Zika Virus in the Male Reproductive Tract
Abstract
1. Emergence of Zika Virus
2. Sexual Transmission of ZIKV
3. Persistent Shedding of ZIKV in Semen
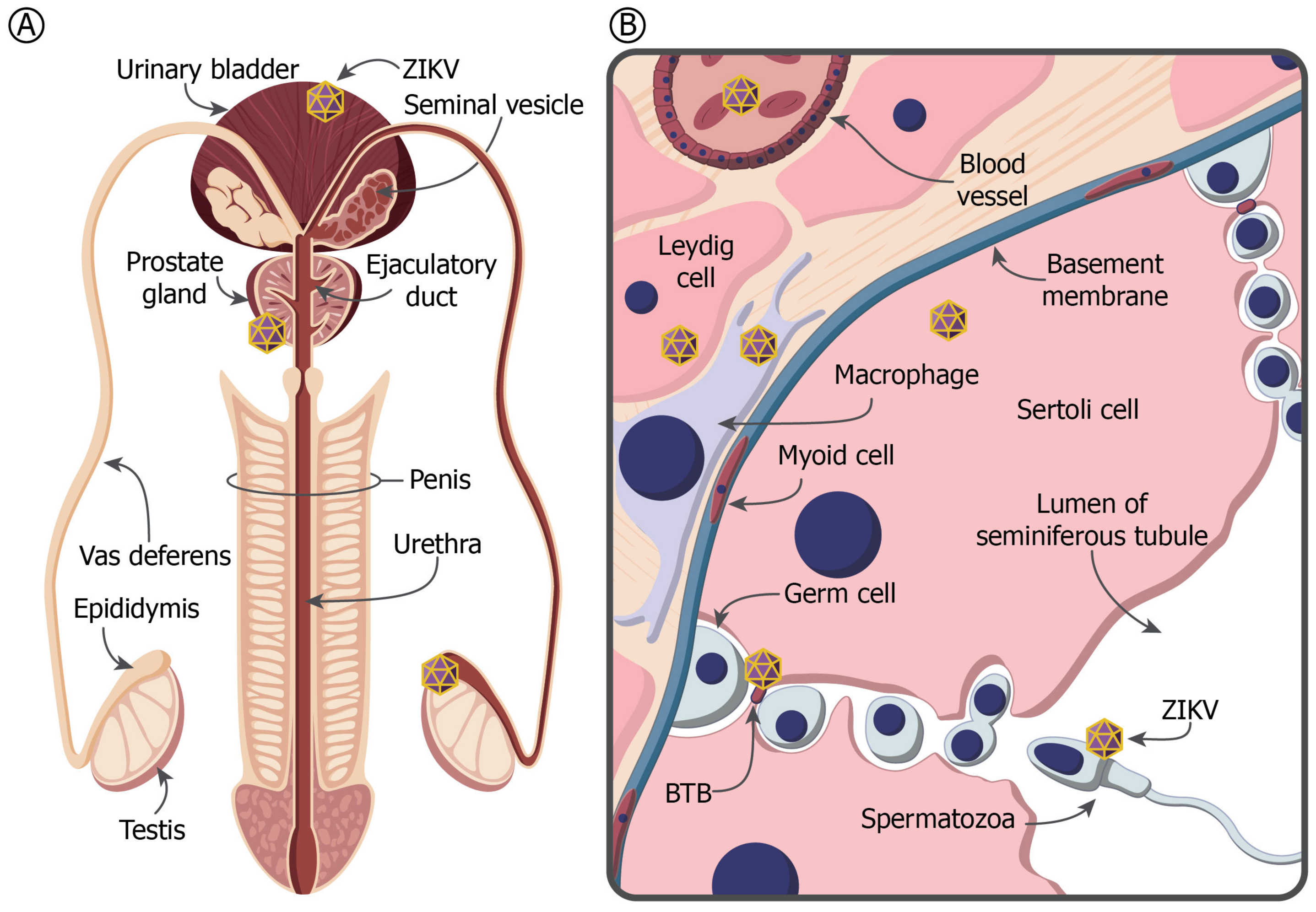
4. The MRT and Immune Privilege
5. ZIKV in the Testis and Prostate Gland
6. Mouse Models of ZIKV in the MRT
7. Primate Models of ZIKV Pathogenesis in the MRT
8. Implications for the Development of Therapeutics and Vaccines
9. Concluding Remarks and Future Prospects
- What are the cellular and molecular mechanisms of ZIKV persistence in the MRT?
- What is the origin of ZIKV in semen?
- What is the ZIKV entry receptor in the MRT?
- Which cells in the MRT are primarily infected following ZIKV attachment and entry?
- What are the viral and host characteristics that influence the infectivity and longevity of ZIKV in semen?
Acknowledgments
Conflicts of Interest
Glossary
| Arthralgia | Non-inflammatory joint pain. |
| Blood-testis-barrier | Physical barrier between blood vessels and the Sertoli cells of the seminiferous tubules in the mammalian testes. |
| Conjunctivitis | Inflammation of the outer layer of the eye and inside of the eyelid that causes the eye to turn pink. |
| Cryptorchidia | Condition in which one or both of the testes fail to descend from the abdomen into the scrotum. |
| Encephalitis | Inflammation of the brain. |
| Epididymis | Highly convoluted duct behind the testis, along which sperm passes to the vas deferens. |
| Guillain-Barré syndrome (GBS) | Autoimmune disease where antibodies and lymphocytes attack and damage the peripheral nerves causing weakness/paralysis and/or abnormal sensations and pain. |
| Hematospermia | Blood in the semen. |
| Hypospadias | A congenital condition in males in which the opening of the urethra is on the underside of the penis. |
| Ifnar1−/− × Ifngr−/− (AG129) mice | Interferon alpha, beta and gamma receptor deficient mice on a 129 background. |
| Ifnar1−/− mice | Interferon alpha and beta receptor deficient mice. |
| Immune-privileged site | Sites that are able to tolerate the introduction of antigens without eliciting an inflammatory immune response. Immune-privileged sites include the central nervous system, the brain, the eye, and regions of the male reproductive tract. |
| Irf3−/− × Irf7−/− mice | Interferon 3 and 7 double knockout mice. |
| Leydig cells | Testosterone-producing cells located in the connective tissue surrounding the seminiferous tubules in the testicle. |
| Male accessory glands | In humans, these are the seminal vesicles, prostate gland, and the bulbourethral glands. |
| Microcephaly | Medical condition in which the brain does not develop properly resulting in a smaller than normal head. |
| Microhematospermia | Hematospermia not evident by macroscopic examinations of the semen, but detected by tests for occult blood. |
| Micropenis | An unusually small penis. |
| Myalgia | Pain in a muscle or group of muscles. |
| Organoids | Three-dimensional cell cultures that incorporate some of the key features of the represented organ. |
| Prostatitis | Inflammation of the prostate gland. |
| Rag1−/− mice | Recombination activating gene 1 (Rag1) deficient mice. |
| Seminal glands | Accessory glands of the MRT, located between the bladder and the rectum that contribute approximately 60–70% of the ejaculate. |
| Seminiferous tubules | The site of the germination, maturation, and transportation of the sperm cells within the male testis. |
| Sertoli cells | Somatic cells of the testis that are part of a seminiferous tubule and facilitate the nourishment and progression of germ cells to spermatozoa. |
| Spermatocytes | Diploid cells formed through the process of spermatogenesis. |
| Spermatogenesis | The origin and development of the sperm cells within the male reproductive organs. |
| Spermatogonia | Undifferentiated male germ cell, formed in the wall of a seminiferous tubule and giving rise by mitosis to spermatocytes. |
| Spermatozoa | The mature motile male sex cell. |
| Testicular atrophy | Medical condition in which the testes diminish in size and may be accompanied by loss of function. |
| Testicular macrophages | Antigen-presenting cells, the most prevalent cell type in the testicular interstitium. They are in close morphological association and functional interaction with Leydig cells. |
| The male reproductive tract (MRT) | The male gonads, associated ducts and glands, and external genitalia that function during procreation. |
| Thrombocytopenia | Condition characterized by abnormally low levels of thrombocytes, also known as platelets, in the blood. This causes bleeding into the tissues, bruising, and slow blood clotting after injury. |
| Vas deferens | Tiny muscular tube in the MRT that carries sperm from the epididymis to the ejaculatory duct. |
References
- Mlakar, J.; Korva, M.; Tul, N.; Popovic, M.; Poljsak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Rus, K.R.; Vipotnik, T.V.; Vodusek, V.F.; et al. Zika virus associated with microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Thurmond, S.; Islas, L.; Hui, K.; Hai, R. Zika virus genome biology and molecular pathogenesis. Emerg. Microbes Infect. 2017, 6, e13. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.I.; Lee, Y.M. Zika virus: An emerging flavivirus. J. Microbiol. 2017, 55, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, M.R. Historical perspectives on Flavivirus research. Viruses 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Weinbren, M.P.; Williams, M.C. Zika virus: Further isolations in the Zika area, and some studies on the strains isolated. Trans. R. Soc. Trop. Med. Hyg. 1958, 52, 263–268. [Google Scholar] [CrossRef]
- Valderrama, A.; Díaz, Y.; López-Vergès, S. Interaction of Flavivirus with their mosquito vectors and their impact on the human health in the Americas. Biochem. Biophys. Res. Commun. 2017, 492, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika virus. 1. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Smithburn, K.C. Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. J. Immunol. 1952, 69, 223–234. [Google Scholar] [PubMed]
- Waggoner, J.J.; Pinsky, B.A. Zika virus: Diagnostics for an emerging pandemic threat. J. Clin. Microbiol. 2016, 54, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kindhauser, M.K.; Allen, T.; Frank, V.; Santhana, R.S.; Dye, C. Zika: The origin and spread of a mosquito-borne virus. Bull. World Health Organ. 2016, 94, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.W.; Choi, G.K.Y.; Yip, C.C.Y.; Cheng, V.C.C.; Yuen, K.Y. Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease. J. Infect. 2016, 72, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.; Teissier, A.; Huart, M.; Merceron, S.; Vanhomwegen, J.; Roche, C.; Vial, A.L.; Teururai, S.; Sicard, S.; Paulous, S.; et al. Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg. Infect. Dis. 2017, 23, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Ioos, S.; Mallet, H.P.; Goffart, I.L.; Gauthier, V.; Cardoso, T.; Herida, M. Current Zika virus epidemiology and recent epidemics. Med. Mal. Infect. 2014, 44, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef] [PubMed]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- Watrin, L.; Ghawche, F.; Larre, P.; Neau, J.P.; Mathis, S.; Fournier, E. Guillain-Barre syndrome (42 cases) occurring during a Zika virus outbreak in French Polynesia. Medicine 2016, 95, e3257. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, M.; Fischer, M.; Staples, J.E. Zika virus spreads to new areas—Region of the Americas, May 2015–January 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Azevedo, R.D.D.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Pereira, J.P.; Moreira, M.E.; Nogueira, R.M.R.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Pomar, L.; Malinger, G.; Benoist, G.; Carles, G.; Ville, Y.; Rousset, D.; Hcini, N.; Pomar, C.; Jolivet, A.; Lambert, V. Association between Zika virus and fetopathy: A prospective cohort study in French Guiana. Ultrasound Obstet. Gynecol. 2017, 49, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, J.; Rabeneck, D.B.; Martines, R.B.; Reagan-Steiner, S.; Ermias, Y.; Estetter, L.B.C.; Suzuki, T.; Ritter, J.; Keating, M.K.; Hale, G.; et al. Zika virus RNA replication and persistence in brain and placental tissue. Emerg. Infect. Dis. 2017, 23, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastere, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Willison, H.J.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barre syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef]
- Soares, C.N.; Brasil, P.; Carrera, R.M.; Sequeira, P.; De Filippis, A.B.; Borges, V.A.; Theophilo, F.; Ellul, M.A.; Solomon, T. Fatal encephalitis associated with Zika virus infection in an adult. J. Clin. Virol. 2016, 83, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Chammard, T.B.; Schepers, K.; Breurec, S.; Messiaen, T.; Destrem, A.L.; Mahevas, M.; Soulillou, A.; Janaud, L.; Curlier, E.; Hermann-Storck, C. Severe thrombocytopenia after Zika virus infection, Guadeloupe, 2016. Emerg. Infect. Dis. 2017, 23, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Vinhaes, E.S.; Santos, L.A.; Dias, L.; Andrade, N.A.; Bezerra, V.H.; de Carvalho, A.T.; de Moraes, L.; Henriques, D.F.; Azar, S.R.; Vasilakis, N.; et al. Transient hearing loss in adults associated with Zika virus infection. Clin. Infect. Dis. 2017, 64, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.D.; Kobylinski, K.C.; Foy, J.L.C.; Blitvich, B.J.; da Rosa, A.T.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef] [PubMed]
- D’Ortenzio, E.; Matheron, S.; Yazdanpanah, Y. Evidence of sexual transmission of Zika virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.M.; Duggal, N.K.; Brault, A.C. Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Negl. Trop. Dis. 2017, 11, e0005990. [Google Scholar] [CrossRef] [PubMed]
- Deckard, D.T.; Chung, W.M.; Brooks, J.T.; Smith, J.C.; Woldai, S.; Hennessey, M.; Kwit, N.; Mead, P. Male-to-male sexual transmission of Zika virus—Texas, January 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.; Slavinski, S.; Komoto, K.; Rakeman, J.; Weiss, D. Suspected female-to-male sexual transmission of Zika virus—New York City, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V. Potential sexual transmission of Zika virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Turmel, J.M.; Abgueguen, P.; Hubert, B.; Vandamme, Y.M.; Maquart, M.; Le Guillou-Guillemette; Leparc-Goffart, I. Late sexual transmission of Zika virus related to persistence in the semen. Lancet 2016, 387, 2501. [Google Scholar] [CrossRef]
- Coelho, F.C.; Durovni, B.; Saraceni, V.; Lemos, C.; Codeco, C.T.; Camargo, S.; de Carvalho, L.M.; Bastos, L.; Arduini, D.; Villela, D.A.M.; et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int. J. Infect. Dis. 2016, 51, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.B.; Carlos, M.P.; Myers, R.A.; White, M.G.; Bobo-Lenoci, T.; Aplan, D.; Blythe, D.; Feldman, K.A. Likely sexual transmission of Zika virus from a man with no symptoms of infection—Maryland, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Freour, T.; Mirallie, S.; Hubert, B.; Splingart, C.; Barriere, P.; Maquart, M.; Leparc-Goffart, I. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill. 2016, 21, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Maxian, O.; Neufeld, A.; Talis, E.J.; Childs, L.M.; Blackwood, J.C. Zika virus dynamics: When does sexual transmission matter? Epidemics 2017, 21, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.Z.; Lou, Y.J.; He, D.H.; Porco, T.C.; Kuang, Y.; Chowell, G.; Ruan, S.G. Prevention and control of Zika as a mosquito-borne and sexually transmitted disease: A mathematical modeling analysis. Sci. Rep. 2016, 6, 28070. [Google Scholar] [CrossRef] [PubMed]
- Allard, A.; Althouse, B.M.; Hebert-Dufresne, L.; Scarpino, S.V. The risk of sustained sexual transmission of Zika is underestimated. PLoS Pathog. 2017, 13, e1006633. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, O.; Beltrán, M.; Nelson, C.A.; Valencia, D.; Tolosa, N.; Farr, S.L.; Padilla, A.V.; Tong, V.T.; Cuevas, E.L.; Espinosa-Bode, A. Zika virus disease in Colombia—Preliminary report. N. Engl. J. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yakob, L.; Kucharski, A.; Hue, S.; Edmunds, W.J. Low risk of a sexually-transmitted Zika virus outbreak. Lancet Infect. Dis. 2016, 16, 1100–1102. [Google Scholar] [CrossRef]
- Haddow, A.D.; Nalca, A.; Rossi, F.D.; Miller, L.J.; Wiley, M.R.; Perez-Sautu, U.; Washington, S.C.; Norris, S.L.; Wollen-Roberts, S.E.; Shamblin, J.D.; et al. High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerg. Infect. Dis. 2017, 23, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, J.M.; Pasquier, C.; Daudin, M.; Chapuy-Regaud, S.; Moinard, N.; Chevreau, C.; Izopet, J.; Mengelle, C.; Bujan, L. Zika virus in semen of a patient returning from a non-epidemic area. Lancet Infect. Dis. 2016, 16, 894–895. [Google Scholar] [CrossRef]
- Matheron, S.; d’Ortenzio, E.; Leparc-Goffart, I.; Hubert, B.; de Lamballerie, X.; Yazdanpanah, Y. Long-lasting persistence of Zika virus in semen. Clin. Infect. Dis. 2016, 63, 1264. [Google Scholar] [CrossRef] [PubMed]
- Nicastri, E.; Castilletti, C.; Liuzzi, G.; Iannetta, M.; Capobianchi, M.R.; Ippolito, G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill. 2016, 21, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Joguet, G.; Mansuy, J.M.; Matusali, G.; Hamdi, S.; Walschaerts, M.; Pavili, L.; Guyomard, S.; Prisant, N.; Lamarre, P.; Dejucq-Rainsford, N.; et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: A prospective observational study. Lancet Infect. Dis. 2017, 17, 1200–1208. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Suberbielle, E.; Chapuy-Regaud, S.; Mengelle, C.; Bujan, L.; Marchou, B.; Delobel, P.; Gonzalez-Dunia, D.; Malnou, C.E.; Izopet, J.; et al. Zika virus in semen and spermatozoa. Lancet Infect. Dis. 2016, 16, 1106–1107. [Google Scholar] [CrossRef]
- Musso, D.; Richard, V.; Teissier, A.; Stone, M.; Lanteri, M.C.; Lantoni, G.; Alsina, J.; Reik, R.; Busch, M. Detection of ZIKV RNA in semen of asymptomatic blood donors. Clin. Microbiol. Infect. 2017, 23, 1001.e1–1001.e3. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bujalance, S.; Gutierrez-Arroyo, A.; De la Calle, F.; Diaz-Menendez, M.; Arribas, J.R.; Garcia-Rodriguez, J.; Arsuaga, M. Persistence and infectivity of Zika virus in semen after returning from endemic areas: Report of 5 cases. J. Clin. Virol. 2017, 96, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Uraki, R.; Jurado, K.A.; Hwang, J.; Szigeti-Buck, K.; Horvath, T.L.; Iwasaki, A.; Fikrig, E. Fetal growth restriction caused by sexual transmission of Zika virus in mice. J. Infect. Dis. 2017, 215, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Bagasra, O.; Addanki, K.C.; Goodwin, G.R.; Hughes, B.W.; Pandey, P.; McLean, E. Cellular targets and receptor of sexual transmission of Zika virus. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.; Thorburn, F.; Petridou, C.; Bailey, D.; Hewson, R.; Simpson, A.J.H.; Brooks, T.J.G.; Aarons, E.J. Presence and persistence of Zika virus RNA in semen, United Kingdom, 2016. Emerg. Infect. Dis. 2017, 23, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Visseaux, B.; Mortier, E.; Houhou-Fidouh, N.; Brichler, S.; Collin, G.; Larrouy, L.; Charpentier, C.; Descamps, D. Zika virus in the female genital tract. Lancet Infect. Dis. 2016, 16, 1220. [Google Scholar] [CrossRef]
- Dejucq-Rainsford, N.; Jegou, B. Viruses in semen and male genital tissues—Consequences for the reproductive system and therapeutic perspectives. Curr. Pharm. Des. 2004, 10, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Meinhardt, A. The testis in immune privilege. Immunol. Rev. 2006, 213, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P.; Hales, D.B. Immunophysiology of the male reproductive tract. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Neil, J.D., Ed.; Elsevier Academic Press: St. Louis, MO, USA, 2006; Volume 1, 2, pp. 1195–1286. ISBN 9780125154000. [Google Scholar]
- Mruk, D.D.; Cheng, C.Y. The mammalian blood-testis barrier: Its biology and regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P. Macrophages and the immune responsiveness of the testis. J. Reprod. Immunol. 2002, 57, 19–34. [Google Scholar] [CrossRef]
- Torres, J.R.; Martinez, N.; Moros, Z. Microhematospermia in acute Zika virus infection. Int. J. Infect. Dis. 2016, 51, 127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huits, R.M.H.G.; De Smet, B.; Ariën, K.K.; Van Esbroeck, M.; de Jong, B.C.; Bottieau, E.; Cnops, L. Kinetics of Zika virus persistence in semen. Bull. World Health Organ. 2016. [Google Scholar] [CrossRef]
- Siemann, D.N.; Strange, D.P.; Maharaj, P.N.; Shi, P.Y.; Verma, S. Zika virus infects human Sertoli cells and modulates the integrity of the in vitro blood-testis barrier model. J. Virol. 2017, 91, e00623-17. [Google Scholar] [CrossRef] [PubMed]
- De Laval, F.; Matheus, S.; Briolant, S. Kinetics of Zika viral load in semen. N. Engl. J. Med. 2017, 377, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika virus infection in human skin cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Retallack, H.; Di Lullo, E.; Arias, C.; Knopp, K.A.; Laurie, M.T.; Sandoval-Espinosa, C.; Leon, W.R.M.; Krencik, R.; Ullian, E.M.; Spatazza, J.; et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. USA 2016, 113, 14408–14413. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.S.; Shim, B.S.; Kwon, Y.C.; Zhang, R.; Otsuka, Y.; Schmitt, K.; Berri, F.; Diamond, M.S.; Choe, H. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jovel, J.; Lopez-Orozco, J.; Limonta, D.; Airo, A.M.; Hou, S.; Stryapunina, I.; Fibke, C.; Moore, R.B.; Hobman, T.C. Human Sertoli cells support high levels of Zika virus replication and persistence. Sci. Rep. 2018, 8, 5477. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Chen, Y.M.; Ge, Y.H.; Ma, P.P.; Ma, Q.H.; Ma, J.; Wang, H.K.; Xue, S.P.; Han, D.S. Immunoexpression of Tyro 3 family receptors—Tyro 3, Axl, and Mer—And their ligand Gas6 in postnatal developing mouse testis. J. Histochem. Cytochem. 2005, 53, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, M.; Takada, A.; Ebihara, H.; Neumann, G.; Fujioka, K.; Irimura, T.; Jones, S.; Feldmann, H.; Kawaoka, Y. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J. Virol. 2006, 80, 10109–10116. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.P.; Horby, P.W. The breadth of viruses in human semen. Emerg. Infect. Dis. 2017, 23, 1922–1924. [Google Scholar] [CrossRef] [PubMed]
- Arsuaga, M.; Bujalance, S.G.; Diaz-Menendez, M.; Vazquez, A.; Arribas, J.R. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect. Dis. 2016, 16, 1107. [Google Scholar] [CrossRef]
- Froeschl, G.; Huber, K.; von Sonnenburg, F.; Nothdurft, H.D.; Bretzel, G.; Hoelscher, M.; Zoeller, L.; Trottmann, M.; Pan-Montojo, F.; Dobler, G.; et al. Long-term kinetics of Zika virus RNA and antibodies in body fluids of a vasectomized traveller returning from Martinique: A case report. BMC Infect. Dis. 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.L.; Lahon, A.; Tran, L.L.; Arya, R.P.; Kneubehl, A.R.; Vogt, M.B.; Xavier, D.; Rowley, D.R.; Kimata, J.T.; Rico-Hesse, R.R. Replication of Zika virus in human prostate cells: A potential source of sexually transmitted virus. J. Infect. Dis. 2017, 217, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Revenig, L.; Leung, A.; Hsiao, W. Ejaculatory physiology and pathophysiology: Assessment and treatment in male infertility. Transl. Androl. Urol. 2014, 3, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.Q.; Li, S.H.; Ma, S.Q.; Jia, L.N.; Zhang, F.C.; Zhang, Y.; Zhang, J.Y.; Wong, G.; Zhang, S.S.; Lu, X.C.; et al. Zika virus causes testis damage and leads to male infertility in mice. Cell 2016, 167, 1511.e10–1524.e10. [Google Scholar] [CrossRef] [PubMed]
- Uraki, R.; Hwang, J.; Jurado, K.A.; Householder, S.; Yockey, L.J.; Hastings, A.K.; Homer, R.J.; Iwasaki, A.; Fikrig, E. Zika virus causes testicular atrophy. Sci. Adv. 2017, 3, e1602899. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.Y.; Gao, N.; Wang, Z.Y.; Cui, X.Y.; Zhou, D.S.; Fan, D.Y.; Chen, H.; Wang, P.G.; An, J. Sertoli cells are susceptible to ZIKV infection in mouse testis. Front. Cell. Infect. Microbiol. 2017, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Kawiecki, A.B.; Mayton, E.H.; Dutuze, M.F.; Goupil, B.A.; Langohr, I.M.; Del Piero, F.; Christofferson, R.C. Tissue tropisms, infection kinetics, histologic lesions, and antibody response of the MR766 strain of Zika virus in a murine model. Virol. J. 2017, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Muruato, A.E.; Jagger, B.W.; Richner, J.; Nunes, B.T.D.; Medeiros, D.B.A.; Xie, X.P.; Nunes, J.G.C.; Morabito, K.M.; Kong, W.P.; et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat. Commun. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.R.; Golden, J.W.; Griffin, B.D.; Warner, B.M.; Ranadheera, C.; Scharikow, L.; Sloan, A.; Frost, K.L.; Kobasa, D.; Booth, S.A. Human polyclonal antibodies produced in transchromosomal cattle prevent lethal Zika virus infection and testicular atrophy in mice. Antivir. Res. 2017, 146, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.W.; Myers, L.M.; Woods, T.A.; Messer, R.J.; Carmody, A.B.; McNally, K.L.; Scott, D.P.; Hasenkrug, K.J.; Best, S.M.; Peterson, K.E. Adaptive immune responses to Zika virus are important for controlling virus infection and preventing infection in brain and testes. J. Immunol. 2017, 198, 3526–3535. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.K.; Ritter, J.M.; Pestorius, S.E.; Zaki, S.R.; Davis, B.S.; Chang, G.J.J.; Bowen, R.A.; Brault, A.C. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep. 2017, 18, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A mouse model of Zika virus pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Hunter, L.; Atkinson, B.; Pearson, G.; Dennis, M.; Hewson, R. Lineage-dependent differences in the disease progression of Zika virus infection in type-I interferon receptor knockout (A129) mice. PLoS Negl. Trop. Dis. 2017, 11, e0005704. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.D.; Muthumani, K.; Warner, B.M.; Majer, A.; Hagan, M.; Audet, J.; Stein, D.R.; Ranadheera, C.; Racine, T.; De La Vega, M.A.; et al. DNA vaccination protects mice against Zika virus-induced damage to the testes. Nat. Commun. 2017, 8, 15743. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.W.; Zhang, A.J.; Chan, C.C.S.; Yip, C.C.Y.; Mak, W.W.N.; Zhu, H.S.; Poon, V.K.M.; Tee, K.M.; Zhu, Z.; Cai, J.P.; et al. Zika virus infection in dexamethasone-immunosuppressed mice demonstrating disseminated infection with multi-organ involvement including orchitis effectively treated by recombinant type I interferons. EBioMedicine 2016, 14, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.S.; Van Wettere, A.J.; Siddharthan, V.; Morrey, J.D.; Julander, J.G. Comparative histopathologic lesions of the male reproductive tract during acute Infection of Zika virus in AG129 and IFNAR-/- mice. Am. J. Pathol. 2018, 188, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Liu, L.; Nakayama, E.; Cooper, T.H.; Yan, K.X.; Eldi, P.; Hazlewood, J.E.; Tang, B.; Le, T.T.; Setoh, Y.X.; et al. A vaccinia-based single vector construct multi-pathogen vaccine protects against both Zika and chikungunya viruses. Nat. Commun. 2018, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.K.; McDonald, E.M.; Ritter, J.M.; Brault, A.C. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci. Rep. 2018, 8, 4510. [Google Scholar] [CrossRef] [PubMed]
- Siddharthan, V.; Van Wettere, A.J.; Li, R.; Miao, J.X.; Wang, Z.D.; Morrey, J.D.; Julander, J.G. Zika virus infection of adult and fetal STAT2 knock-out hamsters. Virology 2017, 507, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Dudley, D.M.; Aliota, M.T.; Mohr, E.L.; Weiler, A.M.; Lehrer-Brey, G.; Weisgrau, K.L.; Mohns, M.S.; Breitbach, M.E.; Rasheed, M.N.; Newman, C.M.; et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun. 2016, 7, 12204. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Dong, H.L.; Huang, X.Y.; Qiu, Y.F.; Wang, H.J.; Deng, Y.Q.; Zhang, N.N.; Ye, Q.; Zhao, H.; Liu, Z.Y.; et al. Characterization of a 2016 clinical isolate of Zika virus in non-human primates. EBioMedicine 2016, 12, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Osuna, C.E.; Lim, S.Y.; Deleage, C.; Griffin, B.D.; Stein, D.; Schroeder, L.T.; Omange, R.W.; Best, K.; Luo, M.; Hraber, P.T.; et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 2016, 22, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.M.; Antony, K.M.; Dudley, D.M.; Kohn, S.; Simmons, H.A.; Wolfe, B.; Salamat, M.S.; Teixeira, L.B.C.; Wiepz, G.J.; Thoong, T.H.; et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017, 13, e1006378. [Google Scholar] [CrossRef] [PubMed]
- Coffey, L.L.; Pesavento, P.A.; Keesler, R.I.; Singapuri, A.; Watanabe, J.; Watanabe, R.; Yee, J.; Bliss-Moreau, E.; Cruzen, C.; Christe, K.L.; et al. Zika virus tissue and blood compartmentalization in acute infection of rhesus macaques. PLoS ONE 2017, 12, e0171148. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.J.; Smith, J.L.; Haese, N.N.; Broeckel, R.M.; Parkins, C.J.; Kreklywich, C.; DeFilippis, V.R.; Denton, M.; Smith, P.P.; Messer, W.B.; et al. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017, 13, e1006219. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Larocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.F.; Ng’ang’a, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Koide, F.; Goebel, S.; Snyder, B.; Walters, K.B.; Gast, A.; Hagelin, K.; Kalkeri, R.; Rayner, J. Development of a Zika virus infection model in cynomolgus macaques. Front. Microbiol. 2016, 7, 2028. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, C.P.; Armitage, C.W.; Kollipara, A.; Andrew, D.W.; Trim, L.; Plenderleith, M.B.; Beagley, K.W. Induction of partial immunity in both males and females is sufficient to protect females against sexual transmission of Chlamydia. Mucosal Immunol. 2016, 9, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Dudley, D.M.; Newman, C.M.; Mohr, E.L.; Gellerup, D.D.; Breitbach, M.E.; Buechler, C.R.; Rasheed, M.N.; Mohns, M.S.; Weiler, A.M.; et al. Heterologous protection against Asian Zika virus challenge in rhesus macaques. PLoS. Negl. Trop. Dis. 2016, 10, e0005168. [Google Scholar] [CrossRef] [PubMed]
- Wahid, B.; Ali, A.; Rafique, S.; Idrees, M. Current status of therapeutic and vaccine approaches against Zika virus. Eur. J. Intern. Med. 2017, 44, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Jenabian, M.A.; Costiniuk, C.T.; Mehraj, V.; Ghazawi, F.M.; Fromentin, R.; Brousseau, J.; Brassard, P.; Belanger, M.; Ancuta, P.; Bendayan, R.; et al. Immune tolerance properties of the testicular tissue as a viral sanctuary site in ART-treated HIV-infected adults. AIDS 2016, 30, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, N.; Soma, R.; Hidano, S.; Watanabe, K.; Umekita, H.; Fukuda, C.; Noguchi, K.; Gendo, Y.; Ozaki, T.; Sonoda, A.; et al. Ribavirin inhibits Zika virus (ZIKV) replication in vitro and suppresses viremia in ZIKV-infected STAT1-deficient mice. Antivir. Res. 2017, 146, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Dejnirattisai, W.; Cao, C.; Scheaffer, S.M.; Supasa, P.; Wongwiwat, W.; Esakky, P.; Drury, A.; Mongkolsapaya, J.; Moley, K.H.; et al. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat. Immunol. 2017, 18, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Larocca, R.A.; Abbink, P.; Peron, J.P.S.; Zanotto, P.M.D.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng’ang’a, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; et al. Safety and immunogenicity of an anti-Zika virus DNA vaccine—Preliminary report. N. Engl. J. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vouga, M.; Baud, D. Imaging of congenital Zika virus infection: The route to identification of prognostic factors. Prenat. Diagn. 2016, 36, 799–811. [Google Scholar] [CrossRef] [PubMed]
| Mouse Genotype (Background). | ZIKV Genotype (Strain) | Inoculation Route | Testis (Infected Cells) | Epididymis | Seminal Vesicles | Vas Deferens | Prostate | Ref. |
|---|---|---|---|---|---|---|---|---|
| Wild Type (BALB/c) Dexamethasone Tx | Asian (PRVABC59) | IP | + (ND) | + | ND | ND | + | [88] |
| Wild Type (C57BL/6) + | Asian (H/FP/2013) | SC | + (SG, PS, ST, LC) | + | ND | ND | ND | [76] |
| anti-IFNαβR mAb | Afr (Dakar 41519) | SC | + (SG, PS, ST, LC) | + | ND | ND | ND | [76] |
| Rag1−/− (C57BL/6) + anti-IFNαβR mAb | Asian (Paraiba_01/2015) | IP | + (SG, PS) | ND | ND | ND | ND | [83] |
| Ifnar1−/− (C57BL/6) | Asian (ZIKV_SMGC-1) | IP | + (LC, GC, PMC, SG) | + | − | ND | − | [77] |
| Asian (Mex2-81) | SC | + (LC) | + | ND | ND | ND | [78] | |
| Asian (PRVABC59) | SC | + (ND) | ND | ND | ND | ND | [82] | |
| Asian (PRVABC59) | SC | + (ST, MSC) | + | ND | ND | ND | [87] | |
| Asian (H/FP/2013) | SC | + (ND) | ND | ND | ND | ND | [85] | |
| Asian (PRVABC59) | SC | + (ST) | + | − | ND | + | [89] | |
| Asian (Mex2-81) | SC | + (SG) | + | ND | ND | ND | [52] | |
| Asian (ZIKVNatal) | SC | +(ND) | ND | ND | ND | ND | [90] | |
| (A129) | Asian (PRVABC59) | SC | + (ND) | − | ND | ND | ND | [86] |
| Asian (PRVABC59) | IP | + (ND) | ND | ND | ND | ND | [81] | |
| African (MP1751) | SC | + (ND) | + | ND | ND | ND | [86] | |
| Ifnar1−/− × Ifngr−/− (AG129) | Asian (PRVABC59) | SC | + (LC) | + | + | ND | + | [89] |
| Asian (PRVABC59/FSS13025/P6-740) | SC | + (ND) | + | + | ND | ND | [31] | |
| Asian (PRVABC59) | IP | + (SG) | + | + | ND | ND | [84] | |
| African (Dakar 41524) | SC | + (ND) | + | + | ND | ND | [31] | |
| (AG6) | Asian (CAS-ZK01) | SC | + (ST, MC) | ND | ND | ND | ND | [79] |
| Irf3−/− × Irf7−/− (C57BL/6) | African (MR766) | SC | + (GC) | + | ND | + | ND | [80] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stassen, L.; Armitage, C.W.; Van der Heide, D.J.; Beagley, K.W.; Frentiu, F.D. Zika Virus in the Male Reproductive Tract. Viruses 2018, 10, 198. https://doi.org/10.3390/v10040198
Stassen L, Armitage CW, Van der Heide DJ, Beagley KW, Frentiu FD. Zika Virus in the Male Reproductive Tract. Viruses. 2018; 10(4):198. https://doi.org/10.3390/v10040198
Chicago/Turabian StyleStassen, Liesel, Charles W. Armitage, David J. Van der Heide, Kenneth W. Beagley, and Francesca D. Frentiu. 2018. "Zika Virus in the Male Reproductive Tract" Viruses 10, no. 4: 198. https://doi.org/10.3390/v10040198
APA StyleStassen, L., Armitage, C. W., Van der Heide, D. J., Beagley, K. W., & Frentiu, F. D. (2018). Zika Virus in the Male Reproductive Tract. Viruses, 10(4), 198. https://doi.org/10.3390/v10040198






