Recovery of Functional Diversity Following Shifting Cultivation in Tropical Monsoon Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.2.1. Plot Investigation
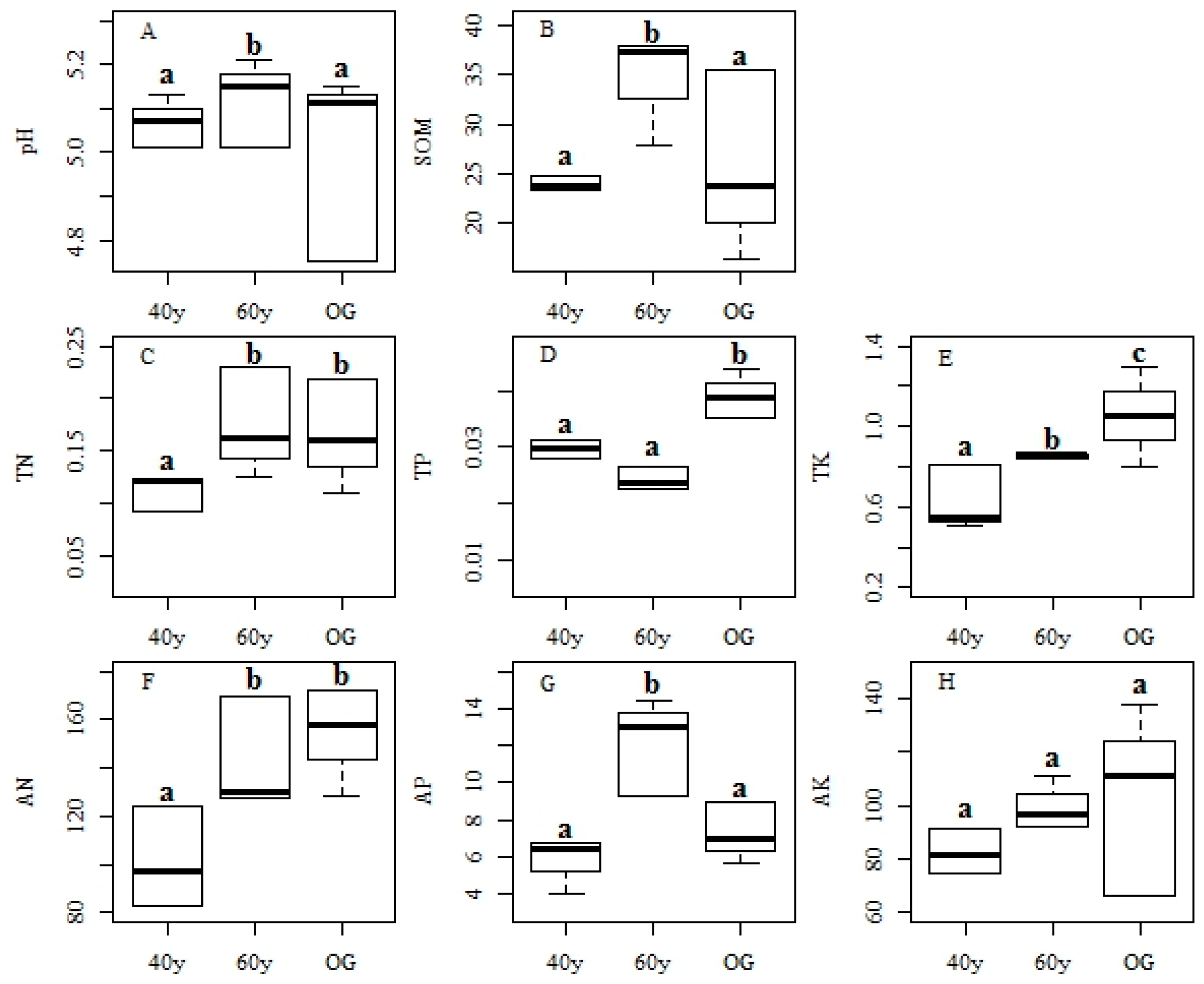
2.2.2. Soil Data
2.2.3. Functional Trait Data
2.3. Statistical Analysis
2.3.1. Forest Structure and Species Diversity
2.3.2. Functional Traits and Functional Diversity
2.3.3. Environmental Effects
3. Results
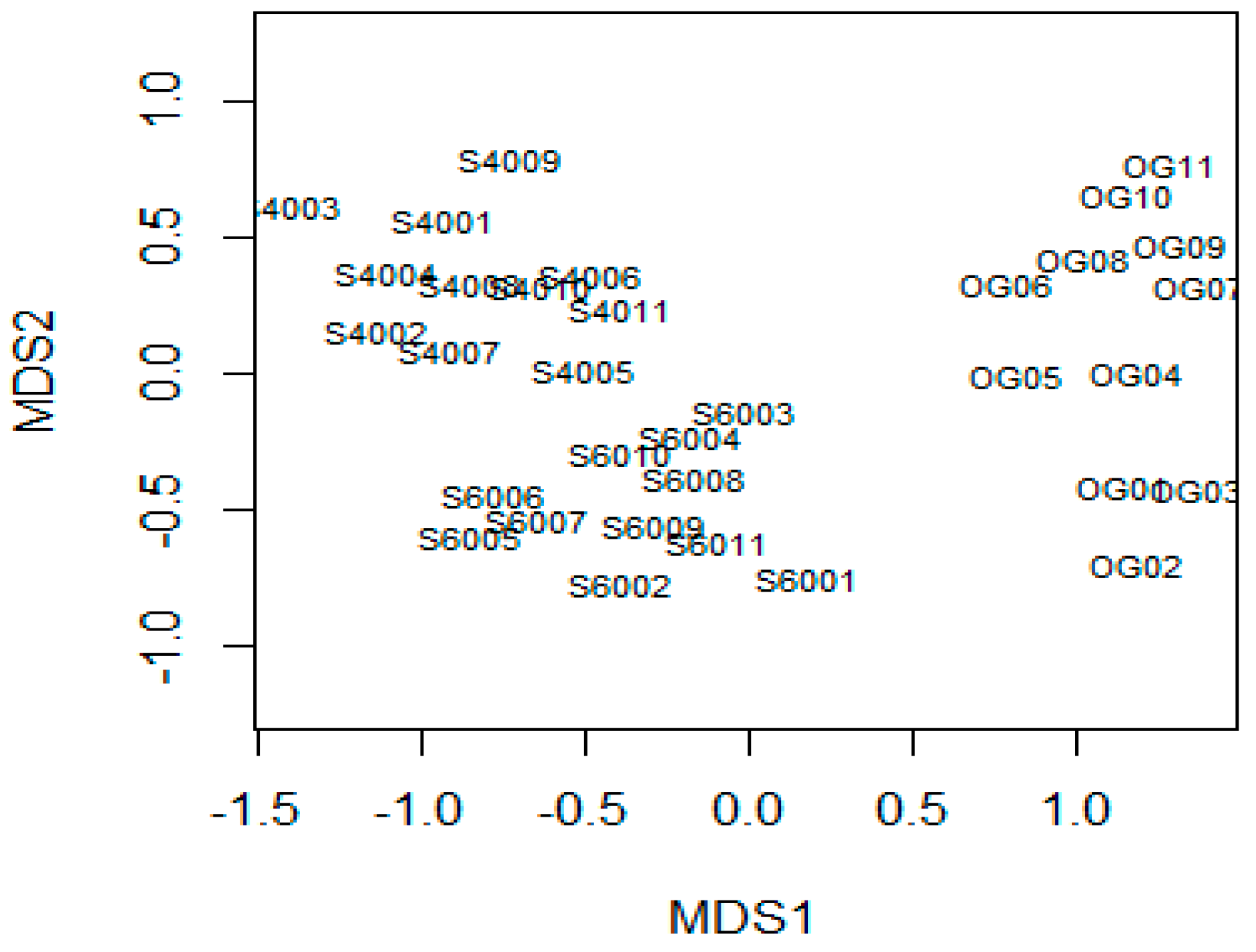
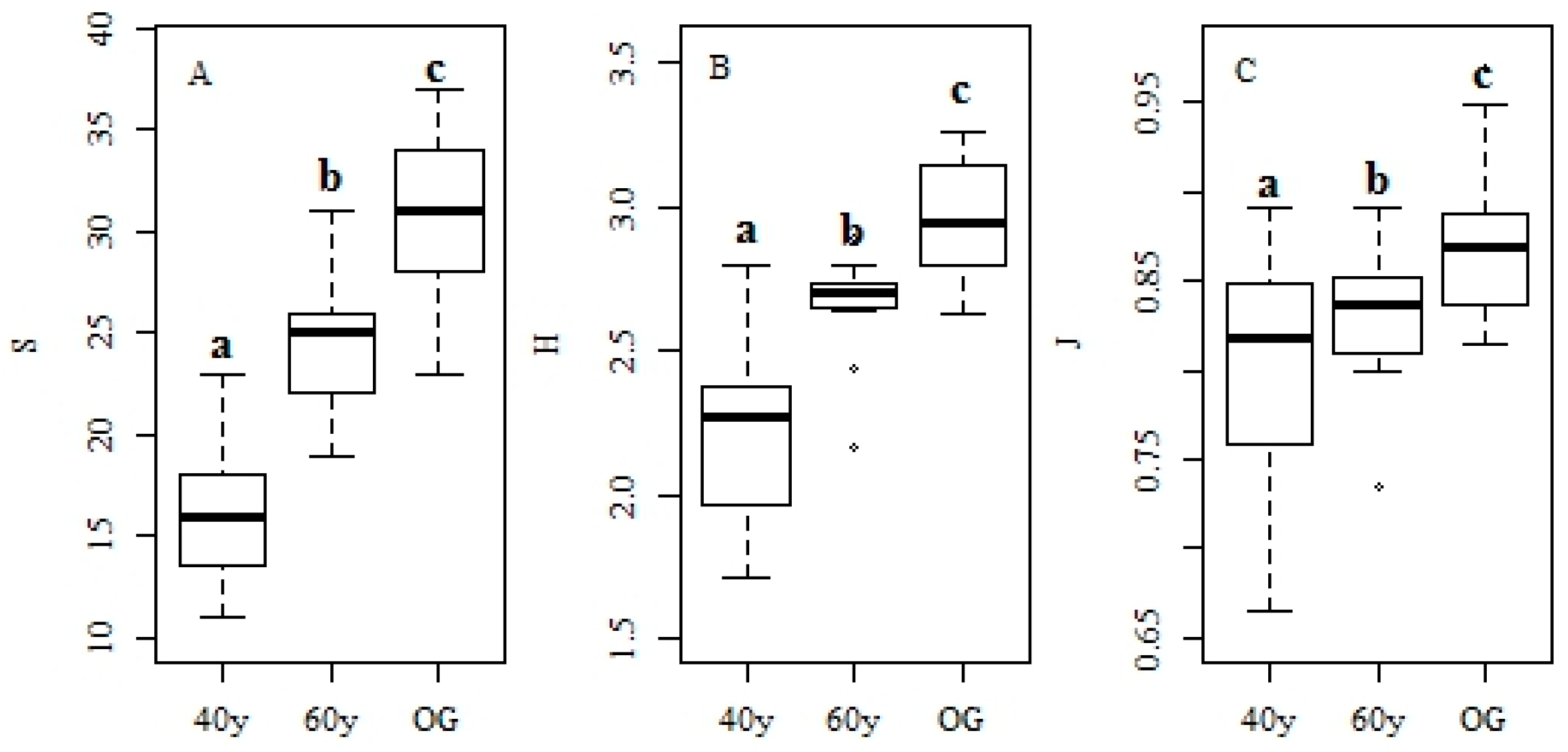
3.1. Changes in Forest Structure and Species Diversity during Succession
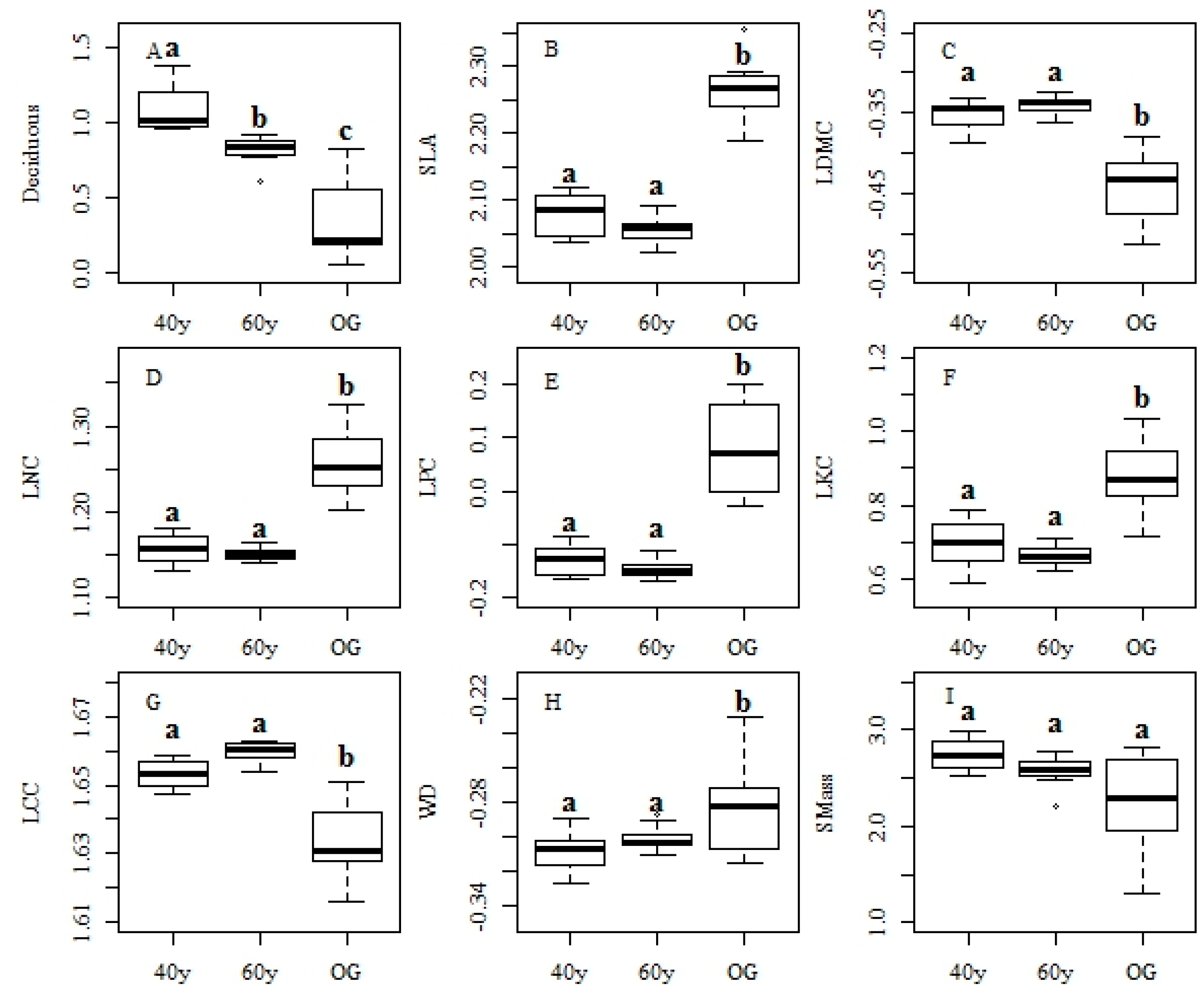
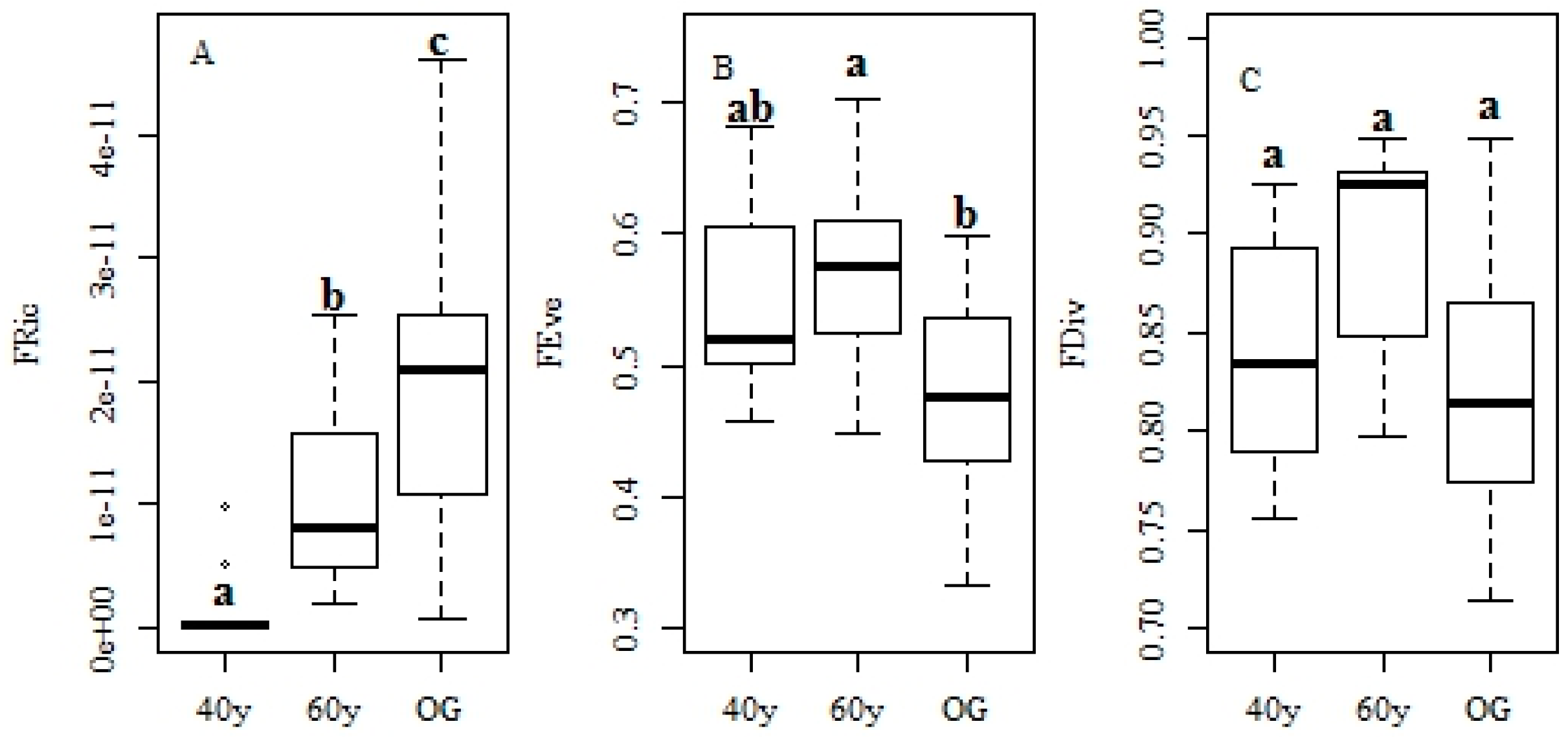
3.2. Changes in Functional Traits and Functional Diversity during Succession
3.3. Changes in Environmental Factors and Effects on Functional Features during Succession
4. Discussion
4.1. Recovery of Forest Structure and Species Diversity in Tropical Monsoon Forests
4.2. Recovery Patterns of Community Level Functional Traits in Tropical Monsoon Forests
4.3. Recovery of Functional Diversity in Tropical Monsoon Forests
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | 40y | 60y | OG | Preferred Time | Indicator Value | p |
|---|---|---|---|---|---|---|
| Castanopsis echinocarpa Miq. | 259 | 201 | 0 | 40y | 56.3 | 0.0022 |
| Aporosa yunnanensis (Pax & K.Hoffm.) F.P.Metcalf | 113 | 65 | 1 | 40y | 63.1 | 0.0004 |
| Cyclobalanopsis fleuryi (Hickel & A.Camus) W.T.Chun | 61 | 0 | 0 | 40y | 72.7 | 0.0002 |
| Castanopsis hystrix Miq. | 37 | 15 | 1 | 40y | 57.1 | 0.0018 |
| Castanopsis mekongensis A.Camus | 31 | 14 | 1 | 40y | 55.1 | 0.0036 |
| Adenanthera pavonina L. | 18 | 1 | 0 | 40y | 68.9 | 0.0002 |
| Ternstroemia gymnanthera (Wight & Arn.) Sprague | 7 | 0 | 0 | 40y | 36.4 | 0.0252 |
| Winchia calophylla A.DC. | 6 | 1 | 0 | 40y | 39 | 0.0272 |
| Machilus austroguizhouensis S.K. Lee & F.N. Wei | 89 | 143 | 11 | 60y | 58.8 | 0.0006 |
| Ardisia tenera Mez | 22 | 63 | 21 | 60y | 48.6 | 0.0376 |
| Garcinia yunnanensis Hu | 20 | 125 | 17 | 60y | 77.2 | 0.0002 |
| Pittosporopsis kerrii Craib | 19 | 155 | 112 | 60y | 54.2 | 0.0056 |
| Engelhardtia spicata Lechen ex Blume | 6 | 19 | 1 | 60y | 39.9 | 0.0442 |
| Metadina trichotoma (Zoll. & Moritzi) Bakh.f. | 5 | 12 | 1 | 60y | 42.4 | 0.0326 |
| Memecylon polyanthum H.L. Li | 2 | 19 | 0 | 60y | 57.6 | 0.001 |
| Syzygium forrestii Merr. & L.M.Perry | 2 | 10 | 0 | 60y | 45.5 | 0.0108 |
| Litchi chinensis Sonn. | 0 | 26 | 10 | 60y | 52.5 | 0.0072 |
| Artocarpus heterophyllus Lam | 0 | 33 | 6 | 60y | 61.5 | 0.0006 |
| Elae spp. | 0 | 30 | 3 | 60y | 49.6 | 0.0062 |
| Alchornea tiliifolia (Benth.) Müll.Arg. | 0 | 25 | 1 | 60y | 69.9 | 0.0002 |
| Cryptocarya densiflora Blume | 0 | 11 | 0 | 60y | 45.5 | 0.0048 |
| Schefflera octophylla (Lour.) Harms | 0 | 10 | 0 | 60y | 54.5 | 0.0012 |
| Barringtonia racemosa (L.) Spreng. | 0 | 9 | 0 | 60y | 54.5 | 0.0016 |
| Xanthophyllum siamense Comm. | 4 | 0 | 12 | Old | 40.9 | 0.0068 |
| Macropanax oreophilus Miq. | 3 | 1 | 15 | Old | 43.1 | 0.021 |
| Saprosma ternatum (Wall.) Hook.f. | 0 | 0 | 71 | Old | 63.6 | 0.0004 |
| Baccaurea ramiflora Lour. | 0 | 1 | 44 | Old | 97.8 | 0.0002 |
| Polyalthia cheliensis Hu | 0 | 0 | 42 | Old | 63.6 | 0.0008 |
| Parashorea chinensis Hsie Wang | 0 | 0 | 39 | Old | 63.6 | 0.0002 |
| Aidia yunnanensis (Hutch.) T.Yamaz. | 0 | 0 | 29 | Old | 63.6 | 0.0002 |
| Gironniera subaequalis Planch. | 0 | 2 | 28 | Old | 67.9 | 0.0002 |
| Diospyros xishuangbannaensis C.Y.Wu & H.Chu | 0 | 0 | 28 | Old | 63.6 | 0.0002 |
| Drypetes indica (Müll.Arg.) Pax & K.Hoffm. | 0 | 6 | 20 | Old | 62.9 | 0.0006 |
| Diospyros atrotricha H.W.Li | 0 | 1 | 13 | Old | 42.2 | 0.008 |
| Chisocheton siamensis Craib | 0 | 0 | 13 | Old | 45.5 | 0.0046 |
| Diospyros nigrocortex C.Y.Wu | 0 | 0 | 13 | Old | 36.4 | 0.025 |
| Knema linifolia (Roxb.) Warb. | 0 | 8 | 12 | Old | 49.1 | 0.0076 |
| Ficus langkokensis Drake | 0 | 0 | 11 | Old | 63.6 | 0.0002 |
| Cinnamomum bejolghota (Buch.-Ham.) Sweet | 0 | 0 | 11 | Old | 45.5 | 0.0056 |
| Dysoxylum excelsum Blume | 0 | 0 | 10 | Old | 54.5 | 0.0022 |
| Antidesma montanum Blume | 0 | 0 | 9 | Old | 63.6 | 0.0008 |
| Dichapetalum gelonioides (Roxb.) Engl. | 0 | 0 | 8 | Old | 63.6 | 0.0006 |
| Pometia tomentosa (Blume) Teijsm. & Binn. | 0 | 0 | 7 | Old | 54.5 | 0.0018 |
| Eurya acromonodontus W.R.Barker | 0 | 0 | 6 | Old | 36.4 | 0.022 |
| Garcinia lancilimba C.Y.Wu ex Y.H.Li | 0 | 0 | 5 | Old | 45.5 | 0.0048 |
| Dysoxylum binectariferum (Roxb.) Hook.f. ex Bedd. | 0 | 0 | 5 | Old | 36.4 | 0.0308 |
| Cryptocarya yunnanensis H.W.Li | 0 | 0 | 4 | Old | 36.4 | 0.0238 |
References
- Vicente-Silva, J.; Bergamin, R.S.; Zanini, K.J.; Pillar, V.D.; Müller, S.C. Assembly patterns and functional diversity of tree species in a successional gradient of Araucaria forest in Southern Brazil. Nat. Conserv. 2016, 14, 67–73. [Google Scholar] [CrossRef]
- Boukili, V.K.; Chazdon, R.L. Environmental filtering, local site factors and landscape context drive changes in functional trait composition during tropical forest succession. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 37–47. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Bongers, F.; Poorter, L. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 2010, 91, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Lasky, J.R.; Uriarte, M.; Boukili, V.K.; Chazdon, R.L. Trait-mediated assembly processes predict successional changes in community diversity of tropical forests. Proc. Natl. Acad. Sci. USA 2014, 111, 5616–5621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiners, S.J.; Cadotte, M.W.; Fridley, J.D.; Pickett, S.T.A.; Walker, L.R. Is successional research nearing its climax? New approaches for understanding dynamic communities. Funct. Ecol. 2015, 29, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Westoby, M.; Wright, I.J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, D.C.; Joshi, C.; van Bodegom, P.M.; Bastow, Z.A.; Fulé, P.Z. A predictive model of community assembly that incorporates intraspecific trait variation. Ecol. Lett. 2012, 15, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Menge, D.N.L.; Chazdon, R.L. Higher survival drives the success of nitrogen-fixing trees through succession in Costa Rican rainforests. New Phytol. 2015, 209, 965–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, D.L.M.; Scariot, A. Principles of natural regeneration of tropical dry forests for restoration. Restor. Ecol. 2006, 14, 11–20. [Google Scholar] [CrossRef]
- Lohbeck, M.; Lebrija-Trejos, E.; Martínez-Ramos, M.; Meave, J.A.; Poorter, L.; Bongers, F. Functional trait strategies of trees in dry and wet tropical forests are similar but differ in their consequences for succession. PLoS ONE 2015, 10, e0123741. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L. Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. New Phytol. 2009, 181, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Plourde, B.T.; Boukili, V.K.; Chazdon, R.L. Radial changes in wood specific gravity of tropical trees: Inter- and intra-specific variation during secondary succession. Funct. Ecol. 2015, 29, 111–120. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Paz, H.; Pla, L.; van Breugel, M.; Martínez-Ramos, M.; Bongers, F. Functional diversity changes during tropical forest succession. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 89–96. [Google Scholar] [CrossRef]
- Bu, W.; Zang, R.; Ding, Y. Field observed relationships between biodiversity and ecosystem functioning during secondary succession in a tropical lowland rainforest. Acta Oecol. 2014, 55, 1–7. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Bongers, F. Biomass is the main driver of changes in ecosystem process rates during tropical forest succession. Ecology 2015, 96, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Lebrija-Trejos, E.; Perez, G.E.A.; Meave, J.A.; Poorter, L.; Bongers, F. Environmental changes during secondary succession in a tropical dry forest in Mexico. J. Trop. Ecol. 2011, 27, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Pineda-Garcı’a, F.; Paz, H.; Meinzer, F.C. Drought resistance in early and late secondary successional species from a tropical dry forest: The interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell Environ. 2013, 36, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Balvanera, P.; Quijas, S.; Perez-Jimenez, A. Distribution patterns of tropical dry forest trees along a mesoscale water availability gradient. Biotropica 2011, 43, 414–422. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R.; Liu, S.; He, F.; Letcher, S.G. Recovery of woody plant diversity in tropical rain forests in southern China after logging and shifting cultivation. Biol. Conserv. 2012, 145, 225–233. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Díaz, S.; et al. Functional traits and the growth—Mortality trade-off in tropical trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y. Vegetation of China; Science Press: Beijing, China, 1980; pp. 363–397. [Google Scholar]
- Editorial Committee of Flora of China. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1988. [Google Scholar]
- Long, W.; Zang, R.; Schamp, B.S.; Ding, Y. Within- and among-species variation in specific leaf area drive community assembly in a tropical cloud forest. Oecologia 2011, 167, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.A.; Geiger, E.L.; Gotsch, S.G.; Rossatto, D.R.; Silva, L.C.R.; Lau, O.L.; Haridasan, M.; Franco, A.C. Ecological thresholds at the savanna-forest boundary: How plant traits, resources and fire govern the distribution of tropical biomes. Ecol. Lett. 2012, 15, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Zang, R.; Ding, Y.; Letcher, S.G.; Long, W.; Huang, Y. Variations and trade-offs in functional traits of tree seedlings during secondary succession in a tropical lowland rain forest. Biotropica 2014, 46, 404–414. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Lewis, O.T.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poorter, L.; Wright, S.J.; Paz, H.; Ackerly, D.D.; Condit, R.; Ibarra-Manríquez, G.; Harms, K.E.; Licona, J.C.; Martínez-Ramos, M.; Mazer, S.J.; et al. Are Functional Traits Good Predictors of Demographic Rates? Evidence from Five Neotropical Forests. Ecology 2008, 89, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Research review. Components of leaf dry mass per area—Thickness and density—Alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999, 144, 35–47. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cheng, K.; Zang, R.; Ding, Y. Environmental filtering of species with different functional traits into plant assemblages across a tropical coniferous-broadleaved forest ecotone. Plant Soil 2014, 380, 361–374. [Google Scholar] [CrossRef]
- Gusmán-M, E.; de la Cruz, M.; Espinosa, C.I.; Escudero, A. Focusing on individual species reveals the specific nature of assembly mechanisms in a tropical dry-forest. Perspect. Plant Ecol. Evol. Syst. 2018. [Google Scholar] [CrossRef]
- Hoeber, S.; Leuschner, C.; Köhler, L.; Arias-Aguilar, D.; Schuldt, B. The importance of hydraulic conductivity and wood density to growth performance in eight tree species from a tropical semi-dry climate. For. Ecol. Manag. 2014, 330, 126–136. [Google Scholar] [CrossRef]
- Chave, J.; Muller-Landau, H.C.; Baker, T.R.; Easdale, T.A.; Steege, H.T.; Webb, C.O. Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol. Appl. 2006, 16, 2356–2367. [Google Scholar] [CrossRef]
- The State Forestry Administration. Seeds of Woody Plants in China; China Forestry Publishing House: Devon, UK, 2001.
- Kew, R.B.G. Seed Information Database (SID), Version 7.1. Available online: http://www.rbgkew.org. uk/data/sid/ (accessed on 1 May 2008).
- Maeshiro, R.; Kusumoto, B.; Fujii, S.; Shiono, T.; Kubota, Y. Using tree functional diversity to evaluate management impacts in a subtropical forest. Ecosphere 2013, 4, 1–17. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D. Causes, consequences and ethics of biodiversity. Nature 2000, 405, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Functional Diversity. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 109–120. [Google Scholar]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.G.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field—Methodology matters! Funct. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Rangel, T.F.; Diniz, J.A.F.; Bini, L.M. SAM: A comprehensive application for spatial analysis in macroecology. Ecography 2010, 33, 46–50. [Google Scholar] [CrossRef]
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–356. [Google Scholar] [CrossRef]
- Denslow, J.S.; Guzman, S. Variation in stand structure, light, and seedling abundance across a tropical moist forest chronosequence, Panama. J. Veg. Sci. 2000, 11, 201–212. [Google Scholar] [CrossRef]
- Letcher, S.G.; Chazdon, R.L. Rapid recovery of biomass, species richness, and species composition in a forest chronosequence in Northeastern Costa Rica. Biotropica 2009, 41, 608–617. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; McGraw-Hill Publishing Company: New York, NY, USA, 1990. [Google Scholar]
- Hu, J.; Herbohn, J.; Chazdon, R.L.; Baynes, J.; Wills, J.; Meadows, J.; Sohel, M.S.I. Recovery of species composition over 46 years in a logged Australian tropical forest following different intensity silvicultural treatments. For. Ecol. Manag. 2018, 409, 660–666. [Google Scholar] [CrossRef]
- Lawrence, D. Erosion of tree diversity during 200 years of shifting cultivation in Bornean rain forest. Ecol. Appl. 2004, 14, 1855–1869. [Google Scholar] [CrossRef]
- Chazdon, R.L. Regenerating tropical forest ecosystems. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 277–286. [Google Scholar]
- Baker, P.J.; Bunyavejchewin, S.; Oliver, C.D.; Ashton, D.H. Disturbance history and historical stand dynamics of a seasonal tropical forest in Wentern Thailand. Ecol. Monogr. 2005, 75, 317–343. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Dent, D.H.; DeWalt, S.J.; Denslow, J.S. Secondary forests of central Panama increase in similarity to old-growth forest over time in shade tolerance but not species composition. J. Veg. Sci. 2013, 24, 530–542. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Rodriguez-Velázquez, J.; van Breugel, M.; Bongers, F.; Dalling, J. Changing drivers of species dominance during tropical forest succession. Funct. Ecol. 2014, 28, 1052–1058. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.-H.; Cao, M.; Yu, G.-R.; Tang, J.-W.; Deng, X.-B.; Song, Q.-H.; Tang, Y.; Zheng, Z.; Liu, W.-J.; Feng, Z.-L.; et al. High sensitivity of a tropical rainforest to water variability: Evidence from 10 years of inventory and eddy flux data. J. Geophys. Res. Atmos. 2013, 118, 9393–9400. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Cao, M.; Stoy, P.C.; Zhang, Y. Assessing self-organization of plant communities—A thermodynamic approach. Ecol. Model. 2009, 220, 784–790. [Google Scholar] [CrossRef]
- Baraloto, C.; Hardy, O.J.; Paine, C.E.T.; Dexter, K.G.; Cruaud, C.; Dunning, L.T.; Gonzalez, M.-A.; Molino, J.-F.; Sabatier, D.; Savolainen, V.; et al. Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities. J. Ecol. 2012, 100, 690–701. [Google Scholar] [CrossRef] [Green Version]
- Carreño-Rocabado, G.; Peña-Claros, M.; Bongers, F.; Alarcón, A.; Licona, J.-C.; Poorter, L. Effects of disturbance intensity on species and functional diversity in a tropical forest. J. Ecol. 2012, 100, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Kattge, J.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—A global database of plant traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef] [Green Version]
- Mason, N.W.H.; Richardson, S.J.; Peltzer, D.A.; de Bello, F.; Wardle, D.A.; Allen, R.B. Changes in coexistence mechanisms along a long-term soil chronosequence revealed by functional trait diversity. J. Ecol. 2012, 100, 678–689. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.S.; Carlos, P.P.; Pinto, V.D.; Meireles, A.; Negreiros, D.; Morellato, L.P.C.; Fernandes, G.W. Connection between tree functional traits and environmental parameters in an archipelago of montane forests surrounded by rupestrian grasslands. Flora 2018, 238, 51–59. [Google Scholar] [CrossRef]
- Lachenbruch, B.; McCulloh, K.A. Traits, properties, and performance how woody plants combine hydraulic and mechanical functions in a cell, tissue, or whole plant—Lachenbruch. New Phytol. 2014, 204, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Padilha, D.L.; De Marco Júnior, P. A gap in the woods: Wood density knowledge as impediment to develop sustainable use in Atlantic. For. Ecol. Manag. 2018, 424, 448–457. [Google Scholar] [CrossRef]
- Ali, A.; Mattsson, E. Wood density is a sustainability indicator for the management of dry zone homegarden agroforests: Evidences from biodiversity–ecosystem function relationships. Ecol. Indic. 2018. [Google Scholar] [CrossRef]
- Zanne, A.E.; Westoby, M.; Falster, D.S.; Ackerly, D.D.; Loarie, S.R.; Arnold, S.E.J.; Coomes, D.A. Angiosperm wood structure: Global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 2010, 97, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.; Firn, J.; Lamb, D.; Herbohn, J. Wood density: A tool to find complementary species for the design of mixed species plantations. For. Ecol. Manag. 2014, 334, 106–113. [Google Scholar] [CrossRef]
- Pratt, R.B.; Jacobsen, A.L.; Ewers, F.W.; Davis, S.D. Relationships among xylem transport, biomechanics and storage in stems and roots. New Phytol. 2007, 174, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Miralles-Wilhelm, F. Biophysical properties and functional significance of stem water storage tissues in Neotropical savanna trees. Plant Cell Environ. 2007, 30, 236–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, S.R.; Rodella, R.A.; Angyalossy, V.; Marcati, C.R. Structural variations in root and stem wood of Styrax (Styracaceae) from Brazilian forest and cerrado. IAWA J. 2007, 28, 173–188. [Google Scholar] [CrossRef]
- Ackerly, Y. Functional strategies of chaparral shubs in relation to seaonal water defict and disturbance. Ecol. Monogr. 2004, 74, 25–44. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worbes, M.; Blanchart, S.; Fichtler, E. Relations between water balance, wood traits and phenological behavior of tree species from a tropical dry forest in Costa Rica—A multifactorial study. Tree Physiol. 2013, 33, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Ball, M.C.; Luly, J.G.; Holtum, J.A.M. Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees 2005, 19, 305–311. [Google Scholar] [CrossRef]
- Niklas, K.J.; Spatz, H.C. Wordwide correlation of mechanical properites and wood density. Am. J. Bot. 2010, 97, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Pittermann, J.; Choat, B.; Jansen, S.; Stuart, S.A.; Lynn, L.; Dawson, T.E. The Relationships between Xylem Safety and Hydraulic Efficiency in the Cupressaceae: The Evolution of Pit Membrane Form and Function. Plant Physiol. 2010, 153, 1919–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lens, F.; Sperry, J.S.; Christman, M.A.; Choat, B.; Rabaey, D.; Jansen, S. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol. 2011, 190, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Markesteijn, L.; Poorter, H.; Paz, H.; Sack, L. Ecological dfferentiation in xylemcavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ. 2011, 34, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Iglesias, B.; Villar, R.; Poorter, L. Functional traits predict drought performance and distribution of Mediterranean woody species. Acta Oecol. 2014, 56, 10–18. [Google Scholar] [CrossRef]
- Ster Steege, H.; Hammond, D.S. Character convergence, diversity, and distrubance in tropical rain forest in Guyana. Ecology 2001, 82, 3197–3212. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Lebrija-Teros, E.; Matrinez-Ramos, M.; Maeave, J.A.; Paz, H.; Perez-Garcia, E.A.; Romero-Perez, I.E.; Tauro, A.; Bongers, F. Successional changes in functional composition contrast for dry and wet tropical forest. Ecology 2013, 94, 1211–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, S.; Janson, C.H. The relationship between seed size and establishment conditions in tropical woody plants. Ecology 1985, 66, 773–780. [Google Scholar] [CrossRef]
- Dalling, J.W.; Hubbel, S.P. Seed size, growth rate and gap microsite conditions as determinants of recruitment success for pioneer species. J. Ecol. 2002, 90, 557–568. [Google Scholar] [CrossRef] [Green Version]
- Hammond, D.S.; Brown, W.K. Seed size of woody plants in relation to disturbance, dispersal, soil type in wet Neotropical forests. Ecology 1995, 76, 2544–2561. [Google Scholar] [CrossRef]
- Jansen, P.A.; Bongers, F.; van der Meer, P.J. Is farther seed dispersal better? Spatial patterns of offspring mortality in three rainforest tree species with different dispersal abilities? Ecography 2008, 31, 43–52. [Google Scholar] [CrossRef]
- Salvador-Van Eysenrode, D.; Bogaert, J.; van Hecke, P.; Impens, I. Forest canopy perforation in time and space in Amazonian Ecuador. Acta Oecol. 2000, 21, 285–291. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
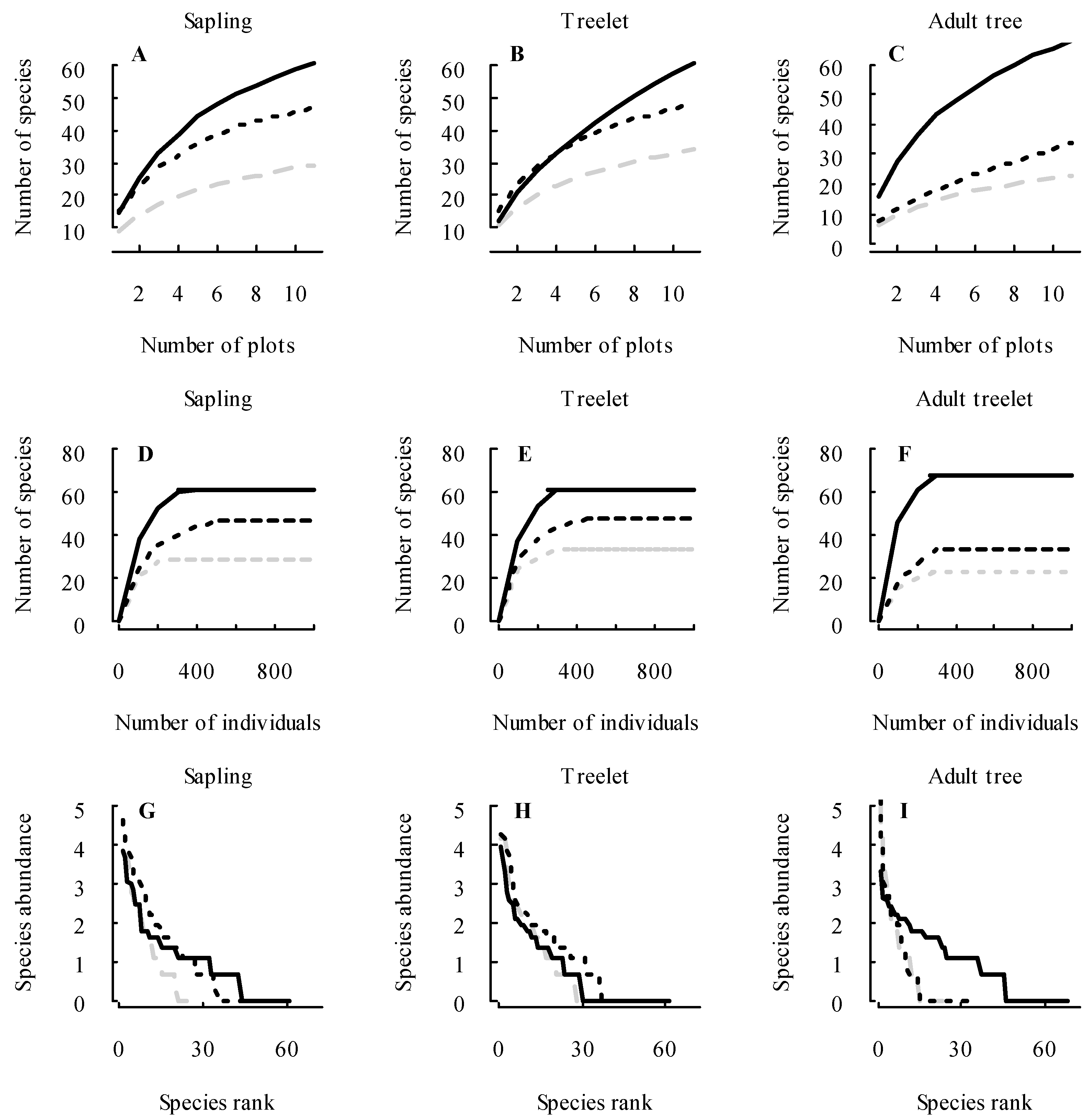
| Tree Size | No. of Stems | No. of Species | No. Species Showing Preference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40y | 60y | OG | Total | 40y | 60y | OG | Total | 40y | 60y | OG | Total | |
| Saplings | 235 | 482 | 308 | 1025 | 29 | 47 | 61 | 97 | 4 | 11 | 9 | 24 |
| Treelets | 312 | 427 | 251 | 990 | 34 | 48 | 61 | 101 | 6 | 9 | 8 | 23 |
| Adult trees | 275 | 296 | 270 | 841 | 23 | 34 | 68 | 94 | 3 | 2 | 12 | 17 |
| Total | 822 | 1205 | 829 | 2856 | 44 | 65 | 102 | 135 | 8 | 15 | 24 | 47 |
| Deciduous | SLA | LDMC | LNC | LPC | LKC | LCC | WD | Smass | S | H | J | Log_FRic | FEve | FDiv | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | #219 | #484 | #493 | #457 | #107 | #363 | #484 | #255 | #194 | #131 | #1 | #384 | #226 | #481 | #380 |
| r_adj | 0.799 | 0.777 | 0.611 | 0.806 | 0.824 | 0.317 | 0.672 | 0.105 | −0.292 | 0.644 | 0.415 | −0.197 | 0.384 | −0.324 | 0.429 |
| Rtime | −0.976 | +0.664 | −0.442 | +0.695 | +0.863 | +0.293 | −0.314 | +0.67 | −0.982 | +0.991 | +0.971 | +0.219 | +0.987 | −0.38 | −0.217 |
| Log_pH | +0.382 | −0.577 | +0.492 | −0.544 | −0.917 | −0.6 | +0.329 | +0.444 | −0.317 | +0.275 | +0.216 | −0.458 | −0.217 | −0.239 | −0.531 |
| Log_SOM | +0.214 | −0.338 | +0.292 | −0.308 | −0.274 | −0.44 | +0.352 | −0.277 | +0.261 | +0.609 | +0.237 | −0.259 | +0.229 | +0.344 | +0.39 |
| Log_TN | −0.959 | +0.267 | +0.289 | +0.514 | +0.422 | −0.319 | +0.482 | +0.399 | +0.324 | −0.525 | +0.205 | +0.236 | −0.283 | −0.26 | −0.377 |
| Log_TP | −0.247 | +0.94 | −0.887 | +0.783 | +0.706 | +0.751 | −0.924 | +0.425 | +0.238 | +0.265 | +0.218 | +0.274 | −0.569 | −0.486 | +0.428 |
| Log_TK | +0.224 | −0.415 | +0.296 | −0.572 | −0.659 | +0.467 | +0.519 | −0.262 | +0.433 | −0.297 | +0.289 | +0.581 | −0.256 | −0.249 | −0.469 |
| Log_AN | +0.966 | +0.434 | −0.777 | −0.418 | −0.307 | +0.522 | −0.921 | −0.262 | +0.226 | +0.254 | +0.197 | +0.204 | +0.214 | −0.388 | −0.797 |
| Log_AP | −0.212 | +0.51 | −0.32 | +0.667 | −0.55 | +0.388 | −0.393 | −0.396 | +0.261 | +0.251 | +0.245 | −0.243 | +0.532 | +0.437 | +0.802 |
| Log_AK | +0.335 | +0.386 | +0.565 | +0.785 | +0.978 | −0.465 | +0.374 | +0.492 | +0.27 | −0.335 | +0.24 | +0.541 | +0.227 | −0.252 | +0.68 |
| DistCntr | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I | Moran’s I |
| 0.199 | 0.023 | −0.187 | −0.156 | −0.169 | −0.189 | −0.105 | −0.207 | 0.036 | −0.048 | −0.057 | −0.072 | −0.057 | −0.075 | −0.046 | 0.187 |
| 0.601 | −0.165 | −0.113 | −0.031 | −0.117 | −0.077 | −0.138 | 0.033 | −0.069 | −0.083 | −0.179 | −0.22 | −0.255 | −0.131 | −0.128 | 0.229 |
| 1.132 | 0.163 | −0.043 | −0.089 | 0.025 | −0.012 | −0.022 | −0.074 | 0.238 | −0.005 | 0.111 | 0.154 | 0.091 | 0.094 | 0.033 | 0.042 |
| 1.6 | 0.005 | 0.259 | 0.112 | 0.235 | 0.18 | 0.148 | 0.069 | −0.046 | 0.058 | 0.019 | 0.007 | 0.036 | −0.074 | −0.154 | −0.115 |
| 1.873 | −0.274 | −0.113 | −0.015 | −0.133 | −0.026 | −0.122 | 0.098 | −0.28 | 0.207 | −0.169 | −0.164 | −0.114 | −0.14 | 0.05 | −0.194 |
| 2.106 | −0.054 | −0.145 | −0.114 | −0.15 | −0.161 | −0.098 | −0.188 | −0.025 | −0.21 | 0.005 | 0.132 | 0.072 | 0.139 | 0.031 | −0.196 |
| 2.396 | −0.122 | 0.176 | 0.064 | 0.121 | 0.099 | 0.146 | 0.078 | −0.022 | −0.073 | −0.156 | −0.173 | 0.014 | −0.24 | −0.025 | −0.244 |
| 2.793 | 0.139 | −0.1 | 0.007 | −0.096 | −0.069 | −0.089 | −0.026 | −0.099 | −0.059 | 0.142 | 0.062 | −0.048 | 0.148 | 0.052 | 0.057 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, F.; He, Y.; Zang, R. Recovery of Functional Diversity Following Shifting Cultivation in Tropical Monsoon Forests. Forests 2018, 9, 506. https://doi.org/10.3390/f9090506
Deng F, He Y, Zang R. Recovery of Functional Diversity Following Shifting Cultivation in Tropical Monsoon Forests. Forests. 2018; 9(9):506. https://doi.org/10.3390/f9090506
Chicago/Turabian StyleDeng, Fuying, Yunling He, and Runguo Zang. 2018. "Recovery of Functional Diversity Following Shifting Cultivation in Tropical Monsoon Forests" Forests 9, no. 9: 506. https://doi.org/10.3390/f9090506
APA StyleDeng, F., He, Y., & Zang, R. (2018). Recovery of Functional Diversity Following Shifting Cultivation in Tropical Monsoon Forests. Forests, 9(9), 506. https://doi.org/10.3390/f9090506





