Genetic Diversity and Population Structure of Black Pine (Pinus nigra Arn.) in Mt. Athos, Northern Greece
Abstract
1. Introduction
2. Materials and Methods
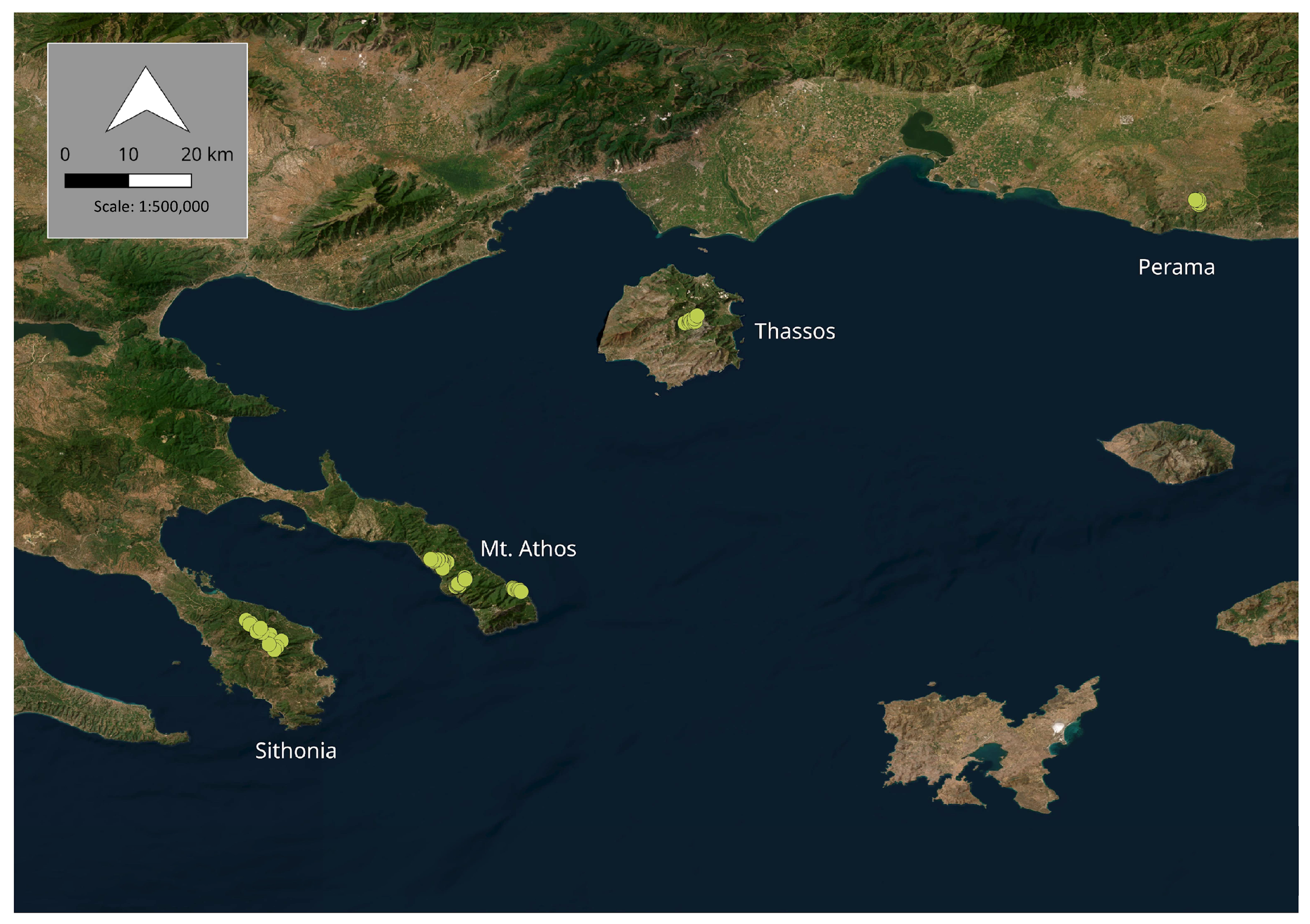
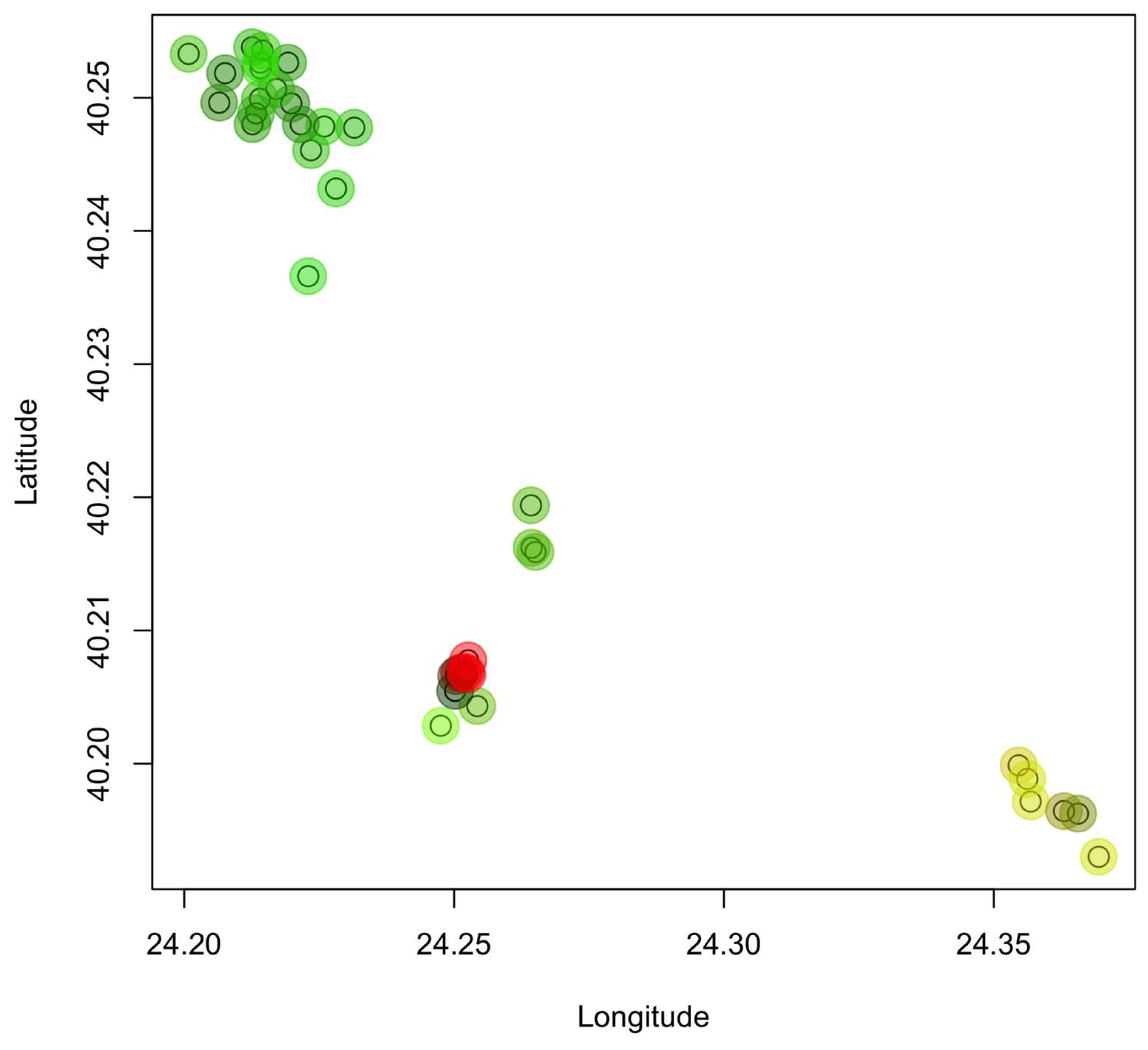
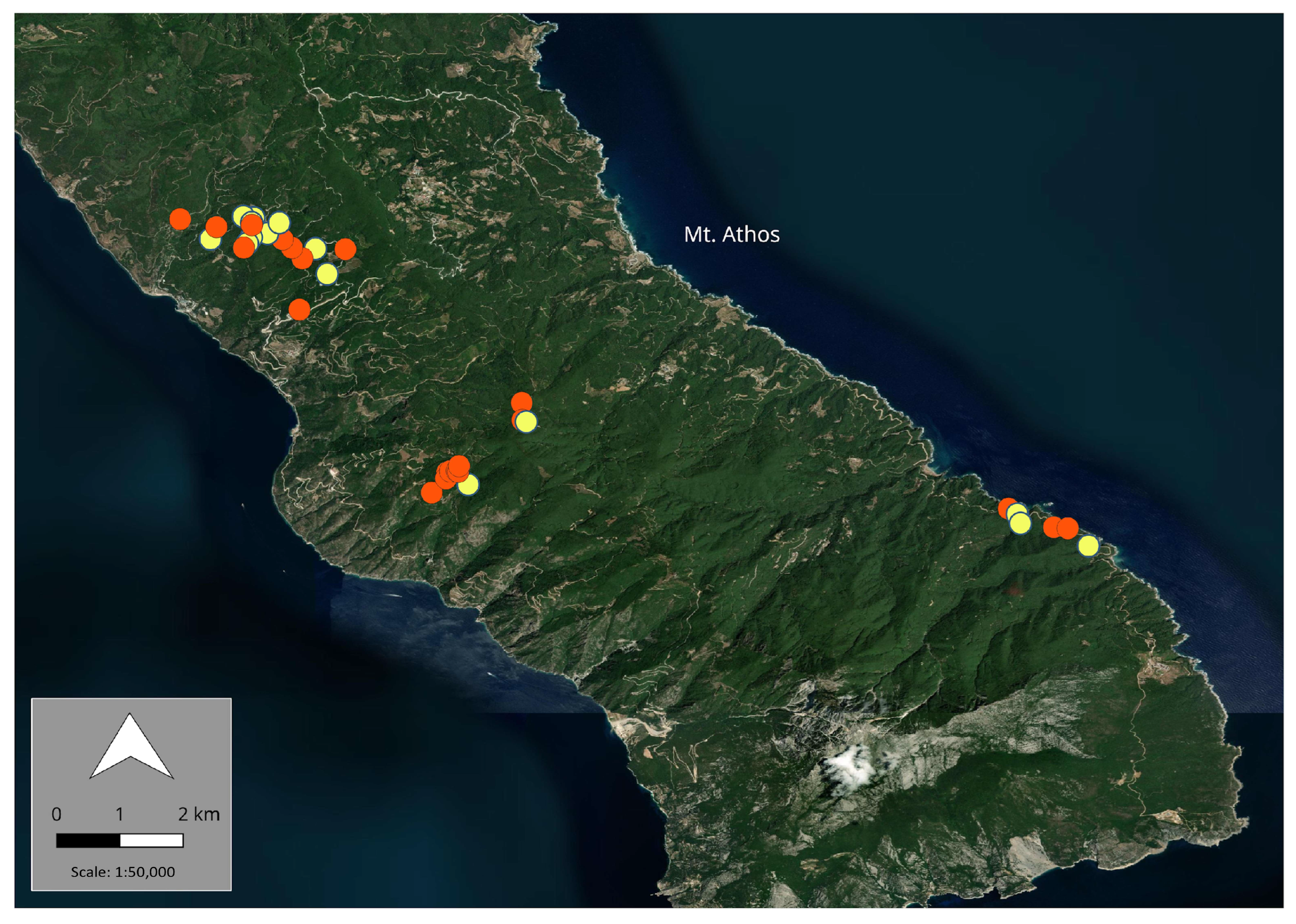
2.1. Sample Collection and Origin
2.2. DNA Extraction and Quantification
2.3. Microsatellite Loci and Multiplex Design
2.4. PCR Amplification
2.5. Data Processing
2.6. Genetic and Statistical Analysis
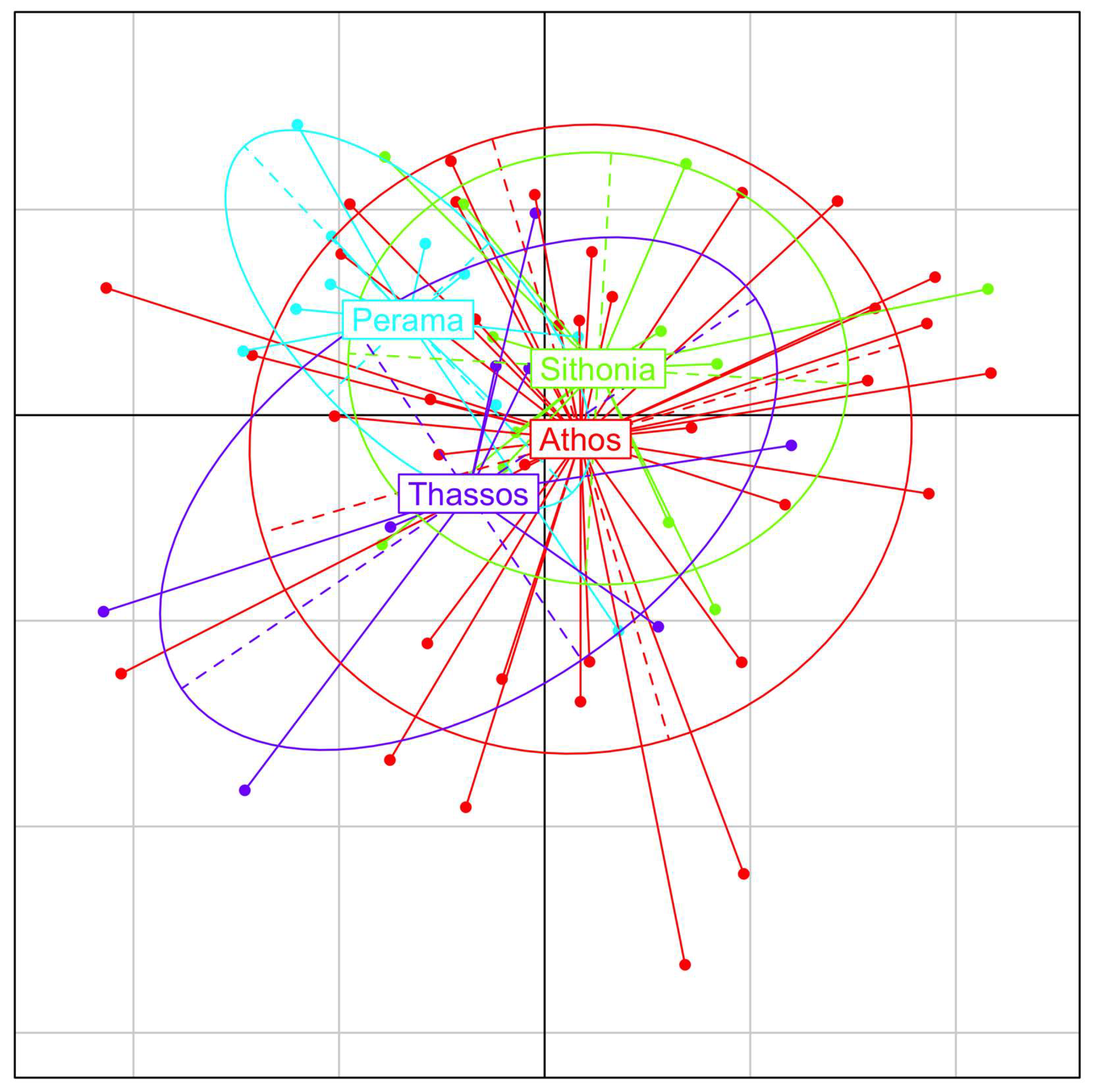
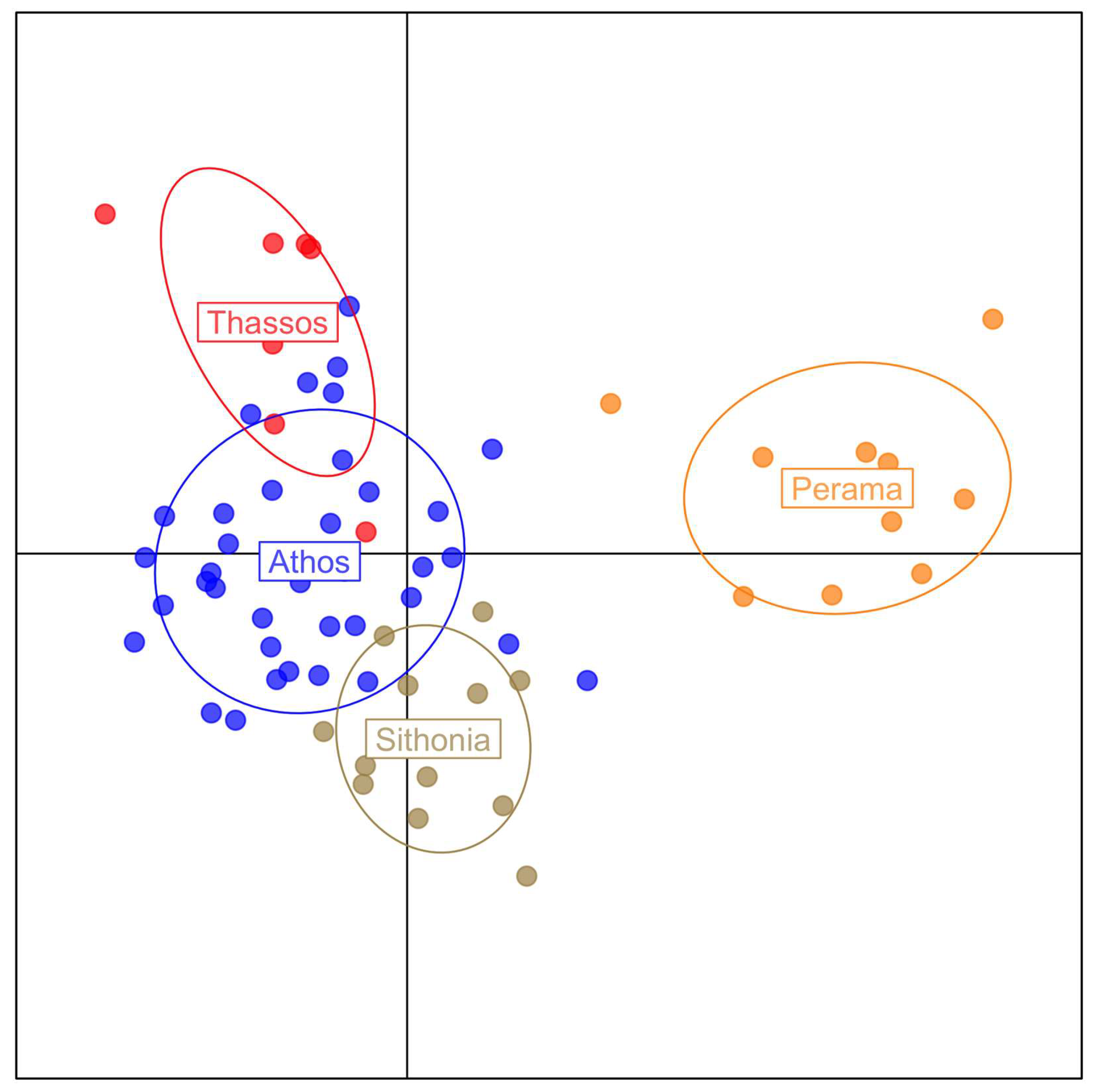
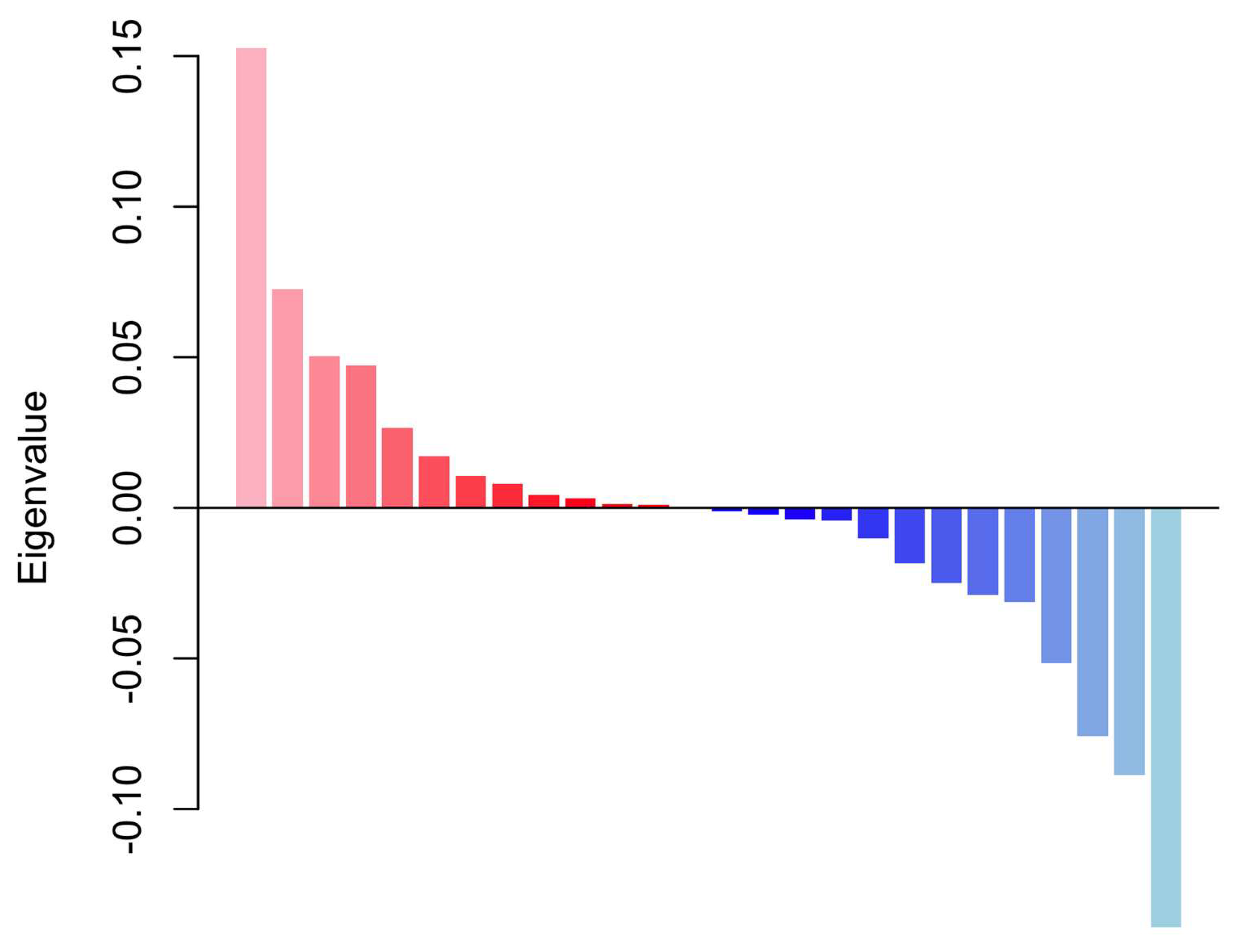
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Skordilis, A.; Thanos, C.A. Comparative Ecophysiology of Seed Germination Strategies in the Seven Pine Species Naturally Growing in Greece. In Current Plant Science and Biotechnology in Agriculture; Springer: Dordrecht, The Netherlands, 1997; pp. 623–632. [Google Scholar] [CrossRef]
- European Commission; Joint Research Centre. European Atlas of Forest Tree Species; Publications Office: Luxembourg, 2016. [Google Scholar]
- Poljanšek, S.; Ballian, D.; Nagel, T.A.; Levanič, T. A 435-Year-Long European Black Pine (Pinus nigra) Chronology for the Central-Western Balkan Region. Tree-Ring Res. 2012, 68, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Mikulová, K.; Jarolímek, I.; Bacigál, T.; Hegedüšová, K.; Májeková, J.; Medvecká, J.; Slabejová, D.; Šibík, J.; Škodová, I.; Zaliberová, M.; et al. The Effect of Non-Native Black Pine (Pinus nigra J. F. Arnold) Plantations on Environmental Conditions and Undergrowth Diversity. Forests 2019, 10, 548. [Google Scholar] [CrossRef]
- Móricz, N.; Garamszegi, B.; Rasztovits, E.; Bidló, A.; Horváth, A.; Jagicza, A.; Illés, G.; Vekerdy, Z.; Somogyi, Z.; Gálos, B. Recent Drought-Induced Vitality Decline of Black Pine (Pinus nigra Arn.) in South-West Hungary—Is This Drought-Resistant Species under Threat by Climate Change? Forests 2018, 9, 414. [Google Scholar] [CrossRef]
- Rubio-Moraga, A.; Candel-Perez, D.; Lucas-Borja, M.E.; Tiscar, P.A.; Viñegla, B.; Linares, J.C.; Gómez-Gómez, L.; Ahrazem, O. Genetic Diversity of Pinus nigra Arn. Populations in Southern Spain and Northern Morocco Revealed By Inter-Simple Sequence Repeat Profiles. Int. J. Mol. Sci. 2012, 13, 5645–5658. [Google Scholar] [CrossRef]
- Scotti-Saintagne, C.; Giovannelli, G.; Scotti, I.; Roig, A.; Spanu, I.; Vendramin, G.G.; Guibal, F.; Fady, B. Recent, Late Pleistocene fragmentation shaped the phylogeographic structure of the European black pine (Pinus nigra Arnold). Tree Genet. Genomes 2019, 15, 76. [Google Scholar] [CrossRef]
- Matziris, D. Genetic Variation and Realized Genetic Gain From Black Pine Tree Improvement. Silvae Genet. 2005, 54, 96–104. [Google Scholar] [CrossRef][Green Version]
- Bergmeier, E. Plant communities and habitat differentiation in the Mediterranean coniferous woodlands of Mt. Parnon (Greece). Folia Geobot. 2002, 37, 309–331. [Google Scholar] [CrossRef]
- Mallinis, G.; Mitsopoulos, I.; Beltran, E.; Goldammer, J. Assessing Wildfire Risk in Cultural Heritage Properties Using High Spatial and Temporal Resolution Satellite Imagery and Spatially Explicit Fire Simulations: The Case of Holy Mount Athos, Greece. Forests 2016, 7, 46. [Google Scholar] [CrossRef]
- EUNIS-Site Factsheet for CHERSONISOS ATHOS. Available online: https://eunis.eea.europa.eu/sites/GR1270003 (accessed on 27 June 2025).
- Goldammer, J.; Mitsopoulos, I.; Mallinis, G.; Woolf, M. Wildfire Hazard and Risk Assessment; UNISDR: Geneva, Switzerland, 2017; pp. 70–79. [Google Scholar]
- Papaioannou, A. Assessment of the Empirical Management Method of Coppice Chestnut (Castanea sativa Mill.) Forests Practiced by the Monks and its Effect on the Availability of Forest Soil Resources in Mount Athos, Greece. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 317–325. [Google Scholar] [CrossRef]
- Afzal-Rafii, Z.; Dodd, R.S. Chloroplast DNA supports a hypothesis of glacial refugia over postglacial recolonization in disjunct populations of black pine (Pinus nigra) in western Europe. Mol. Ecol. 2007, 16, 723–736. [Google Scholar] [CrossRef]
- Dias, A.; Lemos, M.; Pavia, I.; Gaspar, M.J.; Silva, M.E.; Louzada, J.L.; Lima-Brito, J.; Carvalho, A. Genetic characterization of Portuguese allochthonous populations of Pinus nigra using ISSRs and SCoTs and extrapolation of their infraspecific taxonomy. Physiol. Mol. Biol. Plants 2019, 25, 799–805. [Google Scholar] [CrossRef]
- Bogunić, F.; Siljak-Yakovlev, S.; Muratović, E.; Ballian, D. Different karyotype patterns among allopatric Pinus nigra (Pinaceae) populations revealed by molecular cytogenetics. Plant Biol. 2011, 13, 194–200. [Google Scholar] [CrossRef]
- Giovannelli, G.; Roig, A.; Spanu, I.; Vendramin, G.G.; Fady, B. A New Set of Nuclear Microsatellites for an Ecologically and Economically Important Conifer: The European Black Pine (Pinus nigra Arn.). Plant Mol. Biol. Rep. 2017, 35, 379–388. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. micro-checker: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2014. Available online: http://qgis.osgeo.org (accessed on 25 July 2025).
- Patil, P.G.; Jamma, S.M.; Singh, N.V.; Bohra, A.; Parashuram, S.; Injal, A.S.; Gargade, V.A.; Chakranarayan, M.G.; Salutgi, U.D.; Dhinesh Babu, K.; et al. Assessment of genetic diversity and population structure in pomegranate (Punica granatum L.) using hypervariable SSR markers. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2020, 26, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- da Costa, L.S.; Corneleo, N.S.; Stefenon, V.M. Conservation of Forest Biodiversity: How sample size affects the estimation of genetic parameters. An. Acad. Bras. Ciênc. 2015, 87, 1095–1100. [Google Scholar] [CrossRef]
- Huang, S.; Feigs, J.T.; Holzhauer, S.I.; Kramp, K.; Brunet, J.; Decocq, G.; De Frenne, P.; Diekmann, M.; Liira, J.; Spicher, F.; et al. Limited effects of population age on the genetic structure of spatially isolated forest herb populations in temperate Europe. Ecol. Evol. 2024, 14, e10971. [Google Scholar] [CrossRef]
- Lukić, S.; Pantić, D.; Simić, S.B.; Borota, D.; Tubić, B.; Djukić, M.; Djunisijević-Bojović, D. Effects of black locust and black pine on extremely degraded sites 60 years after afforestation—A case study of the Grdelica Gorge (southeastern Serbia). IForest—Biogeosci. For. 2015, 9, 235. [Google Scholar] [CrossRef]
- Young, A.G.; Merriam, H.G.; Warwick, S.I. The effects of forest fragmentation on genetic variation in Acer saccharum Marsh. (sugar maple) populations. Heredity 1993, 71, 277–289. [Google Scholar] [CrossRef][Green Version]
- Crane, P. Conserving our global botanical heritage: The PSESP plant conservation program. Plant Divers. 2020, 42, 319–322. [Google Scholar] [CrossRef]
- Loo, J.; Souvannavong, O.; Dawson, I.K. Seeing the trees as well as the forest: The importance of managing forest genetic resources. For. Ecol. Manag. 2014, 333, 1–8. [Google Scholar] [CrossRef]
- Vajana, E.; Andrello, M.; Avanzi, C.; Bagnoli, F.; Vendramin, G.G.; Piotti, A. Spatial conservation planning of forest genetic resources in a Mediterranean multi-refugial area. Biol. Conserv. 2024, 293, 110599. [Google Scholar] [CrossRef]
- Breman, E.; Ballesteros, D.; Castillo-Lorenzo, E.; Cockel, C.; Dickie, J.; Faruk, A.; O’Donnell, K.; Offord, C.A.; Pironon, S.; Sharrock, S.; et al. Plant Diversity Conservation Challenges and Prospects—The Perspective of Botanic Gardens and the Millennium Seed Bank. Plants 2021, 10, 2371. [Google Scholar] [CrossRef] [PubMed]


| Locus | n | Ho | Ho_adj | He | He_adj | F | F_adj |
|---|---|---|---|---|---|---|---|
| KU953385 | 15.25 ± 6.945 | 0.176 ± 0.104 | 0.524 | 0.717 ± 0.062 | 0.807 | 0.785 ± 0.127 | 0.383 |
| KU953379 | 13 ± 6.868 | 0.347 ± 0.13 | 0.492 | 0.513 ± 0.171 | 0.576 | 0.322 ± 0.109 | - |
| AF333785 | 13.25 ± 5.721 | 0.064 ± 0.04 | 0.139 | 0.189 ± 0.063 | 0.226 | 0.669 ± 0.156 | - |
| AF286619 | 14.75 ± 6.142 | 0.705 ± 0.066 | 0.705 | 0.717 ± 0.014 | 0.717 | 0.017 ± 0.089 | 0.017 |
| KU953384 | 13.25 ± 6.047 | 0.456 ± 0.041 | 0.587 | 0.626 ± 0.047 | 0.677 | 0.264 ± 0.074 | 0.141 |
| KU953382 | 14.25 ± 5.949 | 0.643 ± 0.079 | 0.752 | 0.829 ± 0.012 | 0.850 | 0.224 ± 0.096 | 0.116 |
| KR779884 | 12.25 ± 4.008 | 0.778 ± 0.063 | 0.778 | 0.749 ± 0.033 | 0.749 | −0.043 ± 0.092 | −0.042 |
| Mean | 13.714 ± 5.954 | 0.453 ± 0.075 | 0.568 | 0.62 ± 0.057 | 0.658 | 0.32 ± 0.106 | - |
| Populations | Population Size | p (%) | n/L | Ho | Ho_adj | He | He_adj | F | F_adj |
|---|---|---|---|---|---|---|---|---|---|
| Athos | 37 | 100.00 | 31.286 ± 1.409 | 0.486 ± 0.08 | 0.732 | 0.701 ± 0.075 | 0.757 | 0.308 ± 0.081 | 0.023 |
| Sithonia | 12 | 100.00 | 9.857 ± 0.508 | 0.436 ± 0.078 | 0.55 | 0.666 ± 0.075 | 0.72 | 0.375 ± 0.076 | 0.271 |
| Perama | 10 | 71.43 | 7.143 ± 1.033 | 0.388 ± 0.149 | - | 0.485 ± 0.128 | - | 0.215 ± 0.188 | - |
| Thassos | 8 | 100.00 | 6.571 ± 0.481 | 0.502 ± 0.139 | 0.604 | 0.628 ± 0.077 | 0.668 | 0.301 ± 0.185 | 0.181 |
| Mean | - | 92.86 ± 7.14 | 13.714 ± 0.858 | 0.453 ± 0.112 | - | 0.62 ± 0.089 | - | 0.23 ± 0.133 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulaki Konstantinidou, G.; Giannakopoulos, N.-E.; Pariotis, I.; Mystakidis, E.; Georgiadis, C.; Gounaris, N.; Tegopoulos, K.; Tsifintaris, M.; Georgitsi, M.; Galatsidas, S.; et al. Genetic Diversity and Population Structure of Black Pine (Pinus nigra Arn.) in Mt. Athos, Northern Greece. Forests 2025, 16, 1399. https://doi.org/10.3390/f16091399
Poulaki Konstantinidou G, Giannakopoulos N-E, Pariotis I, Mystakidis E, Georgiadis C, Gounaris N, Tegopoulos K, Tsifintaris M, Georgitsi M, Galatsidas S, et al. Genetic Diversity and Population Structure of Black Pine (Pinus nigra Arn.) in Mt. Athos, Northern Greece. Forests. 2025; 16(9):1399. https://doi.org/10.3390/f16091399
Chicago/Turabian StylePoulaki Konstantinidou, Georgia, Nikolaos-Evangelos Giannakopoulos, Ioannis Pariotis, Eleftherios Mystakidis, Christos Georgiadis, Nikolaos Gounaris, Konstantinos Tegopoulos, Margaritis Tsifintaris, Marianthi Georgitsi, Spyros Galatsidas, and et al. 2025. "Genetic Diversity and Population Structure of Black Pine (Pinus nigra Arn.) in Mt. Athos, Northern Greece" Forests 16, no. 9: 1399. https://doi.org/10.3390/f16091399
APA StylePoulaki Konstantinidou, G., Giannakopoulos, N.-E., Pariotis, I., Mystakidis, E., Georgiadis, C., Gounaris, N., Tegopoulos, K., Tsifintaris, M., Georgitsi, M., Galatsidas, S., & Papageorgiou, A. C. (2025). Genetic Diversity and Population Structure of Black Pine (Pinus nigra Arn.) in Mt. Athos, Northern Greece. Forests, 16(9), 1399. https://doi.org/10.3390/f16091399





