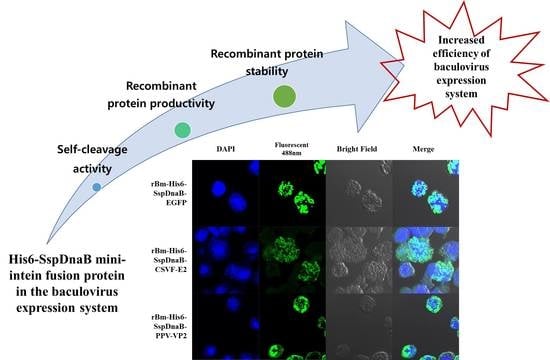
Enhanced Production of Recombinant Protein by Fusion Expression with Ssp DnaB Mini-Intein in the Baculovirus Expression System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
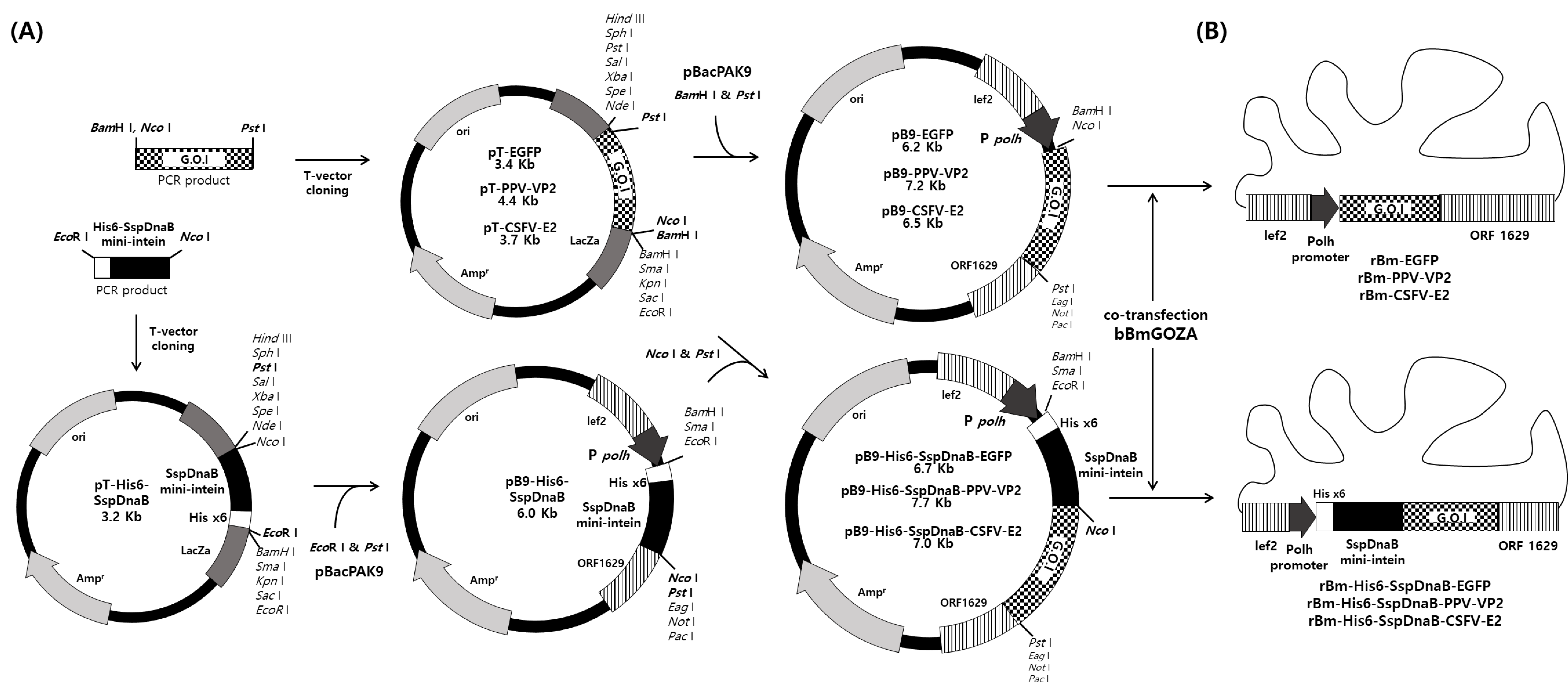
2.2. Construction of Transfer Vector
2.3. Generation of Recombinant Virus
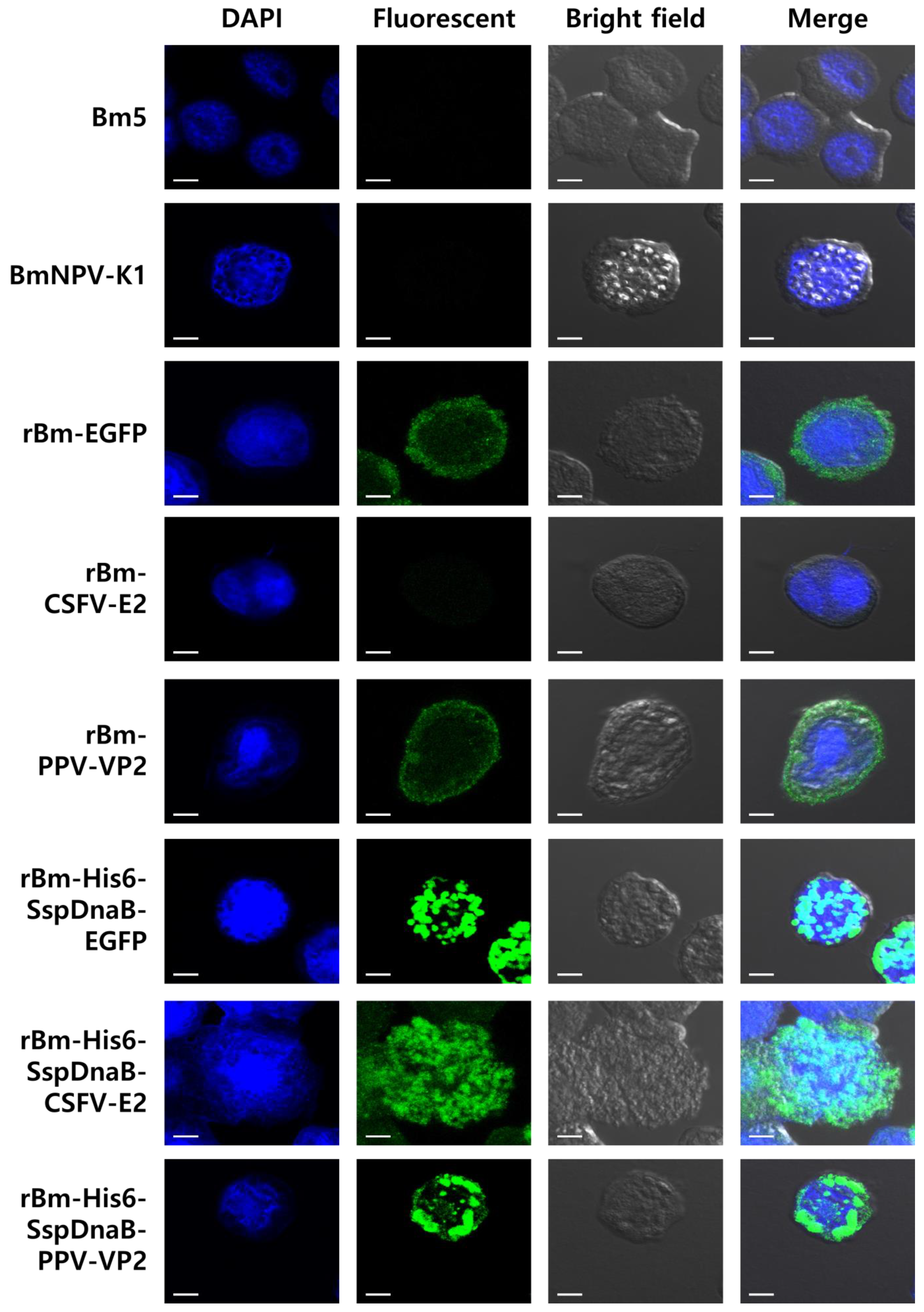
2.4. Measurement of Fluorescence
2.5. Immuno-Fluorescence Assay
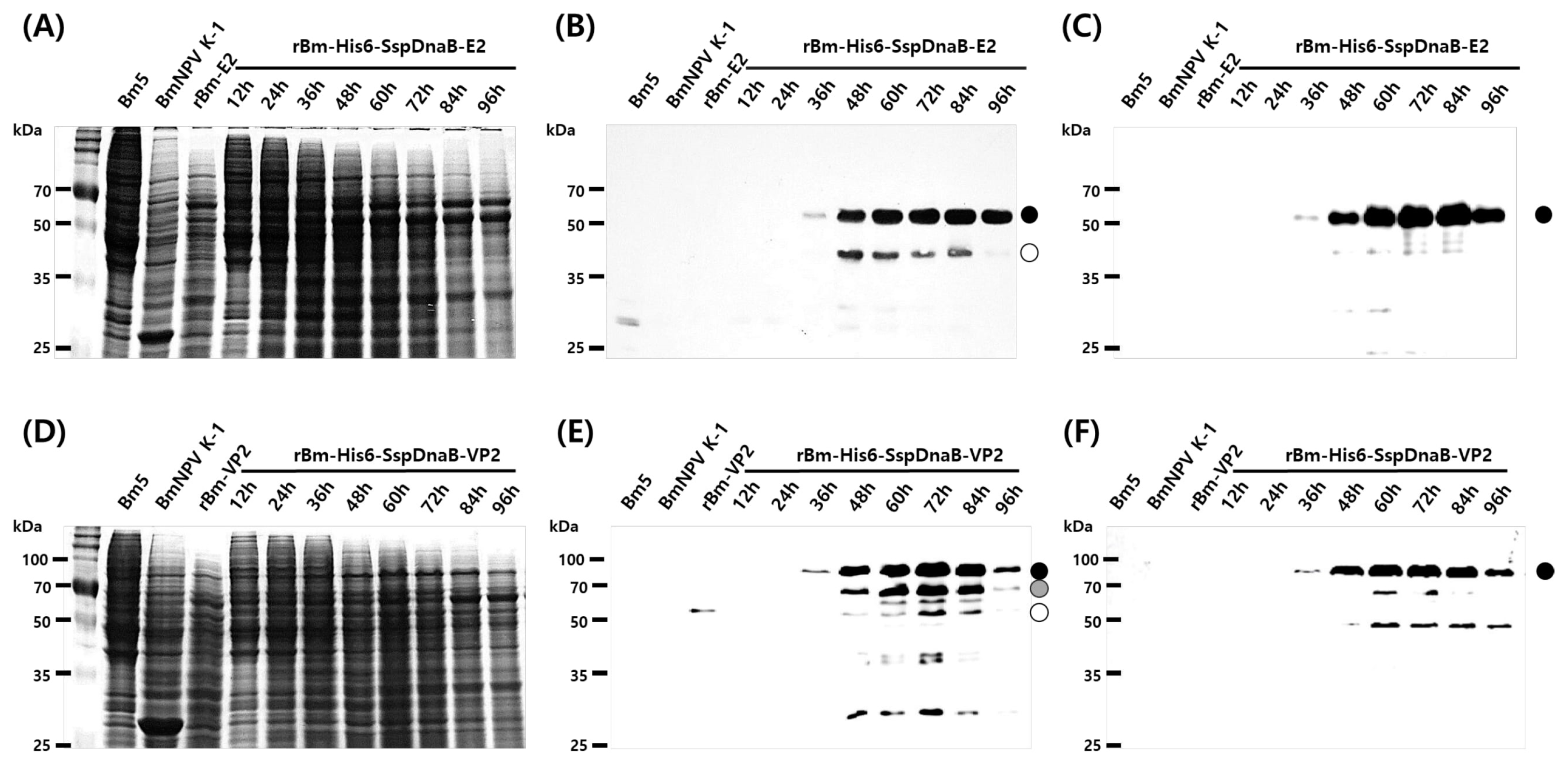
2.6. SDS-PAGE and Western Blot Analysis
2.7. Temperature and pH Inducible Cleavage
3. Results and Discussion
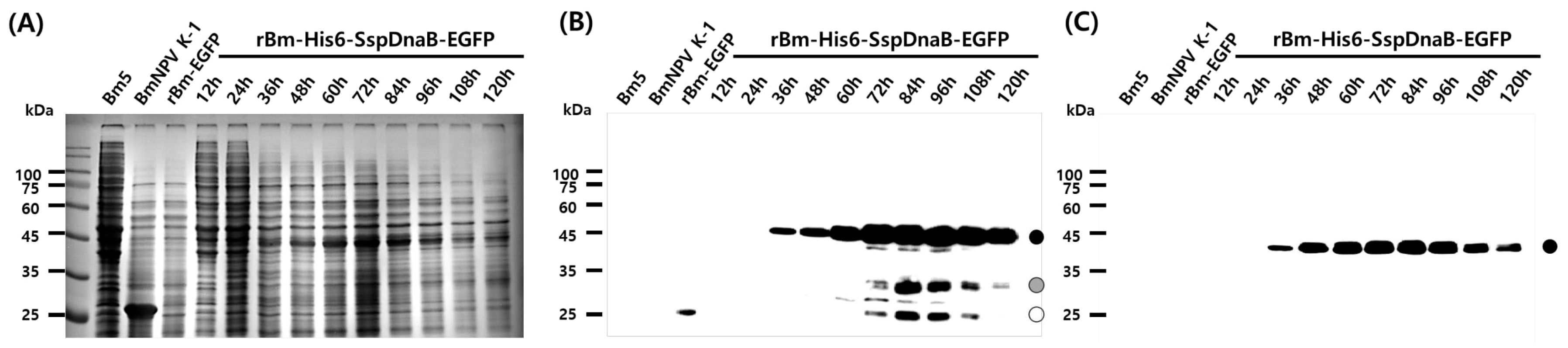
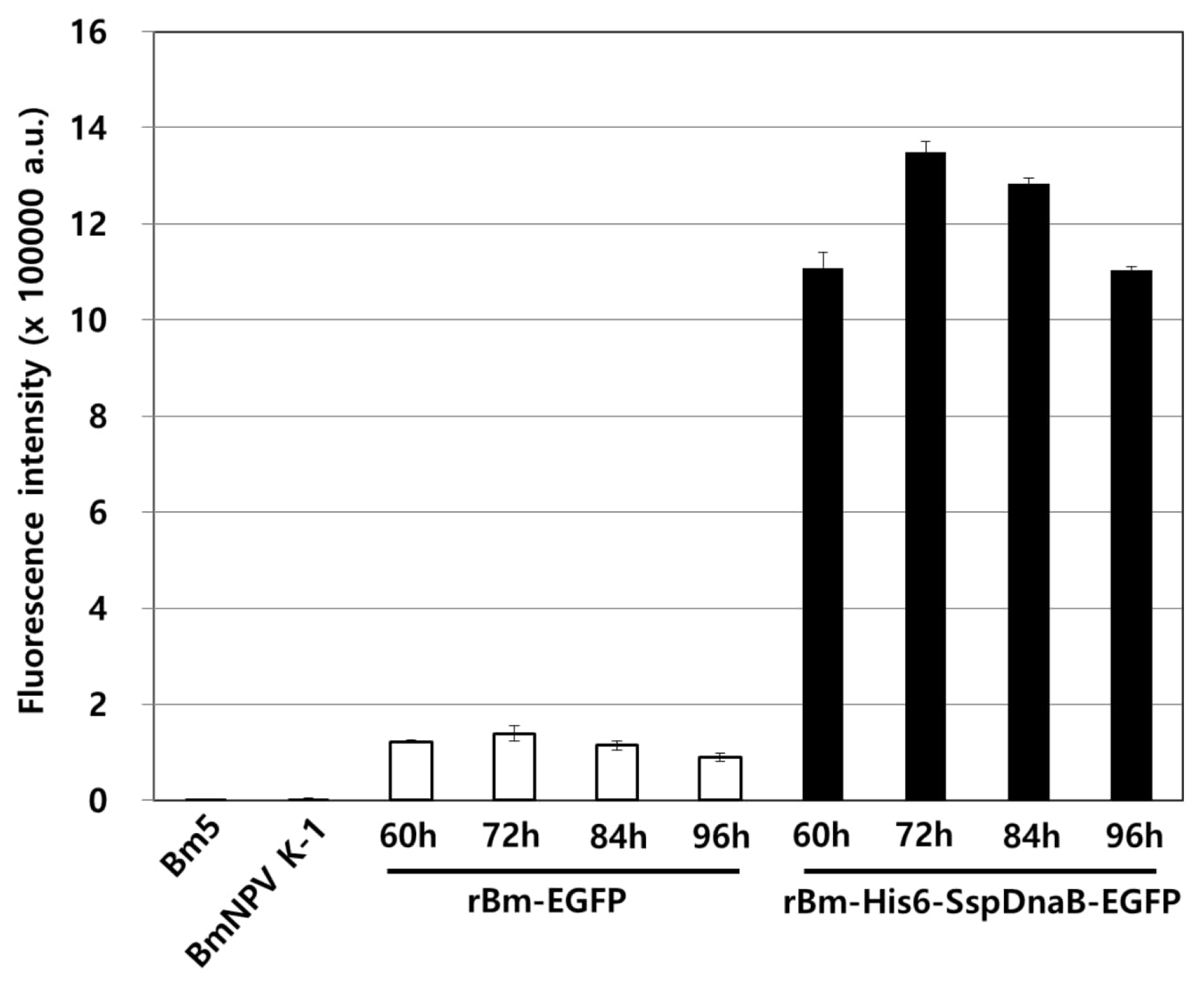
3.1. Effect of Mini-Intein Fusion Expression
3.2. Cleavage Activity of the Mini-Intein
3.3. Status of the Mini-Intein Fusion Protein
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Radner, S.; Celie, P.H.N.; Fuchs, K.; Sieghart, W.; Sixma, T.K.; Stornaiuolo, M. Transient transfection coupled to baculovirus infection for rapid protein expression screening in insect cells. J. Struct. Biol. 2012, 179, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, L.; Kawar, S.; Hollister, R. Engineering N-glycosylation pathways in the baculovirus-insect cell system. Curr. Opin. Biotechnol. 1998, 9, 528–533. [Google Scholar] [CrossRef]
- Kato, T.; Kajikawa, M.; Maenaka, K.; Park, E.Y. Silkworm expression system as a platform technology in life science. Appl. Microbiol. 2010, 85, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Hitchman, R.; Possee, R.; King, L. Baculovirus expression systems for recombinant protein production in insect cells. Recent Pat. Biotechnol. 2009, 3, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Zhang, J.; Dang, T.L.; Hasegawa, H.; Cheng, J.D.; Gianan, I.; O’Neill, J.W.; Wolfson, M.; Siu, S.; Qu, S.; et al. Fusion partners can increase the expression of recombinant interleukins via transient transfection in 2936E cells. Protein Sci. 2010, 19, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waugh, D.S. An overview of enzymatic reagents for the removal of affinity tags. Protein Expr. Purif. 2011, 80, 283–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y. Self-cleaving fusion tags for recombinant protein production. Biotechnol. Lett. 2011, 33, 869–881. [Google Scholar] [CrossRef]
- Phan, J.; Zdanov, A.; Evdokimov, A.G.; Tropea, J.E.; Peters, H.K., 3rd; Kapust, R.B.; Li, M.; Wlodawer, A.; Waugh, D.S. Structural basis for the substrate specificity of tobacco etch virus protease. J. Biol. Chem. 2002, 277, 50564–50572. [Google Scholar] [CrossRef]
- Pedersen, J.; Lauritzen, C.; Madsen, M.T.; Weis Dahl, S. Removal of N-terminal polyhistidine tags from recombinant proteins using engineered aminopeptidases. Protein Expr. Purif. 1999, 15, 389–400. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Zhao, Z. Purification and refolding optimization of recombinant bovine enterokinase light chain overexpressed in Escherichia coli. Protein Expr. Purif. 2007, 56, 40–47. [Google Scholar] [CrossRef]
- Pietrokovski, S. A new intein in cyanobacteria and its significance for the spread of inteins. Trends Genet. 1996, 12, 287–288. [Google Scholar] [CrossRef]
- Wu, H.; Xu, M.Q.; Liu, X.Q. Protein trans-splicing and functional mini-inteins of a cyanobacterial DnaB Intein. Biochim. Biophys. Acta 1998, 1387, 422–432. [Google Scholar] [CrossRef]
- Mathys, S.; Evans, T.C.; Chute, I.C.; Wu, H.; Chong, S.; Benner, J.; Liu, X.-Q.; Xu, M.-Q. Characterization of a self-splicing mini-intein and its conversion into autocatalytic N- and C-terminal cleavage elements: Facile production of protein building blocks for protein ligation. Gene 1999, 231, 1–13. [Google Scholar] [CrossRef]
- Volkmann, G.; Mootz, H.D. Recent progress in intein research: From mechanism to directed evolution and applications. Cell. Mol. Life Sci. 2013, 70, 1185–1206. [Google Scholar] [CrossRef]
- Cooper, M.A.; Taris, J.E.; Shi, C.; Wood, D.W. A convenient split-intein tag method for the purification of tagless target proteins. Curr. Protoc. Protein Sci. 2018, 91, 5.29.1–5.29.23. [Google Scholar] [CrossRef]
- Ji, Y.; Lu, Y.; Yan, Y.; Liu, X.; Su, N.; Zhang, C.; Bi, S.; Xing, X.-H. Design of fusion proteins for efficient and soluble production of immunogenic Ebola virus glycoprotein in Escherichia coli. Biotechnol. J. 2018, 13, e1700627. [Google Scholar] [CrossRef]
- Cui, C.; Zhao, W.; Chen, J.; Wang, J.; Li, Q. Elimination of in vivo cleavage between target protein and intein in the intein-mediated protein purification systems. Protein Expr. Purif. 2006, 50, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.Z.; Lee, K.S.; Kim, B.Y.; Choi, Y.S.; Wei, Y.D.; Choo, Y.M.; Kang, P.D.; Yoon, H.J.; Kim, I.; Je, Y.H.; et al. Functional role of aspartic proteinase cathepsin D in insect metamorphosis. BMC Dev. Biol. 2006, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Hitchman, R.B.; Locanto, E.; Possee, R.D.; King, L.A. Optimizing the baculovirus expression vector system. Methods 2011, 55, 52–57. [Google Scholar] [CrossRef]
- Je, Y.H.; Chang, J.H.; Kim, M.H.; Roh, J.Y.; Jin, B.R.; O’reilly, D.R. The use of defective Bombyx mori nucleopolyhedrovirus genomes maintained in Escherichia coli for the rapid generation of occlusion-positive and occlusion-negative expression vectors. Biotechnol. Lett. 2001, 23, 1809–1817. [Google Scholar] [CrossRef]
- Bae, S.M.; Kim, H.J.; Lee, J.B.; Choi, J.B.; Shin, T.Y.; Koo, H.N.; Choi, J.Y.; Lee, K.S.; Je, Y.H.; Jin, B.R.; et al. Hyper-enhanced production of foreign recombinant protein by fusion with the partial polyhedrin of nucleopolyhedrovirus. PLoS ONE 2013, 8, e60835. [Google Scholar] [CrossRef]
- Ramirez, M.; Guan, D.; Ugaz, V.; Chen, Z. Intein-triggered artificial protein hydrogels that support the immobilization of bioactive proteins. J. Am. Chem. Soc. 2013, 135, 5290–5293. [Google Scholar] [CrossRef]
- Guan, D.; Ramirez, M.; Chen, Z. Split intein mediated ultra-rapid purification of tag less protein (SIRP): Rapid purification of tagless protein using SIRP. Biotechnol. Bioeng. 2013, 110, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Topilina, N.I.; Mills, K.V. Recent advances in in vivo applications of intein-mediated protein splicing. Mob. DNA 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Tsai, T.Y.; Liu, T.T.; Lin, C.C.; Chen, J.H.; Yang, S.C.; Shieh, C.J.; Liu, Y.C. Production of recombinant EGFP via surface display of ice nucleation protein and self-cleavage intein. Biochem. Eng. J. 2011, 54, 158–163. [Google Scholar] [CrossRef]
- Banki, M.R.; Feng, L.; Wood, D.W. Simple bioseparations using self-cleaving elastin-like polypeptide Tags. Nat. Methods 2005, 2, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chen, J.; Yao, H.; Liu, L.; Wang, J.; Zhang, J.; Liu, J.-N. Use of Ssp DnaB derived mini-intein as a fusion partner for production of recombinant human brain natriuretic peptide in Escherichia coli. Protein Expr. Purif. 2005, 43, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Hsuan, S.L.; Chen, Z.W.; Jinn, T.R.; Huang, C.; Liao, J.W.; Chen, T.H.; Liao, C.M.; Lee, W.C.; Wu, T.Y.; et al. Expression and immunological studies of classical swine fever virus glycoprotein E2 in the bi-cistronic baculovirus/larvae expression system. Biosci. Biotechnol. Biochem. 2010, 74, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Gao, J.; Zhou, J.; Yuan, L. Optimizing the method for cleavage of Ssp DnaB mini-intein mediated precursor proteins. J. Anim. Vet. Adv. 2013, 12, 1537–1540. [Google Scholar] [CrossRef]
- Seo, J.H.; Li, L.; Yeo, J.S.; Cha, H.J. Baculoviral polyhedrin as a novel fusion partner for formation of inclusion body in Escherichia coli. Biotechnol. Bioeng. 2003, 84, 467–473. [Google Scholar] [CrossRef]
- Park, J.S.; Ahn, J.Y.; Lee, S.H.; Lee, H.; Han, K.Y.; Seo, H.S.; Ahn, K.Y.; Min, B.H.; Sim, S.J.; Choi, I.S.; et al. Enhanced stability of heterologous proteins by supramolecular self-assembly. Appl. Microbiol. Biotechnol. 2007, 75, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kim, Y.S.; Hwang, D.S.; Seo, J.H.; Jung, H.J.; Du, J.; Cha, H.J. High and compact formation of baculoviral polyhedrin-induced inclusion body by co-expression of baculoviral FP25 in Escherichia coli. Biotechnol. Bioeng. 2007, 96, 1183–1190. [Google Scholar] [CrossRef]
- Ahn, J.Y. Heterologous gene expression using self-assembled supra-molecules with high affinity for hsp70 chaperone. Nucleic Acids Res. 2005, 33, 3751–3762. [Google Scholar] [CrossRef] [PubMed]

| Name of Primer | Primer Sequence * |
|---|---|
| His6-SspDnaB-F | 5′-GAATTCGCCACCATGCACCACCACCACCACCACATCTCTGGCGATAG-3′ |
| SspDnaB-R | 5′-CCATGGCTCTTCCGTTGTGTAC-3′ |
| EGFP-F | 5′-GAATTCCCATGGTGAGCAAGGGCGAGGAGCTG-3′ |
| EGFP-R | 5′-CTGCAGTTACTTGTACAGCTCGTCCATGCCG-3′ |
| PPV-VP2-F | 5′-GAATTCCCATGGCAATGAGTGAAAATGTGGAACAACA-3′ |
| PPV-VP2-R | 5′-CTGCAGCTAGTATAATTTTCTTGGTATAAGTTGTG-3′ |
| CSFV-E2-F | 5′-GAACCTCCATGGCAATGCGGCTAGCCTGCA-3′ |
| CSFV-E2-R | 5′-CTGCAGTCAGTCAGTCACGTCCAGG-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwak, W.S.; Choi, J.B.; Han, B.K.; Bae, S.M.; Woo, S.D. Enhanced Production of Recombinant Protein by Fusion Expression with Ssp DnaB Mini-Intein in the Baculovirus Expression System. Viruses 2018, 10, 523. https://doi.org/10.3390/v10100523
Gwak WS, Choi JB, Han BK, Bae SM, Woo SD. Enhanced Production of Recombinant Protein by Fusion Expression with Ssp DnaB Mini-Intein in the Baculovirus Expression System. Viruses. 2018; 10(10):523. https://doi.org/10.3390/v10100523
Chicago/Turabian StyleGwak, Won Seok, Jae Bang Choi, Beom Ku Han, Sung Min Bae, and Soo Dong Woo. 2018. "Enhanced Production of Recombinant Protein by Fusion Expression with Ssp DnaB Mini-Intein in the Baculovirus Expression System" Viruses 10, no. 10: 523. https://doi.org/10.3390/v10100523
APA StyleGwak, W. S., Choi, J. B., Han, B. K., Bae, S. M., & Woo, S. D. (2018). Enhanced Production of Recombinant Protein by Fusion Expression with Ssp DnaB Mini-Intein in the Baculovirus Expression System. Viruses, 10(10), 523. https://doi.org/10.3390/v10100523