Wildfires and Palm Species Response in a Terra Firme Amazonian Social Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Forest Habitats
2.3. Curuá Leaves, Leaf Litter, and Flammability Potential
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, S.L. Tropical forests and the changing earth system. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 195–210. [Google Scholar] [CrossRef]
- Wees, V.D.; Van der werf, G.R.; Randerson, J.T.; Andela, N.; Chen, Y.; Morton, D.C. The role of fire in global forest loss dynamics. Glob. Change Biol. 2021, 27, 2377–2391. [Google Scholar] [CrossRef] [PubMed]
- Daily, G.C.; Stephen, P.; Goldstein, J.; Kareiva, M.P.; Mooney, A.H.; Pejchar, L.; Ricketts, H.T.; Salzman, J.; Shallenberger, R. Ecosystem services in decision making: Time to deliver. Front. Ecol. Environ. 2009, 7, 21–28. [Google Scholar] [CrossRef]
- Mutoko, M.C.; Hein, L.; Shisanya, C.A. Rainforest conservation versus conversion offsets: Insights from analysis of ecosystem services provided by the Kakamega rainforest in Kenya. Ecosyst. Serv. 2015, 14, 1–11. [Google Scholar] [CrossRef]
- Brondízio, E.S.; Aumeeruddy-Thomas, Y.; Bates, P.; Carino, J.; Fernández-Llamazares, Á.; Ferrari, M.F.; Galvin, K.; Reyes-García, V.; McElwee, P.; Molnár, Z.; et al. Locally based, regionally manifested, and globally relevant: Indigenous and local knowledge, values, and practices for nature. Annu. Rev. Environ. Resour. 2021, 46, 481–509. [Google Scholar] [CrossRef]
- Alves, R.P.; Levis, C.; Bertin, V.M.; Ferreira, M.J.; Cassino, M.F.; Pequeno, P.A.C.L.; Clement, C.R. Local forest specialists maintain traditional ecological knowledge in the face of environmental threats to Brazilian Amazonian protected areas. Front. For. Glob. Chang. 2022, 5, 1028129. [Google Scholar] [CrossRef]
- Lapola, D.M.; Pinho, P.; Barlow, J.; Aragão, L.E.O.C.; Berenguer, É.; Carmenta, R.; Liddy, H.M.; Seixas, H.; Silva, C.V.J.; Walker, W.S.; et al. The drivers and impacts of Amazon forest degradation. Science 2023, 379, 8622. [Google Scholar] [CrossRef]
- Ferraz, S.F.; Ferraz, K.M.; Cassiano, C.C.; Brancalion, P.H.S.; da Luz, D.T.; Azevedo, T.N.; Metzger, J.P. How good are tropical forest patches for ecosystem services provisioning? Landsc. Ecol. 2014, 29, 187–200. [Google Scholar] [CrossRef]
- Flores, B.M.; Holmgren, M. White-sand savannas expand at the core of the Amazon after forest wildfires. Ecosystems 2021, 24, 1624–1637. [Google Scholar] [CrossRef]
- Martínez-Ramos, M.; Ortiz-Rodríguez, I.A.; Piñero, D.; Dirzo, R.; Sarukhán, J. Anthropogenic disturbances jeopardize biodiversity conservation within tropical Rainforest Reserves. Proc. Natl. Acad. Sci. USA 2016, 113, 5323–5328. [Google Scholar] [CrossRef] [PubMed]
- Kahn, F.; Mejia, K. Palm communities in wetland forest ecosystems of Peruvian Amazonia. For. Ecol. Manag. 1990, 33, 169–179. [Google Scholar] [CrossRef]
- Bellot, S.; Lu, Y.; Antonelli, A.; Baker, W.J.; Dransfield, J.; Forest, F.; Kissling, W.D.; Leitch, I.J.; Nic Lughadha, E.; Ondo, I.; et al. The likely extinction of hundreds of palm species threatens their contributions to people and ecosystems. Nat. Ecol. Evol. 2022, 6, 1710–1722. [Google Scholar] [CrossRef]
- Eiserhardt, W.L.; Svenning, J.C.; Kissling, W.D.; Balslev, H. Geographical ecology of the palms (Arecaceae): Determinants of diversity and distributions across spatial scales. Ann. Bot. 2011, 108, 1391–1416. [Google Scholar] [CrossRef]
- de Almeida, G.M.; Ramos, M.A.; Araújo, E.L.; Baldauf, C.; Albuquerque, U.P. Human Perceptions of landscape change: The case of a monodominant forest of Attalea speciosa Mart. ex Spreng (Northeast Brazil). Ambio 2016, 45, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, B.K.; Peres, C.A.; Melo, F.P.; Leal, I.R.; Tabarelli, M. Winner–loser species replacements in human-modified landscapes. Trends Ecol. Evol. 2021, 36, 545–555. [Google Scholar] [CrossRef]
- Mitja, D.; Delaître, E.; Santos, A.M.; Miranda, I.; Coelho, R.F.R.; Macedo, D.J.; Demagistri, L.; Petit, M. Satellite images combined with field data reveal negative changes in the distribution of babassu palms after clearing off Amazonian forests. Environ. Manag. 2018, 61, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Salm, R.; Jalles-Filho, E.; Schuck-Paim, C. A Model for the importance of large arborescent palms in the dynamics of seasonally-dry Amazonian forests. Biota Neotrop. 2005, 5, 151–156. [Google Scholar] [CrossRef]
- Vallejo, M.I.; Galeano, G.; Bernal, R.; Zuidema, P.A. The fate of populations of Euterpe oleracea harvested for palm heart in Colombia. For. Ecol. Manag. 2014, 318, 274–284. [Google Scholar] [CrossRef]
- Soares-Filho, B.; Moutinho, P.; Nepstad, D.; Anderson, A.; Rodrigues, H.; Garcia, R.; Dietzsch, L.; Merry, F.; Bowman, M.; Hissa, L.; et al. Role of Brazilian Amazon protected areas in climate change mitigation. Proc. Natl. Acad. Sci. USA 2010, 107, 10821–10826. [Google Scholar] [CrossRef]
- Pereira, C.A.; Tabarelli, M.; Barros, M.F.; Vieira, I.C.G. Restoring fire-degraded social forests via biocultural approaches: A key strategy to safeguard the Amazon legacy. Restor. Ecol. 2023, 31, e13976. [Google Scholar] [CrossRef]
- Barlow, J.; Berenguer, E.; Carmenta, R.; França, F. Clarifying Amazonia’s burning crisis. Glob. Change Biol. 2020, 26, 319–321. [Google Scholar] [CrossRef]
- Silva Junior, C.H.L.; Aragão, L.E.; Anderson, L.O.; Fonseca, M.G.; Shimabukuro, Y.E.; Vancutsem, C.; Saatchi, S.S. Persistent collapse of biomass in Amazonian forest edges following deforestation leads to unaccounted carbon losses. Sci. Adv. 2020, 6, eaaz8360. [Google Scholar] [CrossRef] [PubMed]
- Aragão, L.E.; Anderson, L.O.; Fonseca, M.G.; Rosan, T.M.; Vedovato, L.B.; Wagner, F.H.; Silva, C.V.; Silva Junior, C.H.; Arai, E.; Aguiar, A.P.; et al. 21st century drought-related fires counteract the decline of Amazon deforestation carbon emissions. Nat. Commun. 2018, 9, 536. [Google Scholar] [CrossRef]
- Barlow, J.; Lennox, G.D.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Mac Nally, R.; Thomson, J.R.; Ferraz, S.F.; Louzada, J.; Oliveira, V.H.; et al. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 2016, 535, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Silva Junior, C.H.L.; Aragão, L.E.O.C.; Fonseca, M.G.; Almeida, C.T.; Vedovato, L.B.; Anderson, L.O. Deforestation-induced fragmentation increases forest fire occurrence in central Brazilian Amazonia. Forests 2018, 9, 305. [Google Scholar] [CrossRef]
- Silvério, D.V.; Brando, P.M.; Bustamante, M.M.; Putz, F.E.; Marra, D.M.; Levick, S.R.; Trumbore, S.E. Fire, fragmentation, and windstorms: A recipe for tropical forest degradation. J. Ecol. 2019, 107, 656–667. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C.A. Fire-mediated dieback and compositional cascade in an Amazonian forest. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Ter Steege, H.; Pitman, N.C.; Sabatier, D.; Baraloto, C.; Salomão, R.P.; Guevara, J.E.; Silman, M.R. Hyperdominance in the Amazonian tree flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef] [PubMed]
- Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Plano de Manejo da Reserva Extrativista Tapajós-Arapiuns; ICMBio: Brasília, Brazil, 2014. [Google Scholar]
- Peres, C.A.; Emilio, T.; Schietti, J.; Desmoulière, S.J.; Levi, T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc. Natl. Acad. Sci. USA 2016, 113, 892–897. [Google Scholar] [CrossRef]
- Withey, K.; Berenguer, E.; Palmeira, A.F.; Espírito-Santo, F.D.B.; Lennox, G.D.; Silva, C.V.J.; Aragão, L.E.O.C.; Ferreira, J.; França, F.; Malhi, Y.; et al. Quantifying immediate carbon emissions from El Niño-mediated wildfires in humid tropical forests. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170312. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Jardim, M.A.; Barros, M.F.; Silva, D.S.; Vieira, I.C.; Tabarelli, M. The role of the soil seed bank in the recovery and restoration of a burned Amazonian terra firme forest. Forests 2024, 15, 1513. [Google Scholar] [CrossRef]
- Pereira, C.A.; Barlow, J.; Tabarelli, M.; Giles, A.L.; de Melo Ferreira, A.E.; Vieira, I.C.G. Recurrent wildfires alter forest structure and community composition of terra firme Amazonian forests. Environ. Res. Lett. 2024, 19, 114051. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. American Society for Testing and Materials: Philadelphia, PA, USA, 2013. [CrossRef]
- Batista, A.C. Incêndios Florestais; UFRPE University Press: Recife, Brazil, 1990; p. 115. [Google Scholar]
- Associação Brasileira de Normas Técnicas (ABNT). Análise Imediata de Carvão Vegetal; NBR 8112; ABNT: Rio de Janeiro, Brazil, 1986. [Google Scholar]
- Gaikwad, H.B.; Kumar, A. Flammability of tropical forest litter with and without fire retardant. Fire Saf. J. 2024, 144, 104106. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A review of the effects of forest fire on soil properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Mondal, N.; Sukumar, R. Fire and soil temperatures during controlled burns in seasonally dry tropical forests of southern India. Curr. Sci. 2014, 107, 1590–1594. Available online: https://www.jstor.org/stable/24107221 (accessed on 24 February 2025).
- Bianchini, L.; Colantoni, A.; Venanzi, R.; Cozzolino, L.; Picchio, R. Physicochemical Properties of Forest Wood Biomass for Bioenergy Application: A Review. Forests 2025, 16, 702. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 24 February 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 5 April 2025).
- Scariot, A. Forest fragmentation effects on palm diversity in central Amazonia. J. Ecol. 1999, 87, 66–76. [Google Scholar] [CrossRef]
- Reis, C.R.; Jackson, T.D.; Gorgens, E.B.; Dalagnol, R.; Jucker, T.; Nunes, M.H.; Ometto, J.P.; Aragão, L.E.; Rodríguez, L.C.E.; Coomes, D.A. Forest disturbance and growth processes are reflected in the geographical distribution of large canopy gaps across the Brazilian Amazon. J. Ecol. 2022, 110, 2971–2983. [Google Scholar] [CrossRef]
- Tabarelli, M.; Aguiar, A.V.; Girão, L.C.; Peres, C.A.; Lopes, A.V. Effects of pioneer tree species hyperabundance on forest fragments in northeastern Brazil. Conserv. Biol. 2010, 24, 1654–1663. [Google Scholar] [CrossRef]
- de Souza, A.F. Aspectos da dinâmica de populações da palmeira Attalea humilis Mart. ex Spreng. em fragmentos de Floresta Atlântica sujeitos a fogo. Master’s Thesis, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, Brazil, 2000; p. 187. [Google Scholar]
- Mertzlufft, C.E.; Madden, M.; Gottdenker, N.L.; Velásquez Runk, J.; Saldaña, A.; Tanner, S.; Calzada, J.E.; Yao, X. Landscape disturbance impacts on Attalea butyracea palm distribution in central Panama. Int. J. Health Geogr. 2020, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Giroldo, A.B.; Nascimento, A.R.T.; Silva, P.P.F.; Pinho Júnior, G.V. Population structure and density of Attalea phalerata Mart. ex Spreng. (Arecaceae) in a semideciduous forest. Rev. Árvore 2012, 36, 637–645. [Google Scholar] [CrossRef]
- Hilário, R.R.; Toledo, J.J. Effects of climate and forest structure on palms, bromeliads and bamboos in Atlantic Forest fragments of Northeastern Brazil. Braz. J. Biol. 2016, 76, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Hernández-ruedas, M.A.; Rodríguez, A.V.; Morante-Filho, J.C.; Meave, J.A.; Ramos, M.M. Fragmentation and matrix contrast favor understory plants through negative cascading effects on a strong competitor palm. Ecol. Appl. 2018, 28, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.B.; May, P.H.; Balick, M.J. The Subsidy from Nature: Palm Forests, Peasantry, and Development on an Amazon Frontier; Columbia University Press: New York, NY, USA, 1993. [Google Scholar]
- Lugo, A.E.; Frangi, J.L. Long-Term Response of Caribbean Palm Forests to Hurricanes. Caribb. Nat. 2016, 1, 157–175. [Google Scholar]
- Kahn, F.; de Castro, A. The palm community in a forest of central Amazonia, Brazil. Biotropica 1985, 17, 210–216. [Google Scholar] [CrossRef]
- Balslev, H.; Kahn, F.; Millán, B.; Svenning, J.C.; Kristiansen, T.; Borchsenius, F.; Pedersen, D.; Eiserhardt, W.L. Species diversity and growth forms in tropical American palm communities. Bot. Rev. 2011, 77, 381–425. [Google Scholar] [CrossRef]
- Rosa, D.C.; Brocardo, C.R.; Rosa, C.; Castro, A.B.; Norris, D.; Fadini, R.F. Species-rich but defaunated: The case of medium and large-bodied mammals in a sustainable use protected area in the Amazon. Acta Amaz. 2021, 51, 323–333. [Google Scholar] [CrossRef]
- Williams-Linera, G. Vegetation structure and environmental conditions of forest edges in Panama. J. Ecol. 1990, 78, 356–373. [Google Scholar] [CrossRef]
- Barot, S.; Mitja, D.; Miranda, I.; Meija, G.D.; Grimaldi, M. Reproductive plasticity in an Amazonian palm. Evol. Ecol. Res. 2005, 7, 1051–1065. [Google Scholar]
- Araújo, F.D.C.; Siqueira, K.N.; Neves, S.M.A.S.; Neves, R.J. Influência antrópica na distribuição espacial de babaçu na região Sudoeste Mato-Grossense. Caminhos Geogr. 2016, 17, 41–60. [Google Scholar]
- Andreazzi, C.S.; Pires, A.S.; Fernandez, F.A. Mamíferos e palmeiras neotropicais: Interações em paisagens fragmentadas. Oecol. Bras. 2009, 13, 554–574. [Google Scholar] [CrossRef]
- Anthelme, F.; Lincango, J.; Gully, C.; Duarte, N.; Montúfar, N. How anthropogenic disturbances affect the resilience of a keystone palm tree in the threatened Andean cloud forest. Biol. Conserv. 2011, 144, 1059–1067. [Google Scholar] [CrossRef]
- Ziccardi, L.G.; Graça, P.M.L.A.; Figueiredo, E.O.; Yanai, A.M.; Fearnside, P.M. Community composition of tree and palm species following disturbance in a forest with bamboo in southwestern Amazonia, Brazil. Biotropica 2021, 53, 1328–1341. [Google Scholar] [CrossRef]
- Prieto, P.V.; Sansevero, J.B.B.; Garbin, M.L.; Braga, J.M.A.; Rodrigues, P.J.F.P. Edge effects of linear canopy openings on understorey communities in a lowland Atlantic Tropical Forest. Appl. Veg. Sci. 2014, 17, 121–128. [Google Scholar] [CrossRef]
- Baez, S.; Balslev, H. Edge effects on palm diversity in rain forest fragments in western Ecuador. Biodivers. Conserv. 2007, 16, 2201–2211. [Google Scholar] [CrossRef]
- Hordijk, I.; Meijer, F.; Nissen, E.; Boorsma, T.; Poorter, L. Cattle affect regeneration of the palm species Attalea princeps in a Bolivian forest-savanna mosaic. Biotropica 2019, 51, 28–38. [Google Scholar] [CrossRef]
- De Cassia Quitete Portela, R.; Colmenares-Trejos, S.L.; De Mattos, E.A. Linking plant functional traits to demography in a fragmented landscape. Front. For. Glob. Chang. 2021, 4, 717406. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- De Paula Protásio, T.; Bufalino, L.; Tonoli, G.H.D.; Couto, A.M.; Trugilho, P.F.; Guimarães Júnior, M. Relationship between higher calorific value and the elemental and mineral components of plant biomass. Pesqui. Florest. Bras. 2011, 31, 113. [Google Scholar]
- Trojanová, K.; Veľková, V.; Kačík, F. Volatile Organic Compounds Arising from Wood Polymers on Thermal Loading of Spruce Wood. Polymers 2025, 17, 875. [Google Scholar] [CrossRef] [PubMed]
- Beard, K.H.; Vogt, K.A.; Vogt, D.J.; Scatena, F.N.; Covich, A.P.; Sigurdardottir, R.; Siccama, T.G.; Crowl, T.A. Structural and functional responses of a subtropical forest to 10 years of hurricanes and droughts. Ecol. Monogr. 2005, 75, 345–361. [Google Scholar] [CrossRef]
- Cochrane, M.A.; Laurance, W.F. Synergisms among fire, land use, and climate change in the Amazon. Ambio A J. Environ. Soc. 2008, 37, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Balch, J.K.; Balch, J.K.; Brando, P.M.; Nepstad, D.C.; Coe, M.T.; Silvério, D.; Massad, T.J.; Davidson, E.A.; Lefebvre, P.; Oliveira-Santos, C.; et al. The susceptibility of southeastern Amazon forests to fire: Insights from a large-scale burn experiment. BioScience 2015, 65, 893–905. [Google Scholar] [CrossRef]
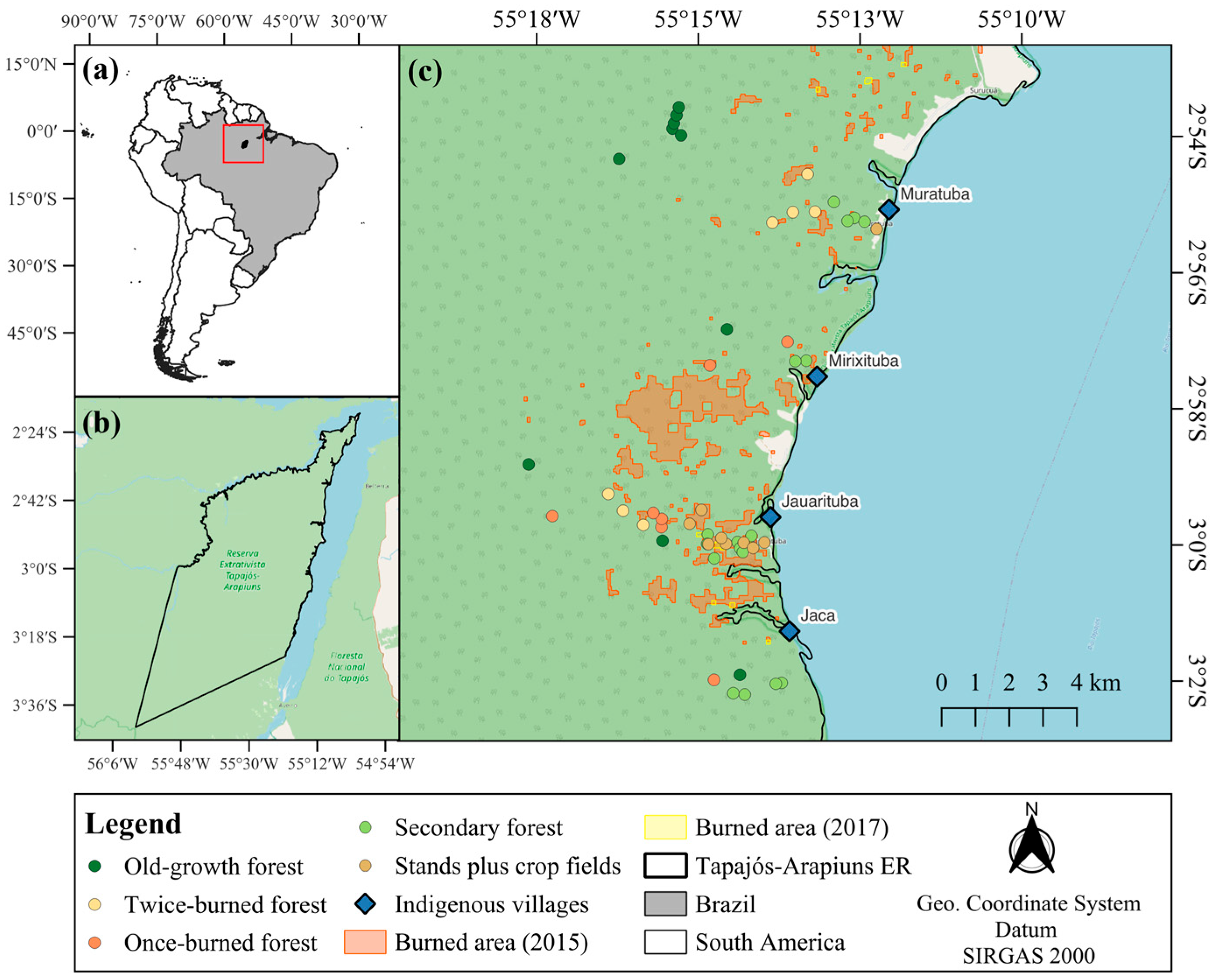
| Species | OGF | BF | SF | CF |
|---|---|---|---|---|
| Astrocaryum aculeatum G.Mey. | 2 | 4 | 1 | 6 |
| Astrocaryum gynacanthum Mart. | 0 | 0 | 21 | 112 |
| Astrocaryum vulgare Mart. | 0 | 0 | 0 | 51 |
| Attalea maripa (Aubl.) Mart. | 39 | 32 | 12 | 34 |
| Attalea spectabilis Mart. | 43 | 518 | 358 | 873 |
| Bactris coccinea Barb. Rodr. | 0 | 0 | 3 | 18 |
| Desmoncus orthacanthos Mart. | 0 | 5 | 0 | 0 |
| Oenocarpus bacaba Mart. | 16 | 13 | 0 | 0 |
| Oenocarpus distichus Mart. | 0 | 3 | 3 | 0 |
| Syagrus cocoides Mart. | 0 | 0 | 1 | 11 |
| Total | 100 | 575 | 399 | 1105 |
| OGF | BF | SF | CF | X2 | df | p Value | p-Value Between Habitats | |
|---|---|---|---|---|---|---|---|---|
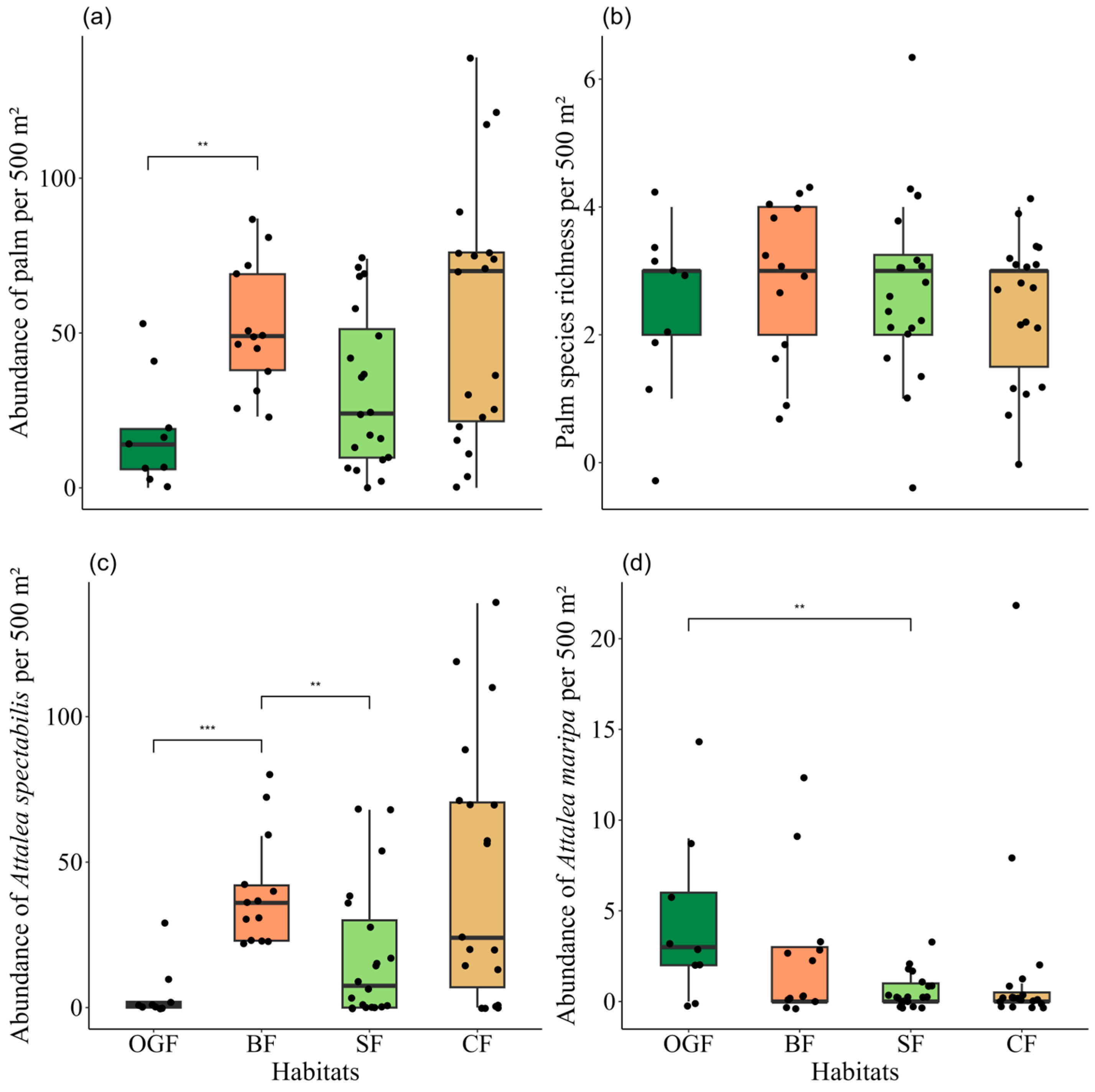
| Abundance of palm | 16.6 ± 18.0 | 51.3 ± 20.4 | 31.5 ± 25.5 | 56.4 ± 42.0 | 12.78 | 3 | <0.01 | OGF x BF = 0.01 |
| Palm species richness | 2.3 ± 1.2 | 2.9 ± 1.1 | 2.7 ± 1.3 | 2.4 ± 1.1 | 2.25 | 3 | 0.52 | - |
| Abundance of Attalea spectabilis | 4.8 ± 9.6 | 39.8 ± 19.1 | 17.9 ± 23.1 | 45.9 ± 44.9 | 15.86 | 3 | <0.01 | BF x OGF = < 0.01 BF x SF = 0.02 |
| Abundance of Attalea maripa | 4.3 ± 4.6 | 2.5 ± 3.8 | 0.6 ± 0.9 | 1.8 ± 5.2 | 10.56 | 3 | 0.01 | CF x OGF = > 0.05 SF x OGF = 0.02 |
| OGF | Curuá | OBF | TBF | SF | F | X2 | df | p Value | p-Value Between Habitats | |
|---|---|---|---|---|---|---|---|---|---|---|
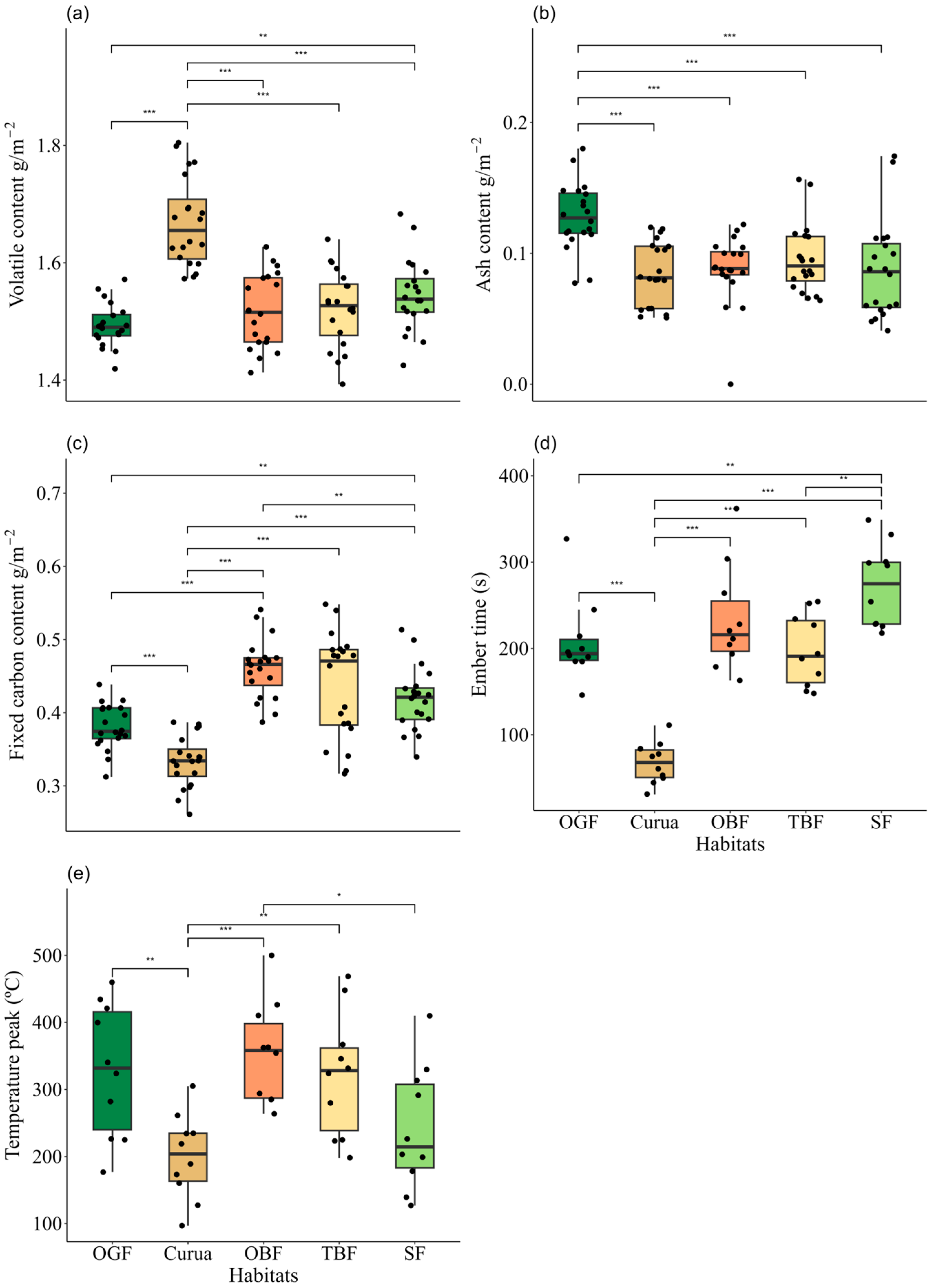
| Volatile content | 1.49 ± 0.04 | 1.67 ± 0.08 | 1.52 ± 0.06 | 1.52 ± 0.07 | 1.54 ± 0.06 | - | 44.76 | 4 | <0.01 | Curua x OGF < 0.01 Curua x OBF < 0.01 Curua x TBF < 0.01 Curua x SF < 0.01 SF x OGF = 0.03 |
| Ash content | 0.13 ± 0.03 | 0.08 ± 0.02 | 0.09 ± 0.03 | 0.10 ± 0.03 | 0.09 ± 0.04 | 8.01 | - | 4 | <0.01 | Curua x SF < 0.01 OGF x OBF < 0.01 SF x OGF < 0.01 OGF x TBF = < 0.01 |
| Fixed carbon content | 0.38 ± 0.03 | 0.33 ± 0.04 | 0.46 ± 0.03 | 0.44 ± 0.07 | 0.42 ± 0.04 | - | 50.13 | 4 | <0.01 | Curua x OBF < 0.01 Curua x OGF = < 0.01 Curua x SF < 0.01 Curua x TBF < 0.01 OGF x OBF < 0.01 SF x OGF = 0.03 |
| Ember time | 208 ± 48.6 | 67.7 ± 24.01 | 233.1 ± 60.93 | 197.5 ± 41.52 | 273.1 ± 48.01 | 28 | - | 4 | <0.01 | Curua x OBF < 0.01 Curua x OGF < 0.01 Curua x SF < 0.01 Curua x TBF < 0.01 SF x OGF = 0.02 SF x TBF = < 0.01 |
| Temperature peak | 328.9 ± 99.21 | 200 ± 63.14 | 354.3 ± 75.28 | 321.2 ± 92.22 | 241.6 ± 91.27 | 5.87 | - | 4 | <0.01 | Curua x OBF = < 0.01 Curua x OGF = 0.01 Curua x TBF = 0.02 SF x OBF = 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, T.T.A.; Oliveira, V.B.; Barros, M.F.; Andrade, F.W.C.; Tabarelli, M.; Vieira, I.C.G. Wildfires and Palm Species Response in a Terra Firme Amazonian Social Forest. Forests 2025, 16, 1271. https://doi.org/10.3390/f16081271
Costa TTA, Oliveira VB, Barros MF, Andrade FWC, Tabarelli M, Vieira ICG. Wildfires and Palm Species Response in a Terra Firme Amazonian Social Forest. Forests. 2025; 16(8):1271. https://doi.org/10.3390/f16081271
Chicago/Turabian StyleCosta, Tinayra T. A., Vynicius B. Oliveira, Maria Fabíola Barros, Fernando W. C. Andrade, Marcelo Tabarelli, and Ima C. G. Vieira. 2025. "Wildfires and Palm Species Response in a Terra Firme Amazonian Social Forest" Forests 16, no. 8: 1271. https://doi.org/10.3390/f16081271
APA StyleCosta, T. T. A., Oliveira, V. B., Barros, M. F., Andrade, F. W. C., Tabarelli, M., & Vieira, I. C. G. (2025). Wildfires and Palm Species Response in a Terra Firme Amazonian Social Forest. Forests, 16(8), 1271. https://doi.org/10.3390/f16081271








