Enhanced Detection of Phytophthora Species at P. pluvialis Outbreak Sites in Commercial Forests Across Britain
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection and Sampling Strategy
2.2. Stream Water Sampling by Baiting
2.3. Stream Water and Rainwater Sampling for Metabarcoding
2.4. Soil Sampling
2.5. Processing of Samples for Metabarcoding
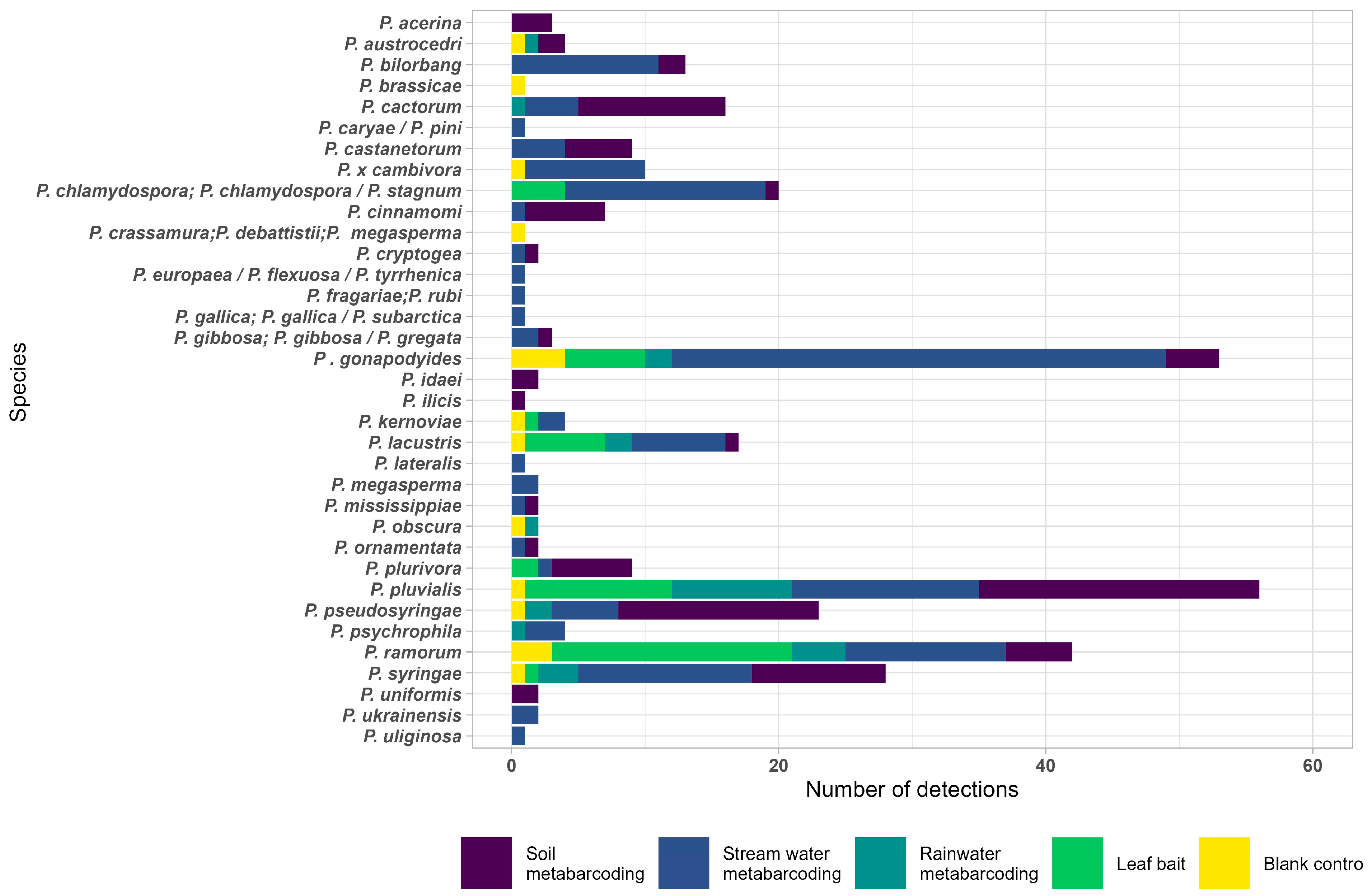
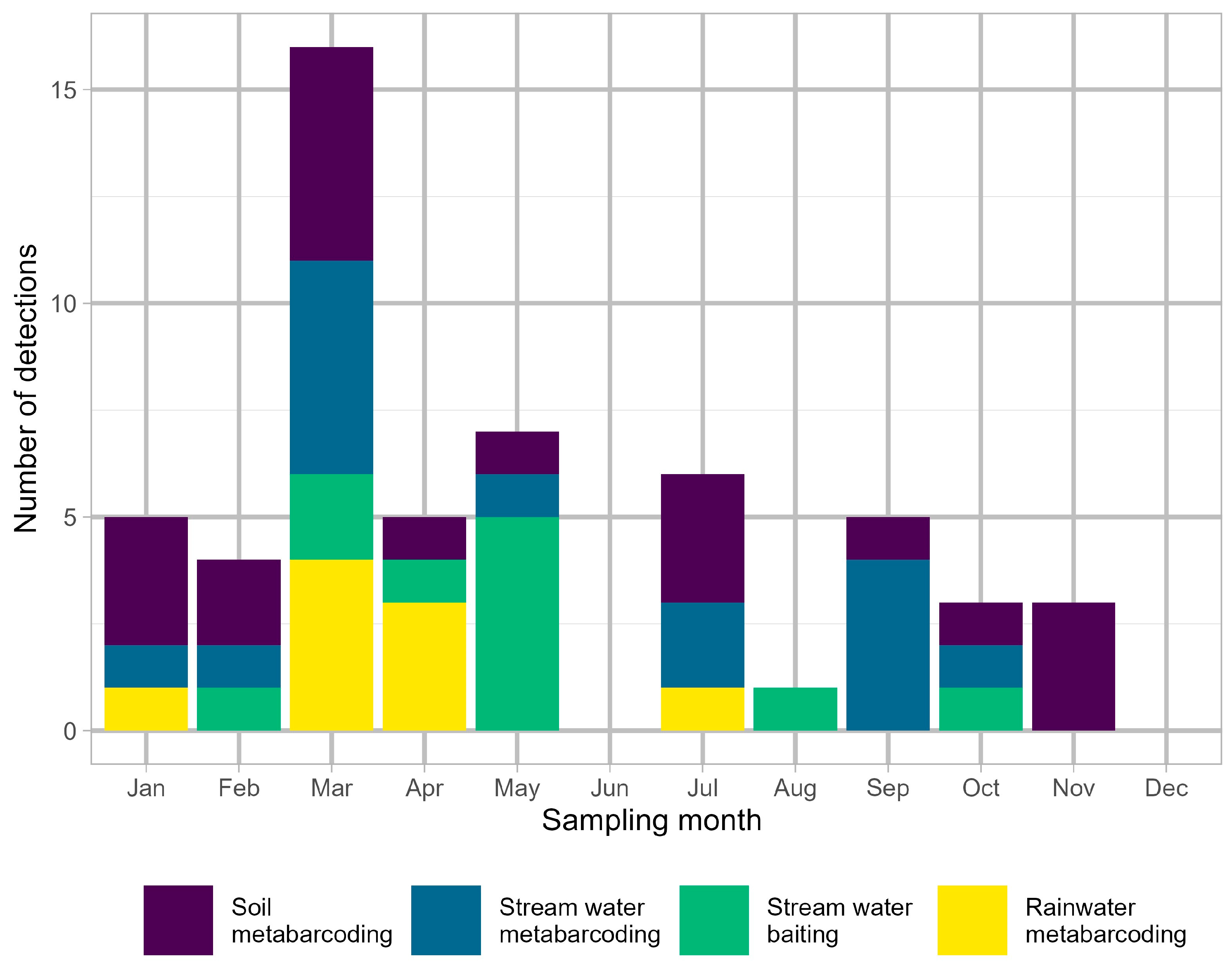
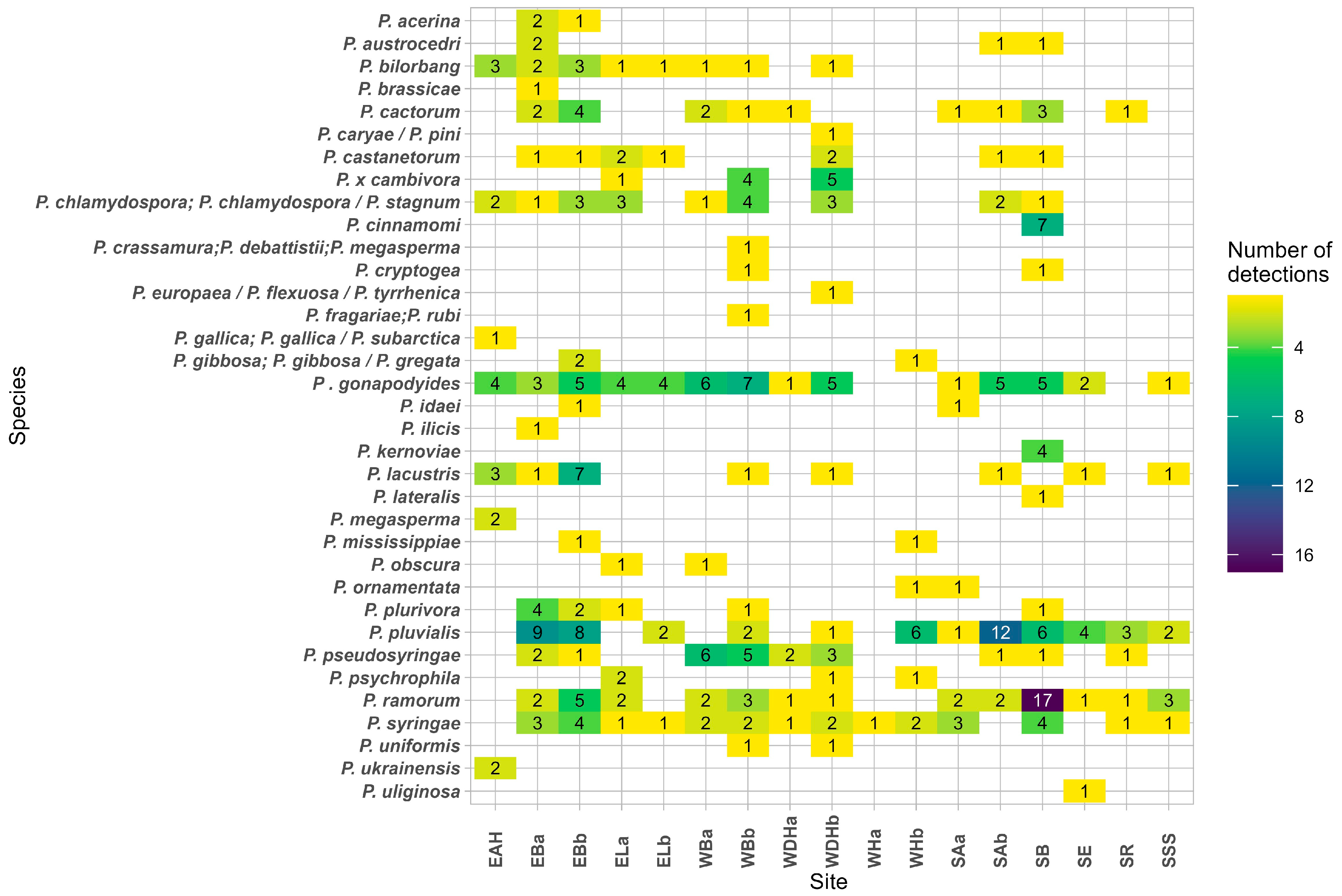
3. Results
3.1. Phytophthora Detection in Stream Water by Baiting
3.2. Phytophthora Detection in Stream Water by Metabarcoding
3.2.1. England—Stream Water
3.2.2. Wales—Stream Water
3.2.3. Scotland—Stream Water
3.3. Phytophthora Detection in Rainwater by Metabarcoding
3.4. Phytophthora Detection in Soil by Metabarcoding
3.4.1. England—Soil
3.4.2. Wales—Soil
3.4.3. Scotland—Soil
3.5. Detections of Unknown Phytophthora spp. and Nothophytophthora spp.
4. Discussion
4.1. Detection of Phytophthora Pluvialis and Implications for Spread and Surveillance
4.2. Other Phytophthora Species Detected and Implications for Forest Health
4.3. Unidentified Phytophthora Species and Other Oomycete Genera
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abad, Z.G.; Burgess, T.I.; Bourret, T.; Bensch, K.; Cacciola, S.O.; Scanu, B.; Mathew, R.; Kasiborski, B.; Srivastava, S.; Kageyama, K.; et al. Phytophthora: Taxonomic and Phylogenetic Revision of the Genus. Stud. Mycol. 2023, 106, 259–348. [Google Scholar] [CrossRef]
- Jung, T.; Milenković, I.; Balci, Y.; Janoušek, J.; Kudláček, T.; Nagy, Z.; Baharuddin, B.; Bakonyi, J.; Broders, K.D.; Cacciola, S.O.; et al. Worldwide Forest Surveys Reveal Forty-Three New Species in Phytophthora Major Clade 2 with Fundamental Implications for the Evolution and Biogeography of the Genus and Global Plant Biosecurity. Stud. Mycol. 2024, 107, 251–388. [Google Scholar] [CrossRef] [PubMed]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Green, S.; Cooke, D.E.L.; Barwell, L.; Purse, B.V.; Cock, P.; Frederickson-Matika, D.; Randall, E.; Keillor, B.; Pritchard, L.; Thorpe, P.; et al. The Prevalence of Phytophthora in British Plant Nurseries; High-Risk Hosts and Substrates and Opportunities to Implement Best Practice. Plant Pathol. 2025, 74, 696–717. [Google Scholar] [CrossRef]
- Webber, J.F.; Mullett, M.; Brasier, C.M. Dieback and Mortality of Plantation Japanese Larch (Larix kaempferi) Associated with Infection by Phytophthora Ramorum. New Dis. Rep. 2010, 22, 19. [Google Scholar] [CrossRef]
- Green, S.; Elliot, M.; Armstrong, A.; Hendry, S.J. Phytophthora Austrocedrae Emerges as a Serious Threat to Juniper (Juniperus communis) in Britain. Plant Pathol. 2015, 64, 456–466. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; Chitty, R.; Eacock, A.; Jones, B.; Biddle, M.; Crampton, M.; Lewis, A.; Olivieri, L.; Webber, J.F. First Report of Phytophthora pluvialis in Europe Causing Resinous Cankers on Western Hemlock. New Dis. Rep. 2022, 45, e12064. [Google Scholar] [CrossRef]
- Reeser, P.; Sutton, W.; Hansen, E.; Reeser, P.; Sutton, W.; Hansen, E. Phytophthora pluvialis, a New Species from Mixed Tanoak-Douglas-Fir Forests of Western Oregon, U.S.A. N Am. Fungi 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Dick, M.A.; Williams, N.M.; Karl-Friedrich, M.B.; Gardner, J.F.; Bulman, L.S. Pathogenicity of Phytophthora pluvialis to Pinus radiata and Its Relation with Red Needle Cast Disease in New Zealand. N. Z. J. For. Sci. 2014, 44, 6. [Google Scholar] [CrossRef]
- Riddell, C.E.; Frederickson-Matika, D.; Armstrong, A.C.; Elliot, M.; Forster, J.; Hedley, P.E.; Morris, J.; Thorpe, P.; Cooke, D.E.L.; Pritchard, L.; et al. Metabarcoding Reveals a High Diversity of Woody Host-Associated Phytophthora spp. In Soils at Public Gardens and Amenity Woodlands in Britain. PeerJ 2019, 7, e6931. [Google Scholar] [CrossRef]
- Landa, B.B.; Arias-Giraldo, L.F.; Henricot, B.; Montes-Borrego, M.; Shuttleworth, L.A.; Pérez-Sierra, A. Diversity of Phytophthora Species Detected in Disturbed and Undisturbed British Soils Using High-Throughput Sequencing Targeting ITS RRNA and COI MtDNA Regions. Forests 2021, 12, 229. [Google Scholar] [CrossRef]
- Riddell, C.E.; Dun, H.F.; Elliot, M.; Armstrong, A.C.; Clark, M.; Forster, J.; Hedley, P.E.; Green, S. Detection and Spread of Phytophthora austrocedri within Infected Juniperus communis Woodland and Diversity of Co-Associated Phytophthoras as Revealed by Metabarcoding. For. Pathol. 2020, 50, e12602. [Google Scholar] [CrossRef]
- Green, S.; Cooke, D.E.L.; Dunn, M.; Barwell, L.; Purse, B.; Chapman, D.S.; Valatin, G.; Schlenzig, A.; Barbrook, J.; Pettitt, T.; et al. PHYTO-THREATS: Addressing Threats to UK Forests and Woodlands from Phytophthora; Identifying Risks of Spread in Trade and Methods for Mitigation. Forests 2021, 12, 1617. [Google Scholar] [CrossRef]
- Català, S.; Pérez-Sierra, A.; Abad-Campos, P. The Use of Genus-Specific Amplicon Pyrosequencing to Assess Phytophthora Species Diversity Using EDNA from Soil and Water in Northern Spain. PLoS ONE 2015, 10, e0119311. [Google Scholar] [CrossRef]
- Schiffer-Forsyth, K.; Frederickson Matika, D.; Hedley, P.E.; Cock, P.J.A.; Green, S. Phytophthora in Horticultural Nursery Green Waste—A Risk to Plant Health. Horticulturae 2023, 9, 616. [Google Scholar] [CrossRef]
- Elliott, C.G.; Hendrie, M.R.; Knights, B.A. The Sterol Requirement of Phytophthora cactorum. J. Gen. Microbiol. 1966, 42, 425–435. [Google Scholar] [CrossRef]
- Brasier, C.M.; Beales, P.A.; Kirk, S.A.; Denman, S.; Rose, J. Phytophthora kernoviae sp. Nov., an Invasive Pathogen Causing Bleeding Stem Lesions on Forest Trees and Foliar Necrosis of Ornamentals in the UK. Mycol. Res. 2005, 109, 853–859. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: London, UK, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A Molecular Phylogeny of Phytophthora and Related Oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Longmire, J.L.; Maltbie, M.; Baker, R.J. Use of Lysis Buffer in DNA Isolation and Its Implication for Museum Collections; Museum of Texas Tech University: Lubbock, TX, USA, 1997. [Google Scholar]
- QIAGEN N.V. DNeasy® Blood and Tissue Handbook; 06/2023 HB-2061-003; QIAGEN N.V.: Hilden, Germany, 2023. [Google Scholar]
- Scibetta, S.; Schena, L.; Chimento, A.; Cacciola, S.O.; Cooke, D.E.L. A Molecular Method to Assess Phytophthora Diversity in Environmental Samples. J. Microbiol. Methods 2012, 88, 356–368. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Cooke, D.E.L.; Thorpe, P.; Pritchard, L. THAPBI PICT—A Fast, Cautious, and Accurate Metabarcoding Analysis Pipeline. PeerJ 2023, 11, e15648. [Google Scholar] [CrossRef] [PubMed]
- Illumina Support Centre. Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System; Illumina Support Centre: San Diego, CA, USA, 2013; pp. 1–28. [Google Scholar]
- Edgar, R.C. UNOISE2: Improved Error-Correction for Illumina 16S and ITS Amplicon Sequencing. bioRxiv 2016, 081257. [Google Scholar] [CrossRef]
- Pirronitto, S.; Paquet, F.; Gaucet, V.; Chandelier, A. First Report of Phytophthora pluvialis in Douglas Fir Plantations in Belgium. New Dis. Rep. 2024, 49, e12244. [Google Scholar] [CrossRef]
- Fraser, S.; Gomez-Gallego, M.; Gardner, J.; Bulman, L.S.; Denman, S.; Williams, N.M. Impact of Weather Variables and Season on Sporulation of Phytophthora pluvialis and Phytophthora kernoviae. For. Pathol. 2020, 50, e12588. [Google Scholar] [CrossRef]
- Watt, M.S.; Holdaway, A.; Watt, P.; Pearse, G.D.; Palmer, M.E.; Steer, B.S.C.; Camarretta, N.; McLay, E.; Fraser, S. Early Prediction of Regional Red Needle Cast Outbreaks Using Climatic Data Trends and Satellite-Derived Observations. Remote Sens. 2024, 16, 1401. [Google Scholar] [CrossRef]
- Harris, A. Sporulation Potential of Phytophthora pluvialis on Epidemiology Significant Conifer Species Grown in the UK. In End of Year Report to DEFRA Future Proofing Plant Health; Department for Environment, Food and Rural Affairs (DEFRA): London, UK, 2024. [Google Scholar]
- Dun, H.F.; MacKay, J.J.; Green, S. Expansion of Natural Infection of Japanese Larch by Phytophthora ramorum Shows Trends Associated with Seasonality and Climate. Plant Pathol. 2024, 73, 419–430. [Google Scholar] [CrossRef]
- Fichtner, E.J.; Lynch, S.C.; Rizzo, D.M. Survival, Dispersal, and Potential Soil-Mediated Suppression of Phytophthora ramorum in a California Redwood-Tanoak Forest. Phytopathology 2009, 99, 608–619. [Google Scholar] [CrossRef]
- Burgess, T.I.; Scott, J.K.; Mcdougall, K.L.; Stukely, M.J.C.; Crane, C.; Dunstan, W.A.; Brigg, F.; Andjic, V.; White, D.; Rudman, T.; et al. Current and Projected Global Distribution of Phytophthora cinnamomi, One of the World’s Worst Plant Pathogens. Glob. Change Biol. 2017, 23, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.E.L. Threats Posed by Phytophthora to Scottish Plant Health; a Review of Previous Findings, Pathways of Entry and Further Spread and the Status of Diagnostic Techniques; Report to Scottish Forestry; Forest Research: Edinburgh, UK, 2015. [Google Scholar]
- Scanu, B.; Linaldeddu, B.T.; Deidda, A.; Jung, T. Diversity of Phytophthora Species from Declining Mediterranean Maquis Vegetation, Including Two New Species, Phytophthora crassamura and P. ornamentata sp. Nov. PLoS ONE 2015, 10, e0143234. [Google Scholar] [CrossRef]
- Schoebel, C.N.; Stewart, J.; Gruenwald, N.J.; Rigling, D.; Prospero, S. Population History and Pathways of Spread of the Plant Pathogen Phytophthora plurivora. PLoS ONE 2014, 9, e85368. [Google Scholar] [CrossRef]
- Jung, T. Beech Decline in Central Europe Driven by the Interaction between Phytophthora Infections and Climatic Extremes. For. Pathol. 2009, 39, 73–94. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, H.; Obwald, W. Involvement of Soilborne Phytophthora Species in Central European Oak Decline and the Effect of Site Factors on the Disease. Plant Pathol. 2000, 49, 706–718. [Google Scholar] [CrossRef]
- Redondo, M.A.; Stenlid, J.; Oliva, J. Genetic Variation Explains Changes in Susceptibility in a Naïve Host against an Invasive Forest Pathogen: The Case of Alder and the Phytophthora alni Complex. Phytopathology 2020, 110, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Jung, M.H.; Cacciola, S.O.; Cech, T.; Bakonyi, J.; Seress, D.; Mosca, S.; Schena, L.; Seddaiu, S.; Pane, A.; et al. Multiple New Cryptic Pathogenic Phytophthora Species from Fagaceae Forests in Austria, Italy and Portugal. IMA Fungus 2017, 8, 219–244. [Google Scholar] [CrossRef]
- Scanu, B.; Jones, B.; Webber, J.F. A New Disease of Nothofagus in Britain Caused by Phytophthora pseudosyringae. New Dis. Rep. 2012, 25, 27. [Google Scholar] [CrossRef]
- Brasier, C.M.; Cooke, D.E.L.; Duncan, J.M.; Hansen, E.M. Multiple New Phenotypic Taxa from Trees and Riparian Ecosystems in Phytophthora gonapodyides-P. Megasperma ITS Clade 6, Which Tend to Be High-Temperature Tolerant and Either Inbreeding or Sterile. Mycol. Res. 2003, 107, 277–290. [Google Scholar] [CrossRef]
- Jung, T.; Stukely, M.J.C.; Hardy, G.E.S.J.; White, D.; Paap, T.; Dunstan, W.A.; Burgess, T.I. Multiple New Phytophthora Species from ITS Clade 6 Associated with Natural Ecosystems in Australia: Evolutionary and Ecological Implications. Persoonia Mol. Phylogeny Evol. Fungi 2011, 26, 13–39. [Google Scholar] [CrossRef]
- Jung, T.; Milenković, I.; Corcobado, T.; Májek, T.; Janoušek, J.; Kudláček, T.; Tomšovský, M.; Nagy, Z.; Durán, A.; Tarigan, M.; et al. Extensive Morphological and Behavioural Diversity among Fourteen New and Seven Described Species in Phytophthora Clade 10 and Its Evolutionary Implications. Persoonia Mol. Phylogeny Evol. Fungi 2022, 49, 1–57. [Google Scholar] [CrossRef]
- Corcobado, T.; Cech, T.L.; Daxer, A.; Ďatková, H.; Janoušek, J.; Patra, S.; Jahn, D.; Hüttler, C.; Milenković, I.; Tomšovský, M.; et al. Phytophthora, Nothophytophthora and Halophytophthora Diversity in Rivers, Streams and Riparian Alder Ecosystems of Central Europe. Mycol. Prog. 2023, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, R.; Destefanis, M.; Milenković, I.; Tomšovsky, M.; Janoušek, J.; Bellgard, S.E.; Weir, B.S.; Ček, T.K.; Jung, M.H.; Jung, T. Two New Nothophytophthora Species from Streams in Ireland and Northern Ireland: Nothophytophthora irlandica and N. Lirii sp. Nov. PLoS ONE 2021, 16, e0250527. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Scanu, B.; Bakonyi, J.; Seress, D.; Kovács, G.M.; Durán, A.; Von Stowasser, E.S.; Schena, L.; Mosca, S.; Thu, P.Q.; et al. Nothophytophthora Gen. Nov., a New Sister Genus of Phytophthora from Natural and Semi-Natural Ecosystems. Persoonia Mol. Phylogeny Evol. Fungi 2017, 39, 143–174. [Google Scholar] [CrossRef]


| Site | Country of Location | Focal Tree Species * and Whether Infected with P. pluvialis (y/n) | Rainwater Trap (RWT) Distance to Canopy of Nearest Focal Tree | Soil Sampling Points in Relation to RWT | Stream Sampling Distance to Focal Tree |
|---|---|---|---|---|---|
| EAH | England, Hampshire | WH (n), DF (n) | Not sampled | Not sampled | 1.4 km from WH and 2.1 km from DF |
| EBa | England, Cumbria | WH (y), DF (y), JL (y) | 0.5 m | 0.5 m from RWT; 5 m from stream sampling point; 2 m from footpath | 5 m |
| EBb | England, Cumbria | WH (y), DF (y) | 0.5 m | 2 m from RWT and footpath; 15 m from stream sampling point | 15 m |
| ELa | England, Cornwall | WH (y), DF (y) | 1 m | 1 m from RWT and under canopy of symptomatic tree | 20 m |
| ELb | England, Cornwall | WH (y), DF (y) | 1 m | 1 m from RWT and under canopy of symptomatic tree | 5 m |
| WBa | Wales, Carmarthenshire | WH (y), | 1 m | 1 m adjacent to RWT and under canopy of symptomatic tree | 101 m downhill from sample |
| WBb | Wales, Carmarthenshire | WH (y), | 1 m | 1 m adjacent to RWT and under canopy of symptomatic tree | 530 m upstream from (a) sample |
| WDHa | Wales, Gwynedd/Powys | WH (y), DF (y) | 1 m | 1 m adjacent to RWT and under canopy of symptomatic tree | 15 m |
| WDHb | Wales, Gwynedd/Powys | WH (y) | 1 m | 1 m adjacent to RWT and under canopy of symptomatic tree | 35 m |
| WHa | Wales, Powys | >4 km upstream from infected WH at Hafren B. No trees in sampling area or upstream. | No focal tree | Within 5 m of RWT | No focal tree |
| WHb | Wales, Powys | WH (y) | 1 m | 1 m adjacent to RWT and under canopy of symptomatic tree | 35 m |
| SAa | Scotland, Argyll | WH (y), DF (y) | 0.5 m | 4 m | 15 m |
| SAb | Scotland, Argyll | WH (y), DF (y) | 0.5 m | 0.5 m–1 m at cardinal points from infected tree | 20 m |
| SB | Scotland, Argyll (public garden) | WH (y) | 1 m | 1 m from base of infected tree and 2–4 m from RWT | 15 m |
| SE | Scotland, Argyll | WH (y) | 5 m | 0.5 m–1 m at cardinal points from infected tree | 40 m |
| SR | Scotland, Invernessshire | WH (n), DF (n) | 4 m | 1 m at cardinal points from base of nearest infected tree | 20 m |
| SSS | Scotland, Ross and Cromarty | WH (y), DF (y) | 3 m | 1 m at cardinal points from base of infected tree | 20 m |
| Site | Species Detected by Baiting | Number of Detections | Month and Year of Sampling Period |
|---|---|---|---|
| England | |||
| EAH | Not tested | - | Not tested |
| EBa | Phytophthora pluvialis | 1 | May 2023 |
| Nothophytophthora sp. | 1 | May 2023 | |
| Phytophthora chlamydospora | 1 | August 2023 | |
| EBb | Phytophthora lacustris | 5 | September and October 2022, June and September (×2) 2023 |
| Phytophthora chlamydospora | 2 | September 2022, November 2022 | |
| Phytophthora ramorum | 4 | October and November 2022, May (×2) 2023 | |
| Phytophthora pluvialis | 2 | May (×2) 2023 | |
| Nothophytophthora sp. | 1 | September 2023 | |
| ELa | Phytophthora ramorum | 1 | September 2022 |
| Phytophthora chlamydospora | 1 | October 2022 | |
| Phytophthora plurivora | 1 | September 2023 | |
| ELb | Phytophthora gonapodyides | 2 | October 2022, September 2023 |
| Phytophthora syringae | 1 | October 2022 | |
| Wales | |||
| WBa | Phytophthora ramorum | 2 | November 2022, April 2023 |
| WBb | Phytophthora ramorum | 2 | February and May 2023 |
| Phytophthora plurivora | 1 | September 2023 | |
| WDHa | No species detected | ||
| WDHb | Phytophthora ramorum | 1 | October 2022 |
| WHa | No species detected | ||
| WHb | No species detected | ||
| Scotland | |||
| SAa | Phytophthora ramorum | 1 | January 2023 |
| Nothophytophthora sp | 1 | October 2023 | |
| SAb | Phytophthora lacustris | 1 | November 2023 |
| Phytophthora gonapodyides | 2 | October 2022, January 2023 | |
| Phytophthora pluvialis | 5 | February, March, April, May (×2) 2023 | |
| Phytophthora ramorum | 1 | April 2023 | |
| Elongisporangium undulatum | 1 | September 2023 | |
| Nothophytophthora sp. | 1 | October 2023 | |
| SB | Phytophthora ramorum | 6 | November and December 2022, February, March, May and June 2023 |
| Phytophthora kernoviae | 1 | September 2022 | |
| SE | Phytophthora pluvialis | 2 | March and August 2023 |
| Phytophthora gonapodyides | 1 | June 2023 | |
| SR | Nothophytophthora sp. | 1 | October 2023 |
| Phytophthora pluvialis | 1 | October 2023 | |
| SSS | No species detected | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacLaren, A.; Frederickson-Matika, D.; Cock, P.J.A.; Crisp, D.; Dun, H.; Pérez-Sierra, A.; Green, S. Enhanced Detection of Phytophthora Species at P. pluvialis Outbreak Sites in Commercial Forests Across Britain. Forests 2025, 16, 1419. https://doi.org/10.3390/f16091419
MacLaren A, Frederickson-Matika D, Cock PJA, Crisp D, Dun H, Pérez-Sierra A, Green S. Enhanced Detection of Phytophthora Species at P. pluvialis Outbreak Sites in Commercial Forests Across Britain. Forests. 2025; 16(9):1419. https://doi.org/10.3390/f16091419
Chicago/Turabian StyleMacLaren, Alastair, Debbie Frederickson-Matika, Peter J. A. Cock, Daniel Crisp, Heather Dun, Ana Pérez-Sierra, and Sarah Green. 2025. "Enhanced Detection of Phytophthora Species at P. pluvialis Outbreak Sites in Commercial Forests Across Britain" Forests 16, no. 9: 1419. https://doi.org/10.3390/f16091419
APA StyleMacLaren, A., Frederickson-Matika, D., Cock, P. J. A., Crisp, D., Dun, H., Pérez-Sierra, A., & Green, S. (2025). Enhanced Detection of Phytophthora Species at P. pluvialis Outbreak Sites in Commercial Forests Across Britain. Forests, 16(9), 1419. https://doi.org/10.3390/f16091419







