Temperature Effects on Forest Soil Greenhouse Gas Emissions: Mechanisms, Ecosystem Responses, and Future Directions
Abstract
1. Introduction
1.1. Global Significance of Forest Soil Greenhouse Gas Emissions
1.2. Main Types and Characteristics of Forest Soil Greenhouse Gas Emissions
1.2.1. Carbon Dioxide (CO2)
1.2.2. Methane (CH4)
1.2.3. Nitrous Oxide (N2O)
1.2.4. Interrelationships Among the Three Greenhouse Gases
1.3. Key Factors Influencing Forest Soil Greenhouse Gas Emissions
1.3.1. Climatic Factors
1.3.2. Nitrogen Deposition
1.3.3. Forest Management
1.3.4. Soil Physicochemical Properties
1.4. Research Objectives and Content Framework
1.4.1. Research Background and Significance
1.4.2. Research Objectives
- To resolve the contrasting outcomes of post-fire restoration, we analyzed the impacts and mechanisms of different restoration methods on forest soil greenhouse gas emissions, providing a scientific basis for managing degraded forest ecosystems [73].
- To clarify the inconsistent effects of forest thinning, we evaluated the regulatory effects of management on soil CH4 and CO2 emissions, offering theoretical support for optimizing forest operational management practices [61].
- To uncover complex biogeochemical interactions, we explored the impact of nitrogen deposition and its interaction with soil fauna on forest soil greenhouse gas emissions, deepening our understanding of nitrogen-cycling processes [75].
- To better predict high-latitude feedback, we elucidated the relationship between temperature sensitivity and greenhouse gas emissions from high-latitude wetland forest soils, assessing future emission trends under climate warming [96].
1.4.3. Content Framework
2. Nitrogen Deposition and Soil Fauna Regulation
2.1. Effects of Nitrogen Deposition on Forest Soil Greenhouse Gas Emissions
2.1.1. Nitrogen Deposition Patterns and Trends
2.1.2. Effects on Soil CO2 Emissions
2.1.3. Effects on Soil CH4 Fluxes
2.1.4. Effects on Soil N2O Emissions
2.2. Interaction of Nitrogen Deposition and Soil Fauna
2.2.1. Effects of Nitrogen Deposition on Soil Fauna Communities
2.2.2. Soil Fauna Mediation of Nitrogen Deposition Effects on CO2 Emissions
2.2.3. Soil Fauna Mediation of Nitrogen Deposition Effects on CH4 Fluxes
2.2.4. Soil Fauna Mediation of Nitrogen Deposition Effects on N2O Emissions
3. Temperature Sensitivity and Greenhouse Gas Emissions from High-Latitude Wetland Forest Soils
3.1. Characteristics of High-Latitude Wetland Forest Ecosystems
3.2. Greenhouse Gas Emission Patterns in High-Latitude Wetland Forests
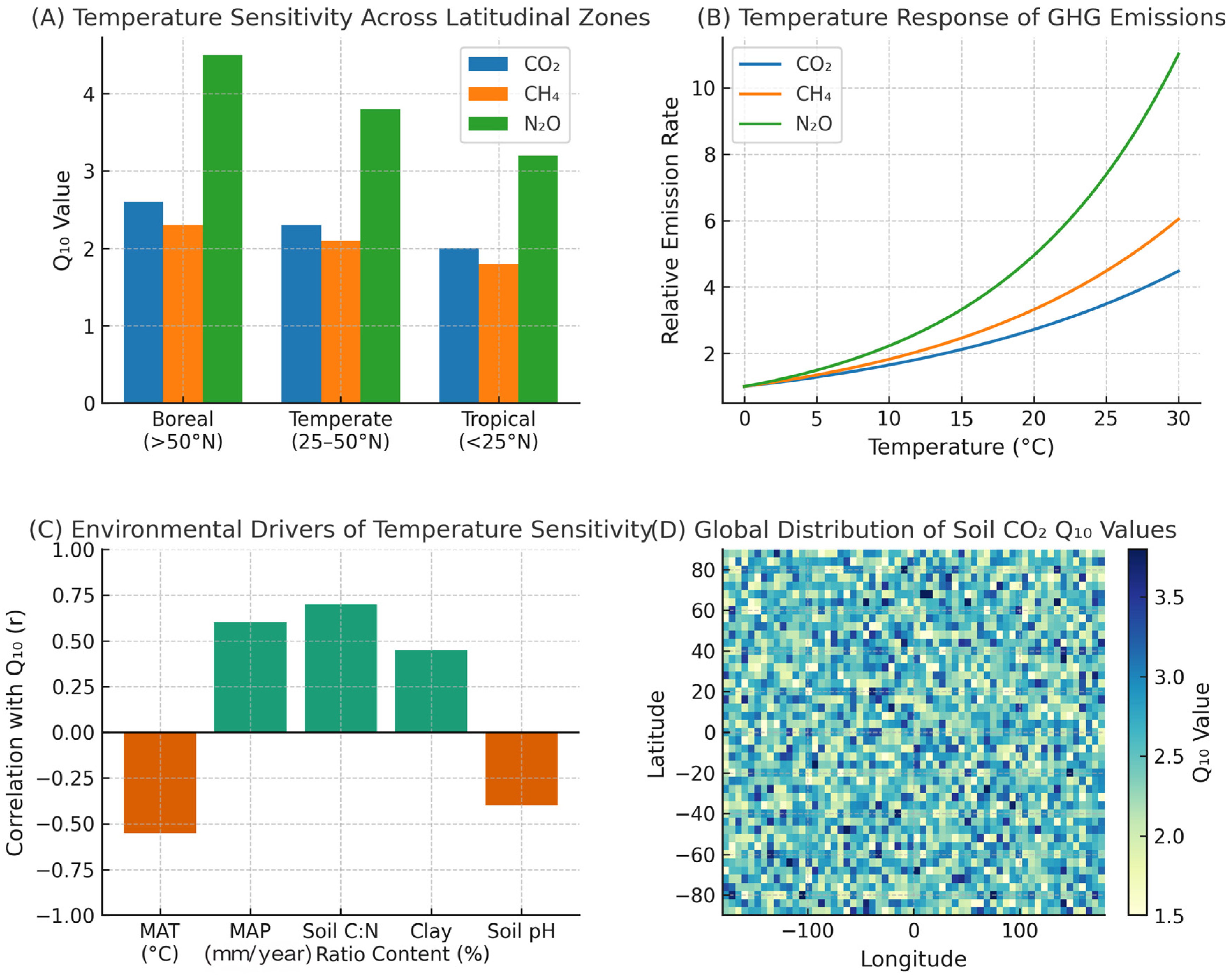
3.3. Temperature Sensitivity (Q10) of Greenhouse Gas Emissions
3.3.1. Q10 of CO2 Emissions
3.3.2. Q10 of CH4 Emissions
3.3.3. Q10 of N2O Emissions
3.4. Factors Influencing Temperature Sensitivity
3.5. Implications of Climate Warming
4. Discussion and Future Directions
4.1. Synthesis of Key Findings
4.2. Knowledge Gaps
4.3. Future Research Directions
4.4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wei, X.; Wu, F.; Van Meerbeek, K.; Desie, E.; Ni, X.; Yue, K.; Heděnec, P.; Yang, J.; An, N. Warming and altered precipitation rarely alter N addition effects on soil GHG fluxes: A meta-analysis. Ecol. Process. 2023, 12, 56. [Google Scholar] [CrossRef]
- Barneze, A.S.; Abdalla, M.; Whitaker, J.; McNamara, N.P.; Ostle, N.J. Predicted Soil Greenhouse Gas Emissions from Climate Warming and Grassland Management Interactions: A Modeling Study. Agronomy 2022, 12, 3055. [Google Scholar] [CrossRef]
- Muhammad, I.; Luo, X. Exploring Synergies: Greenhouse Gas Dynamics, Soil Mechanisms, and Forest Management in a Changing Climate—A Review. Glob. Change Biol. 2024, 30, e70016. [Google Scholar]
- Shen, K.; Li, L.; Wei, S.; Liu, J.; Zhao, Y. A network meta-analysis on responses of forest soil carbon sequestration to management practices. Ecol. Process. 2024, 13, 41. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Dalal, R.C.; Finn, D.; Menzies, N.W. Global changes in soil stocks of carbon, nitrogen, phosphorus, and sulphur as influenced by long-term agricultural production. Glob. Change Biol. 2017, 23, 2509–2519. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Dalal, R.C.; McKenna, B.A.; Smith, P.; Wang, P.; Zheng, Z.; van der Bom, F.J.T.; Menzies, N.W. Soil is a major contributor to global greenhouse gas emissions and climate change. SOIL 2024, 10, 873–885. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Kovács, S. Quantifying global warming potential variations from greenhouse gas emission sources in forest ecosystems. Carbon Res. 2024, 3, 70. [Google Scholar] [CrossRef]
- Walkiewicz, A.; Bulak, P.; Khalil, M.I.; Osborne, B. Assessment of soil CO2, CH4, and N2O fluxes and their drivers, and their contribution to the climate change mitigation potential of forest soils in the Lublin region of Poland. Eur. J. For. Res. 2024, 144, 29–52. [Google Scholar] [CrossRef]
- Sachs, J.D.; Schmidt-Traub, G.; Mazzucato, M.; Messner, D.; Nakicenovic, N.; Rockström, J. Six Transformations to achieve the Sustainable Development Goals. Nat. Sustain. 2019, 2, 805–814. [Google Scholar] [CrossRef]
- Basheer, S.; Wang, X.; Farooque, A.A.; Nawaz, R.A.; Pang, T.; Neokye, E.O. A Review of Greenhouse Gas Emissions from Agricultural Soil. Sustainability 2024, 16, 4789. [Google Scholar] [CrossRef]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Mao, J.; Bachmann, C.M.; Hoffman, F.M.; Koren, G.; Chen, H.; Tian, H.; Liu, J.; Tao, J.; Tang, J.; et al. Soil moisture controls over carbon sequestration and greenhouse gas emissions: A review. NPJ Clim. Atmos. Sci. 2025, 8, 16. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Smith, P.; Soussana, J.F.; Angers, D.; Schipper, L.; Chenu, C.; Rasse, D.P.; Batjes, N.H.; van Egmond, F.; McNeill, S.; Kuhnert, M.; et al. How to measure, report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal. Glob. Change Biol. 2020, 26, 219–241. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Zhu, J.; Zhuang, Q.; Jin, Y.; Tan, Z.; Yan, Z.; Hänninen, H. Soil Methane Oxidation in Boreal Forests Shows Higher Temperature Sensitivity Than in Temperate Regions. Glob. Change Biol. 2022, 28, 6566–6580. [Google Scholar]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Vargas, R.; Carbone, M.S.; Reichstein, M.; Baldocchi, D.D. Frontiers and challenges in soil respiration research: From measurements to model-data integration. Biogeochemistry 2011, 102, 1–13. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M.; et al. Temperature and soil organic matter decomposition rates–synthesis of current knowledge and a way forward. Glob. Change Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Megonigal, J.P.; Guenther, A.B. Methane Emissions from Upland Forest Soils and Vegetation. Tree Physiol. 2008, 28, 491–498. [Google Scholar] [CrossRef]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. Biogeosci. 2010, 115, G00K02. [Google Scholar] [CrossRef]
- AuthorE, F.; AuthorG, H. Responses of soil greenhouse gas fluxes to land management in a subtropical forest. Sci. Total Environ. 2025, 960, 170408. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Dutaur, L.; Verchot, L.V. A global inventory of the soil CH4 sink. Glob. Biogeochem. Cycles 2007, 21, GB4013. [Google Scholar] [CrossRef]
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Luo, G.J.; Kiese, R.; Wolf, B.; Butterbach-Bahl, K. Effects of soil temperature and moisture on methane uptake and nitrous oxide emissions across three different ecosystem types. Biogeosciences 2013, 10, 3205–3219. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Kotowska, A.; Bubier, J.; Dise, N.B.; Crill, P.; Hornibrook, E.R.C.; Minkkinen, K.; Moore, T.R.; Myers-Smith, I.H.; Nykänen, H.; et al. A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Glob. Change Biol. 2014, 20, 2183–2197. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Mosier, A.R.; Halvorson, A.D.; Zhang, F.S. The impact of nitrogen placement and tillage on NO, N2O, CH4 and CO2 fluxes from a clay loam soil. Plant Soil 2006, 280, 177–188. [Google Scholar] [CrossRef]
- Davidson, E.A.; Keller, M.; Erickson, H.E.; Verchot, L.V.; Veldkamp, E. Testing a Conceptual Model of Soil Emissions of Nitrous and Nitric Oxides. BioScience 2000, 50, 667–680. [Google Scholar] [CrossRef]
- Groffman, P.M.; Hardy, J.P.; Driscoll, C.T.; Fahey, T.J. Snow depth, soil freezing, and fluxes of carbon dioxide, nitrous oxide and methane in a northern hardwood forest. Glob. Change Biol. 2006, 12, 1748–1760. [Google Scholar] [CrossRef]
- Tian, H.; Yang, J.; Xu, R.; Lu, C.; Canadell, J.G.; Davidson, E.A.; Jackson, R.B.; Arneth, A.; Chang, J.; Ciais, P.; et al. Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Glob. Change Biol. 2019, 25, 640–659. [Google Scholar] [CrossRef]
- Groffman, P.M.; Butterbach-Bahl, K.; Fulweiler, R.W.; Gold, A.J.; Morse, J.L.; Stander, E.K.; Tague, C.; Tonitto, C.; Vidon, P. Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 2009, 93, 49–77. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Wang, J.; Zhang, Y.; Jiang, L.; Xia, J.; Zhou, L.; Zhou, X. Differential responses of temperature sensitivity of greenhouse gas emissions to warming in forest soils. Environ. Pollut. 2024, 350, 123456. [Google Scholar]
- Tate, K.R. Soil methane oxidation and land-use change—From process to mitigation. Soil Biol. Biochem. 2015, 80, 260–272. [Google Scholar] [CrossRef]
- Lucash, M.S.; Scheller, R.M.; Sturtevant, B.R.; Gustafson, E.J.; Kretchun, A.M.; Foster, J.R. Carbon, climate, and natural disturbance: A review of mechanisms, challenges, and tools for understanding forest carbon stability in an uncertain future. Carbon Balance Manag. 2024, 19, 282. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Steenbergh, A.K. Interactions between methane and the nitrogen cycle in light of climate change. Curr. Opin. Environ. Sustain. 2014, 9–10, 26–36. [Google Scholar] [CrossRef]
- Liang, L.L.; Grantz, D.A.; Jenerette, G.D. Multivariate regulation of soil CO2 and N2O pulse emissions from agricultural soils. Glob. Change Biol. 2016, 22, 1286–1298. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2018, 69, 10–20. [Google Scholar] [CrossRef]
- Bai, E.; Li, S.; Xu, W.; Li, W.; Dai, W.; Jiang, P. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytol. 2013, 199, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Courtois, E.A.; Stahl, C.; Van den Berge, J.; Bréchet, L.; Van Langenhove, L.; Richter, A.; Urbina, I.; Soong, J.L.; Peñuelas, J.; Janssens, I.A. Spatial Variation of Soil CO2, CH4 and N2O Fluxes Across Topographical Positions in Tropical Forests of the Guiana Shield. Ecosystems 2018, 21, 1445–1458. [Google Scholar] [CrossRef]
- Birch, H.F. The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 1958, 10, 9–31. [Google Scholar] [CrossRef]
- Carey, J.C.; Tang, J.; Templer, P.H.; Kroeger, K.D.; Crowther, T.W.; Burton, A.J.; Dukes, J.S.; Emmett, B.; Frey, S.D.; Heskel, M.A.; et al. Temperature response of soil respiration largely unaltered with experimental warming. Proc. Natl. Acad. Sci. USA 2016, 113, 13797–13802. [Google Scholar] [CrossRef]
- Du, B.; Kiese, R.; Butterbach-Bahl, K.; Dirnböck, T.; Rennnenberg, H. Consequences of nitrogen deposition and soil acidification in European forest ecosystems and mitigation approaches. For. Ecol. Manag. 2025, 580, 122523. [Google Scholar] [CrossRef]
- Schwede, D.B.; Simpson, D.; Tan, J.; Fu, J.S.; Dentener, F.; Du, E.; deVries, W. Spatial variation of modelled total, dry and wet nitrogen deposition to forests at global scale. Environ. Pollut. 2018, 243, 1287–1301. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Aronson, E.L.; Helliker, B.R. Methane flux in non-wetland soils in response to nitrogen addition: A meta-analysis. Ecology 2010, 91, 3242–3251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mo, J.; Zhou, G.; Gundersen, P.; Fang, Y.; Lu, X.; Zhang, T.; Dong, S. Methane uptake responses to nitrogen deposition in three tropical forests in southern China. J. Geophys. Res. Atmos. 2008, 113, D11116. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A review of nitrogen enrichment effects on three biogenic GHGs: The CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol. Lett. 2009, 12, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Korkiakoski, M.; Tuovinen, J.-P.; Aurela, M.; Koskinen, M.; Minkkinen, K.; Ojanen, P.; Penttilä, T.; Rainne, J.; Laurila, T.; Lohila, A. Methane exchange at the peatland forest floor-automatic chamber system exposes the dynamics of small fluxes. Biogeosciences 2017, 14, 1947–1967. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B. Nitrogen limitation of decomposition and decay: How can it occur? Glob. Change Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen Saturation in Temperate Forest Ecosystems. BioScience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Ullah, S.; Moore, T.R. Biogeochemical controls on methane, nitrous oxide, and carbon dioxide fluxes from deciduous forest soils in eastern Canada. J. Geophys. Res. Biogeosci. 2011, 116, G03010. [Google Scholar] [CrossRef]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.P.; et al. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Cai, C.; Du, M. Soil respiration and carbon balance in a Moso bamboo (Phyllostachys heterocycla (Carr.) Mitford cv. Pubescens) forest in subtropical China. iForest 2015, 8, 606–614. [Google Scholar]
- Wang, X.F.; Yan, H.; Zeng, J.; Huang, J.X.; Xu, X.Y.; Gao, J.X.; Li, N.; Ding, G.J. Different interval thinning intensities in a Chinese fir plantation affect soil greenhouse gas fluxes and the microbial community. For. Ecol. Manag. 2021, 498, 119542. [Google Scholar] [CrossRef]
- Kulmala, L.; Aaltonen, H.; Berninger, F.; Kieloaho, A.J.; Levula, J.; Bäck, J.; Hari, P.; Kolari, P.; Korhonen, J.F.J.; Kulmala, M.; et al. Changes in biogeochemistry and carbon fluxes in a boreal forest after the clear-cutting and partial burning of slash. Agric. For. Meteorol. 2014, 188, 33–44. [Google Scholar] [CrossRef]
- Mayer, M.; Sandén, H.; Rewald, B.; Godbold, D.L.; Katzensteiner, K. Increase in heterotrophic soil respiration by temperature drives decline in soil organic carbon stocks after forest windthrow in a mountainous ecosystem. Funct. Ecol. 2017, 31, 1163–1172. [Google Scholar] [CrossRef]
- Vesterdal, L.; Clarke, N.; Sigurdsson, B.D.; Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 2013, 309, 4–18. [Google Scholar] [CrossRef]
- Borken, W.; Beese, F. Control of nitrous oxide emissions in European beech, Norway spruce and Scots pine forests. Biogeochemistry 2005, 76, 141–159. [Google Scholar] [CrossRef]
- Schmitz, A.; Sanders, T.G.M.; Bolte, A.; Bussotti, F.; Dirnböck, T.; Johnson, J.; Peñuelas, J.; Pollastrini, M.; Prescher, A.-K.; Sardans, J.; et al. Responses of Forest Ecosystems in Europe to Decreasing Nitrogen Deposition. Environ. Pollut. 2019, 244, 980–994. [Google Scholar] [CrossRef]
- Boeckx, P.; Van Cleemput, O.; Villaralvo, I. Methane oxidation in soils with different textures and land use. Nutr. Cycl. Agroecosyst. 1997, 49, 91–95. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Webster, C.P.; Powlson, D.S. Long-term effects of nitrogen fertilization on methane oxidation in soil of the Broadbalk wheat experiment. Soil Biol. Biochem. 1993, 25, 1307–1315. [Google Scholar] [CrossRef]
- Xu, X.; Sharma, P.; Shu, S.; Lin, T.-S.; Ciais, P.; Tubiello, F.N.; Smith, P.; Campbell, N.; Jain, A.K. Global Greenhouse Gas Emissions from Animal-Based Foods Are Twice Those of Plant-Based Foods. Nat. Food 2021, 2, 724–732. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zhang, Y.; Zhang, J.; Wang, Y.; Guo, L. Comparative Analysis of Forest Soil Carbon Sink and Source Based on Bibliometrics: Development, Hotspots, and Trends. J. Clean. Prod. 2024, 480, 169355. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Crowther, T.W.; Todd-Brown, K.E.O.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ciais, P.; Goll, D.; Huang, Y.; Zhang, Y.; Zhu, Q.; Lauerwald, R.; Guenet, B.; Naipal, V.; Le Noë, J.; et al. The Effects of Nitrogen Deposition on Terrestrial Carbon Sequestration Through Multiple Mechanisms: A Modelling Study. Glob. Change Biol. 2021, 27, 5431–5445. [Google Scholar]
- Wang, Y.S.; Cheng, S.L.; Fang, H.J.; Yu, G.R.; Xu, M.J.; Dang, X.S.; Li, L.S.; Wang, L. Simulated nitrogen deposition reduces CH4 uptake and increases N2O emission from a subtropical plantation forest soil in southern China. PLoS ONE 2014, 9, e93571. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Z.; Wang, Y.; Zhao, W.; Zhang, X.; Ding, M.; Liu, L. Spatial patterns and drivers of soil carbon source greenhouse gas temperature sensitivity across the permafrost region of the Da Xing’an Mountains. Sci. Total Environ. 2023, 858, 159760. [Google Scholar]
- Cen, X.; He, N.; Li, M.; Xu, L.; Yu, X.; Cui, W.; Li, X.; Butterbach-Bahl, K. Suppression of Nitrogen Deposition on Global Forest Soil CH4 Uptake. Glob. Biogeochem. Cycles 2024, 38, e2024GB008098. [Google Scholar] [CrossRef]
- Smith, J.; Jones, A. Microbial community shifts in forest soils under warming: Implications for GHG fluxes. Soil Biol. Biochem. 2024, 180, 108990. [Google Scholar]
- Maaroufi, N.I.; Nordin, A.; Palmqvist, K.; Gundale, M.J. Anthropogenic Nitrogen Deposition Enhances Carbon Sequestration in Boreal Soils. Glob. Change Biol. 2017, 23, 4084–4095. [Google Scholar] [CrossRef]
- He, N.; Cen, X.; Van Sundert, K.; Terrer, C.; Yu, K.; Li, M.; Xu, L.; He, L.; Butterbach-Bahl, K. Global patterns of nitrogen saturation in forests. One Earth 2025, 8, 138–149. [Google Scholar]
- Zhu, J.; Jia, Y.; Yu, G.; Wang, Q.; He, N.; Chen, Z.; He, H.; Zhu, X.; Li, P.; Zhang, F.; et al. Changing patterns of global nitrogen deposition driven by socio-economic and emission changes. Nat. Commun. 2025, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, S. Modeling temperature effects on forest soil N2O emissions: A global perspective. Geoderma 2025, 450, 116780. [Google Scholar]
- Hagedorn, F.; Kluge, B.; Krause, K.; Gavazov, K.; Drollinger, S.; Wiesmeier, M.; Schack-Kirchner, H.; Priesack, E. Soil CH4 and N2O response diminishes during decadal soil warming in a temperate mountain forest. Agric. For. Meteorol. 2023, 329, 109287. [Google Scholar]
- Ahlström, J.; Ahlström, A.; Lindeskog, M.; Smith, B.; Sitch, S.; Arneth, A.; Friedlingstein, P.; Haverd, V.; Ito, A.; Jain, D.; et al. Projected effects of climate change and forest management on carbon fluxes and biomass of a boreal forest. Agric. For. Meteorol. 2024, 348, 109959. [Google Scholar]
- Li, Y.; Ding, M.-D.-B.; Wang, J.; Zhang, X.; Liu, Y.; Chen, X.; Huang, W.; Rillig, M.C. Soil fauna alter the responses of greenhouse gas emissions to changes in water and nitrogen availability. Soil Biol. Biochem. 2023, 180, 108990. [Google Scholar] [CrossRef]
- Yang, J.; Jia, X.; Ma, H.; Chen, X.; Liu, J.; Shangguan, Z.; Yan, W. Effects of warming and precipitation changes on soil GHG fluxes: A meta-analysis. Sci. Total Environ. 2022, 831, 154351. [Google Scholar] [CrossRef]
- Klemedtsson, L.; von Arnold, K.; Weslien, P.; Gundersen, P. Soil CN ratio as a scalar parameter to predict nitrous oxide emissions. Glob. Change Biol. 2005, 11, 1142–1147. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, J.; Cheng, S.; Wang, K.; Kumar, A.; Yu, Z.G.; Zhu, B. Linking soil dissolved organic matter characteristics and the temperature sensitivity of soil organic carbon decomposition in the riparian zone of the Three Gorges Reservoir. Ecol. Indic. 2023, 154, 110768. [Google Scholar] [CrossRef]
- Nishina, K.; Ito, A.; Beerling, D.J.; Cadule, P.; Ciais, P.; Clark, D.B.; Falloon, P.; Friend, A.D.; Kahana, R.; Kato, E.; et al. Quantifying uncertainties in soil carbon responses to changes in global mean temperature and precipitation. Earth Syst. Dyn. 2014, 5, 197–209. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Gao, L.; Shen, L.; Wang, C.; Ma, D. High level of winter warming aggravates soil carbon, nitrogen loss in the Qinghai-Tibet Plateau. Sci. Total Environ. 2023, 905, 167127. [Google Scholar]
- Liu, L.; Zhuang, Q.; Zhu, Q.; Liu, S.; Van Asperen, H.; Pihlatie, M. Global soil consumption of atmospheric carbon monoxide: An analysis using a process-based biogeochemistry model. Atmos. Chem. Phys. 2018, 18, 7913–7931. [Google Scholar] [CrossRef]
- Köster, K.; Berninger, F.; Lindén, A.; Köster, E.; Pumpanen, J. Recovery in Fungal Biomass Is Related to Decrease in Soil Organic Matter after Low-Intensity Fire in Boreal Forest. Forests 2014, 5, 2121–2134. [Google Scholar]
- Gundersen, P.; Christiansen, J.R.; Alberti, G.; Brüggemann, N.; Castaldi, S.; Gasche, R.; Kitzler, B.; Klemedtsson, L.; Lobo-do-Vale, R.; Moldan, F.; et al. The Response of Methane and Nitrous Oxide Fluxes to Forest Change in Europe. Biogeosciences 2012, 9, 3999–4012. [Google Scholar] [CrossRef]
- Luo, Y.; Ahlström, A.; Allison, S.D.; Batjes, N.H.; Brovkin, V.; Carvalhais, N.; Chappell, A.; Ciais, P.; Davidson, E.A.; Finzi, A.; et al. Toward more realistic projections of soil carbon dynamics by Earth system models. Glob. Biogeochem. Cycles 2016, 30, 40–56. [Google Scholar] [CrossRef]
- Lubbers, I.M.; van Groenigen, K.J.; Fonte, S.J.; Six, J.; Brussaard, L.; van Groenigen, J.W. Greenhouse-Gas Emissions from Soils Increased by Earthworms. Nat. Clim. Change 2013, 3, 187–194. [Google Scholar] [CrossRef]
- Limpens, J.; Berendse, F.; Blodau, C.; Canadell, J.G.; Freeman, C.; Holden, J.; Roulet, N.; Rydin, H.; Schaepman-Strub, G. Peatlands and the Carbon Cycle: From Local Processes to Global Implications—A Synthesis. Biogeosciences 2008, 5, 1475–1491. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.; Yu, G. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Change Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef]
- Treat, C.C.; Wollheim, W.M.; Varner, R.K.; Grandy, A.S.; Talbot, J.; Frolking, S. Temperature and Peat Type Control CO2 and CH4 Production in Alaskan Permafrost Peats. Glob. Change Biol. 2014, 20, 2674–2686. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Vesterdal, L.; Gundersen, P. Nitrous Oxide and Methane Exchange in Two Small Temperate Forest Catchments—Effects of Hydrological Gradients and Implications for Global Warming Potentials of Forest Soils. Biogeochemistry 2012, 107, 437–454. [Google Scholar] [CrossRef]
- Bréchet, L.; Ponton, S.; Alméras, T.; Bonal, D.; Epron, D. Does Spatial Distribution of Tree Size Account for Spatial Variation in Soil Respiration in a Tropical Forest? Plant Soil 2011, 347, 293–303. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Jandl, R. Carbon Losses Due to Soil Warming: Do Autotrophic and Heterotrophic Soil Respiration Respond Equally? Glob. Change Biol. 2009, 15, 901–913. [Google Scholar] [CrossRef]
- Snyder, B.A.; Callaham, M.A.; Hendrix, P.F. Spatial Variability of an Invasive Earthworm (Amynthas agrestis) Population and Potential Impacts on Soil Characteristics and Millipedes in the Great Smoky Mountains National Park, USA. Biol. Invasions 2011, 13, 349–358. [Google Scholar] [CrossRef]
| Stand Structure Parameter | CO2 Emissions | CH4 Oxidation | Underlying Mechanisms | Management Applications |
|---|---|---|---|---|
| Tree Density | CO2 emissions are higher in dense stands and decrease with density reduction. | CH4 oxidation is lower in dense stands and optimal at moderate densities. | Underlying mechanisms include the contribution of root respiration, soil temperature regulation, rates of organic matter input, and soil moisture balance. | Management applications involve density management prescriptions, stocking level guidelines, and spacing recommendations. |
| Spatial Arrangement | CO2 emissions are heterogeneous in clustered arrangements and more uniform in regular spacing. | CH4 oxidation is enhanced in gap-cluster arrangements and reduced in uniform spacing. | Mechanisms involve microclimate variation, root distribution patterns, resource competition gradients, and heterogeneity in soil development. | Applications include variable density thinning, gap creation strategies, retention forestry approaches, and enhancement of structural complexity. |
| Vertical Structure | CO2 emissions are higher with complex vertical structures and lower in simplified structures. | CH4 oxidation is variable, depending on understory conditions and soil properties. | Factors at play are light penetration effects, rainfall interception, diversity in litter quality, and variation in rooting depth. | Management strategies encompass multi-cohort management, understory development, canopy stratification, and enhancement of vertical diversity. |
| Species Composition | CO2 emissions are higher in conifer-dominated stands and lower in broadleaf or mixed stands. | CH4 oxidation is higher in mixed stands and lower in pure conifer stands. | Differences arise from litter quality variations, root exudate composition, mycorrhizal associations, and phenological complementarity. | Applications include mixed-species silviculture, conversion strategies, species selection criteria, and enhancement of functional diversity. |
| Age Structure | CO2 emissions are higher in younger stands and more stable in multi-aged stands. | CH4 oxidation is lower in younger stands and higher in mature and multi-aged stands. | This is driven by differences in growth rates, carbon allocation patterns, the stage of soil development, and overall ecosystem stability. | Management approaches involve uneven-aged management, age class distribution considerations, rotation length decisions, and structural retention strategies. |
| Recovery Phase | Timeframe | CO2 Emissions | CH4 Oxidation | Key Mechanisms | Management Implications |
|---|---|---|---|---|---|
| Initial Reduction | 0–2 years | CO2 emissions decrease by 15%–45% depending on thinning intensity. | CH4 oxidation decreases by 10%–40% in trafficked areas, with minimal change in undisturbed areas. | Key mechanisms include reduced root biomass and autotrophic respiration, decreased microbial activity, and altered soil physical conditions. | Management implications involve planning for reduced carbon cycling, monitoring soil physical recovery, and considering timing relative to seasonal cycles. |
| Recovery | 2–5 years | CO2 emissions show a gradual return to baseline, which is faster in lightly thinned stands. | CH4 oxidation shows progressive improvement, especially in well-drained soils. | Mechanisms driving recovery are root system expansion from residual trees, understory development, microbial community adaptation, and residue decomposition. | This phase offers an opportunity for understory management, is a critical period for soil remediation if needed, and serves as an important monitoring phase. |
| Enhancement | 5–8 years | CO2 emissions may exceed baseline by 10%–20% in moderately thinned stands. | CH4 oxidation may exceed baseline by 15%–30% in well-drained soils. | Enhanced individual tree growth, changes in root system architecture, altered resource availability, and priming effects are key mechanisms. | Management should consider the timing of subsequent entries, recognize this as a period of maximum carbon cycling, and note potential trade-offs with carbon sequestration. |
| Stabilization | 8+ years | CO2 emissions converge with unthinned conditions despite structural differences. | CH4 oxidation stabilizes at or slightly above pre-treatment levels. | Stabilization is achieved through stand density recovery, soil organic matter stabilization, ecosystem adaptation, and the establishment of a new equilibrium. | This phase indicates appropriate timing for subsequent thinning, provides a baseline for long-term carbon accounting, and serves as a reference for adaptive management. |
| Fire Severity | CO2 Emissions | CH4 Oxidation | N2O Emissions | Recovery Time | Key Mechanisms |
|---|---|---|---|---|---|
| Low (surface fire, <50% canopy mortality) | Initial decrease (10%–20%) followed by a return to pre-fire levels within 1–2 years | Reduced by 20%–40%, recovery within 3–5 years | Brief pulse (1–3 months) following first rainfall events | 2–5 years | Key mechanisms for low fire severity include partial consumption of the litter layer, limited heating of the soil, and a relatively rapid recovery of vegetation. |
| Moderate (mixed severity, 50%–80% canopy mortality) | Initial decrease (20%–40%) followed by an increase of 10%–30% above pre-fire levels for 2–3 years | Reduced by 40%–70%, recovery within 5–10 years | Elevated for 1–3 years, particularly following precipitation | 5–10 years | Under moderate fire severity, significant combustion of organic matter occurs, accompanied by moderate alterations to the soil structure and an increase in the availability of mineral nitrogen. |
| High (stand-replacing, >80% canopy mortality) | Initial decrease (40%–60%) followed by variable recovery depending on vegetation establishment | Reduced by 70%–95%, recovery may take 10–20 years | Elevated for 3–5 years with high spatial variability | 10–20+ years | High fire severity results in severe loss of organic matter, major alterations to the soil structure, the development of soil hydrophobicity, and a slow process of vegetation recovery. |
| Restoration Method | CO2 Emissions | CH4 Oxidation | N2O Emissions | Carbon Sequestration Rate | Implementation Considerations |
|---|---|---|---|---|---|
| Natural Regeneration | Natural regeneration typically leads to lower initial CO2 emissions, with gradual stabilization occurring over 3–7 years. | CH4 oxidation recovers gradually at a rate of 10%–15% annually, though this process can be limited by the slow recovery of soil structure. | N2O emissions are often elevated for 2–3 years post-fire and are strongly linked to precipitation events. | The initial carbon sequestration rate is slow, around 0.3–0.8 Mg C ha−1 year−1, but this is followed by steady long-term accumulation. | Implementation considerations for natural regeneration include its low cost and the requirement for viable seed sources, alongside the unpredictability of species composition and a longer overall recovery time. |
| Active Reforestation | Active reforestation may cause higher initial CO2 emissions due to site preparation activities, but stabilization is faster, typically within 2–3 years. | Recovery of CH4 oxidation is variable and depends on the intensity of site preparation, with long-term rates being species-dependent. | N2O emissions can be elevated if fertilizers are used, but they tend to decline faster with rapid vegetation establishment. | This method yields a higher carbon sequestration rate (0.5–1.2 Mg C ha−1 year−1), and long-term carbon stocks are species-dependent. | Active reforestation involves higher implementation costs and requires seedling production and planting, but offers greater control over species composition and achieves faster canopy closure. |
| Salvage Logging | Salvage logging results in the highest initial CO2 emissions due to soil disturbance, and stabilization is slower, taking 3–5 years. | Recovery of CH4 oxidation is significantly delayed in trafficked areas, with spatial variability based on equipment impacts. | N2O emissions are variable, depending on soil disturbance and vegetation recovery, and are often elevated in skid trails and landings. | The initial carbon sequestration rate is the lowest (0.2–0.5 Mg C ha−1 year−1), and there is a reduction in long-term potential due to biomass removal. | While allowing for economic timber recovery, salvage logging leads to an increase in soil disturbances, a reduction in coarse woody debris, and an altered microclimate. |
| Soil Rehabilitation (mulching, amendments) | Initial CO2 emissions are variable depending on the type of amendment used, with potential priming effects from labile amendments. | Soil rehabilitation can enhance CH4 oxidation recovery through improved soil structure, and moisture regulation benefits methanotrophs. | N2O emissions can be reduced through careful C/N ratio management, and mulch can create a moisture barrier that reduces emission pulses. | An enhanced carbon sequestration rate (0.6–1.5 Mg C ha−1 year−1) can be achieved with organic amendments, leading to improved long-term stabilization. | This approach has moderate to high costs, faces scalability challenges, and requires consideration of material sourcing, but offers the potential for immediate erosion control. |
| Integrated Approaches | Integrated approaches can lead to optimized CO2 emissions through targeted interventions, with spatial variability based on the treatment mosaic. | CH4 oxidation recovery is enhanced through strategic soil protection and spatial targeting of interventions. | N2O emissions are reduced through strategic nitrogen management and spatial and temporal optimization. | Carbon sequestration rates are optimized through complementary methods, leading to enhanced resilience to future disturbances. | Integrated approaches require detailed planning, incur higher initial assessment costs, necessitate a capacity for adaptive management, and aim for optimized resource allocation. |
| Thinning Intensity | CO2 Emissions | CH4 Oxidation | Soil Temperature | Soil Moisture | Microbial Activity | Root Biomass |
|---|---|---|---|---|---|---|
| Light (20%–30%) | Slight decrease (10%–15%) | Slight increase (5%–10%) | Increase (1–2 °C) | Slight decrease (5%–10%) | Minimal change | Decrease (15%–25%) |
| Moderate (40%–50%) | Moderate decrease (25%–35%) | Maximum increase (15%–20%) | Moderate increase (2–3 °C) | Moderate decrease (15%–25%) | Increase in diversity | Decrease (30%–45%) |
| Heavy (60%–70%) | Strong decrease (40%–50%) | Maximum decrease (5%–10%) | Strong increase (3–5 °C) | Strong decrease (25%–40%) | Shift in community composition | Severe decrease (50%–70%) |
| Forest Ecosystem Type | Dominant Climate Zone | Soil Type | CO2 Emission Q10 | CH4 Emission Q10 | CH4 Uptake Q10 | N2O Emission Q10 |
|---|---|---|---|---|---|---|
| Boreal Coniferous Forest | Boreal | Podzol, Histosol | 2.0–3.5 | 2.5–5.0 (source) | 1.5–2.5 | 2.0–4.0 |
| Temperate Broadleaf Forest | Temperate | Alfisol, Inceptisol | 1.8–3.0 | 1.5–3.0 (source) | 1.8–3.0 | 2.5–4.5 |
| Temperate Coniferous Forest | Temperate | Spodosol, Andosol | 2.2–3.8 | N/A (often sink) | 2.0–3.5 | 2.2–3.8 |
| Tropical Rainforest | Tropical | Oxisol, Ultisol | 1.5–2.5 | 2.0–4.0 (source) | 1.5–2.8 | 1.8–3.5 |
| Montane Forest (High Alt.) | Alpine/Montane | Cambisol, Leptosol | 2.5–4.5 | Variable | 1.2–2.2 | 3.0–5.0 |
| Forested Wetland (Peatland) | Boreal/Temperate | Histosol | 2.0–4.0 | 3.0–10.0+ (source) | 1.0–2.0 | 2.0–3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Wang, Y.; Wang, Y.; Dong, J.; Yu, S. Temperature Effects on Forest Soil Greenhouse Gas Emissions: Mechanisms, Ecosystem Responses, and Future Directions. Forests 2025, 16, 1371. https://doi.org/10.3390/f16091371
Wang T, Wang Y, Wang Y, Dong J, Yu S. Temperature Effects on Forest Soil Greenhouse Gas Emissions: Mechanisms, Ecosystem Responses, and Future Directions. Forests. 2025; 16(9):1371. https://doi.org/10.3390/f16091371
Chicago/Turabian StyleWang, Tiane, Yingning Wang, Yuan Wang, Juexian Dong, and Shaopeng Yu. 2025. "Temperature Effects on Forest Soil Greenhouse Gas Emissions: Mechanisms, Ecosystem Responses, and Future Directions" Forests 16, no. 9: 1371. https://doi.org/10.3390/f16091371
APA StyleWang, T., Wang, Y., Wang, Y., Dong, J., & Yu, S. (2025). Temperature Effects on Forest Soil Greenhouse Gas Emissions: Mechanisms, Ecosystem Responses, and Future Directions. Forests, 16(9), 1371. https://doi.org/10.3390/f16091371





