The 125th Lys and 145th Thr Amino Acids in the GTPase Domain of Goose Mx Confer Its Antiviral Activity against the Tembusu Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Animals
2.2. Immunological Characteristic Research In Vitro
2.3. The Effects of Viruses on goMx mRNA Levels In Vivo
2.4. Plasmid Construction
2.5. Intracellular Location
2.6. Subcellular Colocalization of goMx with the TMUV In Vitro
2.7. Antiviral Activity Assay of goMx
2.8. Real-Time Fluorescence Quantitative PCR (RT-qPCR)
2.9. Western Blot Analysis
2.10. Cell Viability Analysis
2.11. Statistics
3. Results
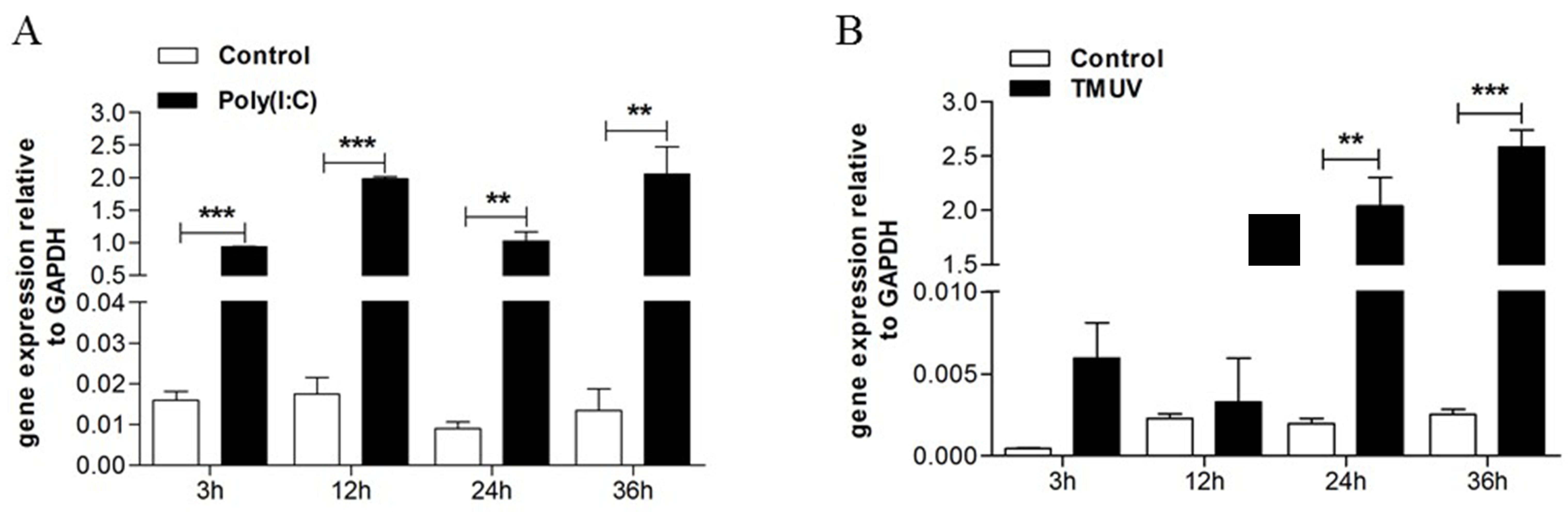
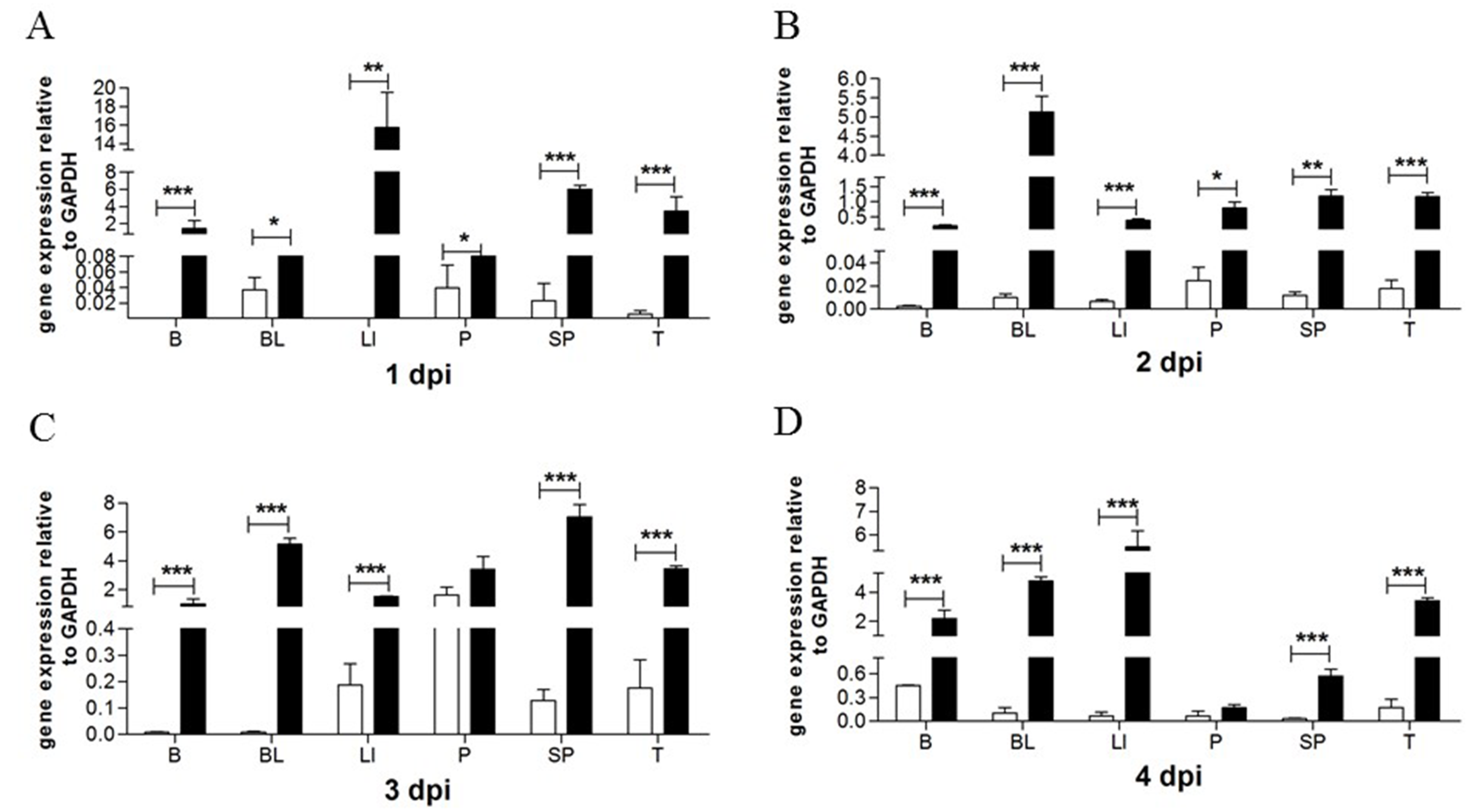
3.1. TMUV Can Stimulate the goMx Gene both In Vitro and In Vivo
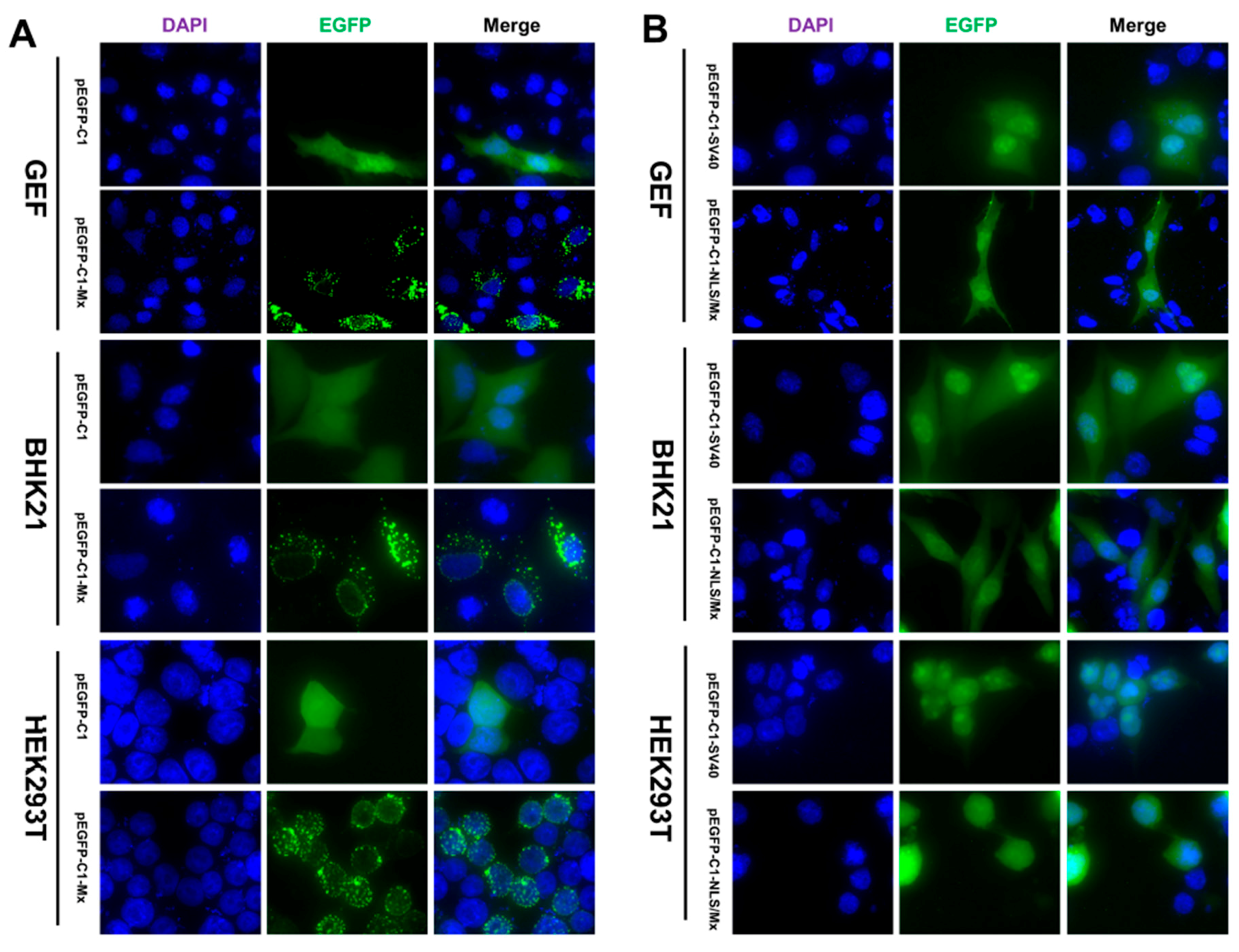
3.2. Intracellular Location of the EGFP Mx Fusion Protein in Different Cell Lines
3.3. Nuclear Localization of goMx Depends on Its Predicted Bipartite NLS
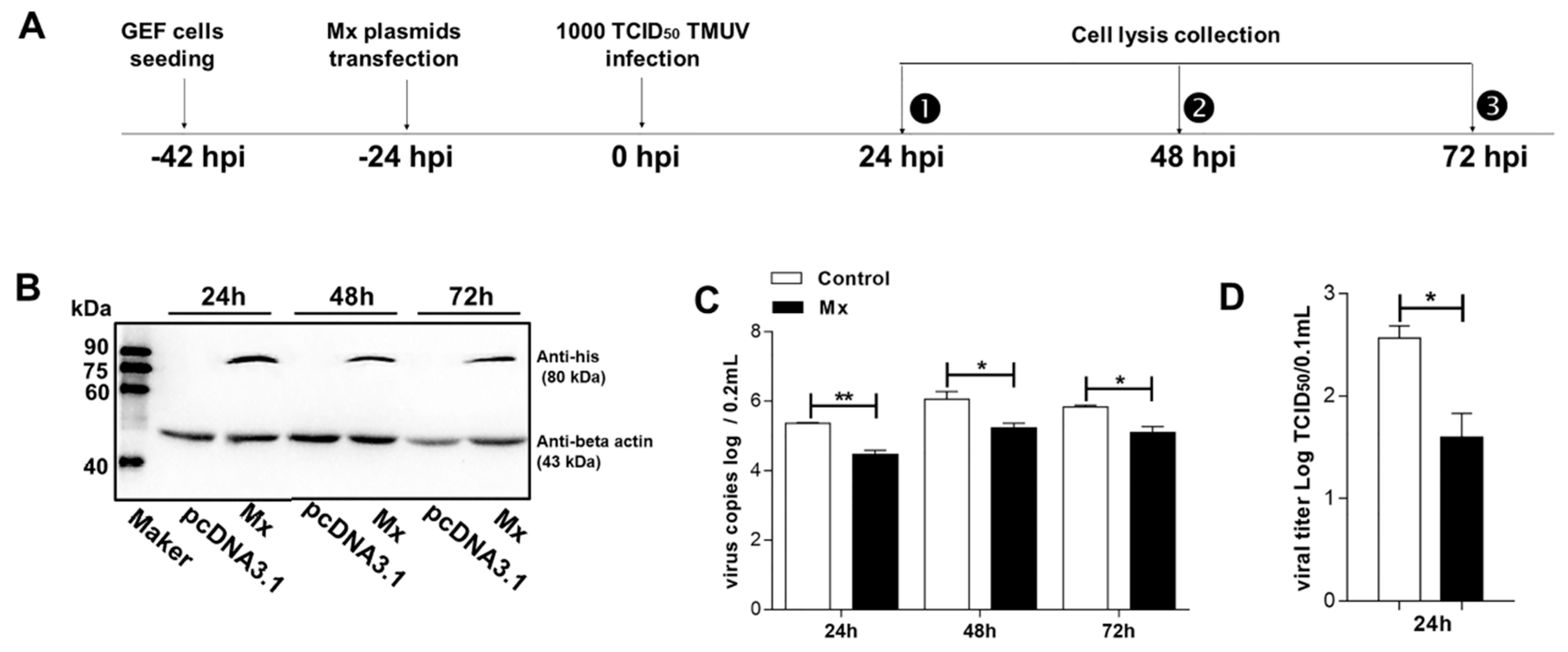
3.4. Antiviral Activity of the goMx
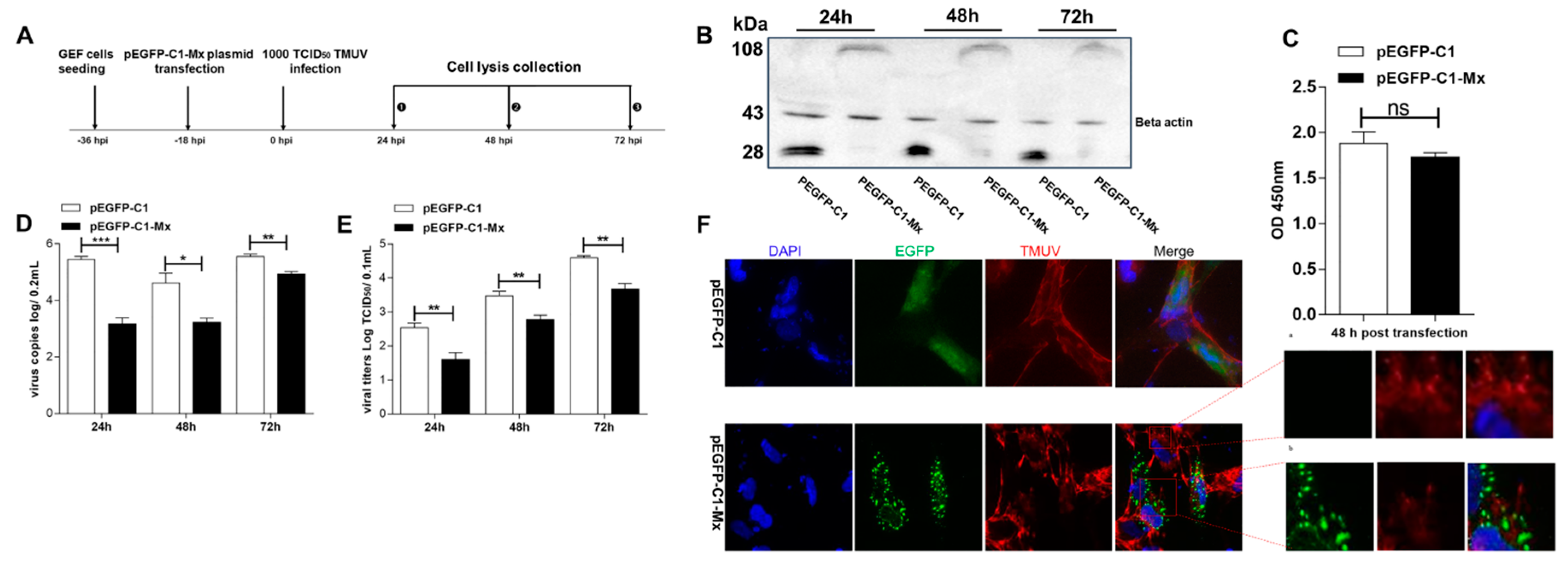
3.5. pEGFP-C1-Mx Fusion Protein Inhibited TMUV Replication
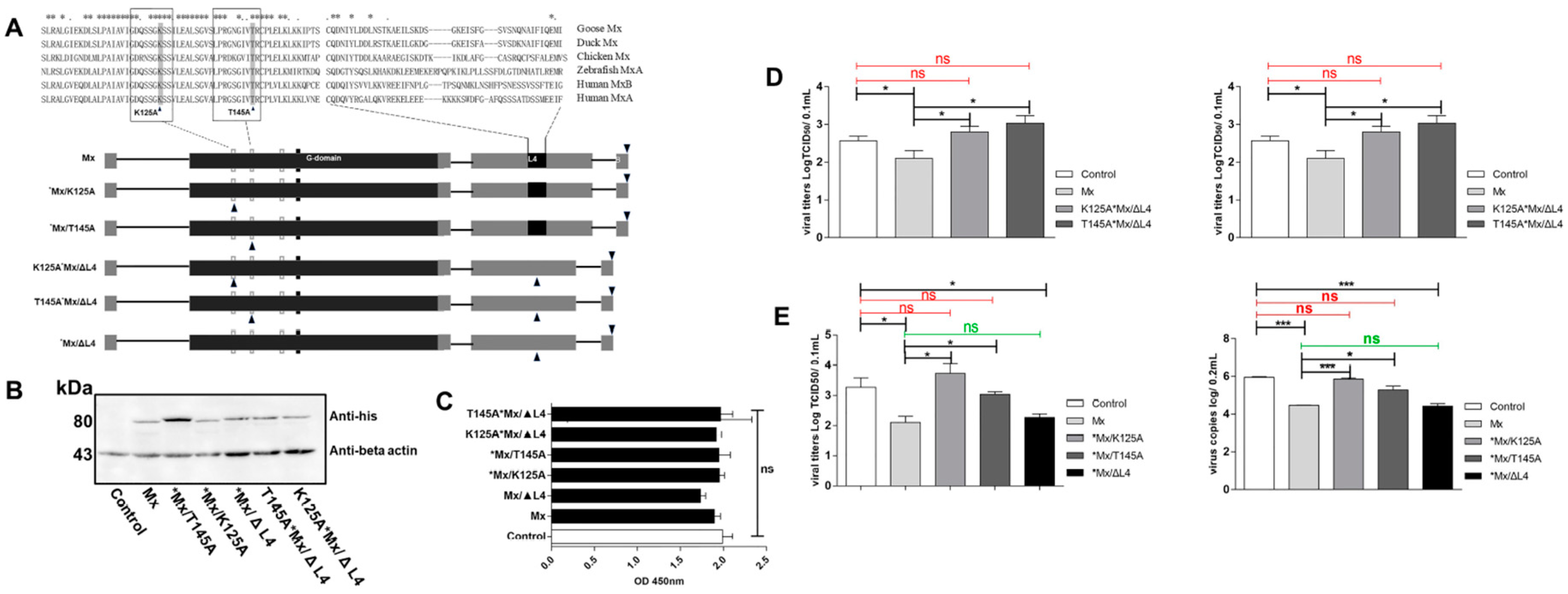
3.6. Antiviral Determinants of goMx
4. Discussion
Ethics Statement
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Holzer, M.; Schilling, M.; Patzina, C.; Schoen, A.; Hoenen, T.; Zimmer, G.; Marz, M.; Weber, F.; Muller, M.A.; et al. Evolution and antiviral specificities of Interferon-induced Mx proteins of bats against Ebola, Influenza, and Other RNA Viruses. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Schusser, B.; Reuter, A.; von der Malsburg, A.; Penski, N.; Weigend, S.; Kaspers, B.; Staeheli, P.; Hartle, S. Mx is dispensable for interferon-mediated resistance of chicken cells against influenza A virus. J. Virol. 2011, 85, 8307–8315. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Tungtrakoolsub, P.; Morozumi, T.; Uenishi, H.; Kawahara, M.; Watanabe, T. A single nucleotide polymorphism of porcine MX2 gene provides antiviral activity against vesicular stomatitis virus. Immunogenetics 2014, 66, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Jin, H.K.; Asano, A.; Takada, A.; Ninomiya, A.; Kida, H.; Hokiyama, H.; Ohara, M.; Tsuzuki, M.; Nishibori, M.; et al. Polymorphisms and the differential antiviral activity of the chicken Mx gene. Genome Res. 2002, 12, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Kochs, G. Human MxA protein: An interferon-induced dynamin-like GTPase with broad antiviral activity. J. Interferon Cytokine Res. 2011, 31, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Young, J.M.; Emerman, M.; Malik, H.S. Evolutionary analyses suggest a function of MxB immunity proteins beyond Lentivirus restriction. PLoS Pathog. 2015, 11, e1005304. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; von der Malsburg, A.; Dick, A.; Faelber, K.; Schroder, G.F.; Haller, O.; Kochs, G.; Daumke, O. Structure of myxovirus resistance protein a reveals intra- and intermolecular domain interactions required for the antiviral function. Immunity 2011, 35, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.L.; Mckelvie, S.A.; Bulloch, E.M.; Kingston, R.L. Transient dimerization of human MxA promotes GTP hydrolysis, resulting in a mechanical power stroke. Structure 2014, 22, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; von der Malsburg, A.; Paeschke, S.; Behlke, J.; Haller, O.; Kochs, G.; Daumke, O. Structural basis of oligomerization in the stalk region of dynamin-like MxA. Nature 2010, 465, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Gao, S.; von der Malsburg, A.; Daumke, O.; Kochs, G. Dynamin-like MxA GTPase: Structural insights into oligomerization and implications for antiviral activity. J. Biol. Chem. 2010, 285, 28419–28424. [Google Scholar] [CrossRef] [PubMed]
- Patzina, C.; Haller, O.; Kochs, G. Structural requirements for the antiviral activity of the human MxA protein against Thogoto and influenza a virus. J. Biol. Chem. 2014, 289, 6020–6027. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Kochs, G. Interferon-induced Mx proteins: Dynamin-like GTPases with antiviral activity. Traffic 2002, 3, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Nigg, P.E.; Pavlovic, J. Oligomerization and GTP-binding requirements of MxA for viral target recognition and antiviral activity against Influenza a Virus. J. Biol. Chem. 2015, 290, 29893–29906. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Graf, L.; Olal, D.; von der Malsburg, A.; Gao, S.; Kochs, G.; Daumke, O. Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity. J. Biol. Chem. 2015, 290, 12779–12792. [Google Scholar] [CrossRef] [PubMed]
- Stertz, S.; Reichelt, M.; Krijnse-Locker, J.; Mackenzie, J.; Simpson, J.C.; Haller, O.; Kochs, G. Interferon-induced, antiviral human MxA protein localizes to a distinct subcompartment of the smooth endoplasmic reticulum. J. Interferon Cytokine Res. 2006, 26, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Von der Malsburg, A.; Abutbul-Ionita, I.; Haller, O.; Kochs, G.; Danino, D. Stalk domain of the dynamin-like MxA GTPase protein mediates membrane binding and liposome tubulation via the unstructured L4 loop. J. Biol. Chem. 2011, 286, 37858–37865. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, A.; Gillespie, L.; Morgan, G.; van der Heide, P.; Khromykh, A. Mackenzie, The West Nile virus assembly process evades the conserved antiviral mechanism of the interferon-induced MxA protein. Virology 2014, 448, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Frese, M.; Kochs, G. Mx proteins: Mediators of innate resistance to RNA viruses. Rev. Sci. Tech. 1998, 17, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Zurcher, T.; Pavlovic, J.; Staeheli, P. Nuclear localization of mouse Mx1 protein is necessary for inhibition of influenza virus. J. Virol. 1992, 66, 5059–5066. [Google Scholar] [PubMed]
- Turan, K.; Mibayashi, M.; Sugiyama, K.; Saito, S.; Numajiri, A.; Nagata, K. Nuclear MxA proteins form a complex with influenza virus NP and inhibit the transcription of the engineered influenza virus genome. Nucleic Acids Res. 2004, 32, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, S.; Mahalingam, S.; Wang, M.; Cheng, A. An updated review of avian-origin Tembusu virus: A newly emerging avian Flavivirus. J. Gen. Virol. 2017, 98, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Zhang, L.; Wang, Y.; Yu, X.; Tian, K.; Su, W.; Han, B.; Su, J. Duck Tembusu virus exhibits neurovirulence in BALB/c mice. Virol. J. 2013, 10, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, G.; Yang, Z.; Lin, S.; Chen, S.; Wang, S.; Goraya, M.U.; Chi, X.; Zeng, X.; Chen, J.L. Avian Tembusu virus infection effectively triggers host innate immune response through MDA5 and TLR3-dependent signaling pathways. Vet. Res. 2016, 47, 74–90. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, A.; Chen, S.; Wu, Z.; Zhang, J.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Yang, Q.; et al. Differential immune-related gene expression in the spleens of duck Tembusu virus-infected goslings. Vet. Microbiol. 2017, 212, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, J.; Jiang, Y.; Zhao, Y.; Li, Q.; Wu, L.; He, X.; Chen, H. The vaccine efficacy of recombinant duck enteritis virus expressing secreted E with or without PrM proteins of duck tembusu virus. Vaccine 2014, 32, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gao, X.; Xiao, Y.; Liu, S.; Peng, S.; Li, X.; Shi, Y.; Zhang, Y.; Yu, L.; Wu, X.; et al. Development of a live attenuated vaccine candidate against duck Tembusu viral disease. Virology 2014, 450–451, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Liu, L.; Li, X.F.; Ye, Q.; Deng, Y.Q.; Qin, E.D.; Qin, C.F. In vitro and in vivo characterization of chimeric duck Tembusu virus based on Japanese encephalitis live vaccine strain SA14-14-2. J. Gen. Virol. 2016, 97, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.L.; Padilla, L.; Castano, J.C. Inhibitors compounds of the flavivirus replication process. Virol. J. 2017, 14, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.; Chen, J.; Zhang, L.; Wang, S.; Goraya, M.U.; Chi, X.; Na, Y.; Shao, W.; Yang, Z.; et al. Avian Interferon-Inducible Transmembrane Protein Family Effectively Restricts Avian Tembusu Virus Infection. Front. Microbiol. 2017, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Tag-El-Din-Hassan, H.T.; Morimatsu, M.; Agui, T. Functional analysis of duck, goose, and ostrich 2′-5′-oligoadenylate synthetase. Infect. Genet. Evol. 2018, 62, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Bazzigher, L.; Schwarz, A.; Staeheli, P. No enhanced influenza virus resistance of murine and avian cells expressing cloned duck Mx protein. Virology 1993, 195, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J.E.; Arango, J.; Ali, R.A.; Bohorquez, E.B.; Lund, A.R.; Ashwell, C.M.; Settar, P.; O’Sullivan, N.P.; Koci, M.D. Genetic variation within the Mx gene of commercially selected chicken lines reveals multiple haplotypes, recombination and a protein under selection pressure. PLoS ONE 2014, 9, e108054. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Chen, S.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Sun, K.; Yang, Q.; Wu, Y.; Chen, X.; et al. Molecular identification and comparative transcriptional analysis of myxovirus resistance GTPase (Mx) gene in goose (Anser cygnoide) after H9N2 AIV infection. Comp. Immunol. Microbiol. Infect. Dis. 2016, 47, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, W.; Wu, Z.; Zhang, J.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Sun, K.; Yang, Q.; et al. Goose Mx and OASL play vital roles in the antiviral effects of Type I, II, and III Interferon against newly emerging avian Flavivirus. Front. Immunol. 2017, 8, 1006–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, S.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Liu, F.; Yang, Q.; Wu, Y.; Sun, K.; et al. Antigen distribution of TMUV and GPV are coincident with the expression profiles of CD8 alpha-positive cells and goose IFN gamma. Sci. Rep. 2016, 6, 25545–25554. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, P.D.; Bosco-Lauth, A.M.; Langevin, S.A.; Anishchenko, M.; Bowen, R.A.; Reisen, W.K.; Brault, A.C. West Nile and St. Louis encephalitis viral genetic determinants of avian host competence. PLoS Negl. Trop. Dis. 2018, 12, e0006302. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sanlés, A.; Ríos-Marco, P.; Romero-López, C.; Berzal-Herranz, A. Functional Information Stored in the Conserved Structural RNA Domains of Flavivirus Genomes. Front. Microbiol. 2017, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- He, D.N.; Zhang, X.M.; Liu, K.; Pang, R.; Zhao, J.; Zhou, B.; Chen, P.Y. In vitro inhibition of the replication of classical swine fever virus by porcine Mx1 protein. Antivir. Res. 2014, 104, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jing, J.; Li, W.; Liu, K.; Shi, B.; Xu, Q.; Ma, Z.; Zhou, B.; Chen, P. Porcine Mx1 fused to HIV tat protein transduction domain (PTD) inhibits classical swine fever virus infection in vitro and in vivo. BMC Vet. Res. 2015, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, S.Q.; Wei, J.C.; Zhang, X.M.; Gao, Z.C.; Liu, K.; Ma, Z.Y.; Chen, P.Y.; Zhou, B. Mx is not responsible for the antiviral activity of interferon-alpha against Japanese Encephalitis Virus. Viruses 2017, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Tazi-Ahnini, R.; di Giovine, F.S.; Mcdonagh, A.J.; Messenger, A.G.; Amadou, C.; Cox, A.; Duff, G.W.; Cork, M. Structure and polymorphism of the human gene for the interferon-induced p78 protein (MX1): Evidence of association with alopecia areata in the down syndrome region. Hum. Genet. 2000, 106, 639–645. [Google Scholar] [PubMed]
- Duc, T.T.; Farnir, F.; Michaux, C.; Desmecht, D.; Cornet, A. Detection of new biallelic polymorphisms in the human MxA gene. Mol. Biol. Rep. 2012, 39, 8533–8538. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, E.; Morozumi, T.; Tsukamoto, K.; Watanabe, T.; Plastow, G.; Mitsuhashi, T. A naturally occurring variant of porcine Mx1 associated with increased susceptibility to influenza virus in vitro. Biochem. Genet. 2007, 45, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brahmakshatriya, V.; Lupiani, B.; Reddy, S.; Okimoto, R.; Li, X.; Chiang, H.; Zhou, H. Associations of chicken Mx1 polymorphism with antiviral responses in avian influenza virus infected embryos and broilers. Poult. Sci. 2012, 91, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yang, C.; Su, J. Protective roles of grass carp Ctenopharyngodon idella Mx isoforms against grass carp reovirus. PLoS ONE 2012, 7, e52142. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, O.G.; Ullrich, E.; Kochs, G.; Haller, O. Interferon-induced antiviral Mx1 GTPase is associated with components of the SUMO-1 system and promyelocytic leukemia protein nuclear bodies. Exp. Cell Res. 2001, 271, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Accola, M.A.; Huang, B.; Al Masri, A.; Mcniven, M.A. The antiviral dynamin family member, MxA, tubulates lipids and localizes to the smooth endoplasmic reticulum. J. Biol. Chem. 2002, 277, 21829–21835. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Asano, A.; Okano, S.; Ko, J.H.; Kon, Y.; Watanabe, T.; Agui, T. Intracellular localization and antiviral property of canine Mx proteins. J. Interferon Cytokine Res. 2005, 25, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Yoneda, A.; Ninomiya, A.; Kawahara, M.; Watanabe, T. Both antiviral activity and intracellular localization of chicken Mx protein depend on a polymorphism at amino acid position 631. Biochem. Biophys. Res. Commun. 2013, 430, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, D.; Schultz, U.; Staeheli, P. The interferon-induced Mx protein of chickens lacks antiviral activity. J. Interferon Cytokine Res. 1995, 15, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Steegmaier, M.; Oorschot, V.; Klumperman, J.; Scheller, R.H. Syntaxin 17 is abundant in steroidogenic cells and implicated in smooth endoplasmic reticulum membrane dynamics. Mol. Biol. Cell 2000, 11, 2719–2731. [Google Scholar] [CrossRef] [PubMed]
- Goujon, C.; Moncorge, O.; Bauby, H.; Doyle, T.; Barclay, W.S.; Malim, M.H. Transfer of the amino-terminal nuclear envelope targeting domain of human MX2 converts MX1 into an HIV-1 resistance factor. J. Virol. 2014, 88, 9017–9026. [Google Scholar] [CrossRef] [PubMed]
- Busnadiego, I.; Kane, M.; Rihn, S.J.; Preugschas, H.F.; Hughes, J.; Blanco-Melo, D.; Strouvelle, V.P.; Zang, T.M.; Willett, B.J.; Boutell, C.; et al. Host and viral determinants of Mx2 antiretroviral activity. J. Virol. 2014, 88, 7738–7752. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, J.; Parthoens, E.; Schepens, B.; Fiers, W.; Saelens, X. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J. Virol. 2012, 86, 13445–13455. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, M.; Stertz, S.; Krijnse-Locker, J.; Haller, O.; Kochs, G. Missorting of LaCrosse virus nucleocapsid protein by the interferon-induced MxA GTPase involves smooth ER membranes. Traffic 2004, 5, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Pitossi, F.; Blank, A.; Schroder, A.; Schwarz, A.; Hussi, P.; Schwemmle, M.; Pavlovic, J.; Staeheli, P. A functional GTP-binding motif is necessary for antiviral activity of Mx proteins. J. Virol. 1993, 67, 6726–6732. [Google Scholar] [PubMed]
- Ponten, A.; Sick, C.; Weeber, M.; Haller, O.; Kochs, G. Dominant-negative mutants of human MxA protein: Domains in the carboxy-terminal moiety are important for oligomerization and antiviral activity. J. Virol. 1997, 71, 2591–2599. [Google Scholar] [PubMed]
| Plasmid Name | Primer Name | Sequence |
|---|---|---|
| Mx | pCDNA3.1-Mx/his F | (CTAGCGTTTAAACTT)AAGCTTGCCACCATGTACCACAGAAGTCCC |
| pCDNA3.1-Mx/his R | (TGCTGGATATCTGCA)GAATTCTCAGTGGTGGTGGTGGTGGTGCAGACAGCTAAAGGTCTT | |
| pEGFP-C1-NLS/Mx | pEGFP-C1-NLS/Mx-F | (TCAGATCTCGAGCTC)AAGCTTCGAATGAAAACAGACTTTTTGC |
| pEGFP-C1-NLS/Mx -R | (GGATCCCGGGCCCGC)GGTACCCTATATGCTTTTTTGAACCTT | |
| pEGFP-C1-Mx | * pEGFP-C1-Mx-F | (TCAGATCTCGAGCTC) ggtggaggaggttctggaggcggtggaagtggtggcggaggtagcATGTACCACAGAAGTCCC |
| pEGFP-C1-Mx-R | (GGATCCCGGGCCCGC) GGTACCAGGTGTTTGTGTGACTATGG | |
| *Mx/K125A | *Mx/K125A-F1 | AGCTCTGGGGCAAGCTCCA |
| pCDNA3.1-Mx/his R | (TGCTGGATATCTGCA)GAATTCTCAGTGGTGGTGGTGGTGGTGCAGACAGCTAAAGGTCTT | |
| pCDNA3.1-Mx/his F | (CTAGCGTTTAAACTT)AAGCTTGCCACCATGTACCACAGAAGTCCC | |
| *Mx/K125A-R1 | TGGAGCTTGCCCCAGAGCT | |
| *Mx/T145A | *Mx/T145A-F1 | GTATCGTTGCACGATGTCC |
| pCDNA3.1-Mx/his R | (TGCTGGATATCTGCA)GAATTCTCAGTGGTGGTGGTGGTGGTGCAGACAGCTAAAGGTCTT | |
| pCDNA3.1-Mx/his F | (CTAGCGTTTAAACTT)AAGCTTGCCACCATGTACCACAGAAGTCCC | |
| *Mx/T145A-R1 | GGACATCGTGCAACGATAC | |
| *Mx/ΔL4 | *Mx/ΔL4-F | TCTCACACGAAGGCCTATT |
| pCDNA3.1-Mx/his R | (TGCTGGATATCTGCA)GAATTCTCAGTGGTGGTGGTGGTGGTGCAGACAGCTAAAGGTCTT | |
| pCDNA3.1-Mx/his F | (CTAGCGTTTAAACTT)AAGCTTGCCACCATGTACCACAGAAGTCCC | |
| *Mx/ΔL4-R | AATAGGCCTTCGTGTGAGAGTATACGATTCTCTCCATT | |
| T145A*Mx/ΔL4 | T145A*Mx/ΔL4-F | TCTCACACGAAGGCCTATT |
| pCDNA3.1-Mx/his R | (TGCTGGATATCTGCA)GAATTCTCAGTGGTGGTGGTGGTGGTGCAGACAGCTAAAGGTCTT | |
| pCDNA3.1-Mx/his F | (CTAGCGTTTAAACTT)AAGCTTGCCACCATGTACCACAGAAGTCCC | |
| T145A*Mx/ΔL4-R | AATAGGCCTTCGTGTGAGAGTATACGATTCTCTCCATT | |
| T145A*Mx/ΔL4-F1 | GTATCGTTGCACGATGTCC | |
| T145A*Mx/ΔL4-R1 | GGACATCGTGCAACGATAC | |
| K125A*Mx/ΔL4 | K125A*Mx/ΔL4-F | TCTCACACGAAGGCCTATT |
| pCDNA3.1-Mx/his R | (TGCTGGATATCTGCA)GAATTCTCAGTGGTGGTGGTGGTGGTGCAGACAGCTAAAGGTCTT | |
| pCDNA3.1-Mx/his F | (CTAGCGTTTAAACTT)AAGCTTGCCACCATGTACCACAGAAGTCCC | |
| K125A*Mx/ΔL4-R | AATAGGCCTTCGTGTGAGAGTATACGATTCTCTCCATT | |
| K125A*Mx/ΔL4-F1 | AGCTCTGGGGCAAGCTCCT | |
| K125A*Mx/ΔL4-R1 | AGGAGCTTGCCCCAGAGCT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zeng, M.; Liu, P.; Yang, C.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Yang, Q.; Wu, Y.; et al. The 125th Lys and 145th Thr Amino Acids in the GTPase Domain of Goose Mx Confer Its Antiviral Activity against the Tembusu Virus. Viruses 2018, 10, 361. https://doi.org/10.3390/v10070361
Chen S, Zeng M, Liu P, Yang C, Wang M, Jia R, Zhu D, Liu M, Yang Q, Wu Y, et al. The 125th Lys and 145th Thr Amino Acids in the GTPase Domain of Goose Mx Confer Its Antiviral Activity against the Tembusu Virus. Viruses. 2018; 10(7):361. https://doi.org/10.3390/v10070361
Chicago/Turabian StyleChen, Shun, Miao Zeng, Peng Liu, Chao Yang, Mingshu Wang, Renyong Jia, Dekang Zhu, Mafeng Liu, Qiao Yang, Ying Wu, and et al. 2018. "The 125th Lys and 145th Thr Amino Acids in the GTPase Domain of Goose Mx Confer Its Antiviral Activity against the Tembusu Virus" Viruses 10, no. 7: 361. https://doi.org/10.3390/v10070361
APA StyleChen, S., Zeng, M., Liu, P., Yang, C., Wang, M., Jia, R., Zhu, D., Liu, M., Yang, Q., Wu, Y., Zhao, X., & Cheng, A. (2018). The 125th Lys and 145th Thr Amino Acids in the GTPase Domain of Goose Mx Confer Its Antiviral Activity against the Tembusu Virus. Viruses, 10(7), 361. https://doi.org/10.3390/v10070361





