Production and Storage of Male-Sterile Somatic Embryos of Sugi (Japanese Cedar, Cryptomeria japonica) at Temperatures Above Freezing
Abstract
1. Introduction
2. Materials and Methods
2.1. SE Initiation, Maintenance, Proliferation, and Selection of Male-Sterile ECLs
2.2. Production of Somatic Embryos
2.3. Somatic Embryo Germination and Conversion
2.4. Storage of Somatic Embryos and Post-Storage Germination Test
2.5. Statistical Analysis
3. Results and Discussion
3.1. Maintenance and Proliferation of Male-Sterile ECLs
3.2. Production of Somatic Embryos from Different Male-Sterile ECLs
3.3. Germination and Conversion of Somatic Embryos
3.4. Germination of Stored Somatic Embryos
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECs | Embryogenic cells |
| ECLs | Embryogenic cell lines |
| EMs | Embryogenic masses |
| GLMM | Generalized linear mixed models |
| SE | Somatic embryogenesis |
References
- FAO. Global Forest Resources Assessment 2020 Main Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; 186p. [Google Scholar]
- Forestry Agency. Statistical Handbook of Forest and Forestry; Forestry Agency, Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2020; pp. 8–9. (In Japanese)
- Matsubara, A.; Sakashita, M.; Gotoh, M.; Kawashima, K.; Matsuoka, T.; Kondo, S.; Yamada, T.; Takeno, S.; Takeuchi, K.; Urashima, M.; et al. Epidemiological Survey of Allergic Rhinitis in Japan 2019. Nippon. Jibiinkoka Gakkai Kaiho 2020, 123, 485–490, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- Panasonic Corporation Communication Design Center. PR Times Press Release. Available online: https://prtimes.jp/main/html/rd/p/000001014.000024101.html (accessed on 2 February 2025).
- Maruyama, E.T.; Ueno, S.; Hirayama, S.; Kaneeda, T.; Moriguchi, Y. Somatic embryogenesis and plant regeneration from sugi (Japanese cedar, Cryptomeria japonica D. Don, Cupressaceae) seed families by marker assisted selection for the male sterility allele ms1. Plants 2020, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, E.T.; Ueno, S.; Hosoi, Y.; Miyazawa, S.; Mori, H.; Kaneeda, T.; Bamba, Y.; Itoh, Y.; Hirayama, S.; Kawakami, K.; et al. Somatic embryogenesis initiation in sugi (Japanese cedar, Cryptomeria japonica D. Don): Responses from male-fertile, male-sterile, and polycross-pollinated-derived seed explants. Plants 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, E.T.; Ueno, S.; Mori, H.; Kaneeda, T.; Moriguchi, Y. Factors influencing somatic embryo maturation in sugi (Japanese cedar, Cryptomeria japonica (Thunb. ex L.f.) D. Don). Plants 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, M.; Maruyama, T.E.; Ueno, S.; Hasegawa, Y.; Moriguchi, Y. Marker-assisted selection for pollen-free somatic plants of sugi (Japanese cedar, Cryptomeria japonica): A simple and effective methodology for selecting male-sterile mutants with ms1-1 and ms1-2. Front. Plant Sci. 2021, 12, 748110. [Google Scholar] [CrossRef]
- Tsuruta, M.; Maruyama, T.E.; Ueno, S.; Kaneeda, T.; Moriguchi, Y. Plant regeneration and in vitro growth performance of male-sterile somatic plantlets of sugi (Japanese cedar, Cryptomeria japonica) derived from different embryogenic cell lines. Forests 2021, 12, 1592. [Google Scholar] [CrossRef]
- Tsuruta, M.; Ueno, S.; Maruyama, T.E.; Hakamata, T.; Moriguchi, Y. Application of STH-PAS, a novel chromatographic visualization system of PCR products for rapid screening of male-sterile lines in Cryptomeria japonica. Bull. For. For. Prod. Res. Inst. 2022, 463, 223–227. [Google Scholar]
- Maruyama, T.E.; Tsuruta, M.; Ueno, S.; Kawakami, K.; Bamba, Y.; Moriguchi, Y. An Improved and Simplified Propagation System for Pollen-Free Sugi (Cryptomeria japonica) via Somatic Embryogenesis. Front. Plant Sci. 2022, 13, 825340. [Google Scholar] [CrossRef]
- FFPRI (Forestry and Forest Products Research Institute). Manual for the Propagation of Pollen-Free Sugi (Cryptomeria japonica) via Somatic Embryogenesis; Department of Forest Molecular Genetics and Biotechnology, Ed.; Asahi Publishers: Ibaraki, Japan, 2024; Version 1.2; pp. 11–15. (In Japanese)
- Klimaszewska, K.; Cyr, D.R. Conifer somatic embryogenesis: I. Development. Dendrobiology 2002, 48, 31–39. [Google Scholar]
- Maruyama, E.; Tanaka, T.; Hosoi, Y.; Ishii, K.; Morohoshi, N. Embryogenic cell culture, protoplast regeneration, cryopreservation, biolistic gene transfer and plant regeneration in Japanese cedar (Cryptomeria japonica D. Don). Plant Biotechnol. 2000, 17, 281–296. [Google Scholar] [CrossRef]
- Ueno, S.; Uchiyama, K.; Moriguchi, Y.; Ujino-Ihara, T.; Matsumoto, A.; Wei, F.J.; Saito, M.; Higuchi, Y.; Futamura, N.; Kanamori, H.; et al. Scanning RNA-Seq and RAD-Seq approach to develop SNP markers closely linked to MALE STERILITY 1 (MS1) in Cryptomeria japonica D. Don. Breed. Sci. 2019, 69, 19–29. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 29 November 2024).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Egertsdotter, U.; von Arnold, S. Classification of embryogenic cell lines of Picea abies as regards protoplast isolation and culture. J. Plant Physiol. 1993, 141, 222–229. [Google Scholar] [CrossRef]
- Bercetche, J.; Pâques, M. Somatic embryogenesis in maritime pine (Pinus pinaster). In Somatic Embryogenesis in Woody Plants, Vol. 3 Gymnosperms; Jain, S., Gupta, P., Newton, R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 222–242. [Google Scholar]
- Filonova, L.H.; Bozhkov, P.V.; von Arnold, S. Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J. Exp. Bot. 2000, 343, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Salajová, T.; Salaj, J. Somatic embryogenesis in Pinus nigra: Embryogenic tissue initiation, maturation and regeneration ability of established cell lines. Biol. Plant. 2005, 49, 333–339. [Google Scholar] [CrossRef]
- Reeves, C.; Hargreaves, C.; Trontin, J.-F.; Lelu-Walter, M.-A. Simple and efficient protocols for the initiation and proliferation of embryogenic tissue of Douglas-fir. Trees 2018, 32, 175–190. [Google Scholar] [CrossRef]
- Klimaszewska, K. Somatic embryogenesis in Picea mariana (Mill.). In Somatic Embryogenesis in Woody Plants, Vol. 3 Gymnosperms; Jain, S., Gupta, P., Newton, R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 67–79. [Google Scholar]
- Klimaszewska, K.; Trontin, J.F.; Becwar, M.R.; Devillard, C.; Park, Y.S.; Lelu-Walter, M.A. Recent progress in somatic embryogenesis of four Pinus spp. Tree For. Sci. Biotechol. 2007, 1, 11–25. [Google Scholar]
- Guo, H.-H.; Wu, J.-F.; Chen, C.-X.; Wang, H.-M.; Zhao, Y.-L.; Zhan, C.-J.; Jia, Y.-H.; Liu, F.; Ning, T.-Y.; Chu, Z.-H.; et al. Identificacion and characterization of cell cultures with various embryogenic/regenerative potential in cotton based on morphological, cytochemical, and cytogenetical assessment. J. Integrat. Agric. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Peng, C.; Gao, F.; Wang, H.; Tretyakova, I.N.; Nosov, A.M.; Shen, S.; Yang, L. Morphological and physiological indicators for screening cell lines with high potential for somatic embryo maturation at an early stage of somatic embryogenesis in Pinus koraiensis. Plants 2022, 11, 1867. [Google Scholar] [CrossRef]
- Lian, Y.; Li, J.; Xu, X.; Yang, Q.; Gao, M.; Chen, Y.; Guan, C. Genotype-dependent induction of embryogenic callus and programmed cell death in Korean pine. BMC Plant Biol. 2025, 25, 741. [Google Scholar] [PubMed]
- Ramarosandratana, A.; Harvengt, L.; Bouvet, A.; Calvayrac, R.; Pâques, M. Effects of carbohydrate source, polyethylene glycol and gellan gum concentration on embryonal-suspensor mass (ESM) proliferation and maturation of maritime pine somatic embryos. Vitr. Cell. Develop. Biol. Plant 2001, 37, 29–34. [Google Scholar] [CrossRef]
- Miguel, C.; Gonçalves, S.; Tereso, S.; Marum, L.; Maroco, J.; Oliveira, M. Somatic embryogenesis from 20 open-pollinated families of Portuguese plus trees of maritime pine. Plant Cell Tissue Organ Cult. 2004, 76, 121–130. [Google Scholar] [CrossRef]
- Lelu-Walter, M.A.; Bernier-Cardou, M.; Klimaszewska, K. Simplified and improved somatic embryogenesis for clonal propagation of Pinus pinaster. Plant Cell Rep. 2006, 25, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Tautorus, T.E.; Fowke, L.C.; Dunstan, D.I. Somatic embryogenesis in conifers. Can. J. Bot. 1991, 69, 1873–1899. [Google Scholar] [CrossRef]
- Stasolla, C.; Kong, L.; Yeung, E.C.; Thorpe, T.E. Maturation of somatic embryos in conifers: Morphogenesis, physiology, biochemistry, and molecular biology. Vitr. Cell Dev. Biol.-Plant 2002, 38, 93–105. [Google Scholar] [CrossRef]
- Stasolla, C.; Yeung, E.C. Recent advances in conifer somatic embryogenesis: Improving somatic embryo quality. Plant Cell Tissue Organ Cult. 2003, 74, 15–35. [Google Scholar] [CrossRef]
- Elmer, K.H.S. Factors regulating somatic embryogenesis in plants. In Somatic Embryogenesis and Gene Expression; Aslam, J., Srivastava, P.S., Sharma, M.P., Eds.; Narosa Publishing House: New Delhi, India, 2013; pp. 56–81. [Google Scholar]
- Egertsdotter, U. Plant physiological and genetical aspects of the somatic embryogenesis process in conifers. Scand. J. For. Res. 2019, 34, 360–369. [Google Scholar] [CrossRef]
- Izuno, A.; Maruyama, T.E.; Ueno, S.; Ujino-Ihara, T.; Moriguchi, Y. Genotype and transcriptome effects on somatic embryogenesis in Cryptomeria japonica. PLoS ONE 2020, 15, e0244634. [Google Scholar] [CrossRef]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Somatic embryo production and plant regeneration of Japanese black pine (Pinus thunbergii). J. For. Res. 2005, 10, 403–407. [Google Scholar] [CrossRef]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Propagation of Japanese red pine (Pinus densiflora Zieb. et Zucc.) via somatic embryogenesis. Prop. Ornam. Plants 2005, 5, 199–204. [Google Scholar]
- Hosoi, Y.; Maruyama, T.E. Plant regeneration from embryogenic tissue of Pinus luchuensis Mayr, and endemic species in Ryukyu Island, Japan. Plant Biotechnol. 2012, 29, 401–406. [Google Scholar] [CrossRef]
- Maruyama, E.T.; Hosoi, Y. Progress in somatic embryogenesis of Japanese pines. Front. Plant Sci. 2019, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.R.; Sutton, B.C.S.; Flinn, B.S. Synchronous and high frequency germination of interior spruce somatic embryos following partial drying at high relative humidity. Can. J. Bot. 1990, 68, 1086–1090. [Google Scholar] [CrossRef]
- Lelu, M.-A.; Klimaszewska, K.; Pflaum, G.; Bastien, C. Effect of maturation duration on desiccation tolerance in hybrid larch (Larix × leptoeuropaea Dengler) somatic embryos. Vitr. Cell Dev. Biol.-Plant 1995, 31, 15–20. [Google Scholar] [CrossRef]
- Lara-Chavez, A.; Flinn, B.S.; Egertsdotter, U. Initiation of somatic embryogenesis from immature zygotic embryos of Oocarpa pine (Pinus oocarpa Schiede ex Schlectendal). Tree Physiol. 2011, 31, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.K.; Juan, I.-P. Improving the germination of somatic embryos of Picea morrisonicola Hayata: Effects of cold storage and partial drying. J. For. Res. 2015, 20, 114–124. [Google Scholar] [CrossRef]
- Pullman, G.S.; Olson, K.; Fisher, T.; Egertsdotter, U.; Frampton, J.; Bucalo, K. Fraser fir somatic embryogenesis: High frequency initiation, maintenance, embryo development, germination and cryopreservation. New For. 2016, 47, 453–480. [Google Scholar] [CrossRef]
- Park, Y.S. Implementation of conifer somatic embryogenesis in clonal forestry: Technical requirements and deployment considerations. Ann. For. Sci. 2002, 59, 651–656. [Google Scholar] [CrossRef]
- Maruyama, E.T.; Ishii, K.; Hosoi, Y. Cryopreservation of sugi embryogenic cells by simple freezing and vitrification methods. In Cryopreservation of Plant Cell and Organs; Takao, N., Hirai, D., Matsumoto, T., Tanaka, D., Eds.; National Institute of Agrobiological Sciences: Ibaraki, Japan, 2006; pp. 145–146. (In Japanese) [Google Scholar]
- Taniguchi, T.; Konagaya, K.; Nanasato, Y. Somatic embryogenesis in artificially pollinated seed families of 2nd generation plus trees and cryopreservation of embryogenic tissue in Cryptomeria japonica D. Don (sugi). Plant Biotechnol. 2020, 37, 239–245. [Google Scholar] [CrossRef]
- Ballesteros, D.; Martínez, M.T.; Sánchez-Romero, C.; Montalbán, I.; Sales, E.; Moncaleán, P.; Arrillaga, I.; Corredoira, E. Current status of the cryopreservation of embryogenic material of woody species. Front. Plant Sci. 2024, 14, 1337152. [Google Scholar] [CrossRef]
- Torres-Viñals, M.; Sabaté-Casaseca, S.; Aktouche, N.; Grenan, S.; Lopez, G.; Porta-Falguera, M.; Torregrosa, L. Large-scale production of somatic embryos as a source of hypocotyl explants for Vitis vinifera micrografting. Vitis 2004, 43, 163–168. [Google Scholar]
- Jayasankar, S.; Van Aman, M.; Cordts, J.; Dhekney, S.; Li, Z.T.; Gray, D.J. Low temperature storage of suspension culture-derived grapevine somatic embryos and regeneration of plants. Vitr. Cell. Dev. Biol. Plant 2005, 41, 752. [Google Scholar] [CrossRef]
- El-Dawayati, M.M.; Abdel baki, M.A.; Abdelgalil, L.M. Effect of Different conservation periods with different sucrose concentrations on conserving somatic embryos clusters of Date Palm (Phoenix dactylifera L.) under minimal growth conditions. Food Nutr. J. 2017, 2, 133. [Google Scholar]
- Shigeta, J.; Mori, T.; Sato, K. Storage of encapsulated somatic embryos of carrot. Biotech. Techn. 1993, 7, 165–168. [Google Scholar] [CrossRef]
- Nadel, B.L.; Altman, A.; Ziv, M. Cold storage and efficient conversion of somatic celery embryos into transplantable plants. Sci. Hort. 1990, 44, 9–16. [Google Scholar] [CrossRef]
- Bornman, C.H.; Dickens, O.S.P.; van der Merwe, C.F.; Coetzee, J.; Botha, A.-M. Somatic embryos of Picea abies behave like isolated zygotic embryos in vitro with greatly reduced physiological vigour. S. Afr. J. Bot. 2003, 69, 176–185. [Google Scholar] [CrossRef]
- Reeves, C.; Tikkinen, M.; Aronen, T.; Krajnakova, J. Application of cold storage and short in vitro germination for somatic embryos of Pinus radiata and P. sylvestris. Plants 2023, 12, 2095. [Google Scholar] [CrossRef] [PubMed]
- Välimäki, S.; Teyssier, C.; Tikkinen, M.; Delile, A.; Boizot, N.; Varis, S.; Lelu-Walter, M.-A.; Aronen, T. Norway spruce somatic embryogenesis benefits from proliferation of embryogenic tissues on filter discs and cold storage of cotyledonary embryos. Front. Plant Sci. 2022, 13, 1031686. [Google Scholar] [CrossRef] [PubMed]

| Stages | Summarized Conditions | Summarized Results | References |
| Embryogenic culture initiation | Culture of megagametophyte explants (isolated from seeds collected in mid-July 2016 from the full-sib seed family ‘Shindai 3’ × ‘Suzu 2’) in the dark at 25 °C. | Initiation rate: 14.1% | Maruyama et al. 2021a [6] |
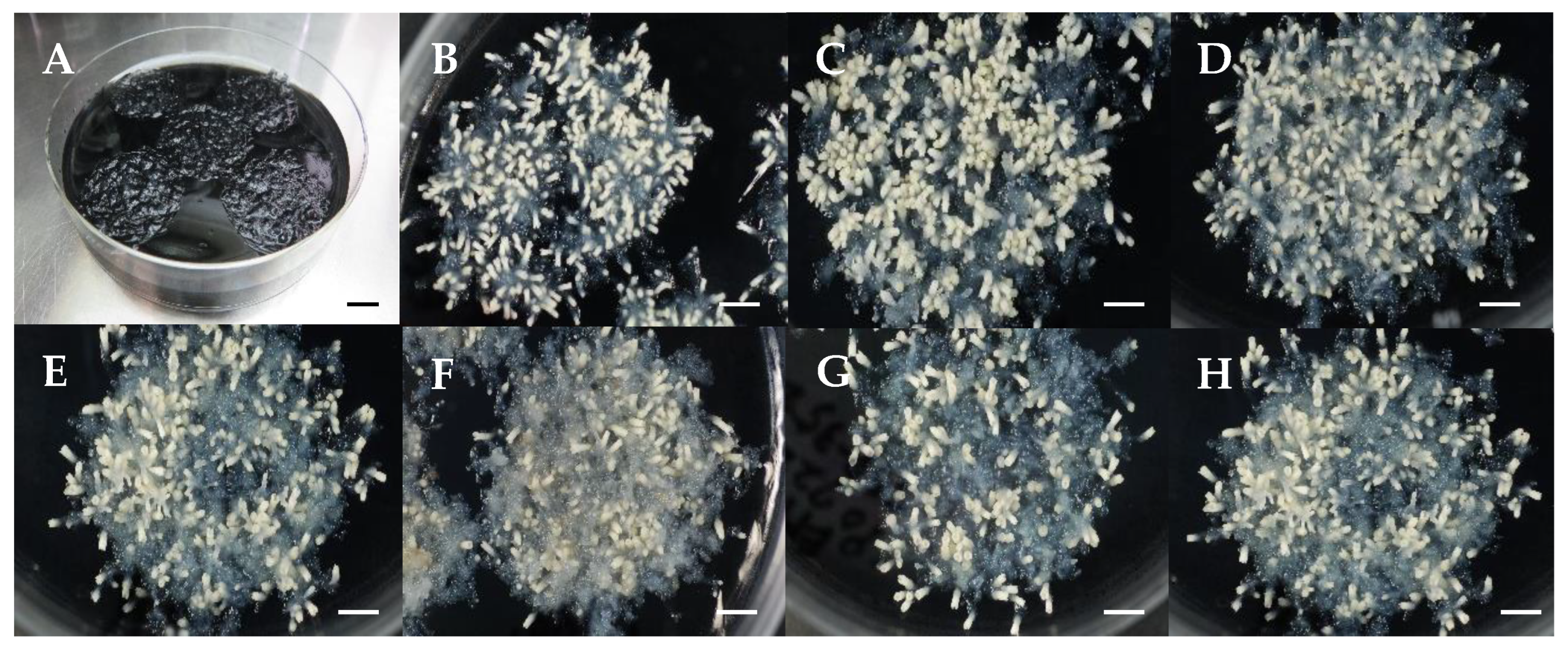
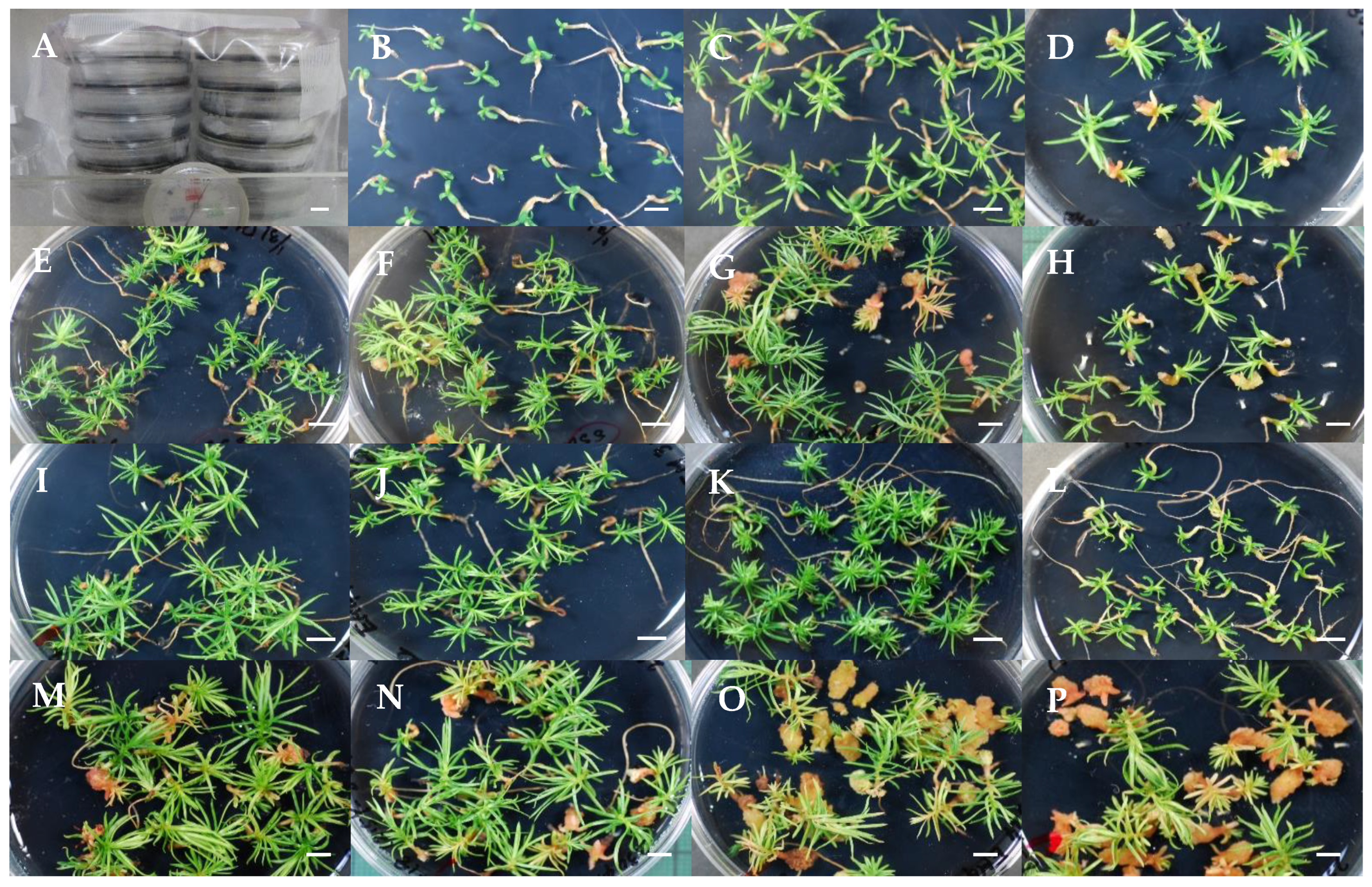
| Maintenance and proliferation of EMs | Subculture EMs on maintenance/proliferation medium in the dark at 25 °C every 2 weeks. | Friable and white EMs: SSD-018, SSD-112, SSD-352; Friable and yellowish-white EMs: SSD-073, SSD-113; Mucilaginous and yellowish-white EMs: SSD-100. | This report (Figure 1) |
| Discrimination of male-sterile ECLs | DNA extraction from embryogenic cells by InstaGene and DNA marker diagnosis of MS1 genotype. | Discrimination probability of male-sterile ECLs: 100%. | Ueno et al. 2019 [5]; Tsuruta et al. 2021a [8] |
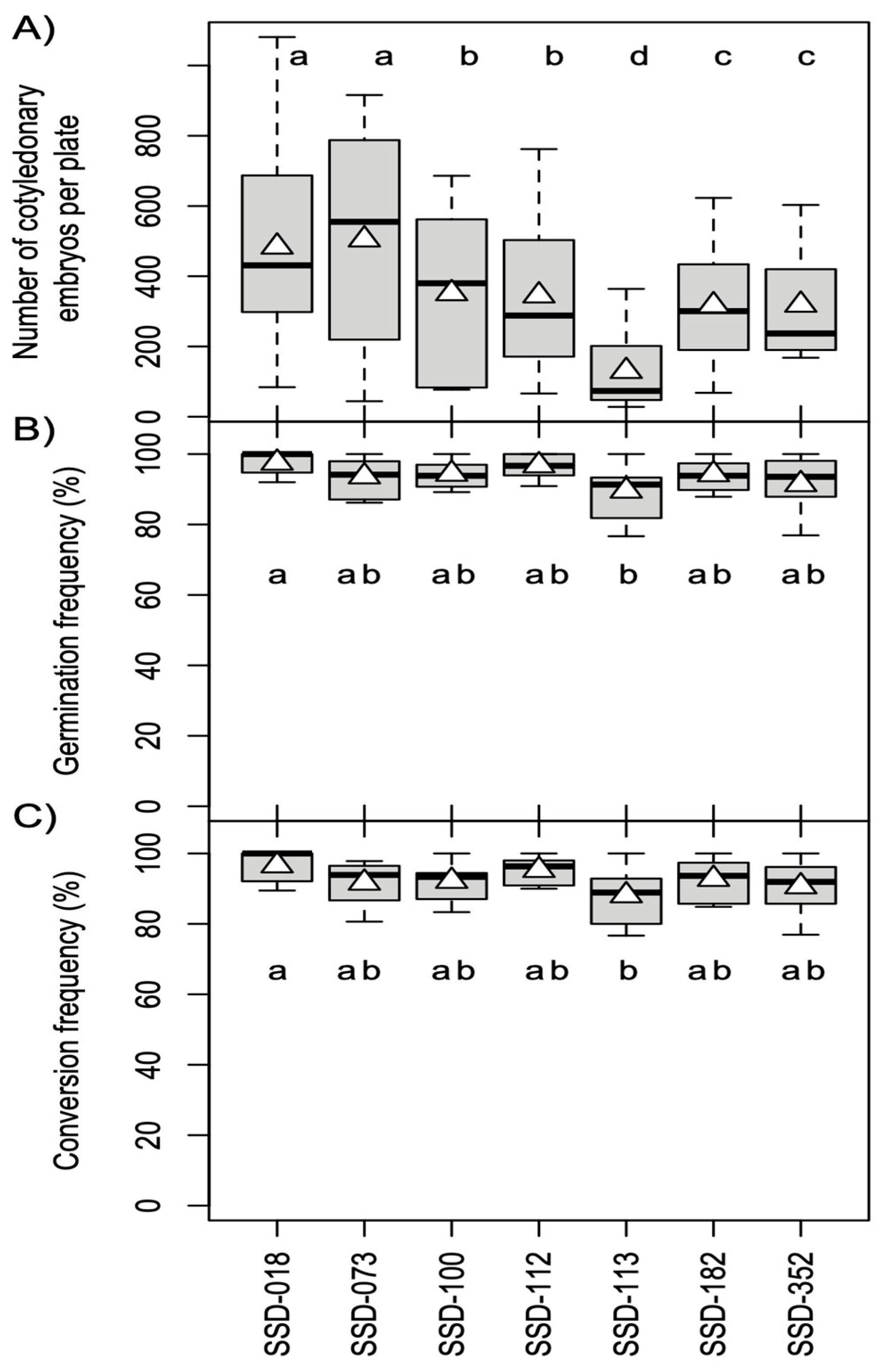
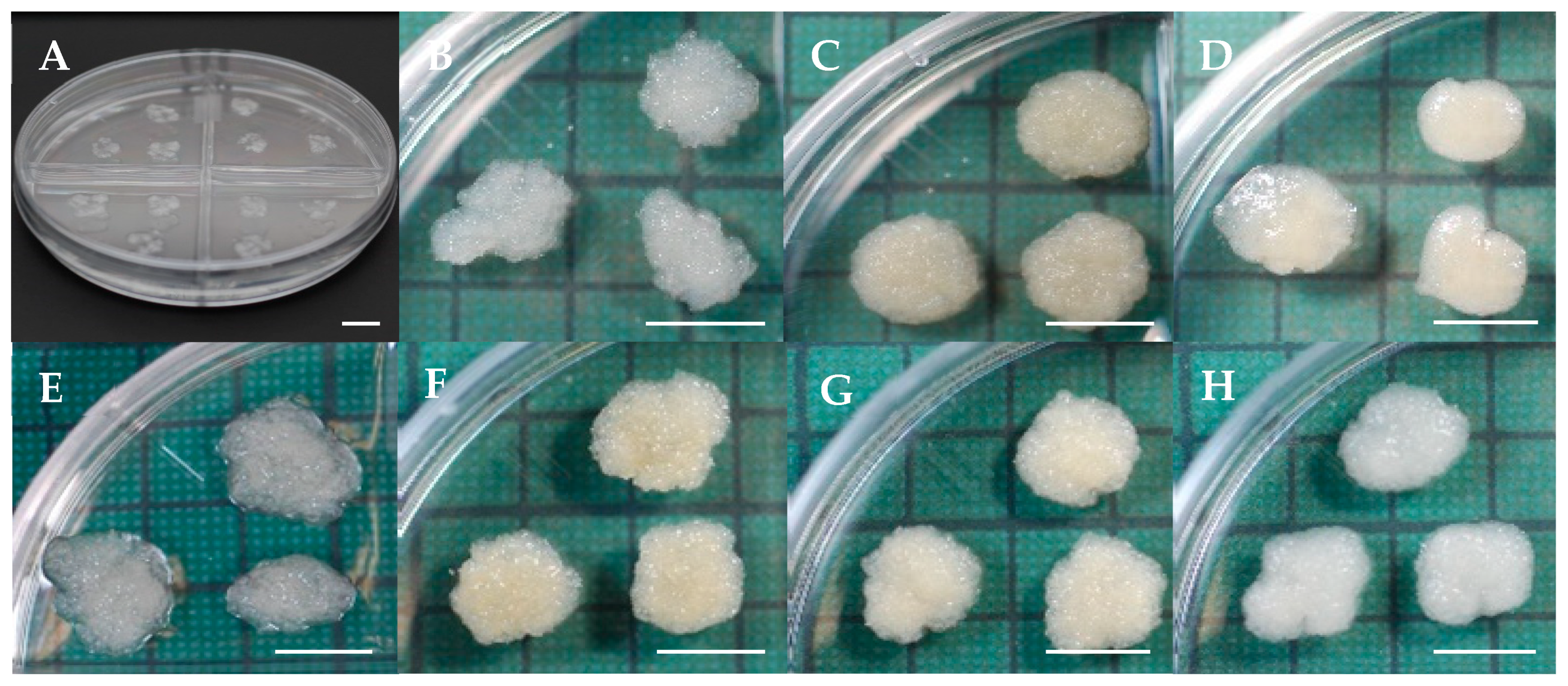
| Production of somatic embryos | Culture of EMs on maturation medium (5 masses per plate, 100 mg each) in the dark at 25 °C. | Average number of mature embryos per plate: 349.8; Range: 129.6–504.1. | This report (Figure 2A) |
| Somatic embryo germination and conversion | Culture somatic embryos on germination medium at 25 °C under a photon flux density of 45–65 µmol m−2 s−1 for 16 h per day. | Average germination rate: 93.9%; Range: 89.6%–97.5%. Average conversion rate: 92.5%; Range: 88.1%–96.6%. | This report (Figure 2B,C) |
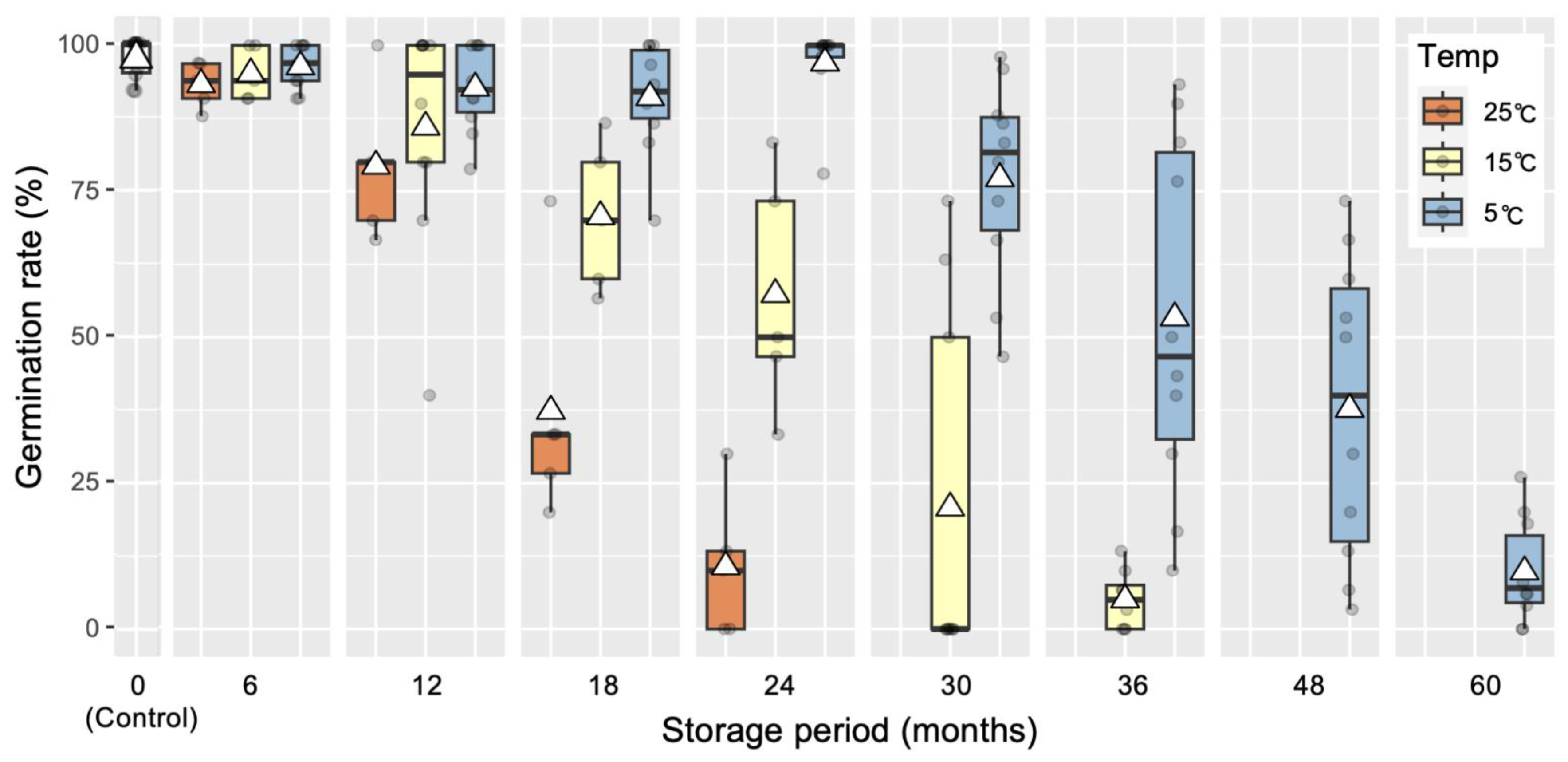
| Storage of somatic embryos and post-storage germination test of embryos derived from line SSD-018 | Plates containing cotyledonary embryos on maturation medium were sealed with three layers of Parafilm®, placed in a Ziploc® freezer bag, and stored in the dark at 25 °C, 15 °C, and 5 °C for 0–60 months (M). Germination test was conducted on germination medium at 25 °C under a photon flux density of 45–65 µmol m−2 s−1 for 16 h per day. | Germination rate after storage at 25 °C for: 6 M (93.3%), 12 M (79.3%), 18 M (37.3%), and 24 M (10.7%). At 15 °C for: 6 M (95.2%), 12 M (86.0%), 18 M (70.7%), 24 M (55.3%), 30 M (20.7%), and 36 M. At 5 °C for: 6 M (96.4%), 12 M (92.7%), 18 M (91.1%), 24 M (97.0%), 30 M (77.2%), 36 M (53.3%), 48 M (37.7%), and 60 M (9.8%). | This report (Figure 5) |
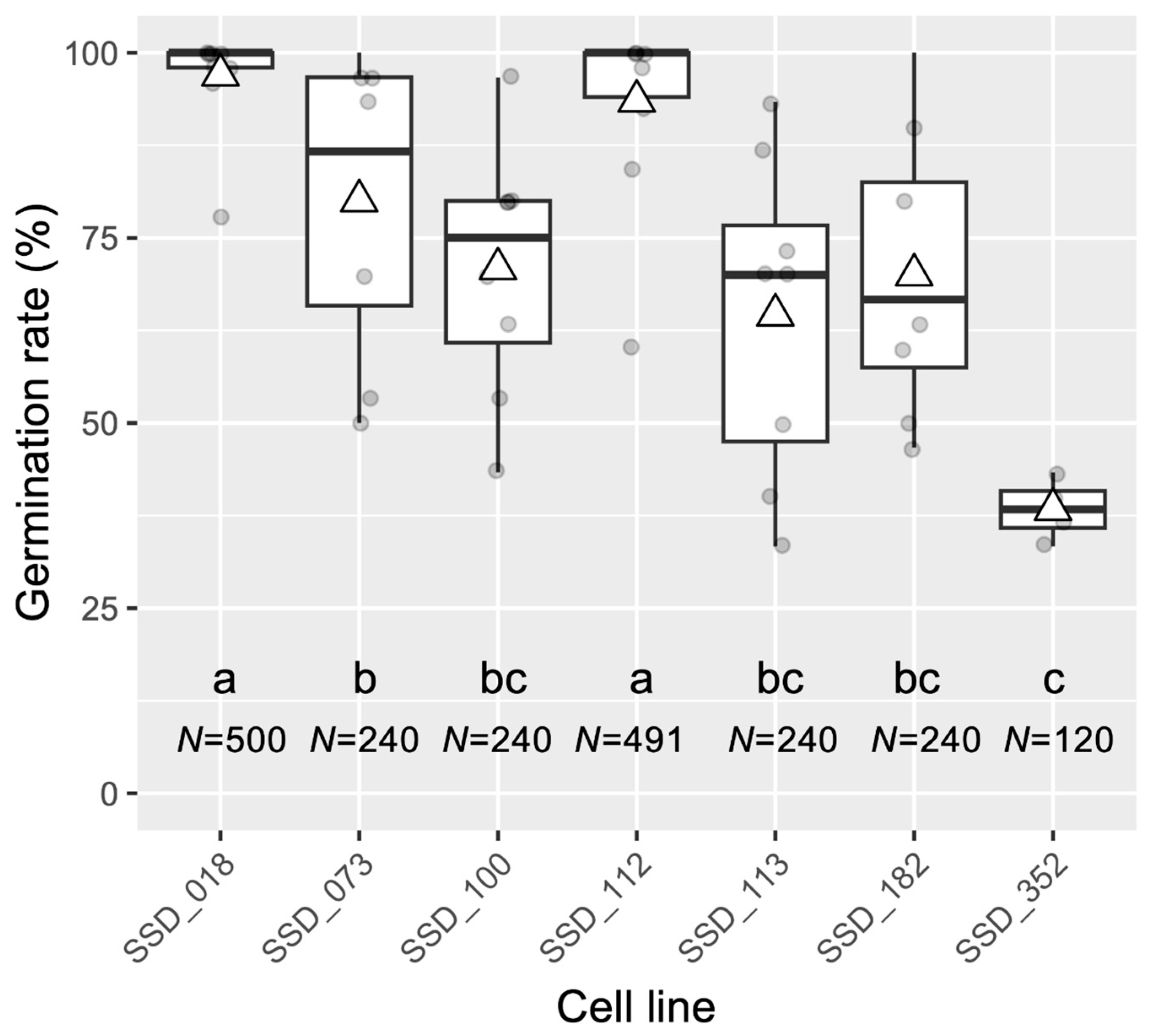
| Storage of somatic embryos and post-storage germination test of embryos derived from different ECLs | The same conditions described above apply to the storage of somatic embryos at 5 °C for 24 months (M). | Germination rate of different ECLs after storage at 5 °C for 24 M: SSD-018 (97,0%), SSD-073 (80%), SSD-100 (70.8%), SSD-112 (93.5%), SSD-113 (64.6%), SSD-182 (70%), and SSD-352 (38.3%). | This report (Figure 6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, T.E.; Tsuruta, M.; Ueno, S.; Moriguchi, Y. Production and Storage of Male-Sterile Somatic Embryos of Sugi (Japanese Cedar, Cryptomeria japonica) at Temperatures Above Freezing. Forests 2025, 16, 1431. https://doi.org/10.3390/f16091431
Maruyama TE, Tsuruta M, Ueno S, Moriguchi Y. Production and Storage of Male-Sterile Somatic Embryos of Sugi (Japanese Cedar, Cryptomeria japonica) at Temperatures Above Freezing. Forests. 2025; 16(9):1431. https://doi.org/10.3390/f16091431
Chicago/Turabian StyleMaruyama, Tsuyoshi E., Momi Tsuruta, Saneyoshi Ueno, and Yoshinari Moriguchi. 2025. "Production and Storage of Male-Sterile Somatic Embryos of Sugi (Japanese Cedar, Cryptomeria japonica) at Temperatures Above Freezing" Forests 16, no. 9: 1431. https://doi.org/10.3390/f16091431
APA StyleMaruyama, T. E., Tsuruta, M., Ueno, S., & Moriguchi, Y. (2025). Production and Storage of Male-Sterile Somatic Embryos of Sugi (Japanese Cedar, Cryptomeria japonica) at Temperatures Above Freezing. Forests, 16(9), 1431. https://doi.org/10.3390/f16091431







