Characterizing Phage Genomes for Therapeutic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Phage Isolation and Genomic DNA Extraction
2.2. Library Preparation and Sequencing
2.3. Genome Assembly
2.4. Resolving Genome Ends
2.5. High Quality Genome Checkpoint
2.6. Phage Lifestyle Checkpoint
2.7. Specialty Genes Checkpoint
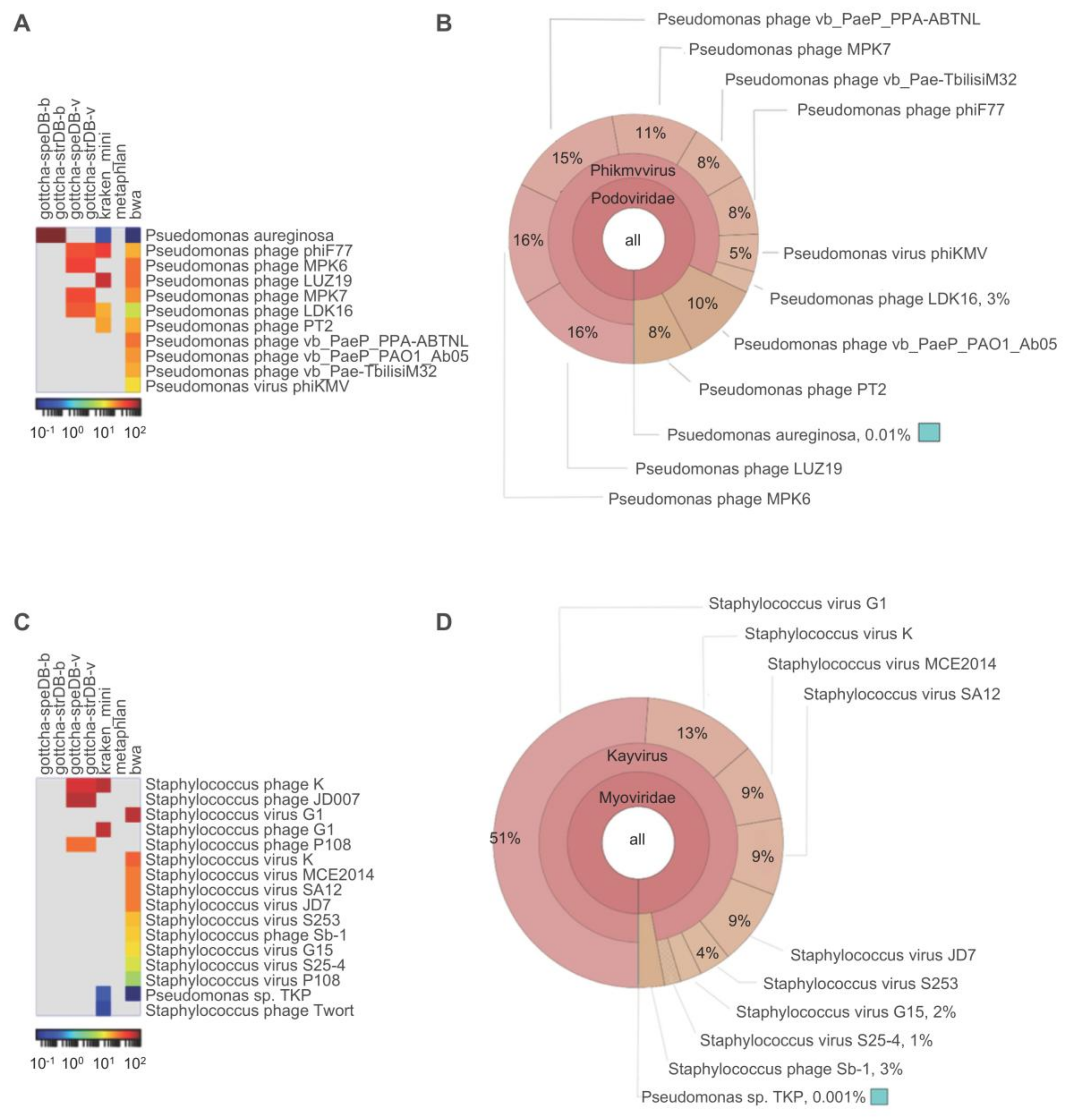
2.8. Contaminant Analysis
2.9. Gene Calling and Functional Annotation
3. Results
3.1. Sequencing and Assembly Statistics
- Check reads for quality, length, nucleotide composition, ambiguous nucleotides.
- Do assemblies agree? If no, is there adequate read-support to resolve differences?
- Is the largest contig a phage sequence? If no, consider contamination analysis.
- Identify (and later, characterize) all contigs >700 bp with >5× coverage.
3.2. High Quality Genomes
- 5.
- Are genome ends resolved?
- 6.
- Is genome supported by adequate even coverage?
3.3. Phage Lifestyle
- 7.
- Do any predicted ORFs present sequence identity to known integrase(s)?
- 8.
- Do classification algorithms (i.e., PHACTS) bolster confidence?
3.4. Specialty Genes Checkpoint
- 9.
- Are any toxins, virulence factors, or antimicrobial resistance genes detected?
3.5. Contaminant Analysis
- 10.
- What percentage of reads are classified as phage?
- 11.
- What is the total assembly size?
- 12.
- What percentage of reads map to non-phage contigs from the assembly?
3.6. Genome Polishing
- 13.
- Were any unsettling genes identified by manual annotation?
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Stratton, C.W. Phages, fitness, virulence, and synergy: A novel approach for the therapy of infections caused by pseudomonas aeruginosa. J. Infect. Dis. 2017, 215, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Bogovazova, G.G.; Voroshilova, N.N.; Bondarenko, V.M.; Gorbatkova, G.A.; Afanas’eva, E.V.; Kazakova, T.B.; Smirnov, V.D.; Mamleeva, A.G.; Glukharev Iu, A.; Erastova, E.I.; et al. Immunobiological properties and therapeutic effectiveness of preparations from klebsiella bacteriophages. Zh. Mikrobiol. Epidemiol. Immunobiol. 1992, 3, 30–33. [Google Scholar]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [PubMed]
- Zschach, H.; Joensen, K.G.; Lindhard, B.; Lund, O.; Goderdzishvili, M.; Chkonia, I.; Jgenti, G.; Kvatadze, N.; Alavidze, Z.; Kutter, E.M.; et al. What can we learn from a metagenomic analysis of a georgian bacteriophage cocktail? Viruses 2015, 7, 6570–6589. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.M.; Hussain, F.A.; Yang, J.; Arevalo, P.; Brown, J.M.; Chang, W.K.; VanInsberghe, D.; Elsherbini, J.; Sharma, R.S.; Cutler, M.B.; et al. A major lineage of non-tailed dsdna viruses as unrecognized killers of marine bacteria. Nature 2018, 554, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Dutilh, B.E.; Cassman, N.; McNair, K.; Sanchez, S.E.; Silva, G.G.; Boling, L.; Barr, J.J.; Speth, D.R.; Seguritan, V.; Aziz, R.K.; et al. A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nat. Commun. 2014, 5, 4498. [Google Scholar] [CrossRef] [PubMed]
- Yosef, I.; Manor, M.; Kiro, R.; Qimron, U. Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proc. Natl. Acad. Sci USA 2015, 112, 7267–7272. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.A. Sequencing, assembling, and finishing complete bacteriophage genomes. Methods Mol. Biol. 2018, 1681, 109–125. [Google Scholar] [PubMed]
- Aziz, R.K.; Ackermann, H.W.; Petty, N.K.; Kropinski, A.M. Essential steps in characterizing bacteriophages: Biology, taxonomy, and genome analysis. Methods Mol. Biol. 2018, 1681, 197–215. [Google Scholar] [PubMed]
- Rihtman, B.; Meaden, S.; Clokie, M.R.; Koskella, B.; Millard, A.D. Assessing illumina technology for the high-throughput sequencing of bacteriophage genomes. PeerJ 2016, 4, e2055. [Google Scholar] [CrossRef] [PubMed]
- Ravn, U.; Didelot, G.; Venet, S.; Ng, K.T.; Gueneau, F.; Rousseau, F.; Calloud, S.; Kosco-Vilbois, M.; Fischer, N. Deep sequencing of phage display libraries to support antibody discovery. Methods 2013, 60, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Mahony, J.; Nauta, A.; van Sinderen, D. Metagenomic approaches to assess bacteriophages in various environmental niches. Viruses 2017, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The rast server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Ladner, J.T.; Beitzel, B.; Chain, P.S.; Davenport, M.G.; Donaldson, E.F.; Frieman, M.; Kugelman, J.R.; Kuhn, J.H.; O’Rear, J.; Sabeti, P.C.; et al. Standards for sequencing viral genomes in the era of high-throughput sequencing. MBio 2014, 5, e01360-14. [Google Scholar] [CrossRef] [PubMed]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- McNair, K.; Bailey, B.A.; Edwards, R.A. Phacts, a computational approach to classifying the lifestyle of phages. Bioinformatics 2012, 28, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Friedman, N.; Molshanski-Mor, S.; Qimron, U. Reversing bacterial resistance to antibiotics by phage-mediated delivery of dominant sensitive genes. Appl. Environ. Microbiol. 2012, 78, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.K.; Collins, J.J. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 4629–4634. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Lamas-Samanamud, G.R. Inhibition of biofilm formation by T7 bacteriophages producing quorum-quenching enzymes. Appl. Environ. Microbiol. 2014, 80, 5340–5348. [Google Scholar] [CrossRef] [PubMed]
- Regeimbal, J.; Jacobs, A.; Corey, B.; Henry, M.; Thompson, M.; Pavlicek, R.; Quinones, J.; Hannah, R.; Ghebremedhin, M.; Crane, N.; et al. Personalized therapeutic cocktail of wild environmental phages rescues mice from acinetobacter baumannii wound infections. Antimicrob. Agents Chemother. 2016, 60, 5806–5816. [Google Scholar] [CrossRef] [PubMed]
- Paithankar, K.R.; Prasad, K.S.N. Precipitation of DNA by polyethylene glycol and ethanol. Nucleic Acids Res. 1991, 19, 1346. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.C.; Chain, P.S. Rapid evaluation and quality control of next generation sequencing data with FaQCs. BMC Bioinform. 2014, 15, 366. [Google Scholar] [CrossRef] [PubMed]
- Seqtk, Toolkit for Processing Sequences in Fasta/q Formats. Available online: https://github.com/lh3/seqtk (accessed on 14 September 2016).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. Spades: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.R.; Depardieu, F.; Fortier, L.C.; Bikard, D.; Monot, M. Phageterm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef] [PubMed]
- Merrill, B.D.; Ward, A.T.; Grose, J.H.; Hope, S. Software-based analysis of bacteriophage genomes, physical ends, and packaging strategies. BMC Genom. 2016, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. Phaster: A better, faster version of the phast phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Pop, M. ARDB—Antibiotic resistance genes database. Nucleic Acids Res. 2009, 37, D443–D447. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.K.; Forsberg, K.J.; Dantas, G. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 2015, 9, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. Card 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Li, P.E.; Lo, C.C.; Anderson, J.J.; Davenport, K.W.; Bishop-Lilly, K.A.; Xu, Y.; Ahmed, S.; Feng, S.; Mokashi, V.P.; Chain, P.S. Enabling the democratization of the genomics revolution with a fully integrated web-based bioinformatics platform. Nucleic Acids Res. 2017, 45, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.A.; Li, P.E.; Scholz, M.B.; Chain, P.S. Accurate read-based metagenome characterization using a hierarchical suite of unique signatures. Nucleic Acids Res. 2015, 43, e69. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genom. Biol. 2014, 15, R46. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Waldron, L.; Ballarini, A.; Narasimhan, V.; Jousson, O.; Huttenhower, C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods 2012, 9, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv, 2013; arXiv:1303.3997v2. [Google Scholar]
- Philipson, C.W.; Davenport, K.; Voegtly, L.; Lo, C.; Li, P.; Xu, Y.; Shakya, M.; Cer, R.Z.; Bishop-Lilly, K.A.; Hamilton, T.; et al. Brief protocol for EDGE bioinformatics: Analyzing microbial and metagenomic NGS data. Bio-Protc. 2017, 7. [Google Scholar] [CrossRef]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- McNair, K.; Zhou, C.; Souza, B.; Edwards, R. Thea: A novel approach to gene identification in phage genomes. bioRxiv 2018. [Google Scholar] [CrossRef]
- Hauser, F.; Chen, W.; Deinlein, U.; Chang, K.; Ossowski, S.; Fitz, J.; Hannon, G.J.; Schroeder, J.I. A genomic-scale artificial microrna library as a tool to investigate the functionally redundant gene space in arabidopsis. Plant Cell 2013, 25, 2848–2863. [Google Scholar] [CrossRef] [PubMed]
- Grazziotin, A.L.; Koonin, E.V.; Kristensen, D.M. Prokaryotic virus orthologous groups (PVOGs): A resource for comparative genomics and protein family annotation. Nucleic Acids Res. 2017, 45, D491–D498. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Xu, D. Quantitative assessment of relationship between sequence similarity and function similarity. BMC Genom. 2007, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Sangar, V.; Blankenberg, D.J.; Altman, N.; Lesk, A.M. Quantitative sequence-function relationships in proteins based on gene ontology. BMC Bioinform. 2007, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Arndt, W.; Miller, B.L.; Wheeler, T.J.; Schreiber, F.; Bateman, A.; Eddy, S.R. Hmmer web server: 2015 update. Nucleic Acids Res. 2015, 43, W30–W38. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Chan, P.P. Trnascan-se on-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canback, B. Aragorn, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No eskape. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No eskape! An update from the infectious diseases society of america. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Tong, Y. Analyzing genome termini of bacteriophage through high-throughput sequencing. Methods Mol. Biol. 2018, 1681, 139–163. [Google Scholar] [PubMed]
- Colavecchio, A.; D’Souza, Y.; Tompkins, E.; Jeukens, J.; Freschi, L.; Emond-Rheault, J.G.; Kukavica-Ibrulj, I.; Boyle, B.; Bekal, S.; Tamber, S.; et al. Prophage integrase typing is a useful indicator of genomic diversity in Salmonella enterica. Front. Microbiol. 2017, 8, 1283. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, T.; Segall, A.M. Characterization of a mutation of bacteriophage λ integrase. Putative role in core binding and strand exchange for a conserved residue. J. Biol. Chem. 2000, 275, 36949–36956. [Google Scholar] [CrossRef] [PubMed]
- Feiner, R.; Argov, T.; Rabinovich, L.; Sigal, N.; Borovok, I.; Herskovits, A.A. A new perspective on lysogeny: Prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 2015, 13, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D.R.; Utter, B.; Fischetti, V.A. Uncovering novel mobile genetic elements and their dynamics through an extra-chromosomal sequencing approach. Mob. Genet. Elem. 2016, 6, e100502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Utter, B.; Deutsch, D.R.; Schuch, R.; Winer, B.Y.; Verratti, K.; Bishop-Lilly, K.A.; Sozhmannan, S.; Fischetti, V.A. Beyond the chromosome: The prevalence of unique extra-chromosomal bacteriophages with integrated virulence genes in pathogenic Staphylococcus aureus. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kallberg, M.; Wang, H.; Wang, S.; Peng, J.; Wang, Z.; Lu, H.; Xu, J. Template-based protein structure modeling using the raptorx web server. Nat. Protoc. 2012, 7, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.; Karplus, K.; Hughey, R.; Chothia, C. Assignment of homology to genome sequences using a library of hidden markov models that represent all proteins of known structure. J. Mol. Biol. 2001, 313, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Picardi, E.; Horner, D.S.; Chiara, M.; Schiavon, R.; Valle, G.; Pesole, G. Large-scale detection and analysis of RNA editing in grape mtDNA by RNA deep-sequencing. Nucleic Acids Res. 2010, 38, 4755–4767. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, V.; Boizeau, L.; Candotti, D.; Vandenbogaert, M.; Servant-Delmas, A.; Caro, V.; Laperche, S. Early minion™ nanopore single-molecule sequencing technology enables the characterization of hepatitis B virus genetic complexity in clinical samples. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Giordano, F.; Ning, Z. Oxford nanopore minion sequencing and genome assembly. Genom. Proteom. Bioinform. 2016, 14, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Aigrain, L.; Quail, M.A.; Coupland, P.; Bonfield, J.K.; Davies, R.M.; Tischler, G.; Jackson, D.K.; Keane, T.M.; Li, J.; et al. De novo yeast genome assemblies from MinION, PacBio and MISeq platforms. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]

| Database | # Of Genes in Database | Last Updated 1 | Database Source |
|---|---|---|---|
| ShortBRED VF 2 | 26,187 | July 2017 | https://huttenhower.sph.harvard.edu/shortbred |
| ShortBRED AR 3 | 932 | July 2017 | https://huttenhower.sph.harvard.edu/shortbred |
| Virulence Factor DataBase (VFDB) | 30,246 | February 2018 | http://www.mgc.ac.cn/VFs/main.htm |
| Comprehensive Antibiotic Resistance Database (CARD) | 2514 | February 2018 | https://card.mcmaster.ca/download |
| Pipeline Output | Pseudomonas Phage vB_PaeP_130_113 | Staphylococcus Phage vB_SauM_0414_108 |
|---|---|---|
| Total Reads | 206,222 | 347,594 |
| Reads Pass FaQCs (%) | 98.82 | 98.38 |
| Reads Pass CLC (%) | 96.97 | 94.03 |
| Reads sub-sampled (#) | 50,000 | 50,000 |
| SPAdes all reads 1 | 1 | 2 |
| CLC all reads 1 | 1 | 1 |
| SPAdes subsampled 1 | 1 | 1 |
| CLC subsampled 1 | 1 | 1 |
| SPAdes all reads 2 | 43,742 | 141,507 |
| CLC all reads 2 | 43,742 | 141,334 |
| SPAdes subsampled 2 | 43,742 | 141,331 |
| CLC subsampled 2 | 43,742 | 141,330 |
| Phage | Class 1 | DTR Region Length | Start, End τ Metric 2 | Coverage in DTR Region | Coverage Outside of DTR Region |
|---|---|---|---|---|---|
| Pseudomonas phage vB_PaeP_130_113 | Short DTR | 463 bp | 0.63, 0.64 | 1018.0× | 634.9× |
| Staphylococcus phage vB_SauM_0414_108 | Long DTR | 10,296 bp | 0.75, 0.55 | 753.1× | 343.9× |
| Phage | PHACTS Lytic Score | PHACTS Temperate Score | PHACTS Standard Deviation | PHASTER Integrase | RAST Integrase | NCBI Annotated Integrase |
|---|---|---|---|---|---|---|
| Pseudomonas phage vB_PaeP_130_113 | 0.66 | 0.34 | 0.073 | No | No | N/A |
| Pseudomonas phage DL62 (GI:KR054031) | 0.73 | 0.26 | 0.117 | No | No | No |
| Pseudomonas phage vB_PaeS_PMG1 (GI:NC_016765) | 0.42 | 0.58 | 0.042 | Yes | Yes | Yes |
| Staphylococcus phage vB_SauM_0414_108 | 0.60 | 0.40 | 0.082 | No | No | N/A |
| Staphylococcus phage K (GI:KF76114) | 0.59 | 0.41 | 0.107 | No | No | No |
| Staphylococcus phage phiSaus-IPLA88 (GI:NC_011614) | 0.28 | 0.72 | 0.048 | Yes | Yes | Yes |
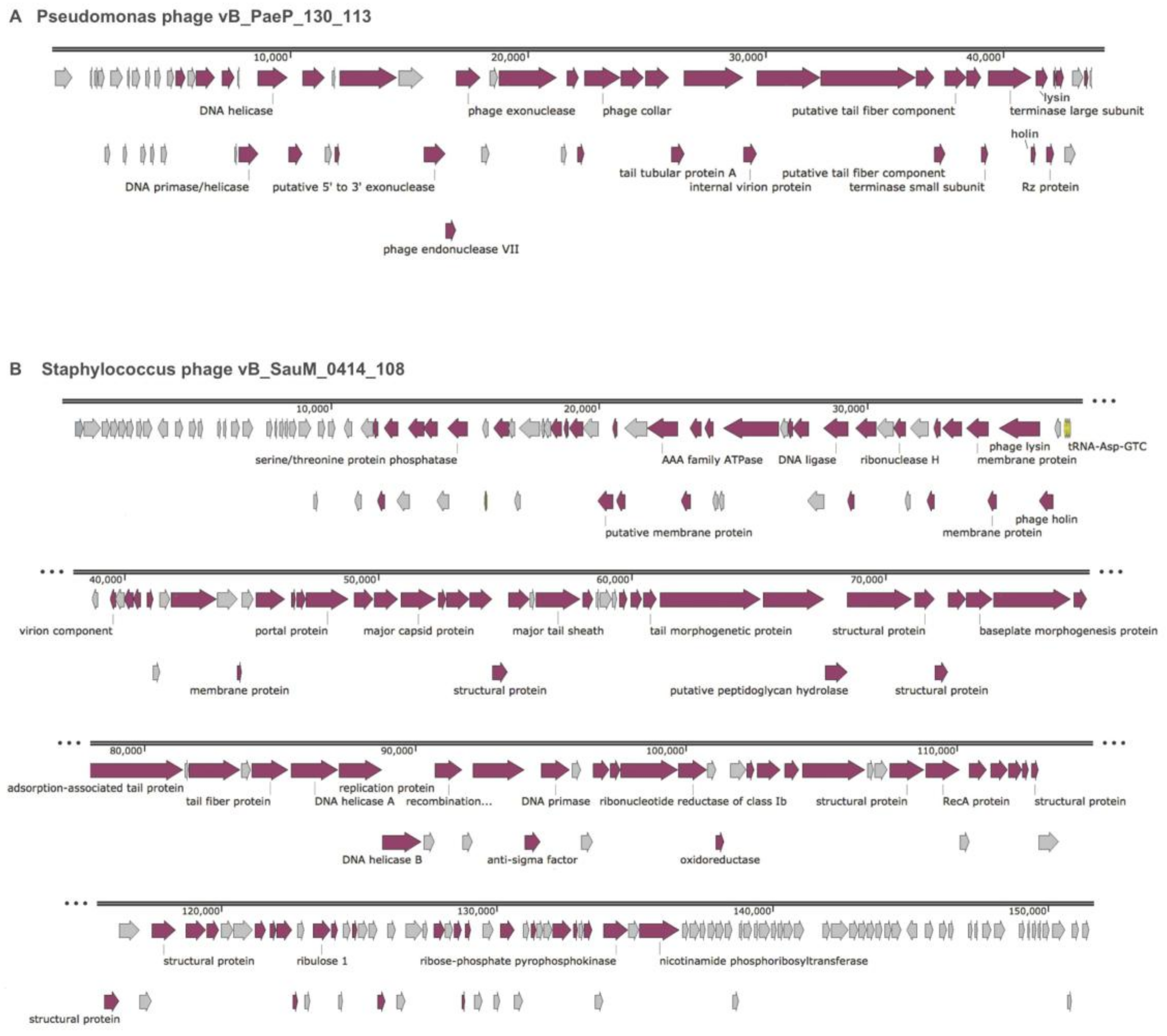
| Phage | Size (bp) | %GC | CDS (#) | Genes with Functional Annotation (#) | Hypothetical Genes (#) | tRNA (#) | Assigned Family |
|---|---|---|---|---|---|---|---|
| Pseudomonas phage vB_PaeP_130_113 | 44,205 | 62.4 | 57 | 35 | 22 | 0 | Podoviridae |
| Staphylococcus phage vB_SauM_0414_108 | 151,627 | 30.4 | 241 | 154 | 87 | 4 | Myoviridae |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philipson, C.W.; Voegtly, L.J.; Lueder, M.R.; Long, K.A.; Rice, G.K.; Frey, K.G.; Biswas, B.; Cer, R.Z.; Hamilton, T.; Bishop-Lilly, K.A. Characterizing Phage Genomes for Therapeutic Applications. Viruses 2018, 10, 188. https://doi.org/10.3390/v10040188
Philipson CW, Voegtly LJ, Lueder MR, Long KA, Rice GK, Frey KG, Biswas B, Cer RZ, Hamilton T, Bishop-Lilly KA. Characterizing Phage Genomes for Therapeutic Applications. Viruses. 2018; 10(4):188. https://doi.org/10.3390/v10040188
Chicago/Turabian StylePhilipson, Casandra W., Logan J. Voegtly, Matthew R. Lueder, Kyle A. Long, Gregory K. Rice, Kenneth G. Frey, Biswajit Biswas, Regina Z. Cer, Theron Hamilton, and Kimberly A. Bishop-Lilly. 2018. "Characterizing Phage Genomes for Therapeutic Applications" Viruses 10, no. 4: 188. https://doi.org/10.3390/v10040188
APA StylePhilipson, C. W., Voegtly, L. J., Lueder, M. R., Long, K. A., Rice, G. K., Frey, K. G., Biswas, B., Cer, R. Z., Hamilton, T., & Bishop-Lilly, K. A. (2018). Characterizing Phage Genomes for Therapeutic Applications. Viruses, 10(4), 188. https://doi.org/10.3390/v10040188





