Remote Sensing Aboveground Biomass Inversion of Four Vegetation Types in the Nanji Wetland
Abstract
1. Introduction
2. Data and Methodology
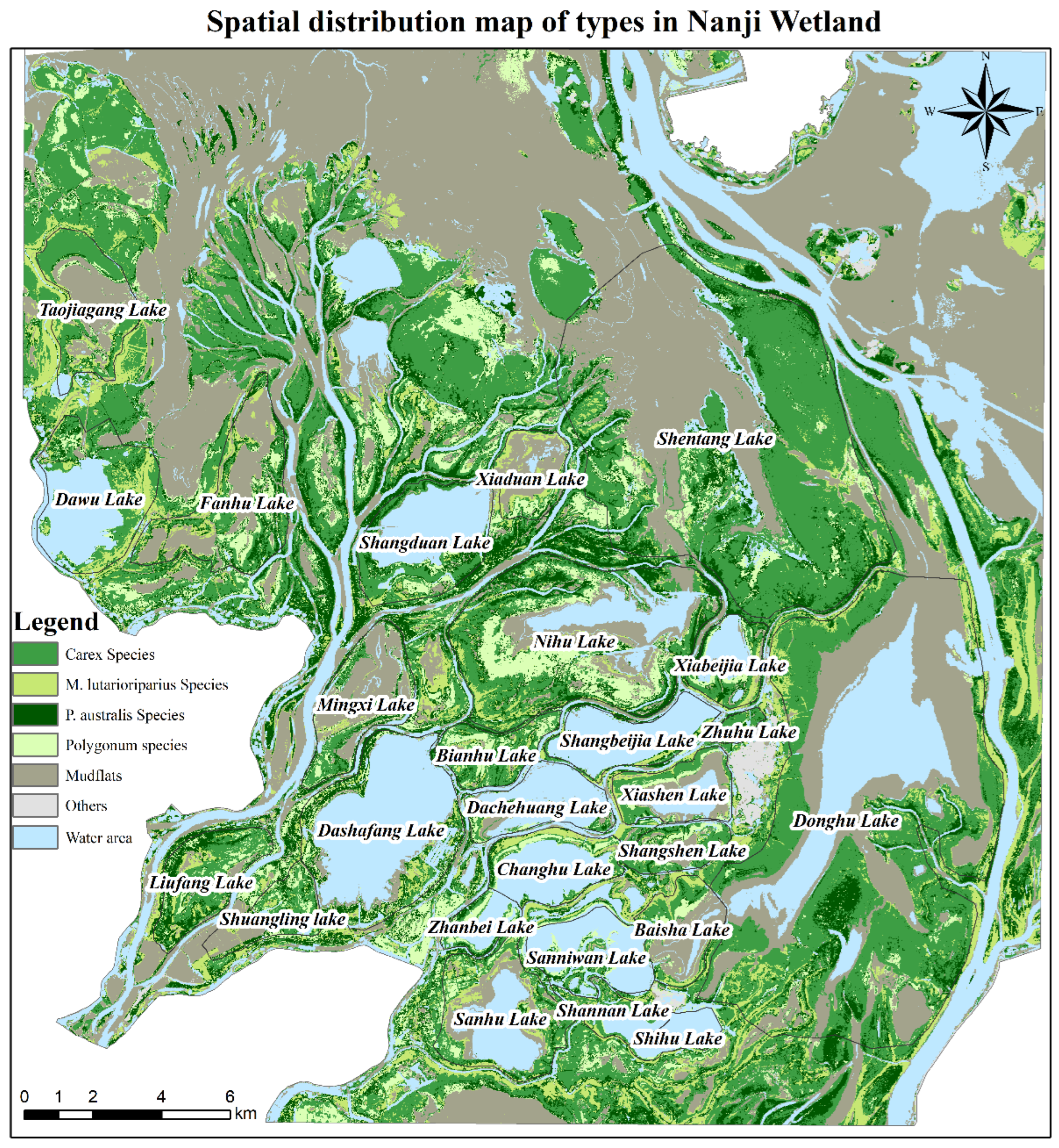
2.1. Study Area
2.2. Data Sources
2.2.1. Sampling Points and Collection of AGB
2.2.2. Preprocessing of Sentinel-2 Images
2.3. Research Methods
2.3.1. Random Forest
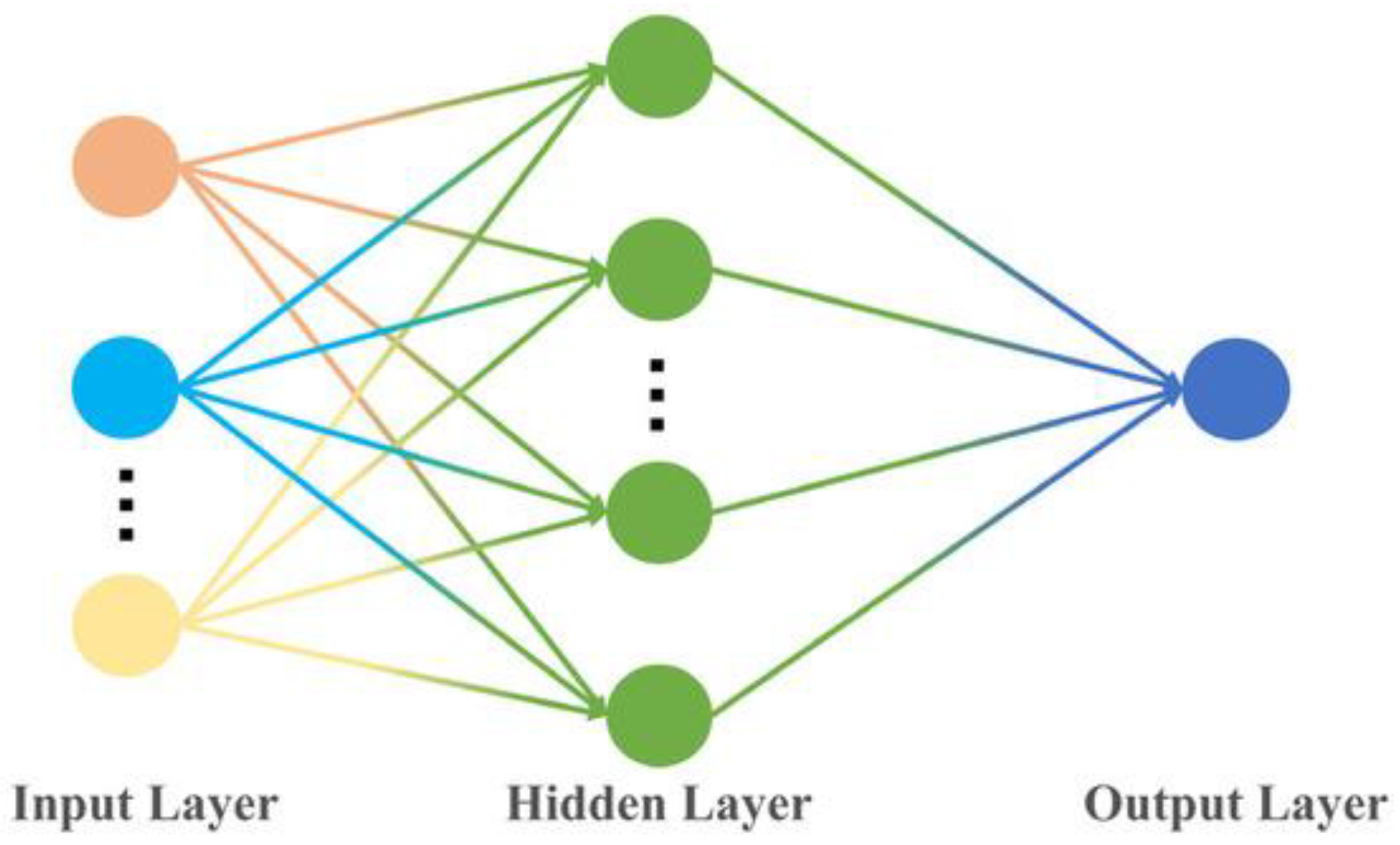
2.3.2. Backpropagation Neural Network
2.3.3. Model Accuracy Evaluation
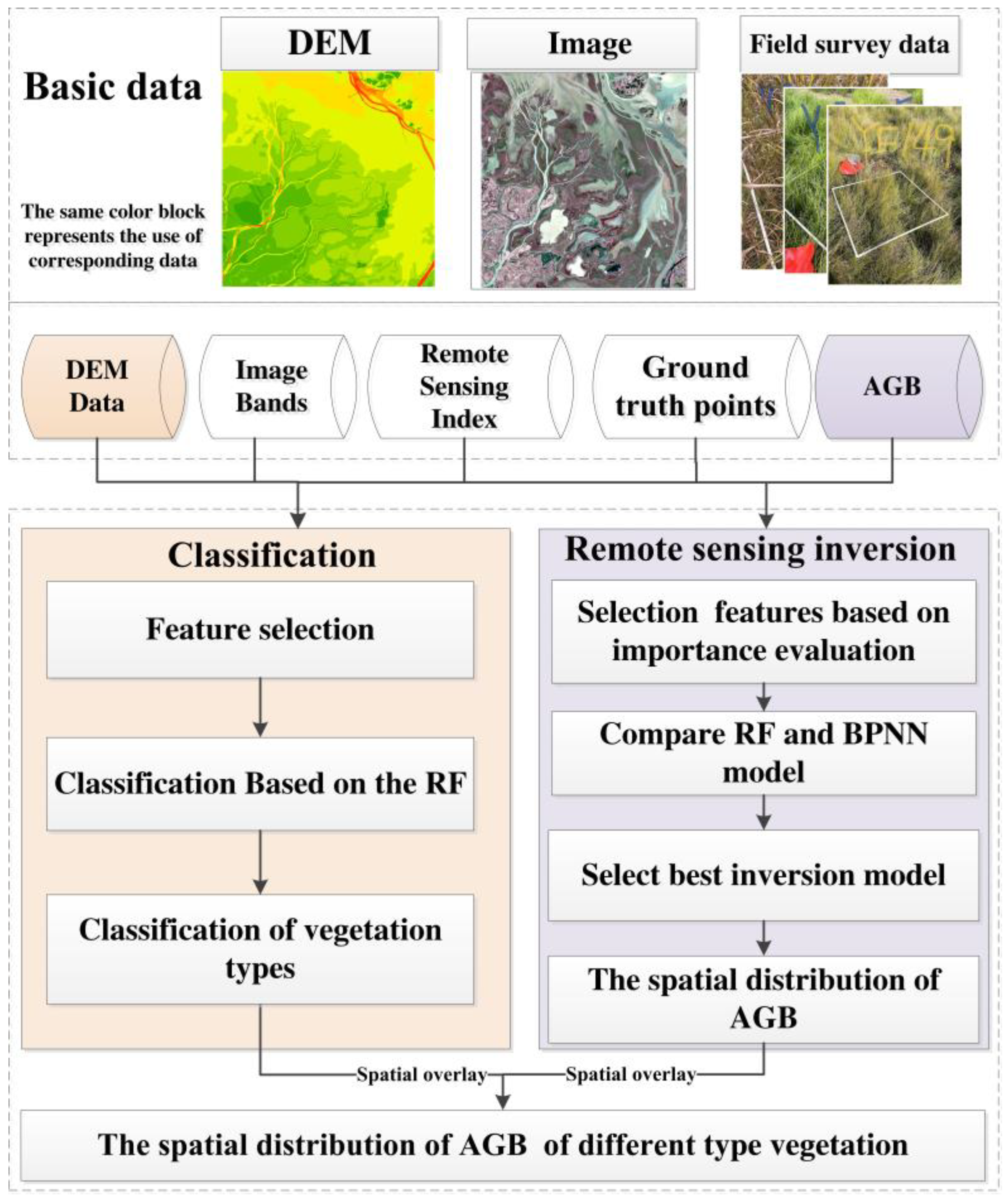
2.3.4. Research Roadmap
3. Results
3.1. Statistical Characteristic Analyses of Vegetation AGB in the Nanji Wetland
3.2. Spatial Distribution Characteristics of Vegetation in the Nanji Wetland
3.3. Remote Sensing Inversion Model and Spatial Distribution
3.3.1. Remote Sensing Inversion
3.3.2. Spatial Feature Analysis of AGB
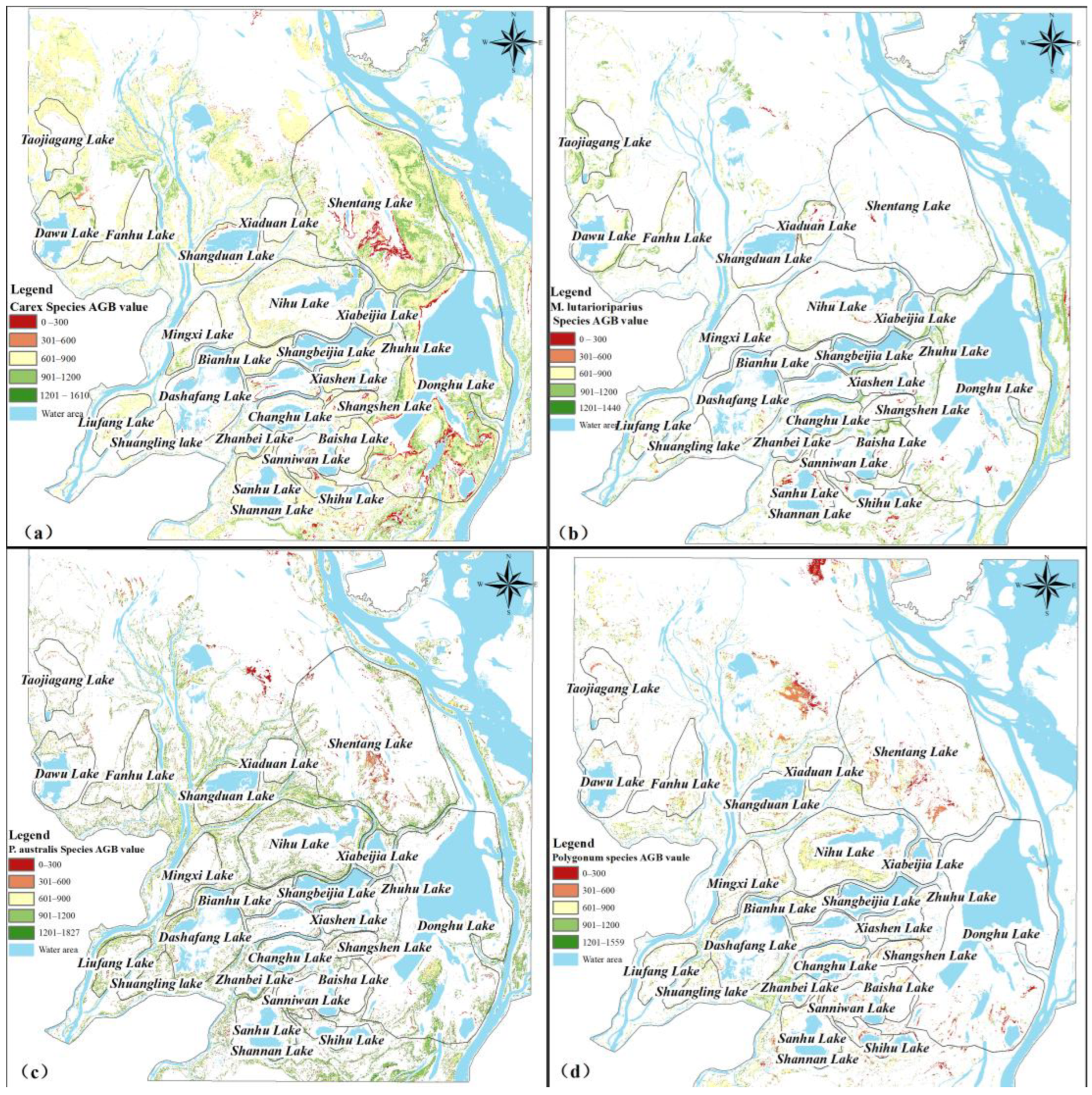
3.3.3. Spatial Distribution Characteristics of Different Vegetation AGB
4. Discussion
4.1. The Influence of Sampling Time and Area on AGB Estimation Result
4.2. AGB Inversion Variables
4.3. Improve the Accuracy of AGB Estimation
5. Conclusions and Outlook
- (1)
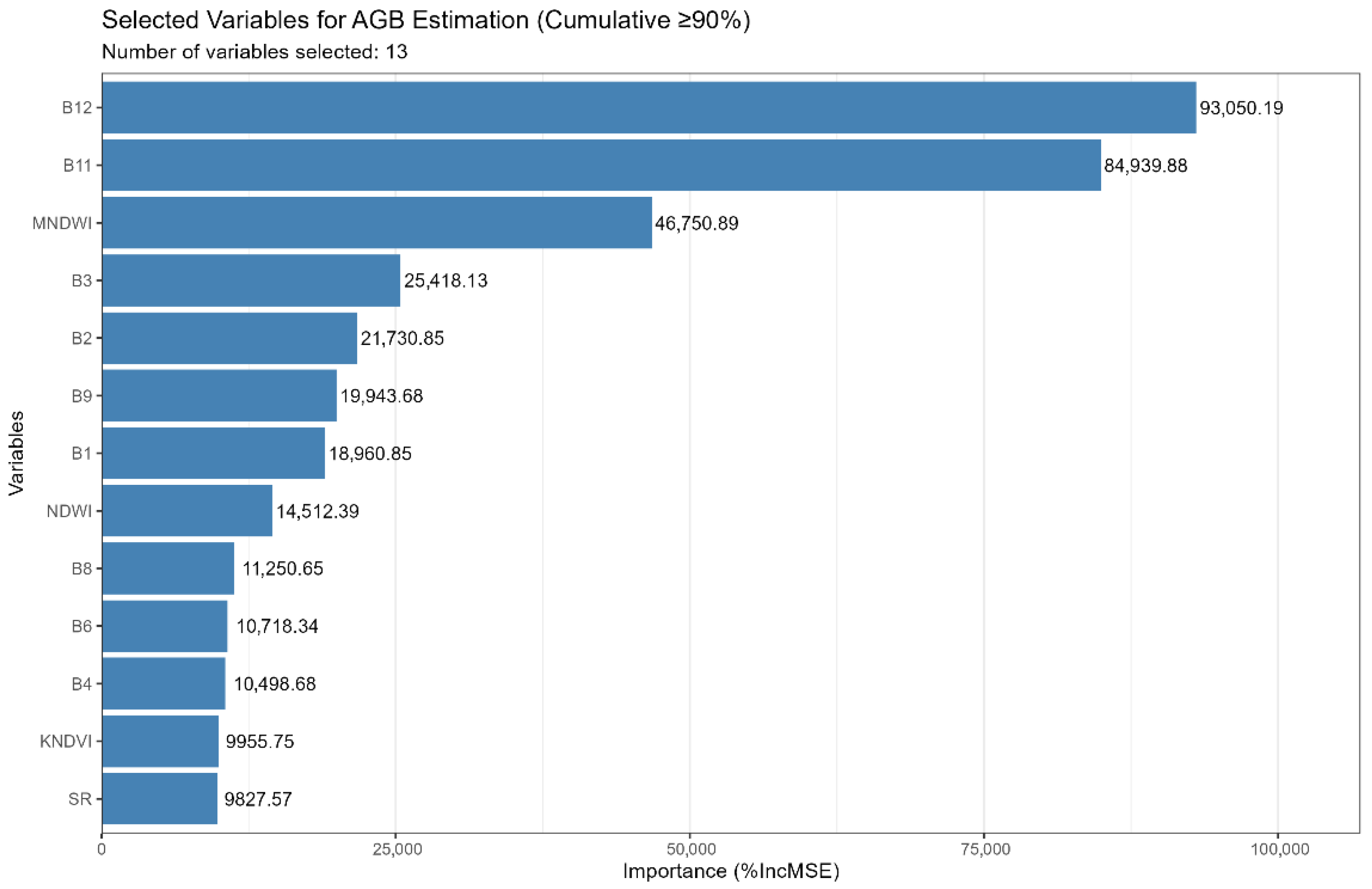
- Through important variable evaluation, the spectral bands and indices B12, B11, MNDWI, B3, B2, B9, B1, NDWI, B8, B6, B4, KNDVI, and SR were identified as key predictors for the RF model.
- (2)
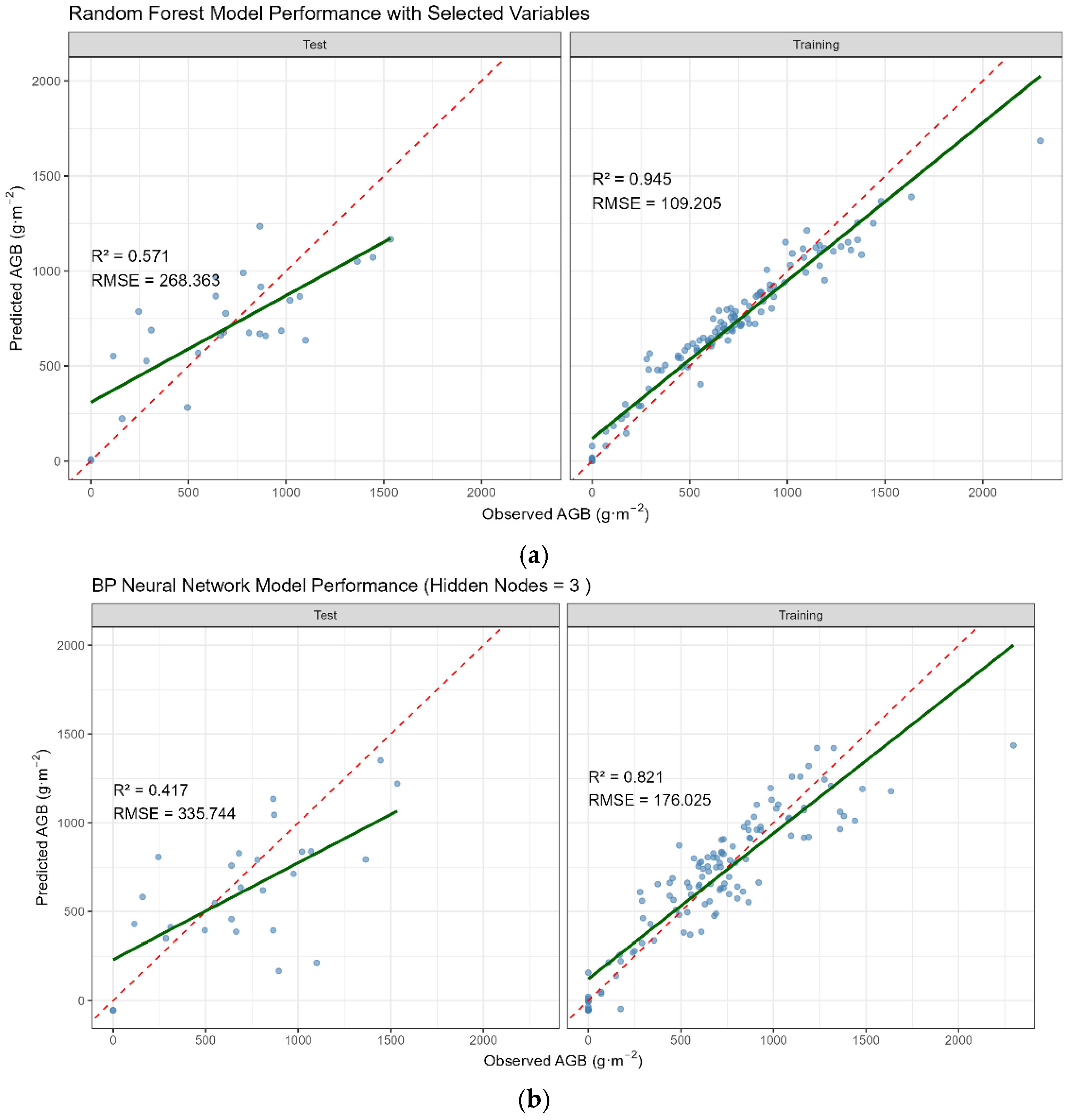
- The key results confirmed RF’s superiority over the BPNN, achieving significantly higher predictive accuracy (R2 = 0.945, RMSE = 109.205 g·m−2) compared to the BPNN (R2 = 0.821, RMSE = 176.025 g·m−2).
- (3)
- During the dry season, the total estimated AGB reached 4.03 × 109 g (mean AGB density = 501.27 g·m−2), exhibiting a clear spatial gradient decreasing from higher elevation areas along the lakeshore towards the central lake regions. Among the vegetation types, Carex spp. dominated AGB accumulation (1.49 × 109 g), significantly surpassing P. australis spp. (6.69 × 108 g), M. lutarioriparius spp. (4.60 × 108 g), and Polygonum spp. (3.61 × 108 g). However, in terms of mean AGB density per unit area, M. lutarioriparius spp. exhibited the highest value (859 g·m−2), slightly exceeding that of P. australis spp. (854 g·m−2), Carex spp. (781 g·m−2), and Polygonum spp. (700 g·m−2).
- (1)
- Exclusion of belowground and aquatic vegetation biomass. BGB and aquatic vegetation biomass were not considered. Future studies aiming for precise regional carbon stock estimation should incorporate both AGB and BGB across terrestrial vegetation, as well as biomass from aquatic vegetation.
- (2)
- Limited environmental variables in the inversion process. The inversion model did not incorporate a broader range of environmental variables known to correlate with biomass. To achieve more accurate biomass estimation in the future, variables influencing biomass dynamics, such as soil properties and hydrological conditions, need to be integrated.
- (3)
- Lack of investigation into hydrological regulation pathways. The pathway through which hydrological regulation policies influence biomass was not explored. Future research should investigate the spatial dynamics of overall Poyang Lake biomass under different hydrological management regimes. This knowledge is essential for enhancing the lake’s carbon sequestration capacity through scientifically informed hydrological regulation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dayathilake, D.D.T.L.; Lokupitiya, E.; Wijeratne, V.P.I.S. Estimation of aboveground and belowground carbon stocks in urban freshwater wetlands of Sri Lanka. Carbon Balance Manag. 2020, 15, 17. [Google Scholar] [CrossRef]
- Ray, R.; Ganguly, D.; Chowdhury, C.; Dey, M.; Das, S.; Dutta, M.K.; Mandal, S.K.; Majumder, N.; De, T.K.; Mukhopadhyay, S.K.; et al. Carbon sequestration and annual increase of carbon stock in a mangrove forest. Atmos. Environ. 2011, 45, 5016–5024. [Google Scholar] [CrossRef]
- Becknell, J.M.; Kucek, L.K.; Powers, J.S. Aboveground biomass in mature and secondary seasonally dry tropical forests: A literature review and global synthesis. For. Ecol. Manag. 2012, 276, 88–95. [Google Scholar] [CrossRef]
- Estrada, G.C.; Soares, M.L. Global patterns of aboveground carbon stock and sequestration in mangroves. An. Acad. Bras. Ciênc. 2017, 89, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, Y.; Song, Z.; Chen, C.; Zhang, Y.; Chen, X.; Chen, W.; Yuan, W.; Wu, X.; Ran, X.; et al. Modelling aboveground biomass carbon stock of the Bohai Rim coastal wetlands by integrating remote sensing, terrain, and climate data. Remote Sens. 2021, 13, 4321. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Tong, S.; Zhang, M.; Jiang, M.; Lu, X. Aboveground biomass of wetland vegetation under climate change in the western Songnen plain. Front. Plant Sci. 2022, 13, 941689. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, W.; Sun, X.; Shao, J.; Zhang, P.; Zhou, G.; He, Y.; Liu, H.; Fu, Y.; Zhou, X. Regulation of climate, soil and hydrological factors on macrophyte biomass allocation for coastal and inland wetlands in China. Sci. Total Environ. 2021, 774, 145317. [Google Scholar] [CrossRef]
- Yao, Y.; Ren, H. Estimation of grassland aboveground biomass on the Qinghai-Tibet Plateau. Acta Ecol. Sin. 2024, 44, 3049–3059. [Google Scholar]
- Zang, P.; Zhang, Y.; Chen, Z.; Hou, G.; Liu, Z.; Lu, X. The inversion modeling and aboveground biomass mapping of withered grass changes in the western grassland of Northeast China. Front. Earth Sci. 2023, 10, 1031098. [Google Scholar] [CrossRef]
- Jin, X.; Lou, Y.; Zhang, P.; Tang, H.; Zhang, Q.; Smith, P. Allometric equations for estimating above-and below-ground biomass of reed (Phragmites australis) marshes. J. Plant Ecol. 2025, 18, rtae113. [Google Scholar] [CrossRef]
- Jensen, D.; Cavanaugh, K.C.; Simard, M.; Christensen, A.; Rovai, A.; Twilley, R. Aboveground biomass distributions and vegetation composition changes in Louisiana’s Wax Lake Delta. Estuar. Coast. Shelf Sci. 2021, 250, 107139. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Wang, Y.; Zhang, J.; Ma, R.; Lu, X.; Jiang, M. Spatiotemporal variation in aboveground biomass and its response to climate change in the marsh of Sanjiang Plain. Front. Plant Sci. 2022, 13, 920086. [Google Scholar] [CrossRef]
- Ren, Y.; Mao, D.; Li, X.; Wang, Z.; Xi, Y.; Feng, K. Aboveground biomass of marshes in Northeast China: Spatial pattern and annual changes responding to climate change. Front. Ecol. Evol. 2022, 10, 1043811. [Google Scholar] [CrossRef]
- Lv, G.; Cui, G.; Wang, X.; Yu, H.; Huang, X.; Zhu, W.; Lin, Z. Signatures of wetland impact: Spatial distribution of forest aboveground biomass in Tumen River Basin. Remote Sens. 2021, 13, 3009. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, Y.; Shen, R.; Cai, Y.; Luo, H.; Jin, B.; Chen, J. Effects of flooding duration on wetland plant biomass: The importance of soil nutrients and season. Freshw. Biol. 2021, 66, 211–222. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Y.; Chen, B. Estimating Spartina alterniflora fractional vegetation cover and aboveground biomass in a coastal wetland using SPOT6 satellite and UAV data. Aquat. Bot. 2018, 144, 38–45. [Google Scholar] [CrossRef]
- Rao, D.; Yu, X.; Li, P.; Xia, S.; Meng, Z.; Liu, Y. Remote sensing estimation of spring Carex biomass in Changhuchi Lake, a shallow sub-lake of Poyang Lake. J. Nat. Resour. 2019, 34, 2001–2011. [Google Scholar] [CrossRef]
- Yang, L.; Xieqin, M.; Li, Q.; Zhang, X.; Zhu, J.; Gao, J. Spatial and temporal dynamics of plant biomass in Poyang Lake Wetland in Spring and Autumn in recent two decades. J. Changjiang River Sci. Res. 2024, 41, 47–54. [Google Scholar]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehman, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef]
- Li, C.; Zhou, L.; Xu, W. Estimating aboveground biomass using Sentinel-2 MSI data and ensemble algorithms for grassland in the Shengjin Lake Wetland, China. Remote Sens. 2021, 13, 1595. [Google Scholar] [CrossRef]
- Wan, R.; Wang, P.; Wang, X.; Yao, X.; Dai, X. Mapping aboveground biomass of four typical vegetation types in the Poyang Lake wetlands based on random forest modelling and Landsat images. Front. Plant Sci. 2019, 10, 1281. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Xiao, J.; Chen, J.; Lin, K.; Bao, G.; Liu, A.; Wei, G. A machine learning scheme for estimating fine-resolution grassland aboveground biomass over China with Sentinel-1/2 satellite images. Remote Sens. Environ. 2024, 311, 114317. [Google Scholar] [CrossRef]
- Lyu, X.; Li, X.; Gong, J.; Li, S.; Dou, H.; Dang, D.; Xuan, X.; Wang, H. Remote-sensing inversion method for aboveground biomass of typical steppe in Inner Mongolia, China. Ecol. Indic. 2021, 120, 106883. [Google Scholar] [CrossRef]
- Yang, X.; Man, W.; Liu, M.; Zhang, Y.; Zheng, H.; Song, J.; Kang, Z. Estimation model of Spartina alterniflora aboveground biomass by remote sensing in Zhejiang Coastal Wetland. Remote Sens. Technol. Appl. 2023, 38, 1445–1454. [Google Scholar]
- Zhou, R.; Yang, C.; Li, E.; Cai, X.; Wang, X. Aboveground biomass estimation of wetland vegetation at the species level using unoccupied aerial vehicle RGB imagery. Front. Plant Sci. 2023, 14, 1181887. [Google Scholar] [CrossRef]
- Adam, E.; Mutanga, O.; Abdel-Rahman, E.M.; Ismail, R. Estimating standing biomass in papyrus (Cyperus papyrus L.) swamp: Exploratory of in situ hyperspectral indices and random forest regression. Int. J. Remote Sens. 2014, 35, 693–714. [Google Scholar] [CrossRef]
- Xi, M.; Kong, F.; Feng, X.; Zi, Y.; Li, Y. Growth dynamics and empirical formula for biomass evaluation of aboveground part of Phragmites australis in Jiaozhou Bay Wetlands. Wetl. Sci. 2016, 14, 816–824. [Google Scholar]
- Wang, S.; Li, S.; Zheng, S.; Gao, W.; Zhang, Y.; Cao, B.; Cui, B.; Shao, D. Estimating biomass and carbon sequestration capacity of Phragmites australis using remote sensing and growth dynamics modeling: A case study in Beijing Hanshiqiao wetland nature reserve, China. Sensors 2022, 22, 3141. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, D.; Zhang, D.; Wang, Z.; Du, B.; Yan, H.; Qiu, Z.; Feng, K.; Wang, J.; Jia, M. Mapping Phragmites australis aboveground biomass in the momoge wetland ramsar site based on Sentinel-1/2 images. Remote Sens. 2022, 14, 694. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, C.; Zhuo, W.; Shi, R.; Zhu, F.; Liu, S. Assessment of the impact of coastal wetland saltmarsh vegetation types on aboveground biomass inversion. Remote Sens. 2024, 16, 4762. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, Y.; Li, B.; Li, J. Estimating vegetation aboveground biomass in Yellow River Delta coastal wetlands using Sentinel-1, Sentinel-2 and Landsat-8 imagery. Ecol. Inform. 2025, 87, 103096. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, R.; Zhang, Z.; Bai, X.; Zeng, Q. Estimation of wetland vegetation aboveground biomass based on remote sensing data: A review. Chin. J. Ecol. 2016, 35, 1936–1946. [Google Scholar]
- Ye, C.; Zhao, X.; Wu, G.; Wang, X.; Liu, Y. Vegetation biomass spatial-temporal variations and the influence of the water level in Poyang Lake National Nature Reserve. J. Lake Sci. 2013, 25, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Huang, Q.; Yi, Q.; Zhu, Z.; Zhou, G.; Tian, L.; Zhou, Y.; Jia, J.; Qian, H. Changes in floodplain vegetation in Poyang Lake wetlands. Acta Ecol. Sin. 2019, 39, 4070–4079. [Google Scholar]
- Tan, Z.; Zhang, Q.; Li, Y.; Xu, X.; Jiang, J. Distribution of typical vegetation communities along elevation in Poyang Lake Wetlands. Wetl. Sci. 2016, 14, 506–515. [Google Scholar]
- Zhang, Q.; Yu, X.; Hu, B. Research on the characteristics of plant communities in the Poyang Nanji Wetlands, China. Resour. Sci. 2013, 35, 42–49. [Google Scholar]
- Guo, R.; Fu, S.; Hou, M.; Liu, J.; Miao, C.; Meng, X.; Feng, Q.; He, J.; Qian, D.; Liang, T. Remote sensing retrieval of nature grassland biomas in Menyuan County, Qinghai Province experimental area based on Sentinel-2 data. Acta Prataculturae Sin. 2023, 32, 15–29. [Google Scholar]
- Ge, G.; Wu, L. Analysis on the Flora of Seed Plants in Nanjishan Nature Reserve Jiangxi. J. Nanchang Univ. 2006, 30, 52–55. [Google Scholar]
- Zhi, Y.; Yi, J.; Liu, W.; Gong, H.; Shao, M.; Dai, N.; Li, Q.; Yang, W. Monitoring of wintering waterbirds in the Nanji Wetland National Nature Reserve of Poyang Lake. Chin. J. Ecol. 2020, 39, 2400–2407. [Google Scholar]
- Forzieri, G.; Dakos, V.; McDowell, N.G.; Ramdane, A.; Cescatti, A. Emerging signals of declining forest resilience under climate change. Nature 2022, 608, 534–539. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Sheykhmousa, M.; Mahdianpari, M.; Ghanbari, H.; Mohammadimanesh, F.; Ghamisi, P.; Homayouni, S. Support vector machine versus random forest for remote sensing image classification: A meta-analysis and systematic review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 6308–6325. [Google Scholar] [CrossRef]
- Schonlau, M.; Zou, R.Y. The random forest algorithm for statistical learning. Stata J. 2020, 20, 3–29. [Google Scholar] [CrossRef]
- Liang, S.; Cheng, J.; Jia, K.; Jiang, B.; Liu, Q.; Liu, S.; Xiao, Z.; Xie, X.; Yao, Y.; Yuan, W. Recent progress in land surface quantitative remote sensing. J. Remote Sens. 2016, 20, 875–898. [Google Scholar]
- Li, H.; Tang, X.; Cui, L.; Zhai, X.; Wang, J.; Zhao, X.; Li, J.; Lei, Y.; Wang, J.; Wang, R.; et al. Estimating aboveground biomass of wetland plant communities from hyperspectral data based on fractional-order derivatives and machine learning. Remote Sens. 2024, 16, 3011. [Google Scholar] [CrossRef]
- Rumelhart, D.; Hinton, G.E.; Williams, R.J. Learning representations by Back Propagating Errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Li, H.; Pei, M.; Zhen, Q.; Zheng, J.; Zhao, H.; Hu, Y.; Fan, J. Estimation of aboveground biomass of Picea schrenkiana forests considering vertical zonality and stand age. Forests 2025, 16, 445. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Xia, Y.; Liu, J.; Chen, Y. Study on the influence of nutritional status of disc lake on Carex. Ecol. Sci. 2024, 43, 85–90. [Google Scholar]
- Zhang, B.; Liu, Q.; Li, X.; Liu, L.; Yang, B.; Husi, L.; Gao, L.; Zhang, W.; Zhang, H.; Bian, Z. The core concepts and fundamental issues of remote sensing science. Natl. Remote Sens. Bull. 2025, 29, 1–48. [Google Scholar] [CrossRef]
- Liu, X.; Guan, Y.; Guo, S.; Zhang, C.; Wang, L. Response on wetland vegetation distribution to hydrology regularity based on harmonic-time series analysis. J. Lake Sci. 2016, 28, 12. [Google Scholar] [CrossRef]
- Wang, R.; Peng, W.; Liu, X.; Wu, W.; Han, Z.; Jiang, C. Analysis of responses of typical vegetation in Nanji Wetland National Nature Reserve of Poyang Lake to water depth and submergence frequency. J. China Inst. Water Resour. Hydropower Res. 2018, 6, 528–535. [Google Scholar]
- Zhao, Y.; Wen, Y.; Wang, F.; Cui, X.; Lan, Z.; Cai, Y.; Zhang, Y.; Lu, S. The effect of flooding duration on the vegetation biomass allocation pattern in shallow lakes of Poyang Lake. J. Jiangxi Norm. Univ. 2024, 48, 572–579. [Google Scholar]
- Zhang, L.; Luo, W.; Zhang, H.; Yin, X.; Li, B. Classification scheme for mapping wetland herbaceous plant communities using time series Sentinel-1 and Sentinel-2 data. Natl. Remote Sens. Bull. 2023, 27, 1362–1375. [Google Scholar] [CrossRef]
- Lin, Y.; Li, X.; Tan, Z.; Song, Y.; Xu, C. Dynamic characteristics of vegetation communities in the floodplain wetland of Lake Poyang based on spatio-temporal fusion of remote sensing data. J. Lake Sci. 2023, 35, 1408–1422. [Google Scholar] [CrossRef]
- Dong, P.; Jing, C.; Wang, G.; Shao, Y.; Gao, Y. The Estimation of Grassland Aboveground Biomass and Analysis of Its Response to Climatic Factors Using a Random Forest Algorithm in Xinjiang, China. Plants 2024, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, C.; Ogaya, R.; Fernández-Martínez, M.; Pugh, T.; Peuelas, J. Increasing climatic sensitivity of global grassland vegetation biomass and species diversity correlates with water availability. New Phytol. 2021, 230, 1761–1771. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Z.; Liu, M. Climate vs. nutrient control: A global analysis of driving environmental factors of wetland plant biomass allocation strategy. J. Clean. Prod. 2023, 406, 136983. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, X.; Zhang, G. Variation characteristics of the decomposition process δ13C and δ15 N of three dominant plant litter in Lake Poyang wetland. J. Lake Sci. 2023, 35, 1694–1704. [Google Scholar]
- Huang, Q.; Fang, C.; Hu, Q. Progresses of wetland ecosystem field monitoring in Nanji Wetland National Nature Reserve, South of Poyang Lake. Wetl. Sci. 2017, 15, 8. [Google Scholar]
- Liu, Y.; Feng, T.; Chen, B. Estimation of multi-scale biomass and carbon storage in the coastal wetlands of Ningbo City through Filed-UAV-Satellite synergy. Natl. Remote Sens. Bull. 2025, 29, 147–166. [Google Scholar] [CrossRef]
- Doughty, C.L.; Ambrose, R.F.; Okin, G.S.; Cavanaugh, K.C. Characterizing spatial variability in coastal wetland biomass across multiple scales using UAV and satellite imagery. Remote Sens. Ecol. Conserv. 2021, 7, 411–429. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, J.; Xu, J.; Wu, W.; Wang, Y.; Guo, H. Assessing the impacts of tidal creeks on the spatial patterns of coastal salt marsh vegetation and its aboveground biomass. Remote Sens. 2022, 14, 1839. [Google Scholar] [CrossRef]
- Naidoo, L.; Van Deventer, H.; Ramoelo, A.; Mathieu, R.; Nondlazi, B.; Gangat, R. Estimating above ground biomass as an indicator of carbon storage in vegetated wetlands of the grassland biome of South Africa. Int. J. Appl. Earth Obs. Geoinf. 2019, 78, 118–129. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Q.; Liu, Y.; Ye, J.; Liu, H.; Huang, L.; Yao, B. Responses of plant biomass and allocation to plant diversity changes in typical wetlands of Poyang Lake. Chin. J. Appl. Environ. Biol. 2023, 29, 1337–1345. [Google Scholar]
- Pan, Y.; Liu, J.; Zhang, M.; Huang, P.; Hipesy, M.; Dai, L.; Ma, Z.; Zhang, F.; Zhang, Z. The below-ground biomass contributes more to wetland soil carbon pools than the above-ground biomass—A survey based on global wetlands. J. Plant Ecol. 2024, 17, rtae017. [Google Scholar] [CrossRef]


| Remote Sensing Index | Calculation Formula |
|---|---|
| NDVI (Normalized Difference Vegetation Index) | |
| kNDVI (Kernel Normalized Difference Vegetation Index) | [40] |
| EVI (Enhanced Vegetation Index) | |
| SAVI (Soil-Adjusted Vegetation Index) | |
| NIRv (Near-Infrared Reflectance of Vegetation) | |
| NDWI (Normalized Difference Water Index) | |
| MNDWI (Modified Normalized Difference Water Index) | |
| SR (Simple Ratio Index) |
| Vegetation Types | Mean Elevation (m) | Mean AGB (g·m−2) |
|---|---|---|
| Carex | 12.77 | 625.32 |
| Carex and J. effusus | 13.21 | 413.33 |
| J. effusus | 12.83 | 197.50 |
| M. lutarioriparius | 13.57 | 1026.67 |
| M. lutarioriparius_ Carex | 13.96 | 914.44 |
| P. australis | 13.40 | 1122.27 |
| P. australis_Carex | 13.48 | 1188.46 |
| P. criopolitanum | 11.92 | 310.00 |
| P. lapathifolium | 13.77 | 868.33 |
| P. lapathifolium_Carex | 13.39 | 603.33 |
| P. orientalis | 12.70 | 1038.33 |
| Z. latifolia | 13.49 | 645.00 |
| Z. latifolia_Carex | 12.53 | 786.67 |
| Unit: km2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Range | Carex | M. lutarioriparius | P. australis | Polygonum | ||||
| Area | Ratio | Area | Ratio | Area | Ratio | Area | Ratio | |
| ≤300 | 8.22 | 4.31% | 2.55 | 4.76% | 2.46 | 3.14% | 3.92 | 7.60% |
| [301, 600] | 7.36 | 3.85% | 1.17 | 2.18% | 4.10 | 5.24% | 7.28 | 14.13% |
| [601, 900] | 125.82 | 65.88% | 24.05 | 44.95% | 35.99 | 45.96% | 32.45 | 62.93% |
| [901, 1200] | 47.78 | 25.02% | 25.65 | 47.95% | 34.07 | 43.52% | 7.74 | 15.02% |
| ≥1201 | 1.79 | 0.94% | 0.08 | 0.16% | 1.68 | 2.15% | 0.17 | 0.32% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, X.; Zhao, X.; Wang, C.; Zeng, H.; Shao, Y. Remote Sensing Aboveground Biomass Inversion of Four Vegetation Types in the Nanji Wetland. Forests 2025, 16, 1376. https://doi.org/10.3390/f16091376
Lai X, Zhao X, Wang C, Zeng H, Shao Y. Remote Sensing Aboveground Biomass Inversion of Four Vegetation Types in the Nanji Wetland. Forests. 2025; 16(9):1376. https://doi.org/10.3390/f16091376
Chicago/Turabian StyleLai, Xiahua, Xiaomin Zhao, Chen Wang, Han Zeng, and Yiwen Shao. 2025. "Remote Sensing Aboveground Biomass Inversion of Four Vegetation Types in the Nanji Wetland" Forests 16, no. 9: 1376. https://doi.org/10.3390/f16091376
APA StyleLai, X., Zhao, X., Wang, C., Zeng, H., & Shao, Y. (2025). Remote Sensing Aboveground Biomass Inversion of Four Vegetation Types in the Nanji Wetland. Forests, 16(9), 1376. https://doi.org/10.3390/f16091376






