Abstract
In this study, we focused on four major coniferous species in the eastern part of Inner Mongolia, namely Larix gmelinii var. principis-rupprechtii (Mayr) Pilg., Larix gmelinii (Rupr.) Kuzen., Pinus tabuliformis Carrière and Pinus sylvestris var. mongolica Litv. and carried out a systematic study on their ectomycorrhiae (EM) fungi. The present study was based on high-throughput sequencing. Based on the high-throughput sequencing data, analyzed by bioinformatics and statistical methods, the results showed that (1) a total of 150 operational taxonomic units (OTUs) were obtained, which belonged to 26 evolutionary branches of Basidiomycota and Ascomycota, respectively. Among them, Tricholoma, Tomentella-thelephora, Suillus-rhizopogon, Wilcoxina, Piloderma, Pustularia, Hygrophorus, Sebacina and Amphinema-tylospora are the EM fungi shared by four conifer species. (2) The species diversity and community composition of EM fungi differed significantly among tree species and sample plots, while soil total nitrogen (N) content and nitrogen/phosphorus (N/P) ratio were the main factors affecting community structure. (3) The Neutral Community Model (NCM) and β-Nearest Taxon Index (β-NTI) showed that stochastic processes dominated the construction of EM fungal communities. The results of this study revealed the geographical distribution pattern and maintenance mechanisms of EM fungal communities of four coniferous species in the eastern part of Inner Mongolia, which provides a scientific basis for the restoration practice of disturbed ecosystems and the sustainable development of the regional economy.
1. Introduction
Ectomycorrhizal (EM) fungi play a crucial role in forest ecosystems, particularly in the growth and nutrient uptake of coniferous tree species. They form symbiotic relationships with plant root systems, helping host plants obtain nutrients and water from the soil while receiving carbon sources from the plants [1]. Additionally, EM fungi significantly enhance host plants’ resistance to environmental stresses through multiple physiological and biochemical mechanisms. Regarding drought tolerance, EM fungi mitigate oxidative damage and maintain cellular turgor pressure by regulating stomatal conductance, promoting photosynthesis, activating antioxidant enzyme systems, and accumulating osmoprotectants [2]. Under salt stress, EM fungi mitigate damage by regulating ion uptake, reducing Na+ accumulation, and enhancing osmotic regulation capacity [3]. Salt stress is a major abiotic stress factor affecting plant growth and productivity, and EM fungi can help plants adapt to this stress [4]. In heavy metal-contaminated environments, mycelium reduces the biotoxicity of heavy metals and their uptake by plants through adsorption, immobilization, formation of mycelium sheaths, and enhanced nutrient absorption. EM fungi enhance plant tolerance to heavy metal stress, with extracellular chelation serving as the primary mechanism for heavy metal tolerance. Studies have also revealed that EM fungi reduce heavy metal accumulation in plant aboveground parts [5]. EM fungi can also induce plant systemic immunity, competing with pathogens for resources and space, altering the rhizosphere microbial community, and producing antimicrobial substances to enhance resistance against pathogens. This induced systemic immunity resembles the immune response triggered by beneficial microorganisms, helping plants resist attacks from multiple pathogens [6,7]. The interaction between plants and EM fungi is a complex process involving the synergistic action of multiple physiological and biochemical mechanisms, enabling plants to better adapt to various environmental stresses [8]. The structure and maintenance mechanisms of EM fungal communities are influenced by various factors, including climate, soil type, ecological drift, and dispersal limitations [9,10,11,12]. Therefore, studying the EM fungal community structure of specific regions and tree species is of great significance for understanding the functions and succession of forest ecosystems.
Understanding the mechanisms underlying microbial community assembly is crucial for comprehending the maintenance of biodiversity and predicting its response to global change. The maintenance of EM fungal communities involves various ecological processes, including deterministic and stochastic processes [13]. The primary factors influencing deterministic processes include abiotic factors (such as climatic conditions and soil physical and chemical properties) and biotic factors (such as host plant specificity) [9], while stochastic processes include ecological drift, dispersal limitations, and EM fungal interspecific interactions (such as competitive exclusion) [10,11,12]. Ecological drift refers to changes in species abundance caused by random birth, death, and migration events. In EM fungal communities, ecological drift may lead to certain species becoming more common or rare without explicit environmental selection [14]. For example, in sorghum systems under drought stress, studies have shown that fungal community assembly is influenced by random forces, particularly during early developmental stages and periods of drought stress [14]. Dispersal limitation refers to the inability of species to reach all potential habitats due to limitations in their dispersal capacity. For EM fungi, spore dispersal may be constrained by factors such as distance, wind, and animal activity, thereby influencing community composition [15]. Studies have shown that at the regional scale, EM fungal community composition is influenced by dispersal limitations, meaning that communities farther apart geographically exhibit lower similarity [16]. However, the specific mechanisms and their interactions in shaping the relative importance of EM fungal communities in different ecosystems require further research to clarify.
The eastern region of Inner Mongolia possesses unique climatic conditions and soil types, providing a special environmental backdrop for studying the diversity and community structure of EM fungi. Studies targeting Pinus sylvestris var. mongolica in Horqin Sandy Land of Inner Mongolia [17], Picea mongolica in the Baiyin’ao Bao Nature Reserve [18], Pinus sylvestris var. mongolica in Honghuaerji [19], Larix principis-rupprechtii in Heili River and Helan Mountain Nature Reserve [20] and Larix gemelinii Rupr. in the Great Khingan Mountains [21] have also yielded some results, but these studies were conducted only at the local scale. For the EM fungal communities of key coniferous tree species in this region (such as Larix gmelinii var. principis-rupprechtii (Mayr) Pilg., Larix gmelinii (Rupr.) Kuzen., Pinus tabuliformis Carrière and Pinus sylvestris var. mongolica Litv.), there is a lack of in-depth research on the systematic structure, geographical distribution patterns, ecological construction processes, and the comprehensive influence of environmental factors at the regional scale.
Given this, this study focuses on the four coniferous tree species mentioned above in the eastern region of Inner Mongolia, utilizing high-throughput sequencing technology combined with bioinformatics and statistical methods, with the aim of: (1) investigating the diversity and community composition of EM fungi in the four coniferous tree species; (2) analyzing the correlation between soil physical and chemical properties, climatic conditions, and EM fungal community structure; identify key influencing factors and their interrelationships; (3) reveal the geographical distribution patterns and maintenance mechanisms of EM fungal communities (i.e., the relative contributions of deterministic and random processes in community assembly). The research findings can provide a scientific basis for ecosystem restoration and regional economic development practices and serve as a reference for studies on the assembly mechanisms and functions of EM fungal communities in different host plants.
2. Materials and Methods
2.1. Study Sites and Sample Collection
The study was conducted in Chifeng City, Inner Mongolia Autonomous Region, with four study sites established: Huanggangliang (E 117°31′, N 43°34′), Heilihé (E 118°27′, N 41°21′), Saihanwula (E 118°14, N 44°17′), and Wangyedian (E 118°10′, N 41°45′). The study area is primarily characterized by hilly and highland terraces, with a northwest-high, southeast-low topography and an average elevation of 1300–2000 m. In these areas, four conifer species serve as the primary woody EM host plants, alongside other EM hosts such as Betulaceae, Fagaceae, and Salicaceae species. Climate information for the sampling sites was extracted from the World Clim dataset [22], revealing that the selected sites are located in semi-arid and cold-temperate climate zones, with mean annual temperatures (MAT) ranging from −0.3 to 5.5 °C and mean annual precipitation (MAP) ranging from 374 to 483 mm in Table S1.
This study selected four secondary forests with an average age of 20–50 years, characterized by dense canopy closure and a thick understory layer of shrubs and saplings, all in a vigorous growth phase. Sampling was completed in July 2021. Due to climatic gradient constraints, Larix gmelinii var. principis-rupprechtii samples were collected from four sites, while samples for the other three conifer species originated from only two sites (Table S1). At each site, 3–7 individual trees were selected from each conifer species (with spacing between trees > 10 m). Fine roots were collected using the drag-and-pull method along three directions of the main trunk, with 1–3 fine roots collected per tree. A total of 12 root samples were collected from Larix gmelinii var. principis-rupprechtii, 11 from Larix gmelinii, 14 from Pinus tabulaeformis, and 14 from Pinus sylvestris var. mongolica. Subsequently, samples were stored in ice packs and immediately transported to the laboratory for cryopreservation at −80 °C. Concurrently with fine root sampling, soil samples (approximately 200 g) were collected from the rhizosphere of each individual, air-dried, and sieved (through a 2 mm mesh) for physicochemical analysis.
2.2. Soil Properties Analysis
The soil physical and chemical properties measured include total nitrogen (N), total phosphorus (P), total calcium (Ca), total magnesium (Mg), and the nitrogen/phosphorus ratio (N/P). The specific operations involved are outlined as follows: N content was determined by the semi-micro Kjeldahl method, P content was determined using a Thermo Fisher Scientific iCAP 6300 (Cambridge, UK) inductively coupled plasma emission spectrometer, the N/P ratio was calculated, and Ca and Mg content were determined using an Optima 8000 PerkinElmer (Shelton, CT, USA) inductively coupled plasma emission spectrometer in Table S1.
2.3. DNA Extraction, PCR, and MiSeq Sequencing
In the laboratory setting, root samples were meticulously washed with tap water to ensure their cleanliness and integrity. Subsequently, intact root samples were meticulously divided into segments measuring approximately 2 cm in length. The selection of intact mycorrhizae for analysis was conducted at random, with 200 slices from each plant selected for examination. Morphological characteristics such as shape, color, dendrites, fungal hyphae, surface texture, transparency, and the presence or absence of vesicles were documented. The 10,200 mycorrhizae samples were then preserved in a refrigerator set at −80 °C. The extraction of DNA was performed using a modified cetyltrimethylammonium bromide (CTAB) method [23]. and the ITS2 region was amplified by polymerase chain reaction (PCR) using a two-step method [24,25,26]. First, the first round of PCR was performed with the fungus-specific primers ITS1f (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4 (5′-TCCTCCCCTCCTTAGAGGAAGTAA-3′) utilized as primers to amplify the entire ITS region, and subsequently, fITS7 (5′-GTGARTCATCGAATCTTTG-3′) and ITS4 with base tag sequences were employed as primers to amplify the fungal ITS2 region. The purified samples were then subjected to sequencing on the Illumina Miseq double-end sequencing platform at the Chengdu Institute of Biology, Chinese Academy of Sciences.
2.4. Bioinformatic Analysis
Initially, low-quality sequences were filtered using the QIIME platform (v.v.1.9.0) [27]. Subsequently, sequences in the ITS2 region of the quality control data were extracted using ITSx software (v. 1.0.3) [28], and sequences in the Unified Fungal Species Database [29] were referenced. Chimeric sequences in the ITS2 region were examined and removed using Usearch v11. The UPARSE pipeline was then used to classify the non-chimeric ITS2 region sequences into OTUs according to 97% sequence similarity [30]. Then, the representative sequences (with the highest number of sequences) of each OTU were classified into OTUs using the National Center for Biotechnology Information (NCBI) and UNITE (v. 8.3) reference databases for the basic local alignment search tool (BLAST) (v. 2.2.31+) [31]. Each OTU was identified to the best taxonomic position by referring to the taxonomic criteria of Tedersoo et al. [32], and the EM fungi were identified according to the method of Kõljalg et al. [29]. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in the National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA028857) which are publicly accessible at CRA028857.
2.5. Statistical Analysis
The classification of EM fungi was performed using R-3.6.1 [33], and alpha diversity metrics, including Chao1 and Shannon indices, were computed with the vegan package [34]. The Chao1 index serves as an estimator for the total number of operational taxonomic units (OTUs) based on the observed OTU count. In contrast, the Shannon index reflects the diversity of OTUs across various samples. This study employed two approaches—cmdscale and metaMDS—to integrate Principal Coordinate Analysis (PCoA) and Non-Metric Multi-Dimensional Scaling (NMDS), avoiding metric-based measurements. By utilizing the ordiellipse function, the 95% confidence intervals for EM fungal communities associated with different coniferous tree species were determined. Furthermore, the envfit command was applied to analyze the influence of geographical locations, climate, and soil factors, thereby identifying the primary drivers shaping the structure of the EM fungal community. The adonis command was used to validate these findings through Permutational Multivariate Analysis of Variance (PERMANOVA) analysis. To investigate the assembly mechanisms of the EM fungal community, the β-Nearest Taxon Index (βNTI) was adopted. Specifically, the comdistnt function in the picante package [35] was utilized to quantify pairwise phylogenetic turnover among communities based on the β-Mean Nearest Taxon Distance (βMNTD). The mntd and ses.mntd functions were employed to calculate the Mean Nearest Taxon Distance (MNTD) and the Beta Taxonomic Index (BTI). A value of |βNTI| > 2 indicates that the phylogenetic turnover rate significantly exceeds (or falls below) the spatial distribution expectation, implying a deterministic process dominates. Conversely, when |βNTI| < 2, no significant difference exists between the phylogenetic turnover rate and the expected spatial distribution, suggesting stochastic processes play a more significant role in community assembly. Additionally, Sloan’s Neutral Community Model (NCM) [36] was applied to assess the assembly process by correlating the migration rate “m” of individual fungal OTUs with their occurrence frequency and abundance (after logarithmic transformation). All results were visualized using ggplot2.
3. Results
3.1. Fungal Database Summary
A total of 626 OTUs were identified from 596,723 sequences. Among them, 200 OTUs were identified as EM fungi. After sample standardization, 150 OTUs were finally obtained for subsequent analysis. These 150 OTUs belonged to 2 phyla, 4 classes, 4 orders, 10 families and 29 genera. Among them, the dominant genera were Tricholoma, Tomentella and Suillus, accounting for 18%, 17% and 14% of the total sequences, respectively (Figure 1).
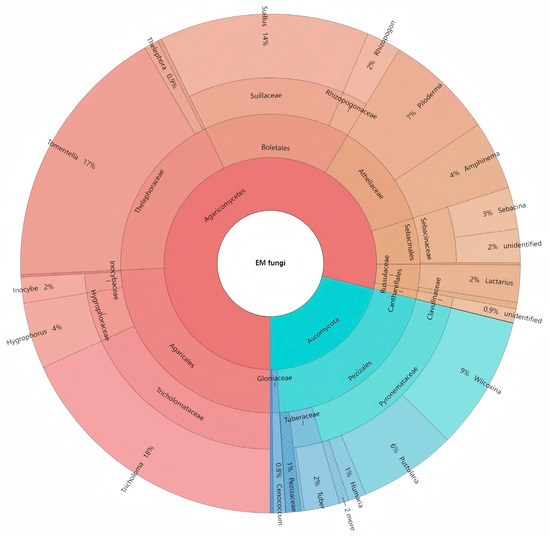
Figure 1.
Krona chart of taxonomic affiliation of ectomycorrhizal fungi and their relative abundances. Inner circles represent higher taxonomic ranks and more detailed taxonomic ranks are presented in outer circles.
The analysis revealed that 72% of phylogenetic lineages belonged to Basidiomycota, while the remaining 28% were affiliated with Ascomycota. Nine dominant ectomycorrhizal (EM) fungal genera were shared among the four coniferous species: Tricholoma, Tomentella/thelephora, Suillus/rhizopogon, Wilcoxina, Piloderma, Pustularia, Hygrophorus, Sebacina, and Amphinema/tylospora (Figure 2). Notably, host-specific distribution patterns emerged: Tricholoma exhibited its highest relative abundance in Larix gmelinii but the lowest in Pinus tabuliformis. Tomentella/thelephora demonstrated peak abundance in Larix principis-rupprechtii while showing minimal presence in L. gmelinii. Suillus/rhizopogon predominated in P. tabuliformis but was least represented in L. principis-rupprechtii. Wilcoxina reached maximum levels in Pinus sylvestris var. mongolica but minimum levels in L. gmelinii. Piloderma displayed contrasting abundance patterns between L. gmelinii (highest) and P. tabuliformis (lowest). Pustularia showed preferential colonization of P. sylvestris var. mongolica over P. tabuliformis. Hygrophorus exhibited host affinity toward L. principis-rupprechtii rather than P. tabuliformis. Sebacina and Amphinema/tylospora both demonstrated significant preference for P. tabuliformis, while registering minimal abundance in L. gmelinii. These findings collectively indicate substantial interspecific variation in EM fungal community composition across host tree species.
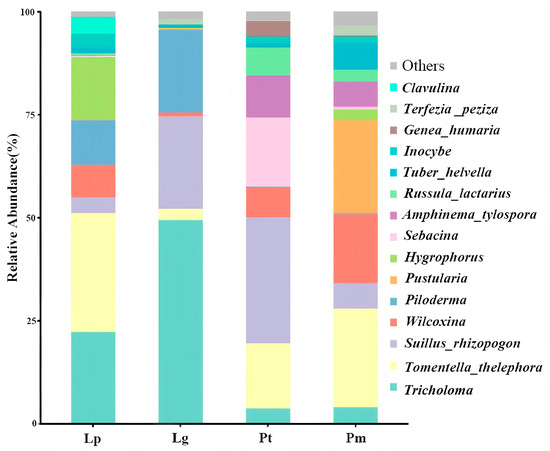
Figure 2.
Species composition of root EM fungi of four conifer species. Note: Lp, Larix gmelinii var. principis-rupprechtii (Mayr) Pilg. Lg, Larix gmelinii (Rupr.) Kuzen. Pt, Pinus tabuliformis Carrière Pm, Pinus sylvestris var. mongolica Litv.
3.2. Diversity of EM Fungi in Four Coniferous Species
The species accumulation curves demonstrated that ectomycorrhizal (EM) fungal diversity associated with roots of four coniferous species failed to reach plateau, indicating that additional sampling would yield new EM fungal taxa. Both Shannon and Chao1 indices revealed distinct EM fungal community compositions among the four coniferous species (Figure 3). Significant interspecific differences in EM fungal diversity were observed, with particularly pronounced disparities between Pinus tabuliformis and Larix gmelinii (p < 0.001), and statistically significant differences between P. tabuliformis and Larix principis-rupprechtii (p < 0.05). Spatial heterogeneity analysis showed significant differences in EM fungal diversity between three sampling sites of L. principis-rupprechtii (Saihanwula vs. Huanggangliang, and Saihanwula vs. Wangyezheng; p < 0.05). Similarly, notable differences emerged between two sampling locations of L. gmelinii (Huanggangliang vs. Saihanwula; p < 0.05).
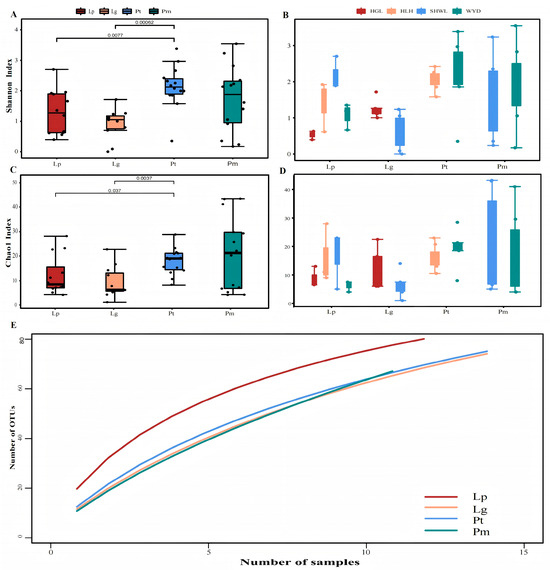
Figure 3.
Differences between two species were tested according to wilcox.test. Cumulative curves (E) of EM fungi Shannon indexes (A,B), Chao1 indexes (C,D) and operational taxonomic units (OTUs) of four conifer species. Note: HGL, Huanggangliang. HLH, Heilihe. SHWL, Saihanwula. WYD, Wangyedian. Lp, Larix gmelinii var. principis-rupprechtii (Mayr) Pilg. Lg, Larix gmelinii (Rupr.) Kuzen. Pt, Pinus tabuliformis Carrière Pm, Pinus sylvestris var. mongolica Litv.
In this study, we conducted PCoA and NMDS ordination analyses to investigate the beta diversity of EM fungi associated with root systems of four coniferous species (Figure 4). The PCoA results revealed that the first principal coordinate explained 29.97% of variance, while the second accounted for 16.76%. Significant differentiation was detected both among tree species (Adonis test: R2 = 0.112, p < 0.001) and across sampling sites (Adonis test: R2 = 0.115, p < 0.001). NMDS ordination combined with envfit analysis further identified soil nitrogen (N) content and N/P ratio as key determinants governing the compositional variation in EM fungal communities in coniferous root systems.
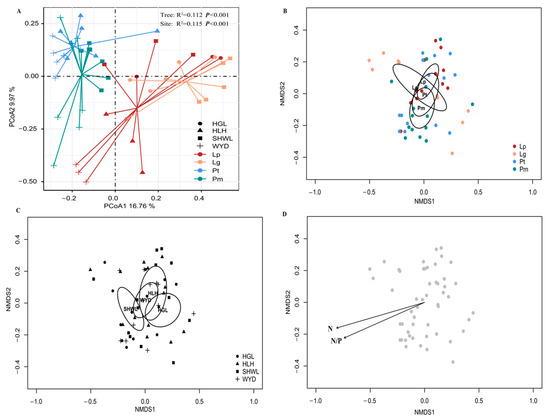
Figure 4.
PCoA (A) and NMDS (B,C) ordination analyses of differences between four conifer EM fungal species and sample sites. Ellipses represent 95% confidence intervals around each site centroid. Soil variables conform to the NMDS ranking (D). N, total nitrogen content, N/P, nitrogen to phosphorus ratio. Note: HGL, Huanggangliang. HLH, Heilihe. SHWL, Saihanwula. WYD, Wangyedian. Lp, Larix gmelinii var. principis-rupprechtii (Mayr) Pilg. Lg, Larix gmelinii (Rupr.) Kuzen. Pt, Pinus tabuliformis Carrière Pm, Pinus sylvestris var. mongolica Litv.
3.3. Ecological Process Analysis of EM Fungi in Four Conifer Species
This study used βNTI to assess the ecological processes driving EM fungal community assembly. Most βNTI values ranged between −2 and 2 (Figure 5A), with only 3.5% of cases exhibiting βNTI values exceeding 2, indicating that stochastic processes dominated. Consistent with these findings, NCM analysis further confirmed that community structure was primarily shaped by stochastic mechanisms (Figure 5B).
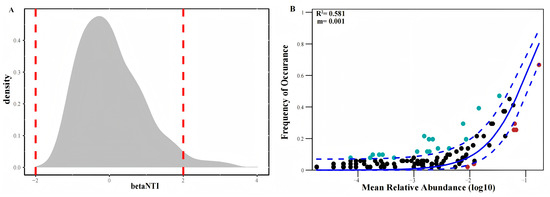
Figure 5.
βNTI of fungal OTUs (A) and NCM Index (B). Dashed red lines indicate the critical values for βNTI, specifically βNTI = −2 and βNTI = 2. Solid blue line represents the predicted occurrence frequency, and dashed blue lines represent 95% confidence intervals around the model prediction. Black dots represent the relationship between the frequency of occurrence and the mean relative abundance (log10 transformed) of microbial communities in different samples. Light blue and red dots indicate the OTUs that occur more and less frequently than given by the model; R2 indicates the fit to the neutral model; m indicates immigration rate.
4. Conclusions
This study indicates that total nitrogen content and the N/P ratio are key soil factors influencing the structure of EM fungal communities. First, nitrogen content directly participates in metabolic synthesis and energy cycling within fungi [37]. High-nitrogen environments typically favor the survival and competitive advantage of fungal groups with efficient nitrogen uptake capabilities (e.g., certain Basidiomycota species) [38]. Second, the N/P ratio reflects soil nutrient balance. Imbalanced N/P ratios may induce host trees to alter carbon allocation strategies, thereby indirectly influencing the types of fungi with which they form symbiosis [39]. This study aligns with findings by Dong Peng et al. [40] in Hulunbuir sandy larch forests, both emphasizing the dominant role of total nitrogen and N/P ratios. However, other research indicates that available nitrogen (rather than total nitrogen) is the primary driver of EM fungal community dynamics [41]. We hypothesize that these discrepancies may stem from differences in nitrogen availability and forms due to varying parent materials, soil formation processes, and organic matter content. For instance, faster leaching of available nitrogen in sandy soils may amplify its influence on community assembly [42,43]. Furthermore, although previous studies have identified mean annual temperature (MAT) as a significant climatic factor influencing EM fungal communities [44,45,46], this study did not detect a significant effect of MAT. This is likely because all four sampling sites are located within a temperate semi-arid zone with relatively small climatic gradient variations, where temperature variability is insufficient to elicit significant community responses. In summary, soil properties collectively shape EM fungal community structure through multiple pathways, including direct nutrient supply, modification of the soil microenvironment, and interactions with hosts.
Clustering of OTUs from four conifer species and EM fungal species composition maps reveal shared dominant EM fungal groups (e.g., Tricholoma, Tomentella-thelephora) performing key ecological functions. Tricholoma promotes nitrogen and phosphorus uptake while enhancing host stress resistance through symbiosis with plant roots [47,48,49,50,51]; Tomentella-thelephora decomposes organic matter, participates in carbon and nitrogen cycling, and maintains ecosystem material flow and stability [49,52,53]. The conservation of host plant phylogenetic niches and variations in root exudates significantly influence EM fungal colonization and community composition [41,44,54,55]. The dominant group composition identified in this study differs from previous research [56,57,58], potentially due to host specificity or regional climate-shaped fungal species pools. Further functional genomics and field control experiments are needed to elucidate their ecological roles.
Shannon index and Chao1 index analyses revealed significant differences in the α diversity of EM fungi among the four conifer species, consistent with previous studies [18,19,20,44]. These α-diversity differences hold significant ecological implications: EM fungal communities with high α diversity decompose organic matter more efficiently, promote nutrient cycling, and contribute to maintaining ecosystem health and balance [49,50,51]. β In diversity, PCoA and NMDS sequencing combined with the PERMANOVA test showed that EM fungal communities differed significantly among tree species and locations. This is consistent with the results of previous studies on plants in the Salicaceae, Betulaceae, and Fagaceae [59,60,61].
The low NCM analysis m value (0.001) and βNTI values falling within the range of −2 to 2 indicate that random processes dominate. One perspective suggests that spatial distance constitutes a barrier to the dispersal of fungal progeny (e.g., fruiting bodies, spores, and mycelium) [32]. Simultaneously, variations in dispersal capacity among fungal species may lead to changes in fungal community composition due to a pioneer effect, demonstrating that pioneer fungi often exhibit a more pronounced advantage in competing for habitat space and resource utilization [62]. Although studies indicate that the mechanisms governing bacterial and fungal community assembly differ across soil types [12], some research highlights deterministic processes as dominant in fungal community formation [63], while others emphasize the importance of stochastic processes [64,65]. These discrepancies may relate to environmental conditions, plant species, and interactions with other microbial groups within the study areas. In tropical forests, fungal community composition exhibits high spatial and temporal dynamics, with stochastic processes playing a significant role in community assembly [66,67]. In drought-stressed agricultural fields, fungal community assembly is also influenced by stochastic processes [68]. Furthermore, interactions between fungi and other microorganisms affect community assembly. For instance, fungal networks can influence bacterial dispersal and community assembly [69]. Within cheese rind microbial communities, bacteria utilize physical networks formed by fungi for dispersal, and such interactions can shape microbial community structure [69]. Collectively, these findings indicate that stochastic processes significantly influence EM fungal community structure. Future research should further explore the interactions between deterministic and stochastic processes and how they jointly affect EM fungal community assembly.
Combining the results of this study with those of previous studies, it can be seen that both deterministic and stochastic factors play a key role in the construction of EM fungal communities in a specific region of eastern Inner Mongolia, where four coniferous species coexist, but stochastic factors play a dominant role. In this study, soil characteristics were more influential than climatic conditions and geographic distance in terms of abiotic factors influencing the structure of EM fungal communities. This conclusion differs from previous research findings. We speculate that this may be related to ecological drift and random processes that generate diversity, but this needs to be investigated in our future research.
This study acknowledges two potential limitations. First, despite extensive sampling, the species accumulation curves indicated that ectomycorrhizal fungal diversity did not reach an asymptote, suggesting that additional sampling may reveal further taxonomic diversity. Second, the relatively narrow climatic gradient across the four sampling sites may have constrained our ability to fully assess the influence of climate variables on community assembly. Future studies incorporating broader climatic gradients and intensified sampling efforts would enhance the comprehensiveness of these findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16091459/s1, Table S1: Information on geographic coordinate, climatic and soil variables in this study.
Author Contributions
Conceptualization, Y.F., F.L. and J.L. (Jinyan Li); methodology, Y.F. and J.L. (Jinyan Li); software, Y.F. and J.L. (Jinyan Li); validation, J.L. (Jinyan Li) and Z.Y.; formal analysis, Y.F. and J.L. (Jinyan Li); investigation, J.L. (Jinyan Li) Z.Y., X.L., L.W. and J.L. (Jiani Lu); resources, Y.F.; data curation, Y.F. and J.L. (Jinyan Li); writing—original draft preparation, J.L. (Jinyan Li).; writing—review and editing, Y.F. and F.L.; visualization, J.L. (Jinyan Li); supervision, Y.F. and J.L. (Jinyan Li); project administration, Y.F. and J.L. (Jinyan Li); funding acquisition, Y.F. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32260006, the Natural Science Foundation of Inner Mongolia Autonomous Region, grant number 2024MS03045, the science and technology project of Inner Mongolia Autonomous Region, grant number 2019GG002, the Science and Technology Project of Ordos, grant number 2022YY008, and Basic Scientific Research Business Fee Project for Directly Affiliated Universities in Inner Mongolia Autonomous Region, grant number 2023RCTD021 and the science and technology project of Inner Mongolia Autonomous Region—2025 Autonomous Region Key R&D and Achievement Transformation Plan Project (Scientific and Technological Support for Ecological Protection and High-Quality Development of the Yellow River Basin, grant number 2025YFHH0153), Natural Science Foundation of Inner Mongolia Autonomous Region, grant number 2025MS03144, and The APC was funded by the Science and Technology Project of Ordos, grant number 2022YY008.
Data Availability Statement
The original data presented in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA028857) at BIG Sub—GSA.
Acknowledgments
During the preparation of this manuscript/study, the authors used QIIME platform (v. v.1.9.0), ITSx software (v. 1.0.3), basic local alignment search tool (BLAST) (v. 2.2.31+), vegan package (v. 2.5.7), Evenn (http://www.ehbio.com/test/venn/#/) (accessed on 20 January 2024), PCNM package (v. 2.1-2), MuMIn package (v. 1.43.17), online Krona tool (v.2.6), randomForest package (v. 4.6-14), rfPermute package (v. 2.1.81), rfUtilities package (v. 2.1-5), pheatmap package (v. 1.0.10), NST package (v. 3.1.9) for the purposes of analyzing and processing data. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Abbreviations
The following abbreviations are used in this manuscript:
| EM | Ectomycorrhiae |
| OTUs | operational taxonomic units |
| NCM | Neutral Community Model |
| β-NTI | β-Nearest Taxon Index |
| MAT | mean annual temperatures |
| MAP | mean annual precipitation |
| CTAB | cetyltrimethylammonium bromide |
| PCR | polymerase chain reaction |
| NCBI | National Center for Biotechnology Information |
| BLAST | basic local alignment search tool |
| PCoA | Principal Coordinate Analysis |
| NMDS | Non-Metric Multi-Dimensional Scaling |
| PERMANOVA | Permutational Multivariate Analysis of Variance |
| βMNTD | β-Mean Nearest Taxon Distance |
| MNTD | Mean Nearest Taxon Distance |
| BTI | Beta Taxonomic Index |
References
- Anderson, I.C.; Cairney, J.W.G. Ectomycorrhizal fungi: Exploring the mycelial frontier. FEMS Microbiol. Rev. 2007, 31, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Zhang, T.Z.; Zhou, Y.B.; Zou, X.M.; Yin, Y.; Li, H.; Liu, L.Y.; Zhang, S.C. Ectomycorrhizal symbioses increase soil calcium availability and water use efficiency of Quercus acutissima seedlings under drought Stress. Eur. J. For. Res. 2021, 140, 1039–1048. [Google Scholar] [CrossRef]
- Nurrahma, A.H.I.; Harsonowati, W.; Putri, H.H.; Iqbal, R. Current Research Trends in Endophytic Fungi Modulating Plant Adaptation to Climate Change-associated Soil Salinity Stress. J. Soil Sci. Plant Nutr. 2024, 24, 6446–6466. [Google Scholar] [CrossRef]
- Gul, H.; Ali, R.; Rauf, M.; Hamayun, M.; Arif, M.; Khan, S.A.; Parveen, Z.; Alrefaei, A.F.; Lee, I.J. Aspergillus welwitschiae BK Isolate Ameliorates the Physicochemical Characteristics and Mineral Profile of Maize under Salt Stress. Plants 2023, 12, 1703. [Google Scholar] [CrossRef]
- Chai, L.; Huang, M.; Cao, X.; Liu, M.J.; Huang, Y. Potential metal-binding ability of proteins in the extracellular slime of Laccaria bicolor exposed to excessive Cu and Cd. Environ. Sci. Pollut. Res. 2019, 26, 20418–20427. [Google Scholar] [CrossRef]
- Tonelli, M.L.; Figueredo, M.S.; Rodríguez, J.; Fabra, A.; Ibañez, F. Induced systemic resistance -like responses elicited by rhizobia. Plant Soil 2020, 448, 1–14. [Google Scholar] [CrossRef]
- Nasehi, A.; Esfahani, M.N.; Esfahani, A.N.; Mohammadbagheri, L.; Yazdi, M.J.; Mohammadi, M. Endophytic fungi as potential inhibitory agents of downy mildews: A review and future prospects. Ecol. Genet. Genom. 2023, 29, 100211. [Google Scholar] [CrossRef]
- Liu, R.; Tang, M.; Chen, Y. Recent advances in the study of mycorrhizal fungi and stress resistance of Plants. J. Fungal Res. 2017, 15, 70–87. [Google Scholar] [CrossRef]
- Liu, N.N.; Hu, H.F.; Ma, W.H.; Deng, Y.; Wang, Q.G.; Luo, A.; Meng, J.H.; Feng, X.J.; Wang, Z.H. Relative importance of deterministic and stochastic processes on soil microbial community assembly in temperate grasslands. Microorganisms 2021, 9, 1929. [Google Scholar] [CrossRef]
- Van Nuland, M.E.; Qin, C.; Pellitier, P.T.; Zhu, K.; Peay, K.G. Climate mismatches with ectomycorrhizal fungi contribute to migration lag in North American tree range Shifts. Proc. Natl. Acad. Sci. USA 2024, 121, e2308811121. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Matsuoka, S.; Ishizuka, W.; Sugai, T. Reduction of the α and β diversity of ectomycorrhizal fungal community under snowmelt: Highlights from a common garden trial using Abies sachalinensis with differing host origins and light Condition. Mycorrhiza 2025, 35, 27. [Google Scholar] [CrossRef]
- Yuan, Y.X.; Li, X.Y.; Liu, F.Q.; Tian, X.Y.; Shao, Y.Z.; Yuan, Z.L.; Chen, Y. Differences in Soil Microbial Communities across Soil Types in China’s Temperate Forests. Forests 2024, 15, 1110. [Google Scholar] [CrossRef]
- Gao, C. Community and Diversity Maintenance Mechanisms of Ectomycorrhizal Fungi and Soil Fungi in a Subtropical Forest. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2013. [Google Scholar]
- Xie, L.L.; Palmroth, S.; Yin, C.; Oren, R. Extramatrical mycelial biomass is mediated by fine root mass and ectomycorrhizal fungal community composition across tree Species. Sci. Total Environ. 2024, 950, 175175. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Toju, H. Mycorrhizal and endophytic fungi structure forest Below-ground symbiosis through contrasting but interdependent assembly Processes. Environ. Microbiome 2024, 19, 84. [Google Scholar] [CrossRef]
- Ibáñez, I.; McPherson, M.R.; Upchurch, R.A.; Zak, D.R. Mycorrhizal fungi influence on mature tree growth: Stronger in high-nitrogen soils for an EMF-associated tree and in low-nitrogen soils for two AMF-associated trees. Plant-Environ. Interact. 2025, 6, e70055. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.S.; Guo, M.S.; Gao, G.L.; Ding, G.D.; Zhang, Y.; Ren, Y. Temporal and spatial variations in root-associated fungi associated with Pinus sylvestris var. mongolica in the Semi-arid and Dry sub-humid desertified regions of northern China. Environ. Sci. 2023, 44, 502–511. [Google Scholar] [CrossRef]
- Bao, Q.L.; Zhang, X.; Liu, Y.G.; Wei, J. High-throughput sequencing analysis of community structure of ectomycorrhizal fungi in the rhizosphere of Picea mongolica in different ages. Mol. Plant Breed. 2021, 19, 4987–4993. [Google Scholar] [CrossRef]
- Zhao, M.; Hao, L.F.; Zhang, M.; Teng, H.; Yan, H.X.; Bai, S.L. Community structure characteristics of ectomycorrhizal fungi in different ages of natural Pinus sylvestris var. mongolica in Honghuaerji. Mycosystema 2019, 38, 1420–1429. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, W.; Wei, J. The community of ectomycorrhizal fungi in the soil of the root zone of Larix principis-rupprechtii in Heili River and Helan Mountain Nature Reserve. Mycosystema 2019, 38, 48–63. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhao, Y.L.; Xu, Y.; Ma, J.J.; Balalola, B.J.; Fan, Y.J. Ectomycorrhizal fungal communities associated with Larix gemelinii Rupr. in the Great Khingan Mountains, China. PeerJ 2021, 9, e11230. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.; Shi, N.N.; Zheng, Y.; Chen, L.; Wubet, T.; Bruelheide, H.; Both, S.; Buscot, F.; Ding, Q.; et al. Community assembly of ectomycorrhizal fungi along a subtropical secondary forest succession. New Phytol. 2015, 205, 771–785. [Google Scholar] [CrossRef]
- Jing, C.Q.; Wang, L.; Wang, T.Y.; Zhang, J.H.; Dong, W.H.; Zhang, X.H. Amplification of deoxyribonucleic acid (DNA) fragment using two-step polymerase chain reaction (PCR). Afr. J. Biotechnol. 2011, 10, 2838–2843. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; Wit, P.D.; Sánchez-García, M.; Ebersberger, I.; Sousa, F.D.; et al. Improved software detection and extraction of ITS1 and ITS 2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Villarreal Ruiz, L.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Core, R.T. R: A Language and Environment for Statistical Computing [J/OL]. 2019. Available online: http://www.Rproject.org (accessed on 29 January 2024).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.0-7. 2013. Available online: http://CRAN.R-project.org/package=vegan (accessed on 12 February 2024).
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Wang, L.X.; Han, M.Z.; Li, X.; Yu, B.B.; Wang, H.; Ginawi, A.; Ning, K.; Yan, Y.J. Mechanisms of niche-neutrality balancing can drive the assembling of microbial community. Mol. Ecol. 2021, 30, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Han, S.J.; Wang, C.G.; Li, M.H. Long-term nitrogen-addition-induced shifts in the ectomycorrhizal fungal community are associated with changes in fine root traits and soil properties in a mixed Pinus koraiensis Forest. Eur. J. Soil Biol. 2022, 112, 103431. [Google Scholar] [CrossRef]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Metcalfe, D.B.; Inselsbacher, E.; Stangl, Z.; Oren, R.; Näsholm, T.; Högberg, P. Greater carbon allocation to mycorrhizal fungi reduces tree nitrogen uptake in a boreal Forest. Ecology 2016, 97, 1012–1022. [Google Scholar] [CrossRef]
- Dong, P.; Ren, Y.; Gao, G.L.; Ding, G.D.; Zhang, Y. Stoichiometry of carbon, nitrogen, and phosphorus in the litter and soil of Pinus sylvestris var. mongolica in the Hulunbuir Sandy Land. Arid Zone Res. 2024, 41, 1354–1363. [Google Scholar] [CrossRef]
- Montesinos-Navarro, A.; Valiente-Banuet, A.; Verdú, M. Plant facilitation through mycorrhizal symbiosis is stronger between distantly related plant species. New Phytol. 2019, 224, 928–935. [Google Scholar] [CrossRef]
- Morgado, L.N.; Semenova, T.A.; Welker, J.M.; Walker, M.D.; Smets, E.; Geml, J. Summer temperature increase has distinct effects on the ectomycorrhizal fungal communities of moist tussock and dry tundra in Arctic Alaska. Glob. Change Biol. 2015, 21, 959–972. [Google Scholar] [CrossRef]
- Orth, R.; Seneviratne, S.I. Analysis of soil moisture memory from observations in Europe. J. Geophys. Res. Atmos. 2012, 117, D15115. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, X.; Xu, Y.; Babalola, B.J.; Xiang, S.M.; Zhao, Y.L.; Fan, Y.J. Fungal diversity and community assembly of ectomycorrhizal fungi associated with five pine species in Inner Mongolia, China. Front. Microbiol. 2021, 12, 646821. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Sakai, A.; Hattori, M.; Nara, K. Strong effect of climate on ectomycorrhizal fungal composition: Evidence from range overlap between two mountains. ISME J. 2015, 9, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Murata, M.; Koizumi, T.; Janowski, D.; Helbert; Nara, K. Climate conditions are primary predictors of the regional-scale spatial diversity patterns of ectomycorrhizal fungi. J. Biogeogr. 2023, 51, 1–13. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Sato, S.; Takizawa, M.; Narimatsu, M. Identification of upregulated genes in tricholoma matsutake mycorrhiza. FEMS Microbiol. Lett. 2022, 369, fnac085. [Google Scholar] [CrossRef]
- Wei, Z.N.; Liu, L.; Lei, Y.D.; Xie, S.S.; Ma, J.M.; Tan, Y.B.; Tang, N.W.; Yang, Z.Q.; Ai, C.B. Establishment of Pinus massoniana–Lactarius hatsudake Symbiosis. Forests 2024, 15, 578. [Google Scholar] [CrossRef]
- Li, F.Q.; Zi, H.Y.; Sonne, C.; Li, X.G. Microbiome sustains forest ecosystem functions across hierarchical scales. Eco Environ. Health 2023, 2, 24–31. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Meng, F.J.; Yin, D.C. Promotion of biomass, photosynthesis, and root growth of seedling biomass, photosynthesis, and root growth of Populus davidiana × P. bolleana by two species of ectomycorrhizal fungi. J. For. Res. 2024, 35, 101. [Google Scholar] [CrossRef]
- Markewitz, D.; Devine, S.; Davidson, E.A.; Brando, P.; Nepstad, D.C. Soil moisture depletion under simulated drought in the Amazon: Impacts on deep root uptake. New Phytol. 2010, 187, 592–607. [Google Scholar] [CrossRef]
- Zhang, X.J.; Shi, F.L.; Zhang, S.C.; Hosen, M.; Zhao, C.L. The diversity and taxonomy of thelephoraceae (Basidiomycota) with descriptions of four species from southwestern China. J. Fungi 2024, 10, 775. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Dragoni, D.; Brown, Z.A.; Phillips, R.P. Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytol. 2015, 206, 1274–1282. [Google Scholar] [CrossRef]
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef]
- Lang, C.; Seven, J.; Polle, A. Host preferences and differential contributions of deciduous tree species shape mycorrhizal species richness in a mixed Central European Forest. Mycorrhiza 2010, 21, 297–308. [Google Scholar] [CrossRef]
- Simard, S.W.; Perry, D.A.; Jones, M.D.; Myrold, D.D. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 1997, 388, 579–582. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae: A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Losos, J.B. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 2008, 11, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L. Research on the Fungal Community of Ectomycorrhizae in the Roots of Common Betulaceae Plants in Chinese Forests. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2019. [Google Scholar]
- Fan, Y.J.; Yan, W.; Zhao, Y.L. Diversity and community constitution of EM fungi in forest communities of Picea crassifolia in Helan Mountains. J. Northeast For. Univ. 2019, 47, 89–93. [Google Scholar] [CrossRef]
- Luo, Z.H.; Wu, C.L.; Wang, Y.L.; Li, C.L.; Wan, L.; Ding, Q. Effects of host identity and leaf traits on foliar endophytic fungal communities in Lauraceae and Fagaceae plants of tropical montane rainforest of Hainan Island. J. Trop. Ecol. 2024, 15, 52–59. [Google Scholar] [CrossRef]
- Yang, J.; Cha, Y.; Oh, S.Y. Habitat prevails over host sex in influencing mycobiome structure of terrestrial isopod, Armadillidium vulgare. Microbiol. Spectr. 2025, 13, e02172-24. [Google Scholar] [CrossRef]
- Birch, J.D.; Lutz, J.A.; Karst, J. Dancing with Douglas-fir: Determinism dominates fungal community assembly Processes. J. Ecol. 2022, 110, 1857–1870. [Google Scholar] [CrossRef]
- Li, M.; Meng, Z.Y.; Li, J.Y.; Zhang, X.; Wang, Y.L.; Li, X.Y.; Yang, Y.Z.; Li, Y.; Yang, X.J.; Chen, X.L.; et al. Stochastic processes dominate the community assembly of ectomycorrhizal fungi associated with Betula platyphylla in Inner Mongolia, China. PeerJ 2025, 13, e19364. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.L.; Xu, Y.; Babalola, B.J.; Xiang, S.M.; Ma, J.M.; Su, Y.; Fan, Y.J. Stochastic processes dominate community assembly of ectomycorrhizal fungi associated with Picea crassifolia in the Helan Mountains, China. Front. Microbiol. 2023, 13, 1061819. [Google Scholar] [CrossRef] [PubMed]
- Buscardo, E.; Geml, J.; Nagy, L. Seasonal dependence of deterministic versus stochastic processes influencing soil fungal community composition in a lowland Amazonian rain Forest. Commun. Earth Environ. 2024, 5, 334. [Google Scholar] [CrossRef]
- Lin, C.P.; Lin, Y.F.; Liu, Y.C.; Lu, M.Y.J.; Ke, H.M.; Tsai, I.J. Spatiotemporal dynamics reveal high turnover and contrasting assembly processes in fungal communities across contiguous habitats of tropical Forests. Environ. Microbiome 2025, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Montoya, L.; Xu, L.; Madera, M.; Hollingsworth, J.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B.; Dahlberg, J.A.; et al. Fungal community assembly in Drought-stressed sorghum shows stochasticity, selection, and universal ecological Dynamics. Nat. Commun. 2020, 11, 34. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Kastman, E.K.; Guasto, J.S.; Wolfe, B.E. Fungal networks shape dynamics of bacterial dispersal and community assembly in cheese rind Microbiomes. Nat. Commun. 2018, 9, 336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).