Non-Structural Carbohydrate Concentration Increases and Relative Growth Decreases with Tree Size in the Long-Lived Agathis australis (D.Don) Lindl.
Abstract
1. Introduction
2. Methods
2.1. Study Site and Sampling
2.2. Chemical Determination of NSC Content
2.3. Data Analysis
3. Results
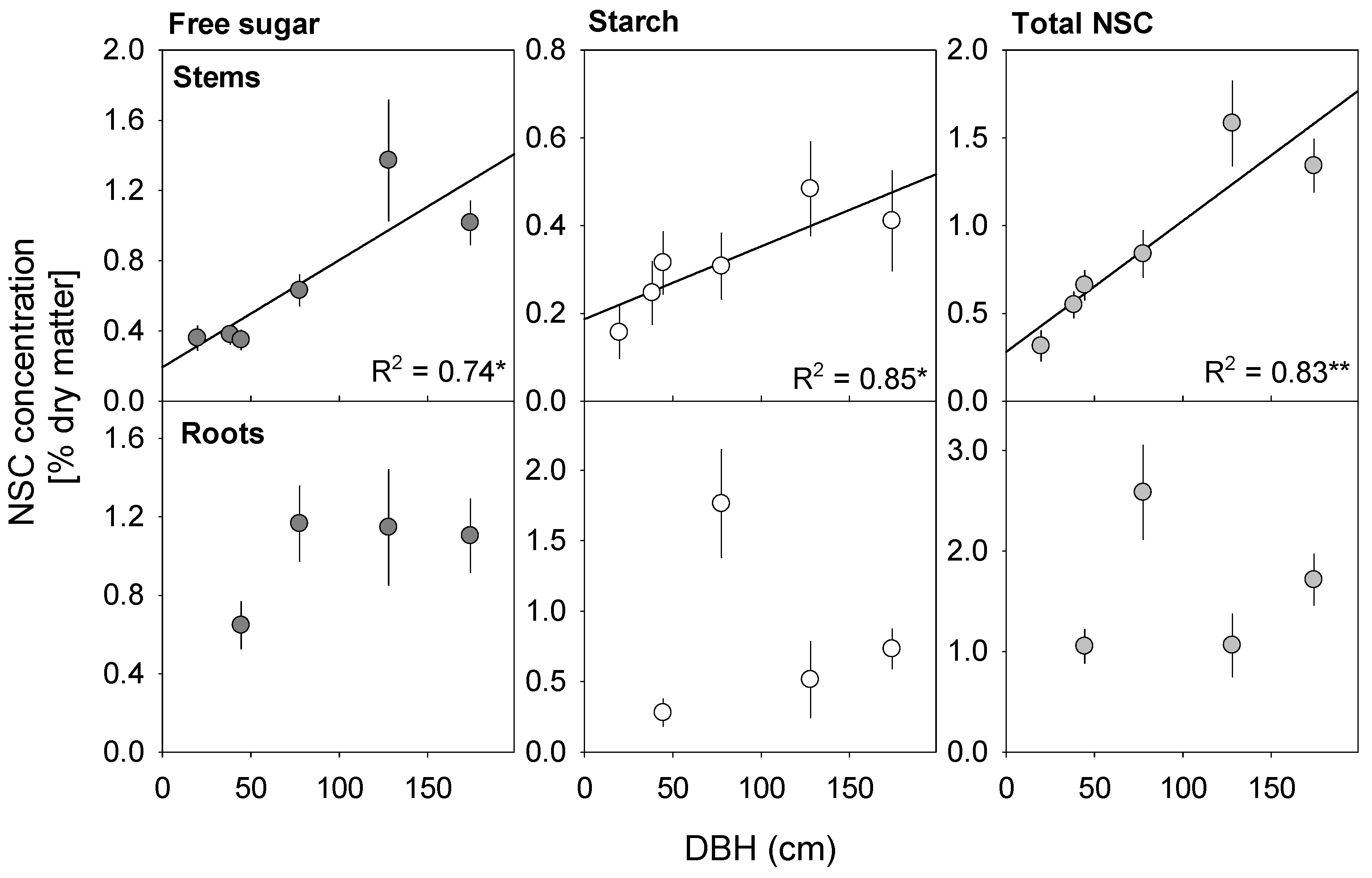
3.1. NSC Concentrations Vary with Tree Size
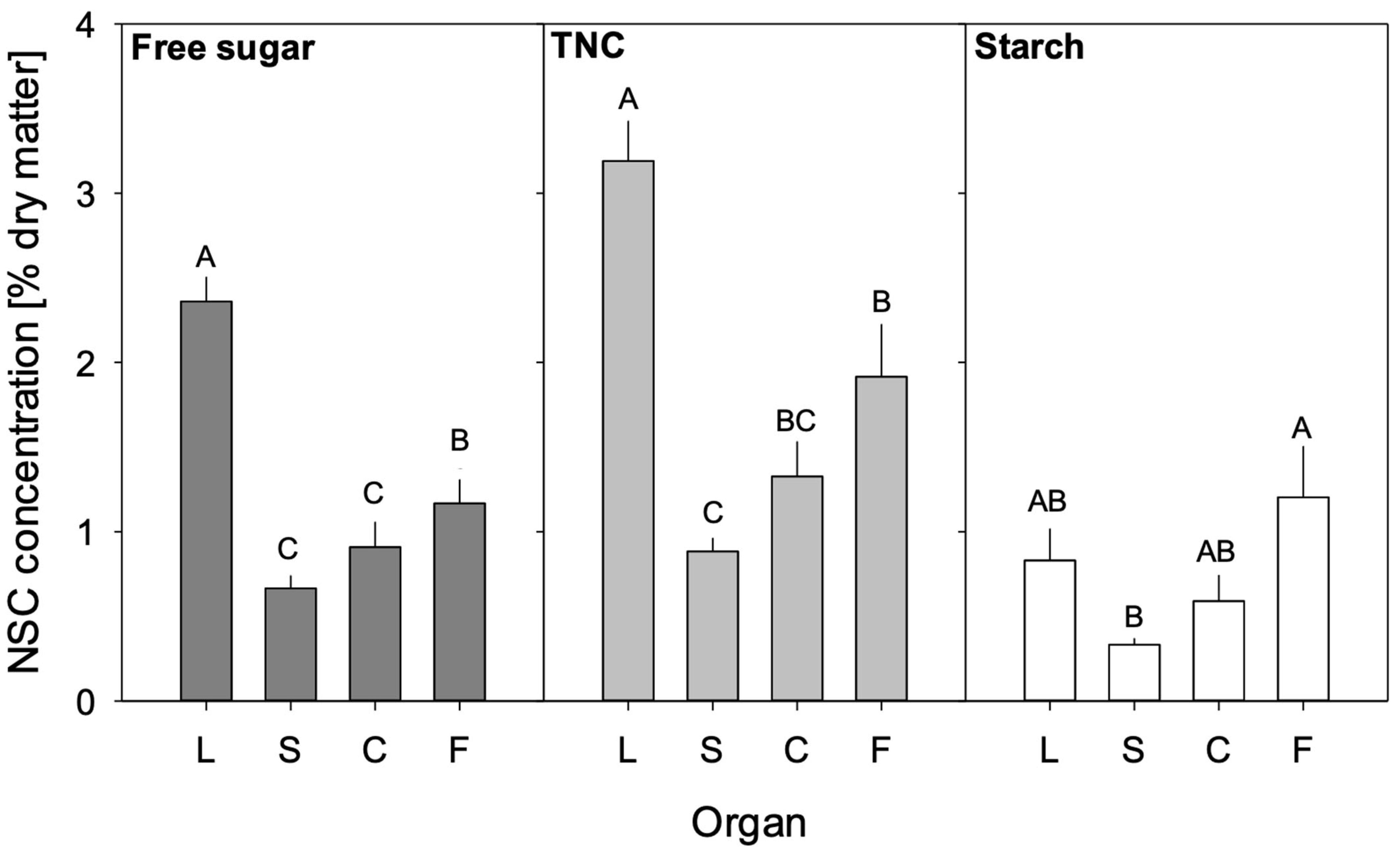
3.2. NSC Concentrations of Different Tree Organs
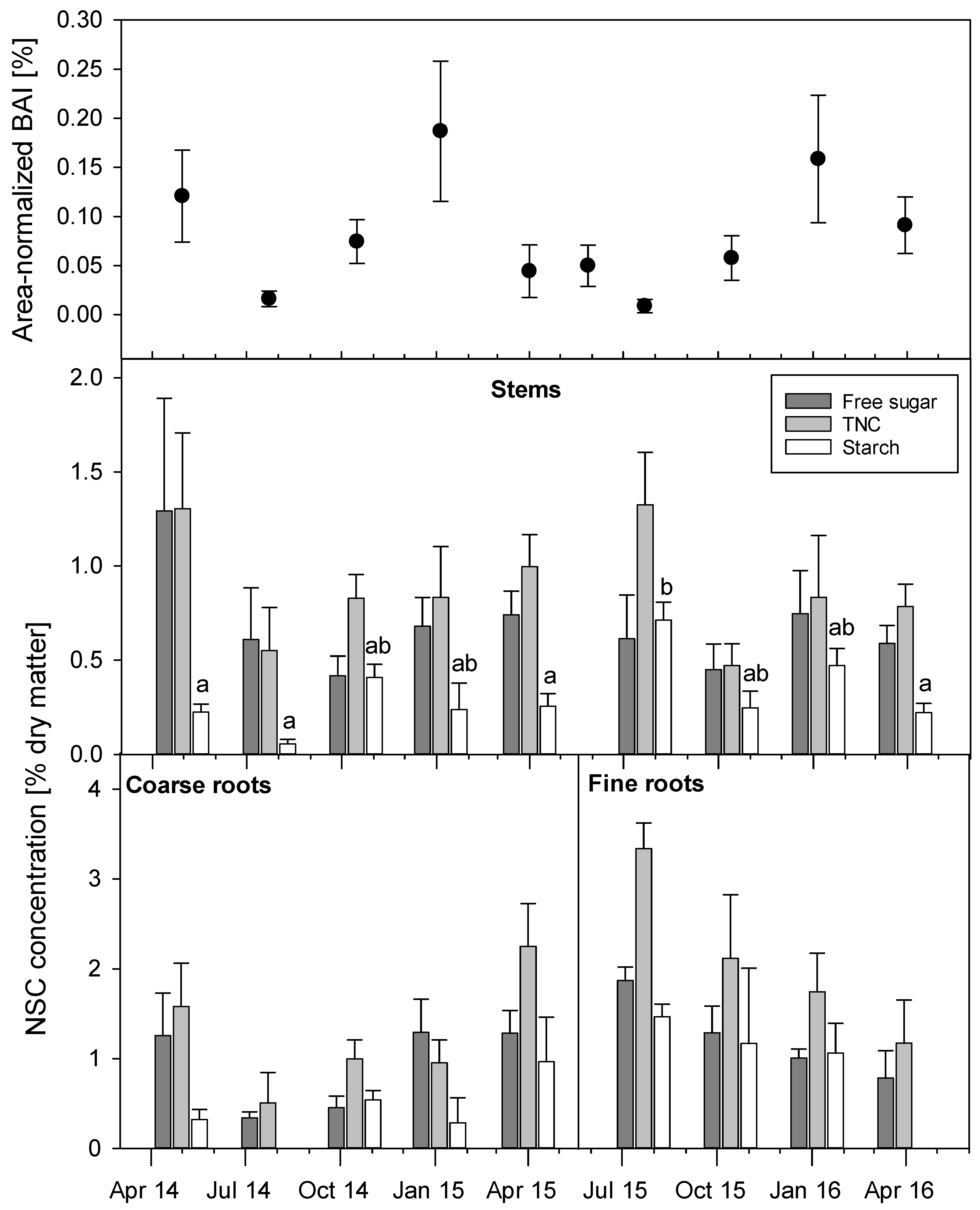
3.3. Seasonal Variations of NSC Concentrations and Growth
4. Discussion
4.1. Methodological Challenges
4.2. NSC Concentrations in Tissues
4.3. Seasonal NSC Dynamics
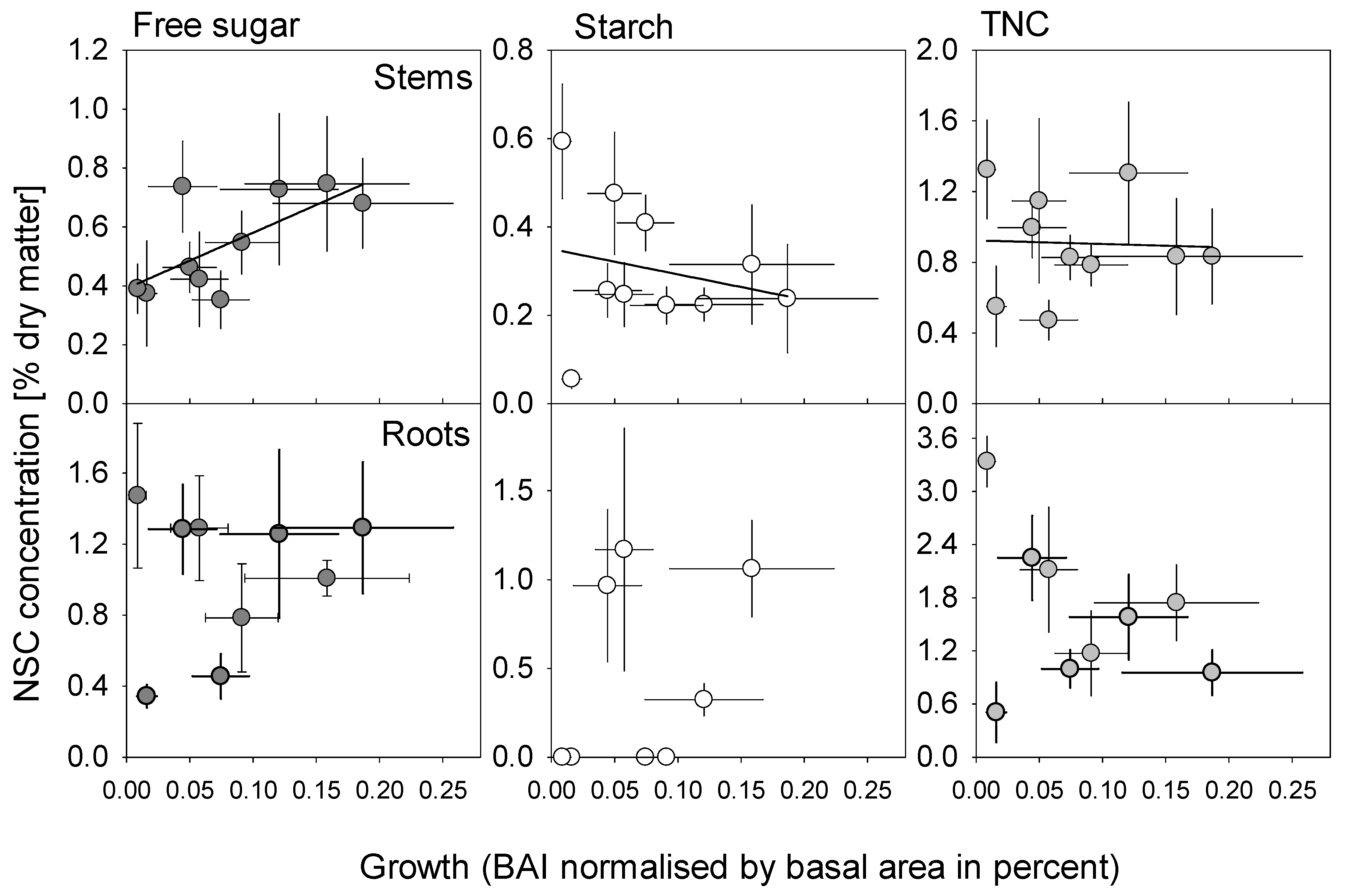
4.4. Relationships Between Tree Size, NSC Concentration and Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Ann. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. The carbon charging of pines at the climatic treeline: A global comparison. Oecologia 2003, 135, 10–21. [Google Scholar] [CrossRef]
- Carbone, M.S.; Czimczik, C.I.; Keenan, T.F.; Murakami, P.F.; Pederson, N.; Schaberg, P.G.; Xu, X.; Richardson, A.D. Age, allocation and availability of nonstructural carbon in mature red maple trees. New Phyolt. 2013, 200, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Piispanen, R.; Saranpää, P. Variation of non-structural carbohydrates in silver birch (Betula pendula Roth) wood. Trees 2001, 15, 444–451. [Google Scholar] [CrossRef]
- Sala, A.; Hoch, G. Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant Cell Environ. 2009, 32, 22–30. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. Global patterns of mobile carbon stores in trees at the high-elevation tree line. Glob. Ecol. Biogeogr. 2012, 21, 861–871. [Google Scholar] [CrossRef]
- Wang, X.; Hu, G.; Zhang, Q.; Quan, X.; Zhang, H.; Aubrey, D.P.; Wang, C. Annual Tree Biomass Increment Is Positively Related to Nonstructural Carbohydrate Pool Size and Depletion: Evidence for Carbon Limitation? Forests 2025, 16, 619. [Google Scholar] [CrossRef]
- Friend, A.D.; Eckes-Shephard, A.H.; Fonti, P.; Rademacher, T.T.; Rathgeber, C.B.K.; Richardson, A.D.; Turton, R.H. On the need to consider wood formation processes in global vegetation models and a suggested approach. Ann. For. Sci. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Hartmann, H.; Bahn, M.; Carbone, M.; Richardson, A.D. Plant carbon allocation in a changing world—Challenges and progress: Introduction to a Virtual Issue on carbon allocation: Introduction to a virtual issue on carbon allocation. New Phytol. 2020, 227, 981–988. [Google Scholar] [CrossRef]
- Fatichi, S.; Leuzinger, S.; Körner, C. Moving beyond photosynthesis: From carbon source to sink-driven vegetation modeling. New Phytol. 2014, 201, 1086–1095. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef] [PubMed]
- El Omari, B. Accumulation versus storage of total non-structural carbohydrates in woody plants. Trees 2022, 36, 869–881. [Google Scholar] [CrossRef]
- Gessler, A.; Zweifel, R. 2024. Beyond source and sink control–toward an integrated approach to understand the carbon balance in plants. New Phytol. 2024, 242, 858–869. [Google Scholar] [CrossRef]
- Begović, K.; Schurman, J.S.; Svitok, M.; Pavlin, J.; Langbehn, T.; Svobodová, K.; Mikoláš, M.; Janda, P.; Synek, M.; Marchand, W.; et al. Large old trees increase growth under shifting climatic constraints: Aligning tree longevity and individual growth dynamics in primary mountain spruce forests. Glob. Change Biol. 2023, 29, 143–164. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Adams, H.D. Getting to the root of carbon reserve dynamics in woody plants: Progress, challenges, and goals. Tree Physiol. 2024, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef]
- Stefaniak, E.Z.; Tissue, D.T.; Dewar, R.C.; Medlyn, B.E. Optimal carbon storage during drought. Tree Physiol. 2024, 44, 34–45. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Change 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Granda, E.; Camarero, J.J. Drought reduces growth and stimulates sugar accumulation: New evidence of environmentally driven non-structural carbohydrate use. Tree Physiol. 2017, 37, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Fajardo, A.; Hoch, G. Single-provenance mature conifers show higher non-structural carbohydrate storage and reduced growth in a drier location. Tree Physiol. 2017, 37, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef]
- Hoch, G.; Popp, M.; Körner, C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 2002, 98, 361–374. [Google Scholar] [CrossRef]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- McDowell, N.G.; Fisher, R.A.; Xu, C.; Domec, J.C.; Hölttä, T.; Mackay, D.S.; Sperry, J.S.; Boutz, A.; Dickman, L.; Gehres, N.; et al. Evaluating theories of drought-induced vegetation mortality using a multimodel–experiment framework. New Phytol. 2013, 200, 304–321. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Wiley, E.; Helliker, B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol. 2012, 195, 285–289. [Google Scholar] [CrossRef]
- Schippers, P.; Vlam, M.; Zuidema, P.A.; Sterck, F. Sapwood allocation in tropical trees: A test of hypotheses. Funct. Plant Biol. 2015, 42, 697–709. [Google Scholar] [CrossRef]
- Silpi, U.; Lacointe, A.; Kasempsap, P.; Thanysawanyangkura, S.; Chantuma, P.; Gohet, E.; Musigamart, N.; Clément, A.; Améglio, T.; Thaler, P. Carbohydrate reserves as a competing sink: Evidence from tapping rubber trees. Tree Physiol. 2007, 27, 881–889. [Google Scholar] [CrossRef]
- Hartmann, H.; Adams, H.D.; Hammond, W.M.; Hoch, G.; Landhäusser, S.M.; Wiley, E.; Zaehle, S. Identifying differences in carbohydrate dynamics of seedlings and mature trees to improve carbon allocation in models for trees and forests. Environ. Exper. Bot. 2018, 152, 7–18. [Google Scholar] [CrossRef]
- Oswald, S.W.; Aubrey, D.P. Modeling starch dynamics from seasonal variations of photosynthesis, growth and respiration. Tree Physiol. 2024, 44, 11–27. [Google Scholar] [CrossRef]
- Blumstein, M.; Sala, A.; Weston, D.J.; Holbrook, N.M.; Hopkins, R. Plant carbohydrate storage: Intra-and inter-specific trade-offs reveal a major life history trait. New Phytol. 2022, 235, 2211–2222. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Steward, G.A.; Beveridge, A.E. A review of New Zealand kauri (Agathis australis (D. Don) Lindl.): Its ecology, history, growth and potential for management for timber. N. Z. J. For. Sci. 2010, 40, 33–59. [Google Scholar]
- Enright, N.J.; Ogden, J. The southern conifers—A synthesis. In Ecology of the Southern Conifers; Enright, N.J., Hill, R.S., Eds.; Melbourne University Press: Melbourne, Australia, 1995; pp. 271–287. [Google Scholar]
- Macinnis-Ng, C.; Wyse, S.; Veale, A.; Schwendenmann, L.; Clearwater, M. Sap flow of the southern conifer, Agathis australis during wet and dry summers. Trees 2016, 30, 19–33. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.; Webb, T.; Lin, Y.-S.; Schwendenmann, L.; Medlyn, B. Leaf age-related and diurnal variation in gas exchange of kauri (Agathis australis). N. Z. J. Bot. 2017, 55, 80–99. [Google Scholar] [CrossRef]
- Pittermann, J.; Sperry, J.S.; Hacke, U.G.; Wheeler, J.K.; Sikkema, E.H. Inter-tracheid pitting and the hydraulic efficiency of conifer wood: The role of tracheid allometry and cavitation protection. Am. J. Bot. 2006, 93, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castillo, M.; Fajardo, A.; Lobos-Catalán, P.; Torres-Morales, P.; Piper, F.I. No carbon shortage in declining trees of the isohydric species Araucaria araucana (Molina) K. Koch under drought. Ann. For. Sci. 2022, 79, 10. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.; Schwendenmann, L. Litterfall, carbon and nitrogen cycling in a southern hemisphere conifer forest dominated by kauri (Agathis australis) during drought. Plant Ecol. 2015, 216, 247–262. [Google Scholar] [CrossRef]
- Thomas, G.; Ogden, J. The scientific reserves of Auckland University. I. General introduction to their history, vegetation, climate and soils. Tane 1983, 29, 143–162. [Google Scholar]
- Wunder, J.; Perry, G.L.W.; McCloskey, S.P.J. Structure and Composition of a Mature Kauri (Agathis australis) Stand at Huapai Scientific Reserve, Waitakere Range; Tree-Ring Laboratory Report; School of Environment, The University of Auckland: Auckland, New Zealand, 2010. [Google Scholar]
- Meinzer, F.; Goldstein, G.; Andrade, J. Regulation of water flux through tropical forest canopy trees: Do universal rules apply? Tree Physiol. 2001, 21, 19–26. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.; Schwendenmann, L.; Clearwater, M.J. Radial variation of sap flow of kauri (Agathis australis) during wet and dry summers. Acta Hortic. 2013, 991, 205–213. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A New Tool for Sampling Microcores from tree stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Beever, R.; Waipara, N.; Ramsfield, T.; Dick, M.; Horner, I. Kauri (Agathis australis) under threat from Phytophthora. Phytophthoras For. Nat. Ecosyst. 2009, 74, 74–85. [Google Scholar]
- Forest, F.; Moat, J.; Baloch, E.; Brummitt, N.; Bachman, S.; Ickert-Bond, S.; Hollingsworth, P.; Liston, A.; Little, D.P.; Mathews, S.; et al. Gymnosperms on the EDGE. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Popp, M.; Lied, W.; Meyer, A.J.; Richter, A.; Schiller, P.; Schwitte, H. Sample preservation for determination of organic compounds: Microwave versus freeze-drying. J. Exp. Bot. 1996, 47, 1469–1473. [Google Scholar] [CrossRef]
- Wong, S.-C. Elevated atmospheric partial pressure of CO2 and plant growth: II. Non-structural carbohydrate content in cotton plants and its effect on growth parameters. Photosynth. Res. 1990, 23, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Bachofen, C.; Moser, B.; Hoch, G.; Ghazoul, J.; Wohlgemuth, T. No carbon “bet hedging” in pine seedlings under prolonged summer drought and elevated CO2. J. Ecol. 2018, 106, 31–46. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.; Wyse, S.V.; Webb, T.; Taylor, D.; Schwendenmann, L. Sustained carbon uptake in a mixed age southern conifer forest. Trees 2017, 31, 967–980. [Google Scholar] [CrossRef]
- Aubrey, D.P.; Teskey, R.O. Stored root carbohydrates can maintain root respiration for extended periods. New Phytol. 2018, 218, 142–152. [Google Scholar] [CrossRef]
- Fajardo, A.; Piper, F.I.; Hoch, G. Similar variation in carbon storage between deciduous and evergreen treeline species across elevational gradients. Ann. Bot. 2013, 112, 623–631. [Google Scholar] [CrossRef]
- Hoch, G.; Richter, A.; Körner, C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Klein, T.; Hoch, G.; Yakir, D.; Körner, C. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol. 2014, 34, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef]
- Newell, E.; Mulkey, S.; Wright, J. Seasonal patterns of carbohydrate storage in four tropical tree species. Oecologia 2002, 131, 333–342. [Google Scholar] [CrossRef]
- Quentin, A.G.; Pinkard, E.A.; Ryan, M.G.; Tissue, D.T.; Baggett, L.S.; Adams, H.D.; Maillard, P.; Marchand, J.; Landhäusser, S.M.; Lacointe, A.; et al. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiol. 2015, 35, 1146–1165. [Google Scholar] [CrossRef]
- Hendrix, D.L.; Peelen, K.K. Artifacts in the Analysis of Plant Tissues for Soluble Carbohydrates. Crop Sci. 1987, 27, 710–715. [Google Scholar] [CrossRef]
- Gruber, A.; Pirkebner, D.; Florian, C.; Oberhuber, W. No evidence for depletion of carbohydrate pools in Scots pine (Pinus sylvestris L.) under drought stress. Plant Biol. 2012, 14, 142–148. [Google Scholar] [CrossRef]
- Würth, M.K.; Pelaez-Riedl, S.; Wright, S.J.; Körner, C. Non-structural carbohydrate pools in a tropical forest. Oecologia 2005, 143, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Barbaroux, C.; Bréda, N.; Dufrêne, E. Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol. 2003, 157, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Burd, A. Seasonal changes in above- and below-ground non-structural carbohydrates (NSC) in Spartina alterniflora in a marsh in Georgia, USA. Aquat. Bot. 2017, 140, 13–22. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Novick, K.A.; Phillips, R.P. Coarse roots prevent declines in whole-tree non-structural carbohydrate pools during drought in an isohydric and an anisohydric species. Tree Physiol. 2018, 38, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Vitasse, Y.; Hoch, G. Coordination between growth, phenology and carbon storage in three coexisting deciduous tree species in a temperate forest. Tree Physiol. 2016, 36, 847–855. [Google Scholar] [CrossRef]
- Smith, M.G.; Miller, R.E.; Arndt, S.K.; Kasel, S.; Bennett, L.T. Whole-tree distribution and temporal variation of non-structural carbohydrates in broadleaf evergreen trees. Tree Physiol. 2018, 38, 570–581. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Meinzer, F.C. Water stress, shoot growth and storage of non-structural carbohydrates along a tree height gradient in a tall conifer. Plant Cell Environ. 2011, 34, 1920–1930. [Google Scholar] [CrossRef]
- Chapin III, F.S.; Schulze, E.-D.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Palacio, S.; Camarero, J.J.; Maestro, M.; Alla, A.Q.; Lahoz, E.; Montserrat-Martí, G. Are storage and tree growth related? Seasonal nutrient and carbohydrate dynamics in evergreen and deciduous Mediterranean oaks. Trees 2018, 32, 777–790. [Google Scholar] [CrossRef]
- Guo, J.S.; Gear, L.; Hultine, K.R.; Koch, G.W.; Ogle, K. Non-structural carbohydrate dynamics associated with antecedent stem water potential and air temperature in a dominant desert shrub. Plant Cell Environ. 2020, 43, 1467–1483. [Google Scholar] [CrossRef]
- Ogden, J.; Ahmed, M. Climate response function analyses of kauri (Agathis australis) tree-ring chronologies in northern New Zealand. J. R. Soc. N. Z. 1989, 19, 205–221. [Google Scholar] [CrossRef]
- Deslauriers, A.; Giovannelli, A.; Rossi, S.; Castro, G.; Fragnelli, G.; Traversi, L. Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiol. 2009, 29, 1223–1235. [Google Scholar] [CrossRef]
- Kaplick, J.; Clearwater, M.J.; Macinnis-Ng, C. Comparative water relations of co-occurring trees in a mixed podocarp-broadleaf forest. J. Plant Ecol. 2018, 12, 163–175. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, P.; Zhu, L.; Wang, Q.; Hu, Y.; Cranston, B.M.; Kaplick, J.; Lei, O.; Chen, X.; Ni, G.; et al. Exploring the influence of biological traits and environmental drivers on water use variations across contrasting forests. Forests 2021, 12, 161. [Google Scholar] [CrossRef]
- Muhr, J.; Angert, A.; Negrón-Juárez, R.I.; Muñoz, W.A.; Kraemer, G.; Chambers, J.Q.; Trumbore, S.E. Carbon dioxide emitted from live stems of tropical trees is several years old. Tree Physiol. 2013, 33, 743–752. [Google Scholar] [CrossRef]
- Muhr, J.; Trumbore, S.; Higuchi, N.; Kunert, N. Living on borrowed time–Amazonian trees use decade-old storage carbon to survive for months after complete stem girdling. New Phytol. 2018, 220, 111–120. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Huggett, B.A.; Furze, M.E.; Czimczik, C.I.; Walker, J.C.; Xu, X.; Schaberg, P.G.; Murakami, P. Distribution and mixing of old and new nonstructural carbon in two temperate trees. New Phytol. 2015, 206, 590–597. [Google Scholar] [CrossRef]
- Furze, M.E.; Huggett, B.A.; Aubrecht, D.M.; Stolz, C.D.; Carbone, M.S.; Richardson, A.D. Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol. 2019, 221, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.; Drake, B.; Hungate, B. Extensive belowground carbon storage supports roots and mycorrhizae in regenerating scrub oaks. Oecologia 2002, 131, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Buscot, F. Why and How Using Micropropagated Trees rather than Germinations for Controlled Synthesis of Ectomycorrhizal Associations? In Mycorrhiza: State of the Art, Genetics and Molecular Biology, Eco-Function, Biotechnology, Eco-Physiology, Structure and Systematics; Varma, A., Ed.; Springer: Berlin, Germany, 2008; pp. 439–465. [Google Scholar]
- Bieleski, R. Factors affecting growth and distribution of kauri (Agathis australis Salisb.) III. Effect of temperature and soil conditions. Aust. J. Bot. 1959, 7, 279–294. [Google Scholar] [CrossRef]
- Wyse, S.V.; Macinnis-Ng, C.M.O.; Burns, B.R.; Clearwater, M.J.; Schwendenmann, L. Species assemblage patterns around a dominant emergent tree are associated with drought resistance. Tree Physiol. 2013, 33, 1269–1283. [Google Scholar] [CrossRef]
- Padamsee, M.; Johansen, R.B.; Stuckey, S.A.; Williams, S.E.; Hooker, J.E.; Burns, B.R.; Bellgard, S.E. The arbuscular mycorrhizal fungi colonising roots and root nodules of New Zealand kauri Agathis australis. Fungal Biol. 2016, 120, 807–817. [Google Scholar] [CrossRef]
- Silvester, W.B. The biology of kauri (Agathis australis) in New Zealand II. Nitrogen cycling in four kauri forest remnants. N. Z. J. Bot. 2000, 38, 205–220. [Google Scholar] [CrossRef]
- Ryan, M.G.; Yoder, B.J. Hydraulic limits to tree height and tree growth. BioScience 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Genet, H.; Bréda, N.; Dufrêne, E. Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiol. 2010, 30, 177–192. [Google Scholar] [CrossRef]
- Sillett, S.C.; Van Pelt, R.; Koch, G.W.; Ambrose, A.R.; Carroll, A.L.; Antoine, M.E.; Mifsud, B.M. Increasing wood production through old age in tall trees. For. Ecol. Manag. 2010, 259, 976–994. [Google Scholar] [CrossRef]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Coomes, D.A.; Lines, E.R.; Morris, W.K.; Rüger, N.; et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 507, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.R.; Bond, B.J.; Meinzer, F.C. Does turgor limit growth in tall trees? Plant Cell Environ. 2004, 27, 229–236. [Google Scholar] [CrossRef]
- Cadwallader BG (2011) The New Zealand Tree Register. Available online: https://register.notabletrees.org.nz/tree/view/800 (accessed on 19 October 2022).
- Kaplick, J.; Clearwater, M.J.; Macinnis-Ng, C. Stem water storage of New Zealand kauri (Agathis australis). Acta Hortic. 2017, 1222, 59–66. [Google Scholar] [CrossRef]
- Cranston, B.M.; Macinnis-Ng, C. Saving some for later: Seasonal variations in sap flow, withdrawal and elastic storage in large trees under throughfall exclusion. Acta Hortic. 2023, 1419, 143–152. [Google Scholar] [CrossRef]
- Zhao, X.; Cranston, B.M.; Macinnis-Ng, C. Water relations of the southern conifer Agathis australis in a throughfall exclusion experiment. Acta Hortic. 2019, 1300, 73–80. [Google Scholar] [CrossRef]
- Cranston, B.M.; Powers, B.F.; Macinnis-Ng, C. Inexpensive throughfall exclusion experiment for single large trees. Appl. Plant Sci. 2020, 8, e11325. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Schwendener, A.; Schmid, S.; Lambert, S.; Wiley, E.; Landhäusser, S.; Hartmann, H.; Hoch, G. Living on next to nothing: Tree seedlings can survive weeks with very low carbohydrate concentrations. New Phytol. 2018, 218, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The possible role of non-structural carbohydrates in the regulation of tree hydraulics. Int. J. Mol. Sci. 2020, 21, 144. [Google Scholar] [CrossRef]


| Tree Species | ID | DBH in cm | Canopy Position | Leaf NSC | Stem Tissue NSC | Root Tissue NSC |
|---|---|---|---|---|---|---|
| Agathis australis | 1 (B079) | 19.8 | Intermediate | x | ||
| 2 (A046) | 38.4 | Co-dominant | x | |||
| 3 (A109) | 44.5 | Co-dominant | x | x | ||
| 4 (A050) | 77.6 | Dominant | x | x | x | |
| 5 (C022) | 128.0 | Dominant | x | x | ||
| 6 (D024) | 174.2 | Dominant | x | x | x |
| Effect | Response Variable | df | F Ratio | p Value | df | F Ratio | p Value | df | F Ratio | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue type | TNC concentration | 3 | 32.88 | <0.001 *** | ||||||
| Free sugar concentration | 3 | 31.66 | <0.001 *** | |||||||
| Starch concentration | 3 | 8.891 | <0.001 *** | |||||||
| Stem tissue | Larger roots (~5 cm diameter) | Smaller roots (1–3 cm diameter) | ||||||||
| Date | TNC concentration | 9 | 1.377 | 0.23 | 4 | 3.148 | 0.06 | 3 | 7.955 | 0.19 |
| Free sugar concentration | 9 | 0.966 | 0.48 | 4 | 3.163 | 0.048 (*) | 4 | 2.419 | 0.13 | |
| Starch concentration | 9 | 4.700 | <0.001 *** | 4 | 0.668 | 0.63 | 2 | 0.103 | 0.90 | |
| Tree | TNC concentration | 5 | 11.77 | <0.001 *** | 3 | 1.179 | 0.36 | 3 | 4.793 | 0.03 * |
| Free sugar concentration | 5 | 8.073 | <0.001 *** | 3 | 0.867 | 0.48 | 3 | 0.757 | 0.55 | |
| Starch concentration | 5 | 1.214 | 0.323 | 3 | 3.328 | 0.07 | 2 | 3.853 | 0.10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplick, J.; Cranston, B.M.; Macinnis-Ng, C. Non-Structural Carbohydrate Concentration Increases and Relative Growth Decreases with Tree Size in the Long-Lived Agathis australis (D.Don) Lindl. Forests 2025, 16, 1270. https://doi.org/10.3390/f16081270
Kaplick J, Cranston BM, Macinnis-Ng C. Non-Structural Carbohydrate Concentration Increases and Relative Growth Decreases with Tree Size in the Long-Lived Agathis australis (D.Don) Lindl. Forests. 2025; 16(8):1270. https://doi.org/10.3390/f16081270
Chicago/Turabian StyleKaplick, Julia, Benjamin M. Cranston, and Cate Macinnis-Ng. 2025. "Non-Structural Carbohydrate Concentration Increases and Relative Growth Decreases with Tree Size in the Long-Lived Agathis australis (D.Don) Lindl." Forests 16, no. 8: 1270. https://doi.org/10.3390/f16081270
APA StyleKaplick, J., Cranston, B. M., & Macinnis-Ng, C. (2025). Non-Structural Carbohydrate Concentration Increases and Relative Growth Decreases with Tree Size in the Long-Lived Agathis australis (D.Don) Lindl. Forests, 16(8), 1270. https://doi.org/10.3390/f16081270







