Influence of Altitude on Biochemical Properties of European Beech (Fagus sylvatica L.) Forest Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soils and Soil Sampling
2.3. Chemical Analysis
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
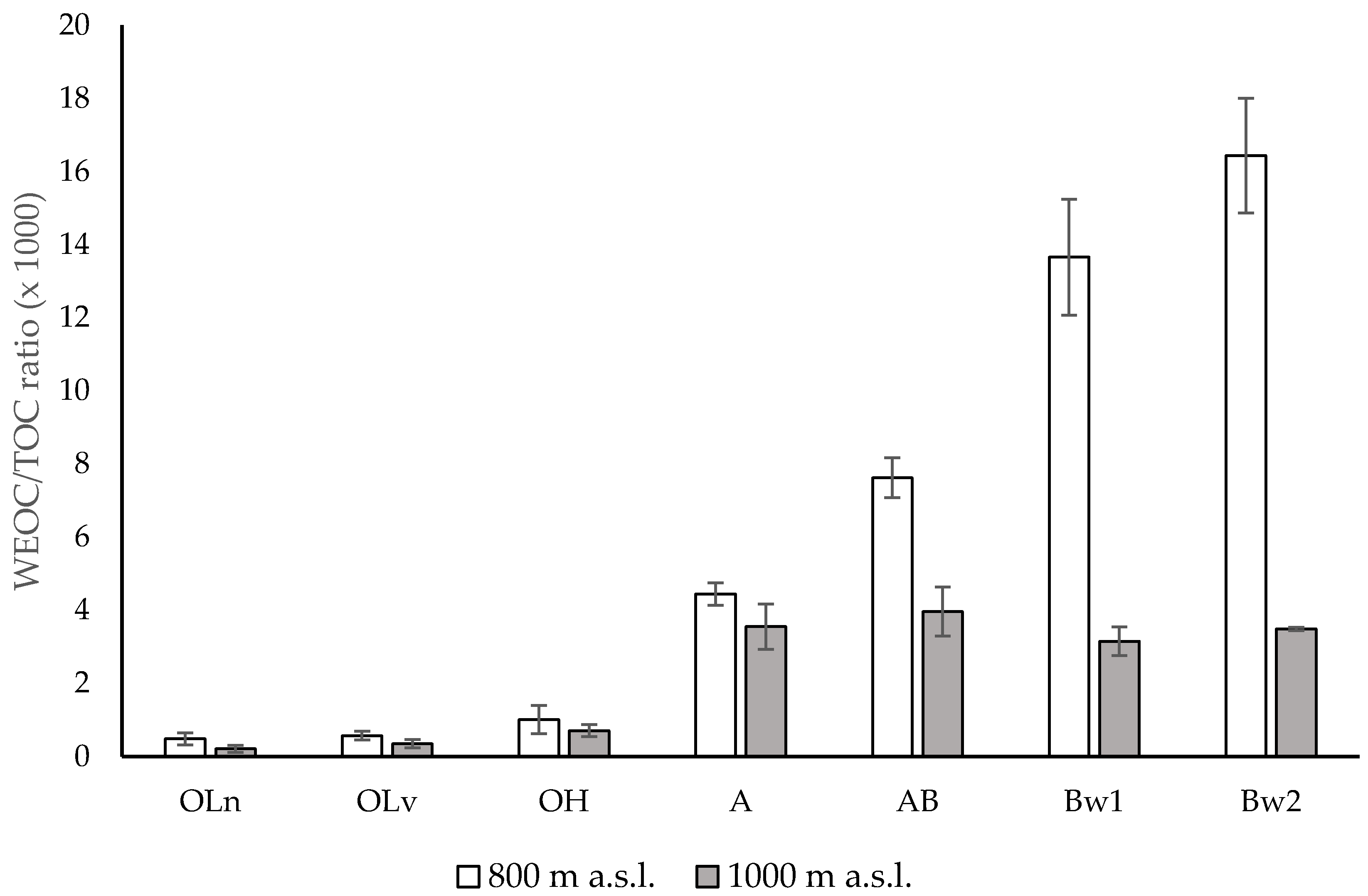
3.1. Chemical Properties
3.2. Microbial Biomass C Content, Basal Respiration and Enzymatic Activity
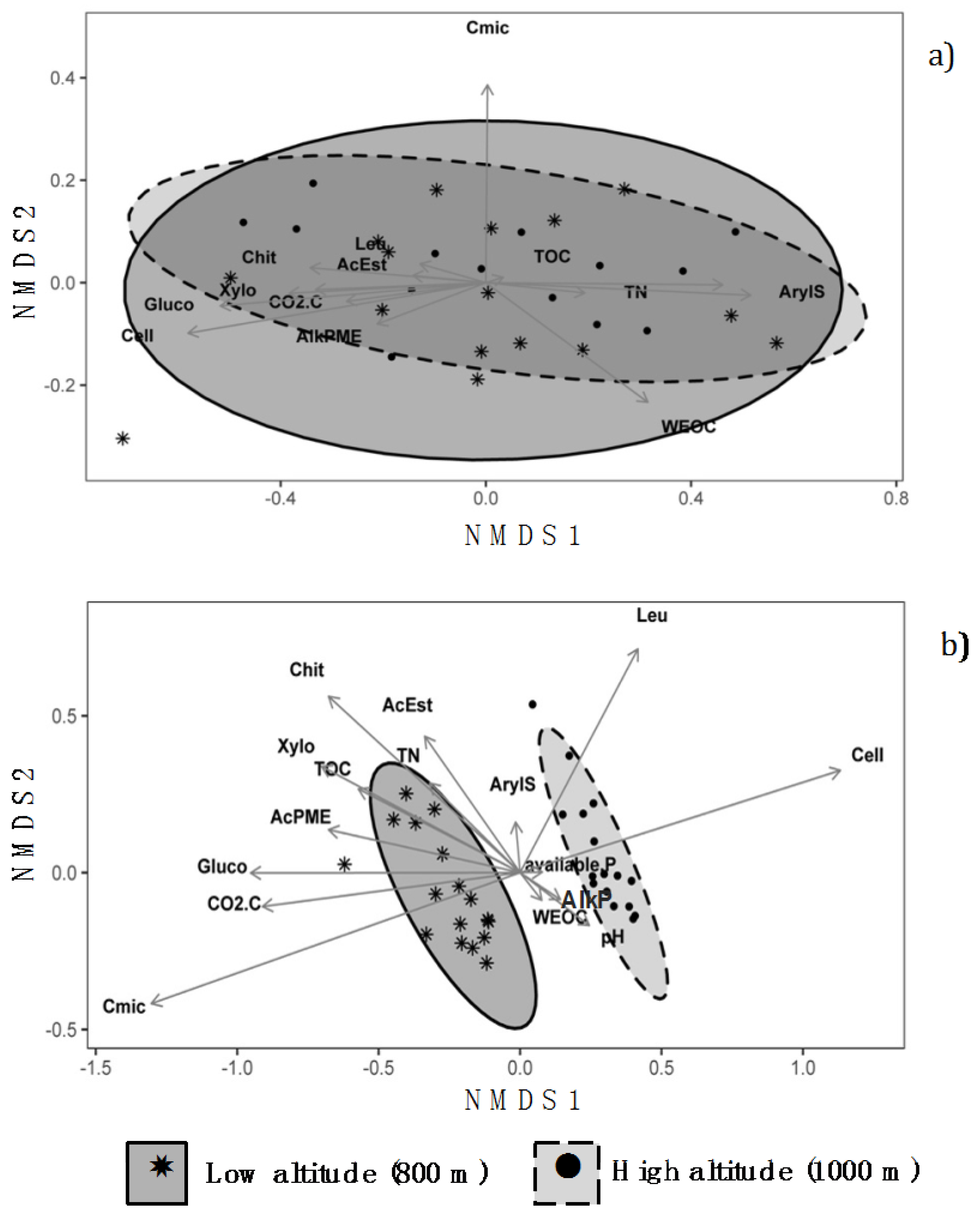
3.3. Non-Metric Multidimensional Scaling Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Summary for policymakers. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 1–30. [Google Scholar]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Di Filippo, A.; Alessandrini, A.; Maugeri, M. Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Chang. Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Forest soils and carbon sequestration. Forest Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Scarascia-Mugnozza, G.; Oswald, H.; Piussi, P.; Radoglou, K. Forests of the Mediterranean region: Gaps in knowledge and research needs. For. Ecol. Manag. 2000, 132, 97–109. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Coleman, D.C.; Crossley, D.A., Jr.; Clark, J.S. Biological indices of soil quality: An ecosystem case study of their use. For. Ecol. Manag. 2000, 138, 357–368. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leiros, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Ludwig, M.; Achtenhagen, J.; Miltner, A.; Eckhardt, K.-U.; Leinweber, P.; Emmerling, C.; Thiele-Bruhn, S. Microbial contribution to SOM quantity and quality in density fractions of temperate arable soils. Soil Biol. Biochem. 2015, 81, 311–322. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Moscatelli, M.C.; Di Tizio, A.; Mancinelli, R.; Greco, S.; Marinari, S. Soil biochemical indicators as a tool to assess the short-term impact of agricultural management on changes in organic C in a Mediterranean environment. Ecol. Indic. 2009, 9, 518–527. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The temperature dependence of organic-matter decomposition—Still a topic of debate. Soil Biol. Biochem. 2006, 38, 2510–2518. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Cox, P.; Betts, R.; Bopp, L.; Von Bloh, W.; Brovkin, V.; Cadule, P.; Doney, S.; Eby, M.; Fung, I. Climate-carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J. Clim. 2006, 19, 3337–3353. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.; Gurevitch, J.; GCTE-NEWS. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hingate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Stone, M.M.; Weiss, M.S.; Goodale, C.L.; Adams, M.B.; Fernandez, I.J.; German, D.P.; Allison, S.D. Temperature sensitivity of soil enzyme kinetics under N-fertilization in two temperate forests. Glob. Chang. Biol. 2012, 18, 1173–1184. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A.; Luo, Y. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Fierer, N.; Colman, B.P.; Schimel, J.P.; Jackson, R.B. Predicting the temperature dependence of microbial respiration in soil: A continental-scale analysis. Glob. Biogeochem. Cycles 2006, 20, GB3026. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Trasar-Cepeda, C.; Leirós, M.D.C.; Seoane, S.; Gil-Sotres, F. Intra-annual variation in biochemical properties and the biochemical equilibrium of different grassland soils under contrasting management and climate. Biol. Fertil. Soils 2011, 47, 633–645. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Miralles, I.; Ortega, R.; Sánchez-Marañón, M.; Leirós, M.C.; Trasar-Cepeda, C.; Gil-Sotres, F. Biochemical properties of range and forest soils in Mediterranean mountain environments. Biol. Fertil. Soils 2007, 43, 721–729. [Google Scholar] [CrossRef]
- Cioci, C.; Corti, G.; Agnelli, A.; Cocco, S. Role of the altitude on the organic matter preservation in soils under a secondary prairie on the Majella massif (Italy). Agrochimica 2008, 52, 313–324. [Google Scholar]
- Margesin, R.; Minerbi, S.; Schinner, F. Long-term monitoring of soil microbiological activities in two forest sites in South Tyrol in the Italian Alps. Microbes Environ. 2014, 29, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yu, G.; Zhang, X.; Ge, J.; He, N.; Wang, Q.; Wang, D. The variations in soil microbial communities, enzyme activities and their relationships with soil organic matter decomposition along the northern slope of Changbai Mountain. Appl. Soil Ecol. 2015, 86, 19–29. [Google Scholar] [CrossRef]
- Vittori Antisari, L.; Falsone, G.; Carbone, S.; Marinari, S.; Vianello, G. Douglas-fir reforestation in North Apennine (Italy): Performance on soil carbon sequestration, nutrients stock and microbial activity. Appl. Soil Ecol. 2015, 86, 82–90. [Google Scholar] [CrossRef]
- Lucas, R.W.; Casper, B.B.; Jackson, J.K.; Balser, T.C. Soil microbial communities and extracellular enzyme activity in the New Jersey Pinelands. Soil Biol. Biochem. 2007, 39, 2508–2519. [Google Scholar] [CrossRef]
- Centro Appenninico “C. Jucci”. Temperature Massime, Minime, Medie Giornaliere, Escursioni Termiche, Precipitazioni Giornaliere e Giorni di Pioggia del Poliennio 1956–2014; Stazione meteorologica di Terminillo; Università degli Studi di Perugia: Perugia, Italy, 2015. [Google Scholar]
- Spina, R.; Stortini, S.; Fusari, R.; Scuterini, C.; Di Marino, M. Caratterizzazione Climatologica delle Marche: Campo Medio della Temperatura per il Periodo 1950–2000; Centro di Ecologia e Climatologia, Osservatorio Geofisico Sperimentale: Macerata, Italy, 2006. [Google Scholar]
- Amici, M.; Spina, R. Campo Medio della Precipitazione Annuale e Stagionale sulle Marche per il Periodo 1950–2000; Centro di Ecologia e Climatologia, Osservatorio Geofisico Sperimentale: Macerata, Italy, 2002. [Google Scholar]
- Pasquale, V.; Verdoya, M.; Chiozzi, P.; Bodri, L.; Bellani, S. Temperature signal in the underground for climate history reconstruction in Italy. Glob. Planet. Chang. 2005, 47, 36–50. [Google Scholar] [CrossRef]
- Desiato, F.; Fioravanti, G.; Fraschetti, P.; Perconti, W.; Piervitali, E. Il Clima Futuro in Italia: Analisi delle Proiezioni dei Modelli Regionali; Stato dell ‘Ambiente 58; Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA): Roma, Italy, 2015.
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C.; Soil Survey Staff. Field Book for Describing and Sampling Soils; Version 3.0.; Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2012.
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA–Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Agnelli, A.; Bol, R.; Trumbore, S.E.; Dixon, L.; Cocco, S.; Corti, G. Carbon and nitrogen in soil and vine roots in harrowed and grass-covered vineyards. Agric. Ecosyst. Environ. 2014, 193, 70–82. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In USDA Circular; U.S. Government Printing Office: Washington, DC, USA, 1954; p. 19. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Fornasier, F.; Margon, A. Bovine serum albumin and Triton X-100 greatly increase phosphomonoesterases and arylsulphatase extraction yield from soil. Soil Biol. Biochem. 2007, 39, 2682–2684. [Google Scholar] [CrossRef]
- Cowie, A.L.; Lonergan, V.E.; Fazle Rabbi, S.M.; Fornasier, F.; Macdonald, C.; Harden, S.; Kawasaki, A.; Singh, B.K. Impact of carbon farming practices on soil carbon in northern New South Wales. Soil Res. 2013, 51, 707–718. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. Analysis of transformations. J. R. Stat. Soc. B (Stat. Methodol.) 1964, 26, 211–252. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: http//www.cran.r-project.org (accessed on 16 May 2016).
- Riebe, C.S.; Kirchner, J.W.; Finkel, R.C. Sharp decrease in long-term chemical weathering rates along an altitudinal transect. Earth Planet. Sci. Lett. 2004, 218, 421–434. [Google Scholar] [CrossRef]
- De Feudis, M.; Cardelli, V.; Massaccesi, L.; Bol, R.; Willbold, S.; Cocco, S.; Corti, G.; Agnelli, A. Effect of beech (Fagus sylvatica L.) rhizosphere on phosphorous availability in soils at different altitudes (central Italy). Geoderma 2016, 276, 53–63. [Google Scholar] [CrossRef]
- Follett, R.F.; Stewart, C.E.; Pruessner, E.G.; Kimble, J.M. Effects of climate change on soil carbon and nitrogen storage in the US Great Plains. J. Soil Water Conserv. 2012, 67, 331–342. [Google Scholar] [CrossRef]
- Zianis, D.; Mencuccini, M. Aboveground net primary productivity of beech (Fagus moesiaca) forest: A case study of Naousa forest, northern Greece. Tree Physiol. 2005, 25, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Pietikäinen, J.; Pettersson, M.; Bååth, E. Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol. Ecol. 2005, 52, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, X.; Cai, X.; Li, X.; Christie, P.; Zhang, J. Soil microbial community structure and activity along a montane elevational gradient on the Tibetan Plateau. Eur. J. Soil Biol. 2014, 64, 6–14. [Google Scholar] [CrossRef]
- Creamer, C.A.; De Menezes, A.B.; Krull, E.S.; Sanderman, J.; Newton-Walters, R.; Farrell, M. Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biol. Biochem. 2015, 80, 175–188. [Google Scholar] [CrossRef]
- Agnelli, A.; Celi, L.; Degl ‘Innocenti, A.; Corti, G.; Ugolini, F.C. The changes with depth of humic and fulvic acids extracted from fine earth and rock fragments of a forest soil. Soil Sci. 2002, 167, 524–538. [Google Scholar] [CrossRef]
- Koch, O.; Tscherko, D.; Kandeler, E. Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Glob. Biogeochem. Cycles 2007, 21, GB4017. [Google Scholar] [CrossRef]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Wallenstein, M.; Allison, S.; Ernakovich, J.; Steinweg, J.M.; Sinsabaugh, R. Controls on the temperature sensitivity of soil enzymes: A key driver of in situ enzyme activity rates. In Soil Enzymology, Soil Biology; Shukla, G., Varma, A., Eds.; Springer: Berlin, Germany, 2011; pp. 245–258. [Google Scholar]
- Lemanowicz, J.; Krzyzaniak, M. Vertical distribution of phosphorus concentrations, phosphatase activity and further soil chemical properties in salt-affected Mollic Gleysols in Poland. Environ. Earth Sci. 2015, 74, 2719–2728. [Google Scholar] [CrossRef]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.A.; Dixon, L.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Haygarth, P.M. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 257–258, 29–39. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Bunemann, E.K., Obreson, A., Frossard, E., Eds.; Springer: Berlin, Germany, 2011; pp. 215–243. [Google Scholar]
- Rejsek, K.; Vranova, V.; Pavelka, M.; Formanek, P. Acid phosphomonoesterase (E.C. 3.1.3.2) location in soil. J. Plant Nutr. Soil Sci. 2012, 175, 196–211. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Greco, A.; Drake, J.E.; Finzi, A.C. Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 2013, 115, 65–76. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. Sugars in soil and sweets for microorganisms: Review of origin, content, composition and fate. Soil Biol. Biochem. 2015, 90, 87–100. [Google Scholar] [CrossRef]
- De Feudis, M.; Cardelli, V.; Massaccesi, L.; Hofmann, D.; Berns, A.E.; Bol, R.; Cocco, S.; Corti, G.; Agnelli, A. Altitude affects the quality of the water-extractable organic matter (WEOM) from rhizosphere and bulk soil in European beech forests. Geoderma 2017, 302, 6–13. [Google Scholar] [CrossRef]
| Horizons | pH | TOC | TN | WEOC | Available P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | Low | High | |
| g kg−1 | mg kg−1 | |||||||||
| OLn | 6.28 (0.11) de | 6.08 (0.05) e | 468.42 (28.56) ab | 540.55 (11.48) a | 12.51 (0.94) b | 16.13 (1.20) ab | 0.19 (0.07) ac | 0.09 (0.04) c | nd | nd |
| OLv | 6.33 (0.10) ce | 6.68 (0.13) bc | 462.19 (27.70) ab | 527.29 (15.51) a | 14.64 (0.74) ab | 17.14 (0.64) a | 0.25 (0.03) ab | 0.16 (0.06) bc | nd | nd |
| OH | 7.02 (0.21) ac | 6.78 (0.30) ad | 387.70 (42.93) b | 424.90 (16.04) b | 16.52 (2.01) ab | 18.61 (1.89) a | 0.30 (0.14) ab | 0.29 (0.10) ab | nd | nd |
| A | 6.94 (0.30) be | 7.33 (0.22) ab | 71.36 (6.57) d | 95.79 (15.24) c | 3.47 (0.53) d | 8.22 (1.00) c | 0.33 (0.02) ab | 0.32 (0.05) a | 17.7 (1.9) ab | 20.2 (1.0) a |
| AB | 6.77 (0.48) be | 7.72 (0.15) a | 40.26 (5.83) e | 62.27 (8.90) d | 2.86 (0.63) e | 5.44 (0.77) d | 0.30 (0.03) ab | 0.23 (0.03) ab | 11.0 (2.1) cd | 15.8 (1.0) b |
| Bw1 | 6.27 (0.33) de | 7.50 (0.12) ab | 22.41 (4.11) f | 62.38 (3.20) d | 1.14 (0.08) f | 5.61 (0.37) cd | 0.31 (0.06) ab | 0.19 (0.03) ac | 8.0 (1.8) d | 15.0 (1.4) bc |
| Bw2 | 6.63 (0.45) be | 7.75 (0.02) a | 12.64 (1.19) g | 52.79 (2.55) de | 1.04 (0.11) f | 5.43 (2.28) d | 0.20 (0.02) ac | 0.18 (0.01) ac | 7.5 (1.6) d | 12.7 (0.3) bc |
| Horizons | Cmic | ΣCO2–C | ||
|---|---|---|---|---|
| Low | High | Low | High | |
| mg kg−1 | ||||
| OLn | 169.00 (46.80) ab | 302.37 (17.98) a | 18,539 (3843) ab | 20,088 (1993) a |
| OLv | 189.60 (47.76) a | 108.41 (23.25) ab | 14,082 (2731) bc | 12,967 (1772) cd |
| OH | 56.99 (33.19) b | 186.51 (27.08) ab | 8128 (2023) de | 7073 (1869) e |
| A | 0.07 (0.01) cd | 0.19 (0.02) c | 1984 (561) f | 697 (67) f |
| AB | 0.07 (0.02) de | 0.19 (0.02) c | 768 (276) f | 421 (84) f |
| Bw1 | 0.03 (0.01) ef | 0.19 (0.01) c | 308 (117) f | 584 (323) f |
| Bw2 | 0.02 (0.01) f | 0.12 (0.06) cd | 265 (93) f | 480 (57) f |
| Horizons | Cell | Xylo | β-Gluco | Chit | AcPME | AlkPME | AcEst | ArylS | Leu |
|---|---|---|---|---|---|---|---|---|---|
| Nanomoles of 4-methylumbelliferone (7-amino-4-methyl coumarine) • g−1 dry soil • hour−1 | |||||||||
| Low Altitude (800 m) | |||||||||
| OLn | 71.3 (25.1) a | 54.5 (11.6) a | 509.1 (142.4) a | 196.8 (25.6) a | 408.1 (116.8) a | 294.5 (49.7) b | 3278.8 (258.0) ab | 6.5 (1.8) d | 335.4 (54.8) a |
| OLv | 38.3 (11.7) b | 35.2 (5.2) ab | 215.8 (82.2) b | 134.5 (15.4) ab | 291.1 (43.1) ac | 318.9 (64.5) b | 3421.3 (239.8) ab | 8.7 (2.4) cd | 357.0 (511.9) a |
| OH | 4.4 (1.5) d | 6.8 (2.2) c | 34.1 (15.2) c | 38.6 (5.1) d | 129.1 (21.3) de | 362.9 (62.6) ab | 1807.3 (548.0) cd | 19.4 (8.9) b | 110.0 (29.0) b |
| A | 0.2 (0.1) ef | 1.2 (0.2) d | 5.1 (0.6) d | 5.6 (1.7) e | 105.4 (11.1) df | 143.7 (17.7) cd | 1810.9 (358.0) c | 67.5 (5.6) a | 25.6 (2.3) d |
| AB | 0.1 (0.0) f | 0.6 (0.1) e | 2.3 (0.2) e | 3.4 (1.6) ef | 72.0 (15.4) eg | 66.4 (41.7) d | 1074.1 (345.5) df | 86.84 (31.4) a | 14.1 (2.8) d |
| Bw1 | 0.0 (0.0) f | 0.4 (0.1) ef | 0.8 (0.2) fg | 1.0 (0.2) fg | 47.8 (11.8) gh | 11.3 (6.1) e | 777.6 (197.3) f | 56.4 (18.0) a | 5.7 (0.7) e |
| Bw2 | 0.0 (0.0) f | 0.2 (0.0) f | 0.5 (0.2) g | 0.7 (0.2) g | 32.3 (9.9) h | 20.4 (10.6) e | 578.8 (195.6) f | 26.0 (5.2) b | 4.3 (1.3) e |
| High Altitude (1000 m) | |||||||||
| OLn | 96.6 (23. 6) a | 63.7 (6.8) a | 581.7 (106.3) a | 202.8 (32.0) a | 338.3 (16.2) ab | 456.0 (70.0) a | 4143.1 (301.6) a | 7.3 (1.8) cd | 364.6 (60.5) a |
| OLv | 15.8 (4.7) c | 20.5 (3.5) b | 118.0 (32.2) b | 92.6 (21.28) bc | 236.9 (42.5) bc | 408.6 (48.6) ab | 2741.4 (354.7) b | 13.7 (3.3) bc | 280.4 (95.2) a |
| OH | 0.8 (1.0) e | 3.8 (2.1) c | 17.2 (8.6) c | 50.7 (15.4) cd | 175.1 (28.8) cd | 278.5 (35.5) b | 1372.8 (212.3) ce | 25.8 (9.1) b | 73.7 (10.1) bc |
| A | 0.0 (0.0) f | 0.5 (0.2) e | 3.3 (0.6) de | 2.5 (0.9) ef | 70.3 (10.5) fg | 165.3 (20.4) bc | 1000.2 (232.3) ef | 62.7 (4.0) a | 19.2 (5.2) d |
| AB | 0.0 (0.0) f | 0.3 (0.2) ef | 2.0 (0.6) ef | 2.4 (1.1) f | 46.7 (9.6) gh | 115.3 (7.8) c | 660.5 (173.4) f | 62.0 (11.5) a | 17.5 (7.5) d |
| Bw1 | 0.1 (0.0) ef | 0.8 (0.4) de | 3.1 (0.8) de | 3.4 (3.1) ef | 57.6 (10.5) fh | 104.5 (29.3) cd | 935.5 (422.0) ef | 77.4 (20.5) a | 30.7 (18.7) cd |
| Bw2 | 0.1 (0.1) ef | 0.7 (0.6) de | 2.1 (1.2) ef | 2.3 (2.2) fg | 42.9 (21.6) gh | 82.8 (30.0) cd | 731.9 (552.0) f | 82.4 (19.6) a | 21.7 (17.5) d |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Feudis, M.; Cardelli, V.; Massaccesi, L.; Lagomarsino, A.; Fornasier, F.; Westphalen, D.J.; Cocco, S.; Corti, G.; Agnelli, A. Influence of Altitude on Biochemical Properties of European Beech (Fagus sylvatica L.) Forest Soils. Forests 2017, 8, 213. https://doi.org/10.3390/f8060213
De Feudis M, Cardelli V, Massaccesi L, Lagomarsino A, Fornasier F, Westphalen DJ, Cocco S, Corti G, Agnelli A. Influence of Altitude on Biochemical Properties of European Beech (Fagus sylvatica L.) Forest Soils. Forests. 2017; 8(6):213. https://doi.org/10.3390/f8060213
Chicago/Turabian StyleDe Feudis, Mauro, Valeria Cardelli, Luisa Massaccesi, Alessandra Lagomarsino, Flavio Fornasier, Danielle Janaina Westphalen, Stefania Cocco, Giuseppe Corti, and Alberto Agnelli. 2017. "Influence of Altitude on Biochemical Properties of European Beech (Fagus sylvatica L.) Forest Soils" Forests 8, no. 6: 213. https://doi.org/10.3390/f8060213
APA StyleDe Feudis, M., Cardelli, V., Massaccesi, L., Lagomarsino, A., Fornasier, F., Westphalen, D. J., Cocco, S., Corti, G., & Agnelli, A. (2017). Influence of Altitude on Biochemical Properties of European Beech (Fagus sylvatica L.) Forest Soils. Forests, 8(6), 213. https://doi.org/10.3390/f8060213







