Identification and Molecular Characterization of a Novel Partitivirus from Trichoderma atroviride NFCF394
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Isolation and Purification of Virus Particles and Transmission Electron Microscopy
2.3. Nucleic Acid Extraction and Viral Genome Sequencing
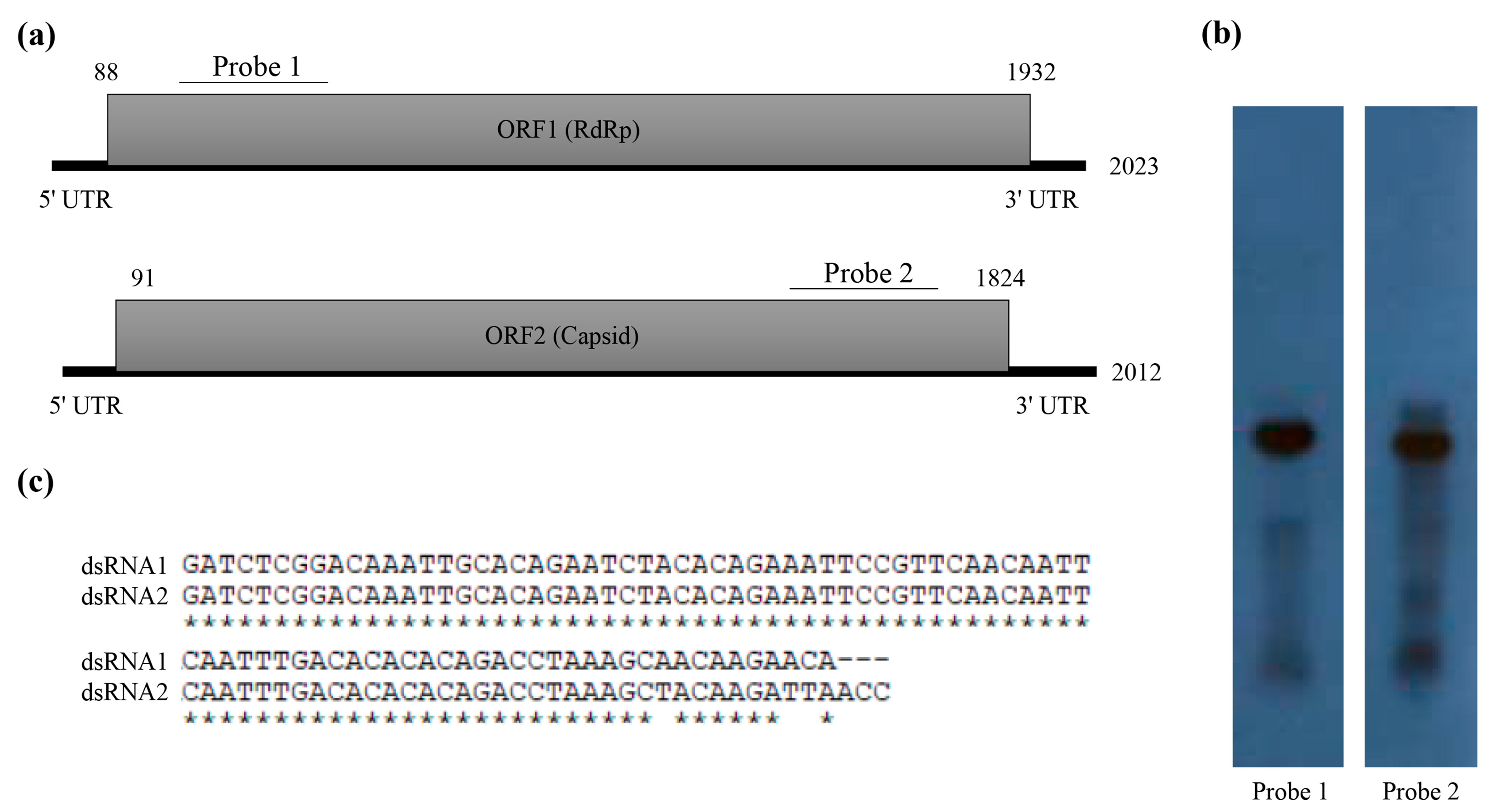
2.4. Rapid Amplification of cDNA Ends (RACE) Analysis
2.5. Sequence Analysis
2.6. Assays of Chitinase and β-1,3-Glucanase Activity
3. Results and Discussion
3.1. Profile of Virus Particles from T. atroviride NFCF394
3.2. Molecular Characterization of Novel Partitivirus from T. atroviride NFCF394
3.3. Phylogenic Analysis of Amino Acid Sequences Encoded by the ORFs
3.4. Phenotypic Characteristics of Mycovirus-Cured and -Containing Strains
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sivasithamparam, K.; Ghisalberti, E.L. Trichoderma and Gliocladium: Basic Biology, Taxonomy and Genetics, 1st ed.; Taylor and Francis: London, UK, 1998; pp. 139–191. [Google Scholar]
- Kubicek, C.P.; Penttila, M.E. Trichoderma and Gliocladium: Enzymes, Biological Control and Commercial Applications, 1st ed.; Taylor and Francis: London, UK, 1998; pp. 49–71. [Google Scholar]
- Gupta, V.K.; Schmoll, M.; Herrera-Estrella, A.; Upadhyay, R.S.; Druzhinina, I.; Tuohy, M.G. Biotechnology and Biology of Trichoderma; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef] [PubMed]
- Papavizas, G.C. Trichoderma and Gliocladium: Biology, ecology, and potential for biocontrol. Annu. Rev. Phytopathol. 1985, 23, 23–54. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Castón, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-plus years of fungal viruses. Virology 2015, 479–480, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Wickner, R.B. Double-stranded and single-stranded RNA viruses of Saccharomyces cerevisiae. Annu. Rev. Microbiol. 1992, 46, 347–375. [Google Scholar] [CrossRef] [PubMed]
- The Online (10th) Report of the International Committee on Taxonomy of Viruses. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report (accessed on 12 December 2016).
- Roossinck, M.J. Metagenomics of plant and fungal viruses reveals an abundance of persistent lifestyles. Front. Microbiol. 2015, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Lee, S.H.; So, K.K.; Kim, J.M.; Kim, D.H. Incidence of diverse dsRNA mycoviruses in Trichoderma spp. causing green mold disease of shiitake Lentinula edodes. FEMS Microbiol. Lett. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yun, S.H.; Chun, J.; Kim, D.H. Characterization of a novel dsRNA mycovirus of Trichoderma atroviride NFCF028. Arch. Virol. 2017, 162, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Yang, H.E.; Kim, D.H. Identification of a novel partitivirus of Trichoderma harzianum NFCF319 and evidence for the related antifungal activity. Front. Plant Sci. under review.
- Kim, J.M.; Jung, J.E.; Park, J.A.; Park, S.M.; Cha, B.J.; Kim, D.H. Biological function of a novel chrysovirus, CnV1-Bs122, in the Korean Cryphonectria nitschkei BS122 strain. J. Biosci. Bioeng. 2013, 115, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, J.; Wang, Y.; Hong, N.; Zhang, F.; Xu, W.; Wang, G. Hypovirulence of the phytopathogenic fungus Botryosphaeria dothidea: Association with a coinfecting chrysovirus and a partitivirus. J. Virol. 2014, 88, 7517–7527. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Kim, J.M.; Chung, H.J.; Lim, J.Y.; Kwon, B.R.; Lim, J.G.; Kim, J.A.; Kim, M.J.; Cha, B.J.; Lee, S.H.; et al. Occurrence of diverse dsRNA in a Korean population of the chestnut blight fungus, Cryphonectria parasitica. Mycol. Res. 2008, 112, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1987, 25, 4876–4882. [Google Scholar] [CrossRef]
- Nibert, M.L.; Ghabrial, S.A.; Maiss, E.; Lesker, T.; Vainio, E.J.; Jiang, D.; Suzuki, N. Taxonomic reorganization of family Partitiviridae and other recent progress in partitivirus research. Virus Res. 2014, 88, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Van Alfen, N.K. Hypovirulence of Endothia (Cryphonectria) parasitica and Rhizoctonia solani. In Fungal Virology, 1st ed.; Buck, K.W., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 143–162. [Google Scholar]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant. Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef] [PubMed]


| Virus | Identity (%) | Overlap |
|---|---|---|
| ORF1 search | ||
| RnPV7 (LC076694) | 60.5 | 376/622 |
| RsPV1 (AND83003) | 52.6 | 328/623 |
| RnPV5 (BAM36403) | 52.8 | 344/652 |
| SsPV-S (GQ280377) | 42.1 | 263/625 |
| ORF2 search | ||
| RnPV7 (BAT32943) | 26.7 | 178/666 |
| SsPV-S (GQ280378) | 21.9 | 138/630 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, J.; Yang, H.-E.; Kim, D.-H. Identification and Molecular Characterization of a Novel Partitivirus from Trichoderma atroviride NFCF394. Viruses 2018, 10, 578. https://doi.org/10.3390/v10110578
Chun J, Yang H-E, Kim D-H. Identification and Molecular Characterization of a Novel Partitivirus from Trichoderma atroviride NFCF394. Viruses. 2018; 10(11):578. https://doi.org/10.3390/v10110578
Chicago/Turabian StyleChun, Jeesun, Han-Eul Yang, and Dae-Hyuk Kim. 2018. "Identification and Molecular Characterization of a Novel Partitivirus from Trichoderma atroviride NFCF394" Viruses 10, no. 11: 578. https://doi.org/10.3390/v10110578
APA StyleChun, J., Yang, H.-E., & Kim, D.-H. (2018). Identification and Molecular Characterization of a Novel Partitivirus from Trichoderma atroviride NFCF394. Viruses, 10(11), 578. https://doi.org/10.3390/v10110578




