Land-Use Change Impacts on Glomalin-Related Soil Protein and Soil Organic Carbon in Huangshan Mountain Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling Approach
2.2. Extraction and Quantification of GRSP
2.3. Determination of Soil Variables
2.4. Statistical Analyses
3. Results
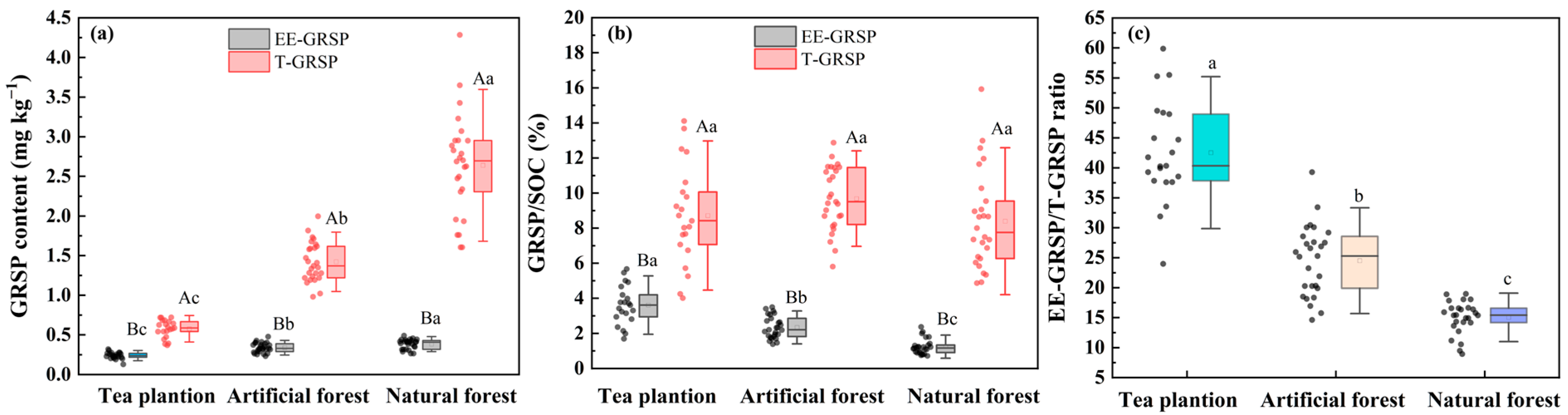
3.1. SOC and GRSP Contents in Tea Plantation and Forest Soils
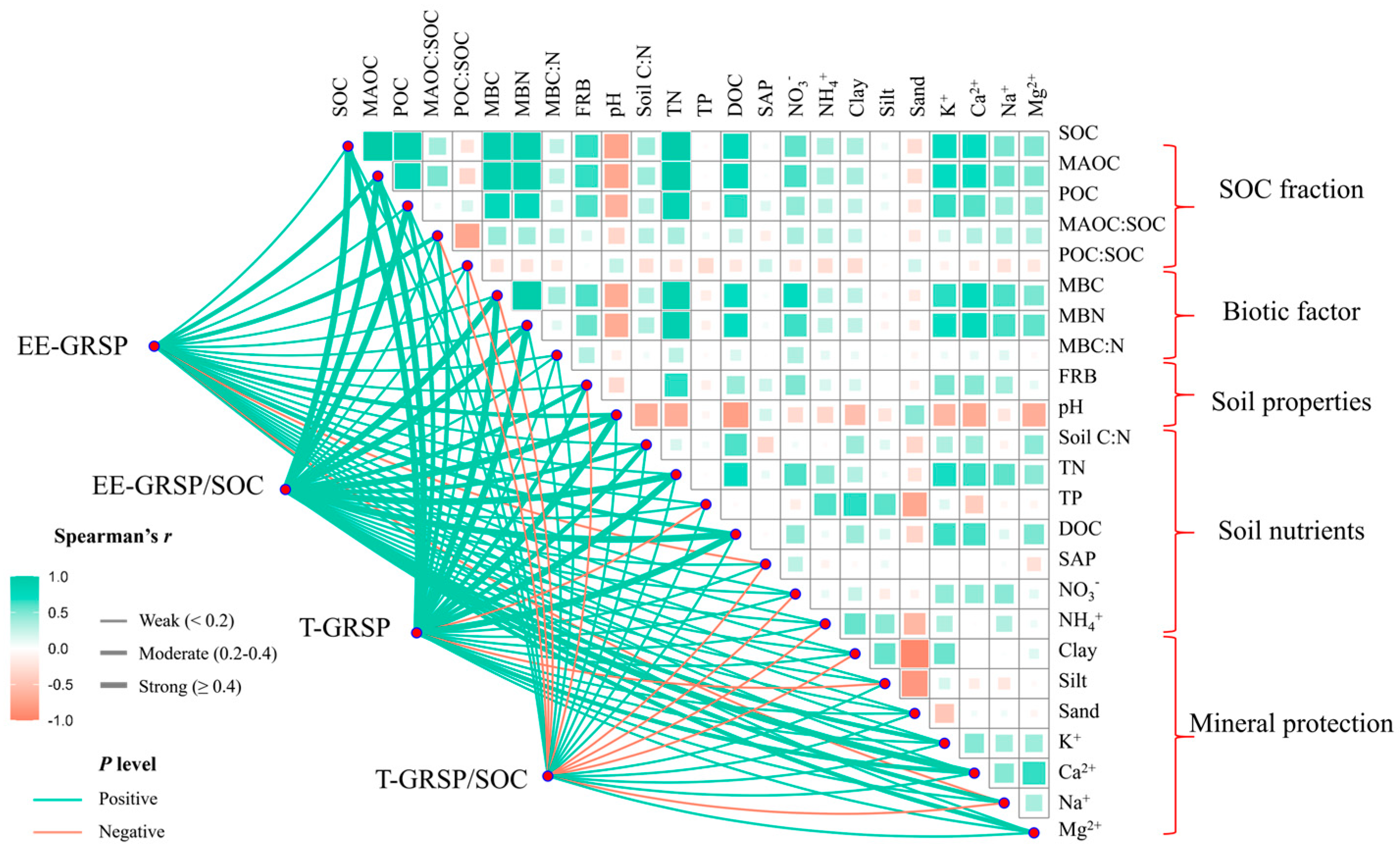
3.2. Relationships Between GRSP and Soil Biotic and Abiotic Factors
3.3. Controlling Factors on GRSP Accumulation Across Three Land-Use Types
4. Discussion
4.1. Changes in SOC and GRSP Content Across Three Land-Use Types
4.2. Factors Affecting T-GRSP Accumulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Sun, G.-X.; Jiang, J. Soil Organic Carbon Responses to Different Land Use Change: A Meta-Analysis. Land Degrad. Dev. 2025. Early View. [Google Scholar] [CrossRef]
- Shuai, Q.; Xue, J.; Dai, L.J.; Huang, Y.Y.; Jin, D.H.; Chen, Z.X.; Li, M.W.; Shi, Z.; Chen, S.C. The effects of land use change on soil organic carbon stock in China: A meta-analysis with the empirical modeling approach. Geoderma Reg. 2024, 36, e00774. [Google Scholar] [CrossRef]
- Shao, P.S.; Liang, C.; Lynch, L.; Xie, H.T.; Bao, X.L. Reforestation accelerates soil organic carbon accumulation: Evidence from microbial biomarkers. Soil Biol. Biochem. 2019, 131, 182–190. [Google Scholar] [CrossRef]
- England, J.R.; Paul, K.I.; Cunningham, S.C.; Madhavan, D.B.; Baker, T.G.; Read, Z.; Wilson, B.R.; Cavagnaro, T.R.; Lewis, T.; Perring, M.P.; et al. Previous land use and climate influence differences in soil organic carbon following refor-estation of agricultural land with mixed-species plantings. Agric. Ecosyst. Environ. 2016, 227, 61–72. [Google Scholar] [CrossRef]
- Chen, G.P.; Ma, S.H.; Tian, D.; Xiao, W.; Jiang, L.; Xing, A.J.; Zou, A.L.; Zhou, L.H.; Shen, H.H.; Zheng, C.Y.; et al. Patterns and determinants of soil microbial residues from tropical to boreal forests. Soil Biol. Biochem. 2020, 151, 108059. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Rillig, M.C.; Maestre, F.T.; Lamit, L.J. Microsite differences in fungal hyphal length, glomalin, and soil aggregate stability in semiarid Mediterranean steppes. Soil Biol. Biochem. 2003, 35, 1257–1260. [Google Scholar] [CrossRef]
- Singh, G.; Pankaj, U.; Chand, S.; Verma, R.K. Arbuscular Mycorrhizal Fungi-Assisted Phytoextraction of Toxic Metals by Zea mays L. From Tannery Sludge. Soil Sediment. Contam. 2019, 28, 729–746. [Google Scholar] [CrossRef]
- Wright, S.F.; FrankeSnyder, M.; Morton, J.B.; Upadhyaya, A. Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant Soil 1996, 181, 193–203. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Nichols, K.A.; Schmidt, W.F.; Torn, M.S. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil 2001, 233, 167–177. [Google Scholar] [CrossRef]
- Li, T.; Yuan, Y.; Mou, Z.; Li, Y.; Kuang, L.; Zhang, J.; Wu, W.; Wang, F.; Wang, J.; Lambers, H.; et al. Faster accumulation and greater contribution of glomalin to the soil organic carbon pool than amino sugars do under tropical coastal forest restoration. Glob. Chang. Biol. 2022, 29, 533–546. [Google Scholar] [CrossRef]
- Agnihotri, R.; Sharma, M.P.; Prakash, A.; Ramesh, A.; Bhattacharjya, S.; Patra, A.K.; Manna, M.C.; Kurganova, I.; Kuzyakov, Y. Glycoproteins of arbuscular mycorrhiza for soil carbon sequestration: Review of mechanisms and controls. Sci. Total Environ. 2022, 806, 150571. [Google Scholar] [CrossRef]
- Koide, R.T.; Peoples, M.S. Behavior of Bradford-reactive substances is consistent with predictions for glomalin. Appl. Soil Ecol. 2013, 63, 8–14. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, H.L.; Chen, J.Y.; Hong, H.L.; Liu, J.C.; Li, J.W.; Yan, C.L. Spatial distribution of glomalin-related soil protein and its relationship with sediment carbon sequestration across a mangrove forest. Sci. Total Environ. 2018, 613–614, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Ma, L.; He, X.; Liu, Z.; Wang, F.; Chu, G.; Tang, X. Accumulation of glomalin-related soil protein benefits soil carbon sequestration: Tropical coastal forest restoration experiences. Land. Degrad. Dev. 2022, 33, 1541–1551. [Google Scholar] [CrossRef]
- Zhang, M.; Che, R.; Cheng, Z.; Zhao, H.; Wu, C.; Hu, J.; Zhang, S.; Liu, D.; Cui, X.; Wu, Y. Decades of reforestation significantly change microbial necromass, glomalin, and their contributions to soil organic carbon. Agric. Ecosyst. Environ. 2023, 346, 108362. [Google Scholar] [CrossRef]
- Gu, R.; Xiao, K.; Zhu, Z.; He, X.; Li, D. Afforestation enhances glomalin-related soil protein content but decreases its contri-bution to soil organic carbon in a subtropical karst area. J. Environ. Manag. 2024, 356, 120754. [Google Scholar] [CrossRef]
- Holátko, J.; Brtnický, M.; Kučerík, J.; Kotianová, M.; Elbl, J.; Kintl, A.; Kynický, J.; Benada, O.; Datta, R.; Jansa, J. Glomalin—Truths, myths, and the future of this elusive soil glycoprotein. Soil Biol. Biochem. 2021, 153, 108116. [Google Scholar] [CrossRef]
- Song, L.; Yang, T.; Xia, S.; Yin, Z.; Liu, X.; Li, S.; Sun, R.; Gao, H.; Chu, H.; Ma, C. Soil depth exerts stronger impact on bacterial community than elevation in subtropical forests of Huangshan Mountain. Sci. Total Environ. 2022, 852, 158438. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Wang, N.; Xie, L.; Chen, S.; Zhao, R.; Feng, Y.; Li, Y.; Ding, H.; Fang, Y. Environmental Heterogeneity Affecting Commu-nity Assembly Patterns and Phylogenetic Diversity of Three Forest Communities at Mt. Huangshan, China. Forests 2022, 13, 133. [Google Scholar] [CrossRef]
- Carter, M.; Gregorich, E. Soil Sampling and Methods of Analysis; Taylor & Francis: London, UK, 2007. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Y.; Du, H.; Wang, K.; Li, D. Increasing plant species diversity enhances microbial necromass carbon content but does not alter its contribution to soil organic carbon pool in a subtropical forest. Soil Biol. Biochem. 2023, 187, 109183. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; van Bodegom, P.M.; Terrer, C.; Zelfde, M.V.T.; McCallum, I.; McCormack, M.L.; Fisher, J.B.; Brundrett, M.C.; de Sá, N.C.; Tedersoo, L. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.T.; Zhang, L.; Cao, Y.P.; Yang, Y.R.; Wang, P.; Li, Z.X.; Lin, Y. Effects of land-use type on soil organic carbon and carbon pool management index through arbuscular mycorrhizal fungi pathways. Glob. Ecol. Conserv. 2023, 43, e02432. [Google Scholar] [CrossRef]
- Huang, B.B.; Yan, G.Y.; Liu, G.C.; Sun, X.Y.; Wang, X.C.; Xing, Y.J.; Wang, Q.G. Effects of long-term nitrogen addition and precipitation reduction on glomalin-related soil protein and soil aggregate stability in a temperate forest. CATENA 2022, 214, 106284. [Google Scholar] [CrossRef]
- Zhu, R.H.; Zheng, Z.C.; Li, T.X.; He, S.Q.; Zhang, X.Z.; Wang, Y.D.; Liu, T. Effect of tea plantation age on the distribution of glomalin-related soil protein in soil water-stable aggregates in southwestern China. Environ. Sci. Pollut. Res. 2018, 26, 1973–1982. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, M.; Tripathi, B.N. Glomalin: An arbuscular mycorrhizal fungal soil protein. Protoplasma 2013, 250, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; He, X.H.; Zou, Y.N.; He, K.P.; Sun, Y.H.; Cao, M.Q. Spatial distribution of glomalin-related soil protein and its rela-tionships with root mycorrhization, soil aggregates, carbohydrates, activity of protease and β-glucosidase in the rhizosphere of Citrus unshiu. Soil Biol. Biochem. 2012, 45, 181–183. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Change Biol. 2018, 25, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.T.; Zheng, Q.; Noll, L.; Zhang, S.S.; Wanek, W. Direct measurement of the in situ decomposition of microbial-derived soil organic matter. Soil Biol. Biochem. 2020, 141, 107660. [Google Scholar] [CrossRef]
- Yang, Y.R.; He, C.J.; Huang, L.; Ban, Y.H.; Tang, M. The effects of arbuscular mycorrhizal fungi on glomalin-related soil pro-tein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS ONE 2017, 12, e0182264. [Google Scholar] [CrossRef]
- Bhople, P.; Keiblinger, K.; Djukic, I.; Liu, D.; Zehetner, F.; Zechmeister-Boltenstern, S.; Joergensen, R.G.; Murugan, R. Micro-bial necromass formation, enzyme activities and community structure in two alpine elevation gradients with different bedrock types. Geoderma 2021, 386, 114922. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xiao, Y.; Chen, W.; Wang, B.; Qian, Z. Land-Use Change Impacts on Glomalin-Related Soil Protein and Soil Organic Carbon in Huangshan Mountain Region. Forests 2025, 16, 1362. https://doi.org/10.3390/f16091362
Zhao Y, Xiao Y, Chen W, Wang B, Qian Z. Land-Use Change Impacts on Glomalin-Related Soil Protein and Soil Organic Carbon in Huangshan Mountain Region. Forests. 2025; 16(9):1362. https://doi.org/10.3390/f16091362
Chicago/Turabian StyleZhao, Yuan, Yuexin Xiao, Wei Chen, Buqing Wang, and Zongyao Qian. 2025. "Land-Use Change Impacts on Glomalin-Related Soil Protein and Soil Organic Carbon in Huangshan Mountain Region" Forests 16, no. 9: 1362. https://doi.org/10.3390/f16091362
APA StyleZhao, Y., Xiao, Y., Chen, W., Wang, B., & Qian, Z. (2025). Land-Use Change Impacts on Glomalin-Related Soil Protein and Soil Organic Carbon in Huangshan Mountain Region. Forests, 16(9), 1362. https://doi.org/10.3390/f16091362





