabel, B.; Salts, Y.; Barg, R. Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol. J. 2017, 15, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Burko, Y.; Shleizer-Burko, S.; Yanai, O.; Shwartz, I.; Zelnik, I.D.; Jacob-Hirsch, J.; Kela, I.; Eshed-Williams, L.; Ori, N. A role for APETALA1/Fruitfull transcription factors in tomato leaf development. Plant Cell 2013, 25, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Pabon-Mora, N.; Ambrose, B.A.; Litt, A. Poppy APETALA1/FRUITFUL orthologs control flowering time, branching, perianth identity, and fruit development. Plant Physiol. 2012, 158, 1685–1704. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Lenhard, M. Genetic control of plant organ growth. New Phytol. 2011, 191, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, C.; Zhang, C.; Shi, B.; Cao, X.; Zhang, T.Q.; Zhao, Z.; Wang, J.W.; Jiao, Y. Cytokinin signaling activates WUSCHEL expression during axillary meristem initiation. Plant Cell 2017, 29, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, M.; Jurgens, G.; Laux, T. The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 2002, 129, 3195–3206. [Google Scholar] [PubMed]
- Li, H.; Qi, M.; Sun, M.; Liu, Y.; Liu, Y.; Xu, T.; Li, Y.; Li, T. Tomato transcription factor SlWUS plays an important role in tomato flower and locule development. Front. Plant Sci. 2017, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Omidvar, V.; Mohorianu, I.; Dalmay, T.; Fellner, M. Identification of miRNAs with potential roles in regulation of anther development and male sterility in 7B-1 male-sterile mutant. BMC Genom. 2015, 16, 878. [Google Scholar] [CrossRef] [PubMed]
- Karlova, R.; van Haarst, J.C.; Maliepaard, C.; van de Geest, H.; Bovy, A.G.; Lammers, M.; Angenent, G.C.; de Maagd, R.A. Identification of microRNA targets in tomato fruit development using high-throughput sequencing and degradome analysis. J. Exp. Bot. 2013, 64, 1863–1878. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Li, C.; Han, X.; Shen, F. Identification of conserved microRNAs and their target genes in tomato (Lycopersicon esculentum). Gene 2008, 414, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Tian, Q.; Reed, J.W. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 2006, 113, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Serra, P.; Minoia, S.; Di Serio, F.; Navarro, B. Viroids from genotype to phenotype just relying on RNA sequence and structural motifs. Front. Microbiol. 2012, 3, 217. [Google Scholar] [CrossRef] [PubMed]
- Itaya, A.; Bundschuh, R.; Archual, A.J.; Joung, J.-G.; Fei, A.; Dai, X.; Zhao, P.X.; Tang, Y.; Nelson, R.S.; Ding, B. Small RNAs in tomato fruit and leaf development. Biochem. Biophys. Acta 2008, 1779, 99–107. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aviña-Padilla, K.; Rivera-Bustamante, R.; Kovalskaya, N.Y.; Hammond, R.W. Pospiviroid Infection of Tomato Regulates the Expression of Genes Involved in Flower and Fruit Development. Viruses 2018, 10, 516. https://doi.org/10.3390/v10100516
Aviña-Padilla K, Rivera-Bustamante R, Kovalskaya NY, Hammond RW. Pospiviroid Infection of Tomato Regulates the Expression of Genes Involved in Flower and Fruit Development. Viruses. 2018; 10(10):516. https://doi.org/10.3390/v10100516
Chicago/Turabian StyleAviña-Padilla, Katia, Rafael Rivera-Bustamante, Natalia Y. Kovalskaya, and Rosemarie W. Hammond. 2018. "Pospiviroid Infection of Tomato Regulates the Expression of Genes Involved in Flower and Fruit Development" Viruses 10, no. 10: 516. https://doi.org/10.3390/v10100516
APA StyleAviña-Padilla, K., Rivera-Bustamante, R., Kovalskaya, N. Y., & Hammond, R. W. (2018). Pospiviroid Infection of Tomato Regulates the Expression of Genes Involved in Flower and Fruit Development. Viruses, 10(10), 516. https://doi.org/10.3390/v10100516





