Spatial Distribution of Mangrove Forest Carbon Stocks in Marismas Nacionales, Mexico: Contributions to Climate Change Adaptation and Mitigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection: Field and Laboratory Protocols
2.2.1. Aboveground Carbon Assessment
2.2.2. Belowground Carbon Assessment
- Rb = root biomass; ρ = wood density; DBH = Diameter at Breast Height (cm).
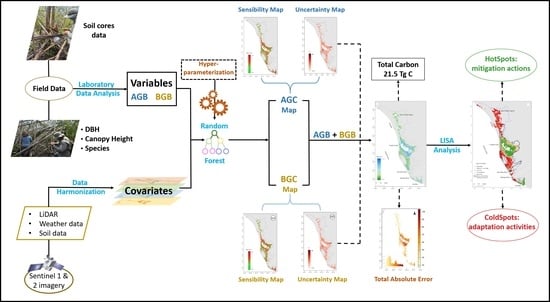
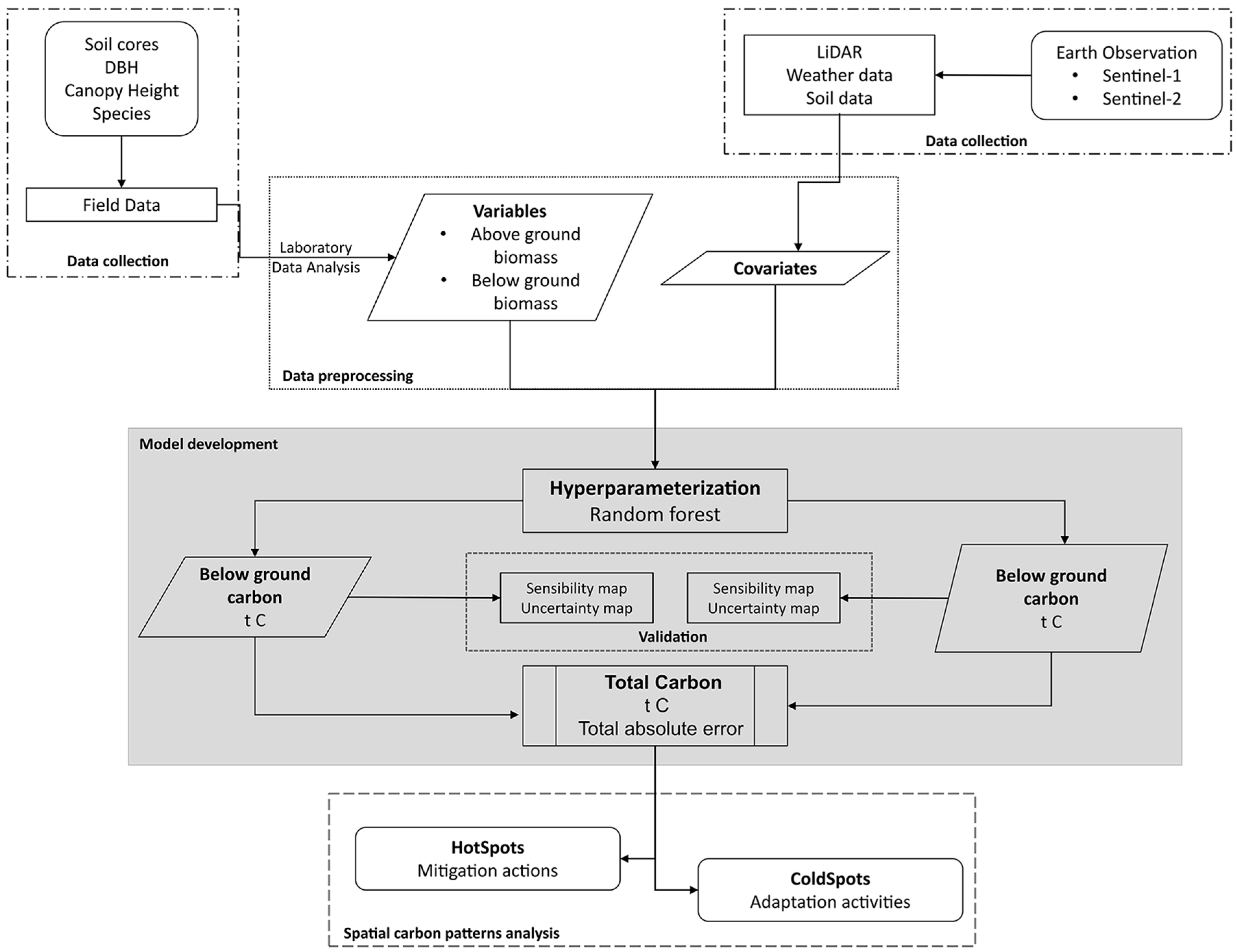
2.3. Data Preprocessing
2.4. Model Development
2.5. Validation
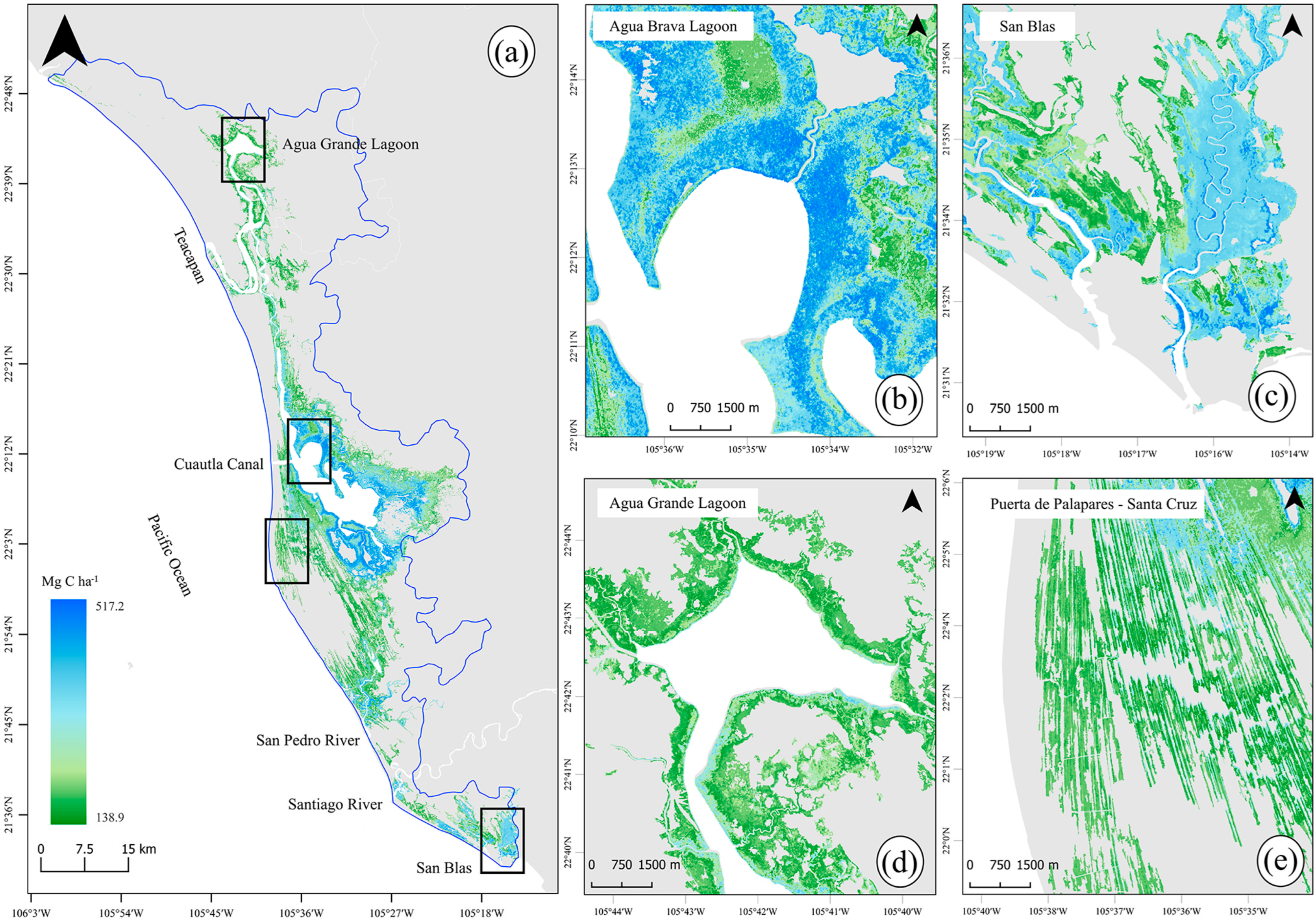
2.6. Spatial Carbon Storage Patterns
3. Results
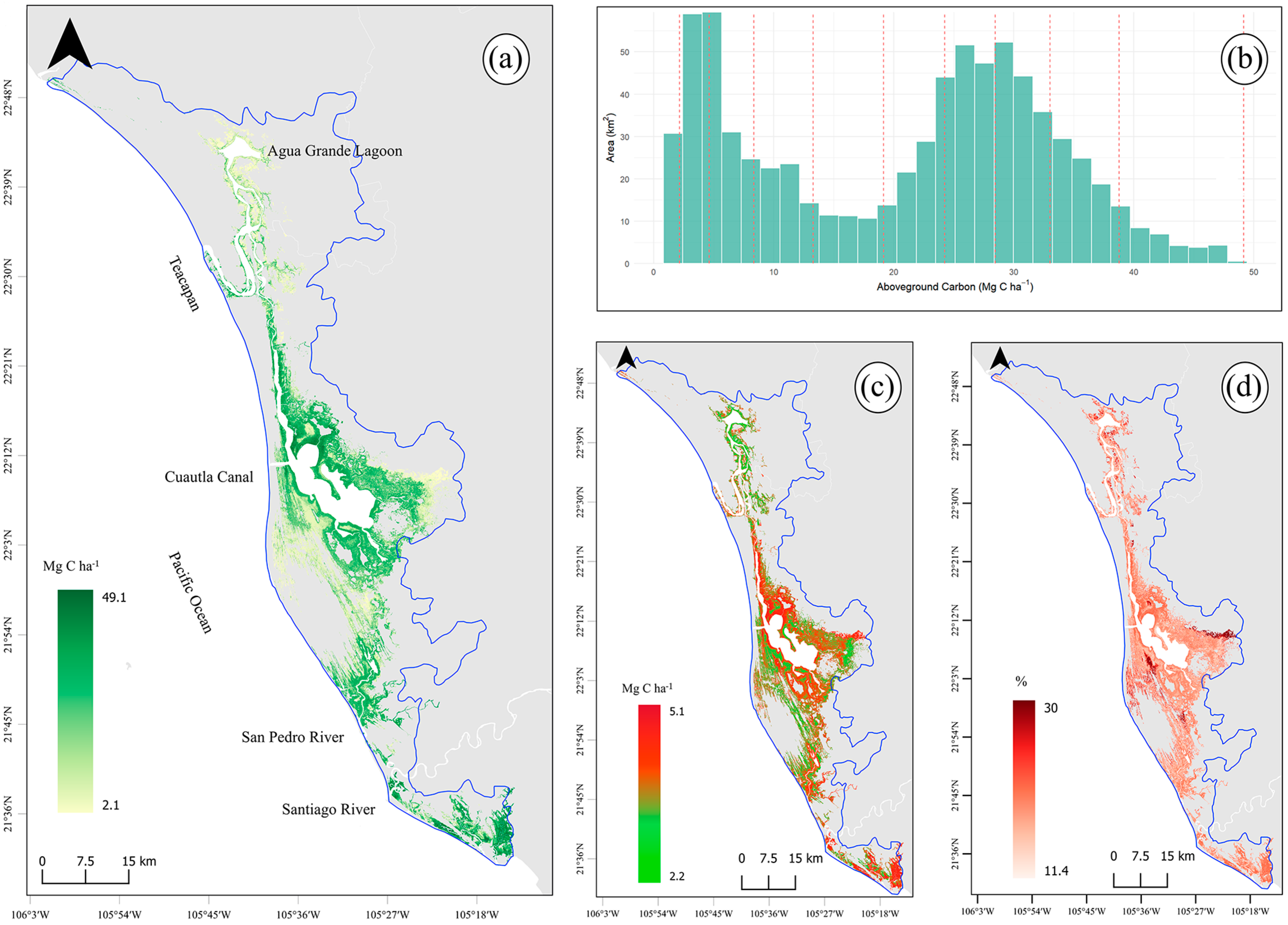
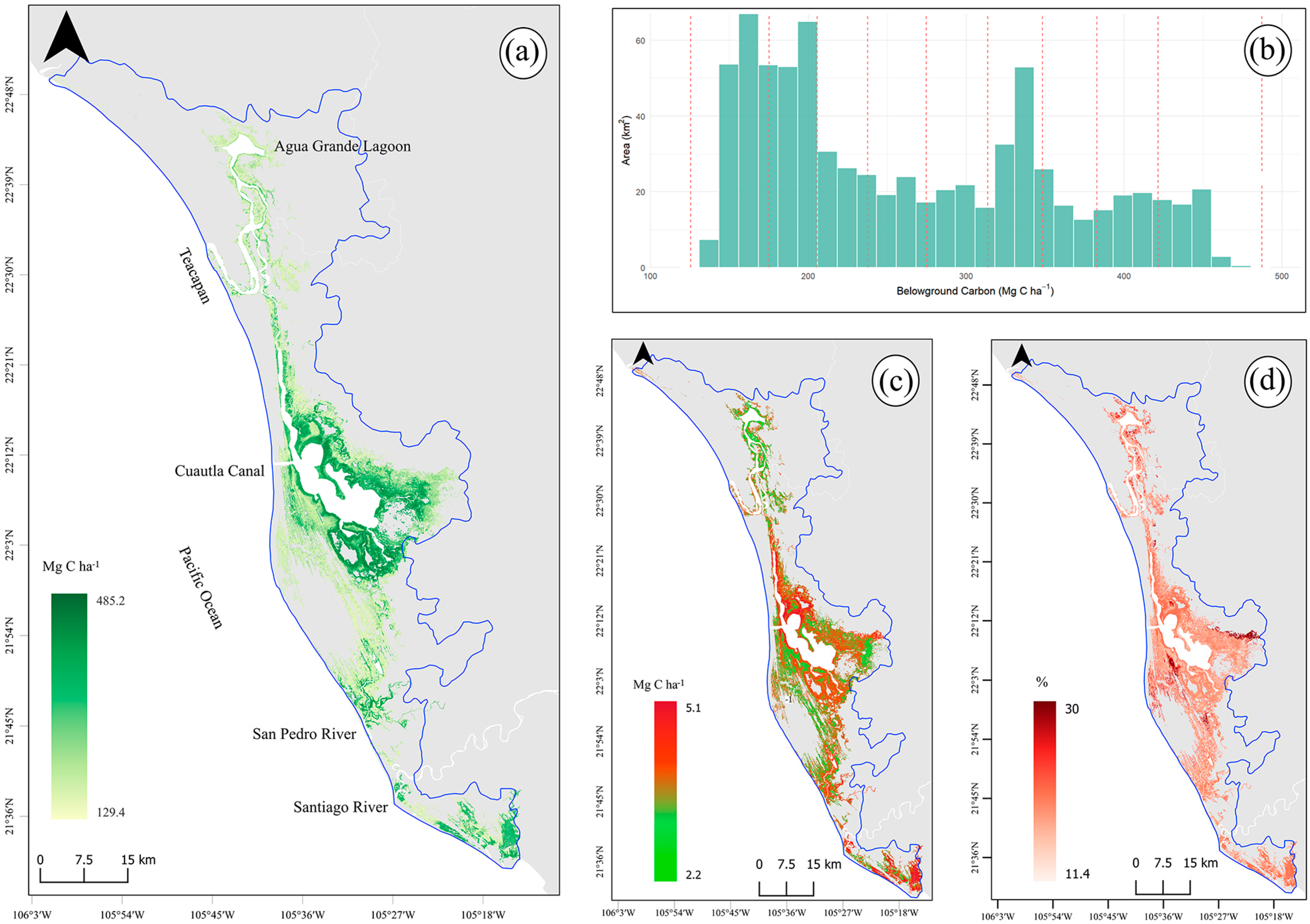
3.1. Above- and Belowground Carbon
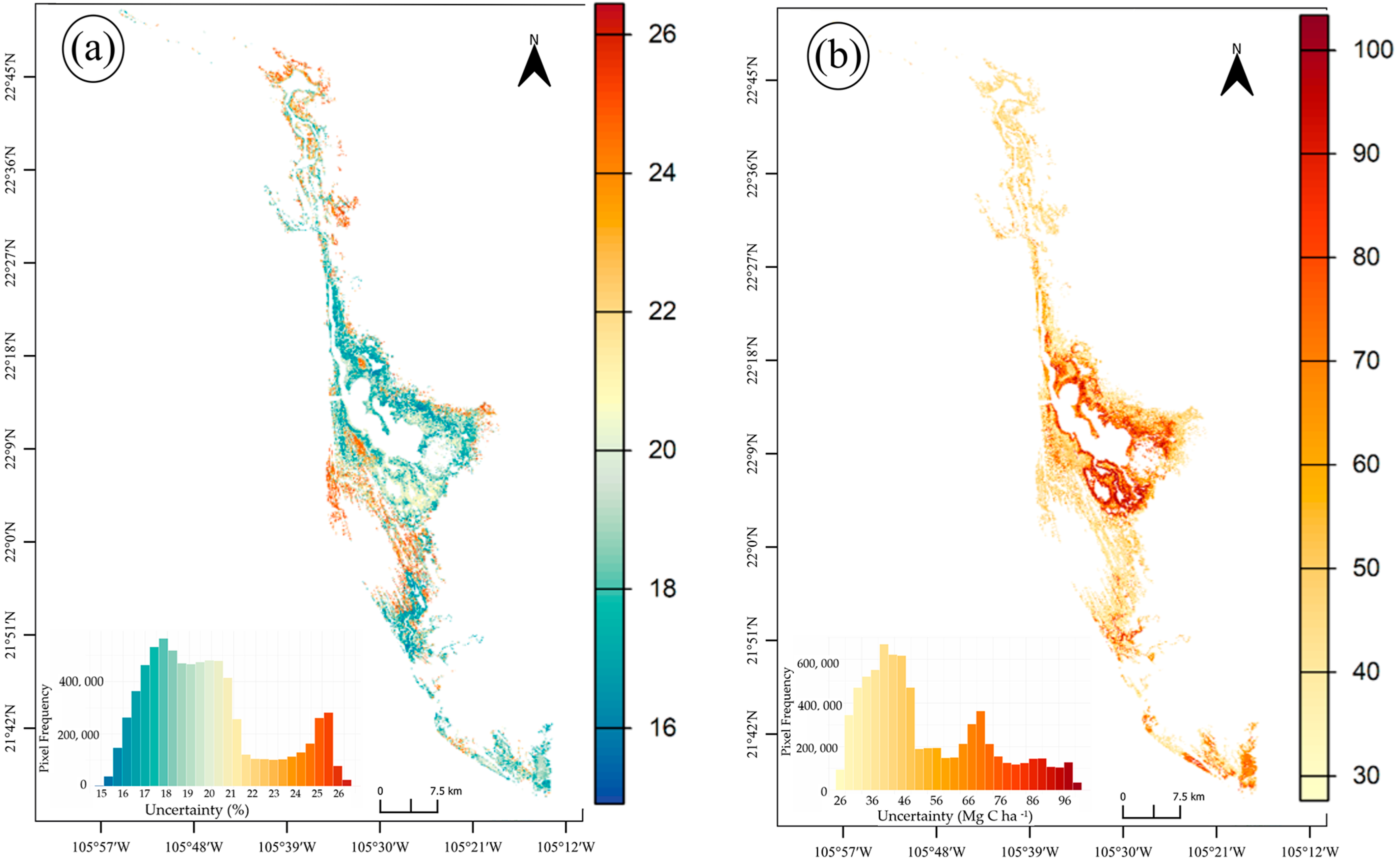
3.2. Total Carbon Stocks and Integrated Uncertainty
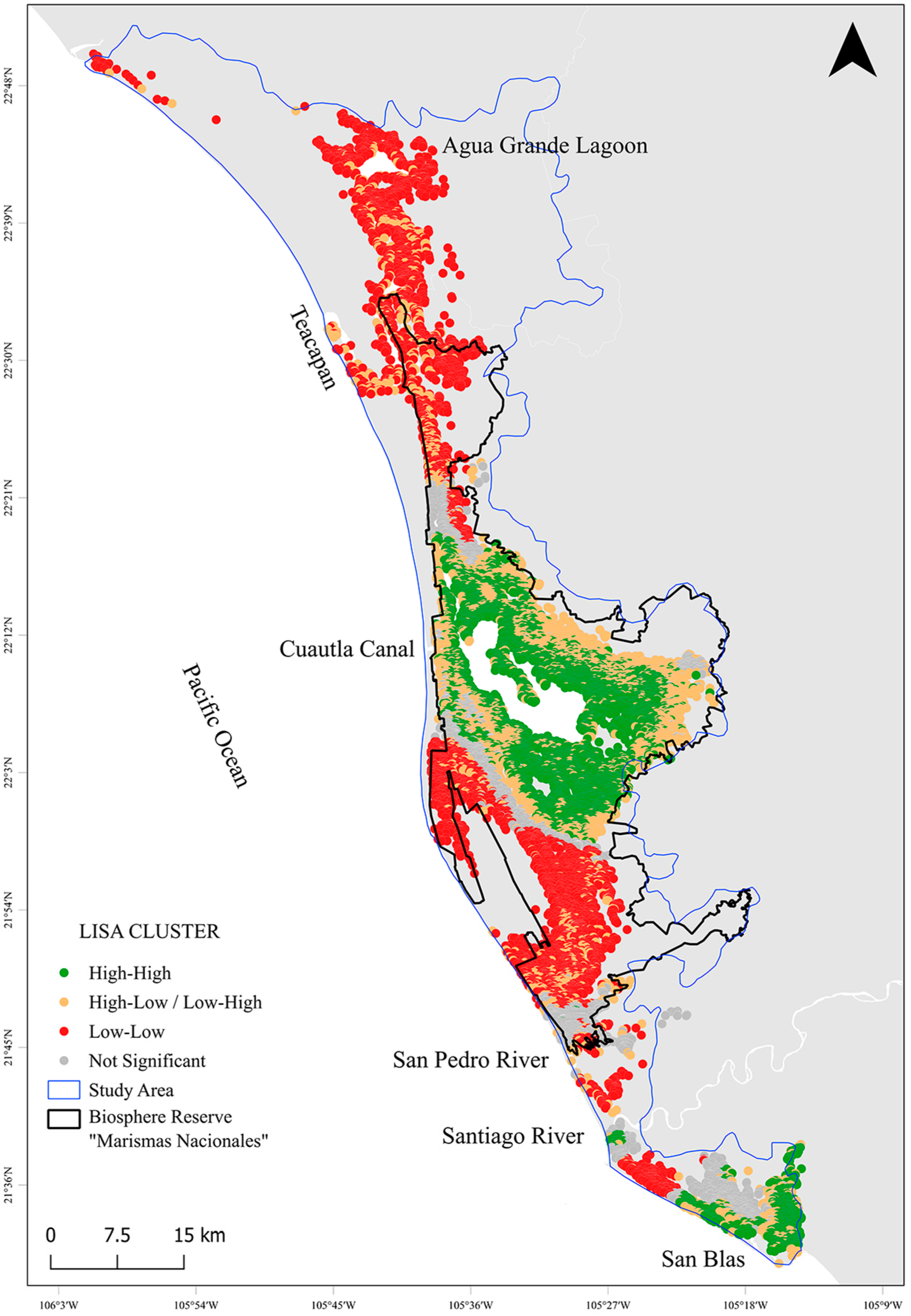
3.3. Mangrove Carbon Hotspots for Targeted Climate Action
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGC | Aboveground carbon |
| BGC | Belowground carbon |
| LiDAR | Light Detection and Ranging |
| UAV | Unoccupied/unmanned aerial vehicle |
| SAR | Synthetic Aperture Radar |
| SMMM | Mexican Mangrove Monitoring System |
| DBH | Diameter at breast height |
| GEE | Google Earth Engine |
| LISA | Local indicators of spatial association |
References
- Beselly, S.M.; Van Der Wegen, M.; Reyns, J.; Grueters, U.; Dijkstra, J.T.; Roelvink, D. Strategic Mangrove Restoration Increases Carbon Stock Capacity Check for Updates. Commun. Earth Environ. 2025, 6, 422. [Google Scholar] [CrossRef]
- Abelardo Gonzalez-Ocampo, H.; Parra-Olivas, M.C.; Pérez-González, E.; Rodríguez-Meza, G.D. Rhizophora mangle L. Bioindicator of Environmental Exposure to Heavy Metals in the Navachiste Lagoon Complex, Sinaloa, Mexico. Mar. Pollut. Bull. 2024, 209, 117131. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Ann. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Fanous, M.; Eden, J.M.; Remesan, R.; Daneshkhah, A. Challenges and Prospects of Climate Change Impact Assessment on Mangrove Environments through Mathematical Models. Environ. Model. Softw. 2023, 162, 105658. [Google Scholar] [CrossRef]
- Flores-Verdugo, F.; Zebadua-Penagos, F.; Flores-de-Santiago, F. Assessing the Influence of Artificially Constructed Channels in the Growth of Afforested Black Mangrove (Avicennia germinans) within an Arid Coastal Region. J. Environ. Manag. 2015, 160, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Martinez, T.; Ruiz-Fernández, A.C.; Sanchez-Cabeza, J.A.; Pérez-Bernal, L.; López-Mendoza, P.G.; Carnero-Bravo, V.; Agraz-Hernández, C.M.; van Tussenbroek, B.I.; Sandoval-Gil, J.; Cardoso-Mohedano, J.G.; et al. Temporal Records of Organic Carbon Stocks and Burial Rates in Mexican Blue Carbon Coastal Ecosystems throughout the Anthropocene. Glob. Planet. Change 2020, 192, 103215. [Google Scholar] [CrossRef]
- Cobacho, S.P.; Janssen, S.A.R.; Brekelmans, M.A.C.P.; van de Leemput, I.A.; Holmgren, M.; Christianen, M.J.A. High Temperature and Eutrophication Alter Biomass Allocation of Black Mangrove (Avicennia germinans L.) Seedlings. Mar. Environ. Res. 2024, 193, 106291. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Aurélio, F.; Freire, M.; Vitor, J.; Rodrigues, M.; Bezerra, L.E.A. Mangrove Recovery in Semiarid Coast Shows Increase of Ecological Processes from Biotic and Abiotic Drivers in Response to Hydrological Restoration. Wetlands 2022, 42, 80. [Google Scholar] [CrossRef]
- Shih, S.S.; Huang, Z.Z.; Hsu, Y.W. Nature-Based Solutions on Floodplain Restoration with Coupled Propagule Dispersal Simulation and Stepping-Stone Approach to Predict Mangrove Encroachment in an Estuary. Sci. Total Environ. 2022, 851, 158097. [Google Scholar] [CrossRef]
- Troche-Souza, C.; Priego-Santander, A.; Equihua, J.; Vázquez-Balderas, B. Spatial Distribution of Carbon Stocks Along Protected and Non-Protected Coastal Wetland Ecosystems in the Gulf of Mexico. Ecosystems 2024, 27, 724–738. [Google Scholar] [CrossRef]
- Velasco-Bautista, E.; Gonzalez-Hernandez, A.; Romero-Sanchez, M.E.; Guerra-De La Cruz, V.; Perez-Miranda, R. Does It Matter Whether to Use Circular or Square Plots in Forest Inventories? A Multivariate Comparison. Forests 2024, 15, 1847. [Google Scholar] [CrossRef]
- Flores-de-Santiago, F.; Flores-Verdugo, F. A Comparison of Forest Structural Methods of Semiarid Mangrove Species Using a Field-Based Approach. Cienc. Mar. 2024, 50, e3432. [Google Scholar] [CrossRef]
- Kovacs, J.M.; Liu, Y.; Zhang, C.; Flores-Verdugo, F.; de Santiago, F.F. A Field Based Statistical Approach for Validating a Remotely Sensed Mangrove Forest Classification Scheme. Wetl. Ecol. Manag. 2011, 19, 409–421. [Google Scholar] [CrossRef]
- Sanam, H.; Thomas, A.A.; Kumar, A.P.; Lakshmanan, G. Multi-Sensor Approach for the Estimation of Above-Ground Biomass of Mangroves. J. Indian Soc. Remote Sens. 2024, 52, 903–916. [Google Scholar] [CrossRef]
- Ou, G.; Zhang, Y.; Withaningsih, S.; Malik, A.D.; Parikesit, P. Aboveground Spatiotemporal Carbon Storage Model in the Changing Landscape of Jatigede, West Java, Indonesia. Forests 2024, 15, 874. [Google Scholar] [CrossRef]
- Peralta-Carreta, C.; Medrano-Pérez, O.R.; Alcudia-Aguilar, A.; Pham, Q.B.; Megia-Vera, H.J. Spatial Distribution of Relict Inland Mangrove (Rhizophora mangle L.) in the San Pedro River Basin: A Transboundary Analysis between Mexico and Guatemala. Estuaries Coasts 2025, 48, 59. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The Role of Coastal Plant Communities for Climate Change Mitigation and Adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Ball, M.C.; Brothers, N.; Pearse, A.; Reef, R. Dynamics of Surface Accretion and Surface Elevation Differ between River and Tide Dominated Settings in Tropical Mangroves. Limnol. Oceanogr. Limnol. Ocean. 2025, 70, 1424–1437. [Google Scholar] [CrossRef]
- Valderrama-Landeros, L.H.; López-Portillo, J.; Velázquez-Salazar, S.; Alcántara-Maya, J.A.; Troche-Souza, C.; Rodríguez-Zúñiga, M.T.; Vázquez-Balderas, B.; Villeda-Chávez, E.; Cruz-López, M.I.; Ressl, R. Regional Distribution and Change Dynamics of Mangroves in México between 1970/80 and 2015. Wetlands 2020, 40, 1295–1305. [Google Scholar] [CrossRef]
- Hao, Q.; Song, Z.; Zhang, X.; He, D.; Guo, L.; van Zwieten, L.; Yu, C.; Wang, Y.; Wang, W.; Fang, Y.; et al. Organic Blue Carbon Sequestration in Vegetated Coastal Wetlands: Processes and Influencing Factors. Earth Sci. Rev. 2024, 255, 104853. [Google Scholar] [CrossRef]
- Zhu, Y.; Myint, S.W.; Liu, K.; Liu, L.; Cao, J. Integration of UAV LiDAR and WorldView-2 Images for Modeling Mangrove Aboveground Biomass with GA-ANN Wrapper. Ecol. Process. 2024, 13, 85. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, C.; Wu, K.; Gao, W.; Yang, N.; Lin, J. Advancements and Trends in Mangrove Species Mapping Based on Remote Sensing: A Comprehensive Review and Knowledge Visualization. Glob. Ecol. Conserv. 2025, 57, e03408. [Google Scholar] [CrossRef]
- Abu Bakar, N.A.; Wan Mohd Jaafar, W.S.; Abdul Maulud, K.N.; Muhmad Kamarulzaman, A.M.; Saad, S.N.M.; Mohan, M. Monitoring Mangrove-Based Blue Carbon Ecosystems Using UAVs: A Review. Geocarto Int. 2024, 39, 2405123. [Google Scholar] [CrossRef]
- Price, C.A.; Papeş, M.; Sgouralis, I.; Schroeder, T.A. Surface Area to Volume Scaling of Alpha Shapes and Convex Hulls Fit to Lidar Derived Mangrove Island Point Clouds. Remote Sens. Lett. 2024, 15, 1259–1269. [Google Scholar] [CrossRef]
- Sunkur, R.; Kantamaneni, K.; Bokhoree, C.; Rathnayake, U.; Fernando, M. Mangrove Mapping and Monitoring Using Remote Sensing Techniques towards Climate Change Resilience. Sci. Rep. 2024, 14, 6949. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Yokoya, N.; Bui, D.T.; Yoshino, K.; Friess, D.A. Remote Sensing Approaches for Monitoring Mangrove Species, Structure, and Biomass: Opportunities and Challenges. Remote Sens. 2019, 11, 230. [Google Scholar] [CrossRef]
- Shinkarenko, S.S.; Bartalev, S.A. Application of Remote Sensing Data in Large-Scale Monitoring of Wetlands. Sovrem. Probl. Distantsionnogo Zondirovaniya Zemli Iz Kosmosa 2023, 20, S100–S114. [Google Scholar] [CrossRef]
- Ávila-Flores, G.; Juárez-Mancilla, J.; Hinojosa-Arango, G.; Cruz-Chávez, P.; López-Vivas, J.M.; Arizpe-Covarrubias, O. A Practical Index to Estimate Mangrove Conservation Status: The Forests from La Paz Bay, Mexico as a Case Study. Sustainability 2020, 12, 858. [Google Scholar] [CrossRef]
- Espinoza-Tenorio, A.; Millán-Vásquez, N.I.; Vite-García, N.; Alcalá-Moya, G. People and Blue Carbon: Conservation and Settlements in the Mangrove Forests of Mexico. Hum. Ecol. 2019, 47, 877–892. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove Forest. In Blue Carbon. SpringerBriefs in Climate Studies; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Bukoski, J.J.; Elwin, A.; Mackenzie, R.A.; Sharma, S.; Purbopuspito, J.; Kopania, B.; Apwong, M.; Poolsiri, R.; Potts, M.D. The Role of Predictive Model Data in Designing Mangrove Forest Carbon Programs. Environ. Res. Lett. 2020, 15, 084019. [Google Scholar] [CrossRef]
- Malerba, M.E.; Duarte de Paula Costa, M.; Friess, D.A.; Schuster, L.; Young, M.A.; Lagomasino, D.; Serrano, O.; Hickey, S.M.; York, P.H.; Rasheed, M.; et al. Remote Sensing for Cost-Effective Blue Carbon Accounting. Earth Sci. Rev. 2023, 238, 104337. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Chiu, C.Y. Biogeochemical Processes of C and N in the Soil of Mangrove Forest Ecosystems. Forests 2020, 11, 492. [Google Scholar] [CrossRef]
- Blanco-Sacristán, J.; Johansen, K.; Elías-Lara, M.; Tu, Y.H.; Duarte, C.M.; McCabe, M.F. Quantifying Mangrove Carbon Assimilation Rates Using UAV Imagery. Sci. Rep. 2024, 14, 4648. [Google Scholar] [CrossRef] [PubMed]
- Padonou, E.A.; Avocèvou-Ayisso, C.M.A.; Sewade, C.; Hovozounkou, G.M.; Akakpo, B.A.; Sinsin, B. Plant Diversity, Population Structures and Carbon Stock of Mangrove Ecosystems of Ramsar Site 1017 in Benin. Wetl. Ecol. Manag. 2025, 33, 24. [Google Scholar] [CrossRef]
- Blanco y Correa, M.; Flores-Verdugo, F.; Ortiz-Pérez, M.A.; de la Lanza-Espino, G.; López-Portillo, J.; Valdez-Hernández, I. Diagnostico Funcional de Marismas Nacionales; Universidad Autónoma de Nayarit and Secretaría de Medio Ambiente y Recursos Naturales: Nayarit, Mexico, 2011. [Google Scholar]
- Berlanga-Robles, C.A. Trends in Mangrove Canopy and Cover in the Teacapan-Agua Brava Lagoon System (Marismas Nacionales) in Mexico: An Approach Using Open-Access Geospatial Data Mapping Mangrove Dynamics in Teacapan-Agua Brava. Wetlands 2025, 45, 1. [Google Scholar] [CrossRef]
- Godwyn-Paulson, P.; Jonathan, M.P.; Roy, P.D.; Rodríguez-Espinosa, P.F.; Muthusankar, G.; Muñoz-Sevilla, N.P.; Lakshumanan, C. Evolution of Southern Mexican Pacific Coastline: Responses to Meteo-Oceanographic and Physiographic Conditions. Reg. Stud. Mar. Sci. 2021, 47, 101914. [Google Scholar] [CrossRef]
- Serrano-Rubio, J.P.; Ruiz, M.D.M.; Vidal-Espitia, U. Integrating Remote Sensing and Image Processing to Test for Disturbance Effects in a Post-Hurricane Mangrove Ecosystem. Signal Image Video Process 2021, 15, 351–359. [Google Scholar] [CrossRef]
- Rodríguez-Sobreyra, R.; Valderrama-Landeros, L.; Flores-Verdugo, F.; Green-Ruiz, C.; Flores-de-Santiago, F. The Influence of Hydrological Variability, Geomorphology, and Salinity Determines the Allocation of Two Dominant Fringe Mangrove Species in Semiarid Coastal Systems. Discov. Conserv. 2025, 2, 10. [Google Scholar] [CrossRef]
- Hernández-Guzmán, R.; Ruiz-Luna, A. Combining Multisensor Images and Social Network Data to Assess the Area Flooded by a Hurricane Event. PeerJ 2024, 12, e17319. [Google Scholar] [CrossRef]
- Pérez-Ceballos, R.; Echeverría-Ávila, S.; Zaldívar-Jiménez, A.; Zaldívar-Jiménez, T.; Herrera-Silveira, J. Contribution of Microtopography and Hydroperiod to the Natural Regeneration of Avicennia germinans in a Restored Mangrove Forest. Cienc. Mar. 2017, 43, 55–67. [Google Scholar] [CrossRef]
- Alvarez-Fuentes, C.J.; Tovar-Ávila, J.; Payan-Alejo, J.; Chávez-Arrenquín, D.A.; Salgado-Ugarte, I.H.; Amezcua, F. Reproductive Ecology of the Chilean Round Ray (Urotrygon chilensis, Günther, 1872) in the Southern Gulf of California. Fishes 2023, 8, 193. [Google Scholar] [CrossRef]
- Rodríguez-Veiga, P.; Saatchi, S.; Tansey, K.; Balzter, H. Magnitude, Spatial Distribution and Uncertainty of Forest Biomass Stocks in Mexico. Remote Sens. Environ. 2016, 183, 265–281. [Google Scholar] [CrossRef]
- Serrano, D.; Flores-Verdugo, F.; Ramírez-Félix, E.; Kovacs, J.M.; Flores-de-Santiago, F. Modeling Tidal Hydrodynamic Changes Induced by the Opening of an Artificial Inlet within a Subtropical Mangrove Dominated Estuary. Wetl. Ecol. Manag. 2020, 28, 103–118. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, H.; Ling, J. A Review of Mangrove Degradation Assessment Using Remote Sensing: Advances, Challenges, and Opportunities. GIsci Remote Sens. 2025, 62, 2491920. [Google Scholar] [CrossRef]
- Pranchai, A.; Jenke, M.; Thongmee, J.; Sutthinon, P.; Karuna, S.; Wongkamhaeng, K.; Berger, U. Artificial Macropores Enhance Mangrove Establishment in Hypersaline Environments. Restor. Ecol. 2025, 33, e70015. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Donato, D.C. Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests; Working Paper 86; CIFOR: Bogor, Indonesia, 2012. [Google Scholar]
- Rodríguez-Zúñiga, M.T.; Troche-Souza, C.; Cruz-López, M.I.; Rivera-Monroy, V.H. Development and Structural Organization of Mexico’s Mangrove Monitoring System (SMMM) as a Foundation for Conservation and Restoration Initiatives: A Hierarchical Approach. Forests 2022, 13, 621. [Google Scholar] [CrossRef]
- CONAFOR. Programa Institucional de La Comisión Nacional Forestal 2020–2024; CONAFOR: Mexico City, Mexico, 2021. [Google Scholar]
- Hiraishi, T.; Krug, T.; Tanabe, K.; Srivastava, N.; Jamsranjav, B.; Fukuda, M.; Troxler, T. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Day, J.W.; Conner, W.H.; Ley-Lou, F.; Day, R.H.; Navarro, A.M. The Productivity and Composition of Mangrove Forests, Laguna de Términos, Mexico. Aquat. Bot. 1987, 27, 267–284. [Google Scholar] [CrossRef]
- Fromard, F.; Puig, H.; Mougin, E.; Marty, G.; Betoulle, J.L.; Cadamuro, L. Structure, above-Ground Biomass and Dynamics of Mangrove Ecosystems: New Data from French Guiana. Oecologia 1998, 115, 39–53. [Google Scholar] [CrossRef]
- Smith, T.J.; Whelan, K.R.T. Development of allometric relations for three mangrove species in South Florida for use in the Greater Everglades Ecosystem restoration. Wetl. Ecol. Manag. 2006, 14, 409–419. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree Allometry and Improved Estimation of Carbon Stocks and Balance in Tropical Forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Hughes, R.; Boone Kauffman, J.; Jaramillo, V.J. Biomass, Carbon, and Nutrient Dynamics of Secondary Forests in a Umid Tropical Region of Mexico. Ecology 1999, 80, 1892–1907. [Google Scholar] [CrossRef]
- Komiyama, A.; Poungparn, S.; Kato, S. Common Allometric Equations for Estimating the Tree Weight of Mangroves. J. Trop. Ecol. 2005, 21, 471–477. [Google Scholar] [CrossRef]
- Howard, J.; Hoyt, S.; Isensee, K.; Pidgeon, E.; Telszewski, M. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrass Meadows; Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature: Arlington, VA, USA, 2014. [Google Scholar]
- Flores, L.; Blas, G.; Hernández, G.; Alcalá, R. Distribution and Sequential Extraction of Some Heavy Metals from Soils Irrigated with Wastewater from Mexico City. Water Air Soil. Pollut. 1997, 98, 105–117. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning, 2nd ed.; Springer: New York, NY, USA, 2009; Volume 27. [Google Scholar]
- Probst, P.; Wright, M.N.; Boulesteix, A.-L. Hyperparameters and Tuning Strategies for Random Forest. WIREs Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef]
- Beselly, S.M.; Grueters, U.; van Der Wegen, M.; Reyns, J.; Dijkstra, J.; Roelvink, D. Modelling Mangrove-Mudflat Dynamics with a Coupled Individual-Based-Hydro-Morphodynamic Model. Environ. Model. Softw. 2023, 169, 105814. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Louppe, G.; Wehenkel, L.; Sutera, A.; Geurts, P. Understanding Variable Importances in Forests of Randomized Trees. In Proceedings of the Advances in Neural Information Processing Systems; Curran Associates Inc.: Red Hook, NY, USA, 2013. [Google Scholar]
- Belgiu, M.; Drăgu, L. Random Forest in Remote Sensing: A Review of Applications and Future Directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Cavalli, R.M. Remote Data for Mapping and Monitoring Coastal Phenomena and Parameters: A Systematic Review. Remote Sens. 2024, 16, 446. [Google Scholar] [CrossRef]
- Efron, B. Jackknife-After-Bootstrap Standard Errors and Influence Functions. J. R. Stat. Soc. Ser. B Stat. Methodol. 1992, 54, 83–111. [Google Scholar] [CrossRef]
- Thiesen, S.; Ehret, U. Assessing Local and Spatial Uncertainty with Nonparametric Geostatistics. Stoch. Environ. Res. Risk Assess. 2022, 36, 173–199. [Google Scholar] [CrossRef]
- Ayushi, K.; Babu, K.N.; Ayyappan, N.; Nair, J.R.; Kakkara, A.; Reddy, C.S. A Comparative Analysis of Machine Learning Techniques for Aboveground Biomass Estimation: A Case Study of the Western Ghats, India. Ecol. Inf. 2024, 80, 102479. [Google Scholar] [CrossRef]
- Ameca, E.I.; Nie, Y.; Wu, R.; Mittermeier, R.A.; Foden, W.; Wei, F. Identifying Protected Areas in Biodiversity Hotspots at Risk from Climate and Human-Induced Compound Events for Conserving Threatened Species. Sci. Total Environ. 2024, 938, 173192. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, T.; Glamore, W.; Felder, S. Restoring Estuarine Ecosystems Using Nature-Based Solutions: Towards an Integrated Eco-Engineering Design Guideline. Sci. Total Environ. 2023, 873, 162362. [Google Scholar] [CrossRef]
- Manzano Sarabia, M.M.; Aguilar, O.G.M.; Cruz, I.C.; Arredondo, L.E.R.; Murillo, E.V.; Domínguez, M.R.; Oliva, M.A.H.; del P, M. Impacto de la Variabilidad Climática y Actividades Humanas en la Estructura de Manglares de Marismas Nacionales; Universidad Autónoma de Sinaloa. Facultad de Ciencias del Mar: Ciudad de México, México, 2023; Informe final SNIBCONABIO, proyecto No. LM019. [Google Scholar]
- Valdés Velarde, E.; Hernández, E.H.; Rodríguez, C.G.S.; Aguilar, J.S.Y.; Jurado, O.G.; Roblero, R.L.S.; Carrillo, C.G. Monitoreo de Carbono azul y Nutrimientos en Agua, Biomasa y Suelo en Manglares de Marismas Nacionales; Universidad Autónoma Chapingo. Centro de Agroforestería para el Desarrollo Sostenible: Ciudad de México, México, 2025; Informe Final SNIBCONABIO, proyectos No. UM002 y LM019. [Google Scholar]
- CONANP, CIIIE Mar Bermejo SC. Restauración Hidrológica de Manglares en El Rabón, dentro del ejido Francisco Villa, Municipio Rosamorada, Nayarit. Adaptación de Ecosistemas Costeros al Cambio Climático en Áreas Naturales Protegidas de México, con Énfasis en la Captura y Reducción de Emisiones de Carbono en Humedales; Informe Final Proyecto; Centro de Investigación e Innovación Integral Empresarial Mar Bermejo SC: Ciudad de México, México, 2017. [Google Scholar]
- Mutanga, O.; Kumar, L. Google Earth Engine Applications. Remote Sens. 2019, 11, 591. [Google Scholar] [CrossRef]
- Velazquez-Salazar, S.; Rodrıguez-Zuniga, M.T.; Alcantara-Maya, J.A.; Villeda-Chavez, E.; Valderrama-Landeros, L.; Troche-Souza, C.; Vazquez-Balderas, B.; Perez-Espinosa, I.; Cruz-López, M.I.; Ressl, R.; et al. Manglares deMexico. In Actualizacion y Analisis De Los Datos 2020; Comision Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2021; p. 168. [Google Scholar]
- Fan, C.; Hou, X.; Zhang, Y.; Li, D. Satellite Data Reveal Concerns Regarding Mangrove Restoration Efforts in Southern China. Remote Sens. 2023, 15, 4151. [Google Scholar] [CrossRef]
- Valderrama-Landeros, L.; Troche-Souza, C.; Alcántara-Maya, J.A.; Velázquez-Salazar, S.; Vázquez-Balderas, B.; Villeda-Chávez, E.; Cruz-López, M.I.; Ressl, R.; Flores-Verdugo, F.; Flores-De-Santiago, F. An Assessment of Mangrove Forest in Northwestern Mexico Using the Google Earth Engine Cloud Computing Platform. PLoS ONE 2024, 19, e0315181. [Google Scholar] [CrossRef]
- Lassalle, G.; Ferreira, M.P.; La Rosa, L.E.C.; de Souza Filho, C.R. Deep Learning-Based Individual Tree Crown Delineation in Mangrove Forests Using Very-High-Resolution Satellite Imagery. ISPRS J. Photogramm. Remote Sens. 2022, 189, 220–235. [Google Scholar] [CrossRef]
- Floriano, B.R.O.; Hanson, B.; Bewley, T.; Ishihara, J.Y.; Ferreira, H.C. A Novel Policy for Coordinating a Hurricane Monitoring System Using a Swarm of Buoyancy-Controlled Balloons Trading off Communication and Coverage. Eng. Appl. Artif. Intell. 2025, 139, 109495. [Google Scholar] [CrossRef]
- Herrera-Silveira, J.A.; Rico, A.C.-; Pech, E.; Pech, M.; Ramírez, J.R.-; Hernández, C.T. Carbon Dynamics (Stocks and Fluxes) in Mangroves of Mexico. Terra Latinoam. 2016, 34, 61–72. [Google Scholar]
- Robles-Archundia, D.A.; López-Vivas, J.M.; León-Cisneros, K.; Vargas-Betancourt, F.; Lara-Uc, M.M.; Hernández-Martínez, L.A.; Hernández-Stefanoni, J.L. Assessment of the Impact of Hurricane Lorena (2019) on the Mangrove Forest of Espiritu Santo Archipelago, Mexico Using Sentinel-2 Imagery. Rev. De. Teledetección 2025, 65, 21747. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, H.; Wang, M.; Wang, W. Ordered Mortality of Mangroves under Extreme Flooding in a Tropical Microtidal Estuarine Lagoon in China. Estuar. Coast. Shelf Sci. 2025, 318, 109233. [Google Scholar] [CrossRef]
- Carol, E.; del Pilar Alvarez, M.; Candanedo, I.; Saavedra, S.; Arcia, M.; Franco, A. Surface Water–Groundwater Interactions in the Matusagaratí Wetland, Panama. Wetl. Ecol. Manag. 2020, 28, 971–982. [Google Scholar] [CrossRef]
- Prados, F.J.; Pérez, M.A.; De la Fuente Roselló, A.L.; MahEng, J.M.; Mosqueda, G. Assessment of the geoecological degradation of the Coastal Plain geosystem of the San Pedro River, Nayarit (Mexico). Rev. De Geogr. Norte Gd. 2025, 91. [Google Scholar] [CrossRef]
- Shu, S.; Yang, J.; Jing, W.; Yang, C.; Wu, J. Mangrove Extraction from Compact Polarimetric Synthetic Aperture Radar Images Based on Optimal Feature Combinations. Forests 2024, 15, 2047. [Google Scholar] [CrossRef]
- Evagorou, E.; Hasiotis, T.; Petsimeris, I.T.; Monioudi, I.N.; Andreadis, O.P.; Chatzipavlis, A.; Christofi, D.; Kountouri, J.; Stylianou, N.; Mettas, C.; et al. A Holistic High-Resolution Remote Sensing Approach for Mapping Coastal Geomorphology and Marine Habitats. Remote Sens. 2025, 17, 1437. [Google Scholar] [CrossRef]
- Tysiąc, P.; Moskalewicz, D.; Janowski, Ł. Development of UAV-Based LiDAR and Multispectral Measurement Techniques for Monitoring Sediment Intrusion in Coastal Wetlands. Measurement 2025, 253, 117459. [Google Scholar] [CrossRef]
- Lombard, F.; Soumaré, S.; Andrieu, J.; Josselin, D. Mangrove Zonation Mapping in West Africa, at 10-m Resolution, Optimized for Inter-Annual Monitoring. Ecol. Inf. 2023, 75, 102027. [Google Scholar] [CrossRef]
- Valderrama-Landeros, L.; Flores-de-Santiago, F. Assessing Coastal Erosion and Accretion Trends along Two Contrasting Subtropical Rivers Based on Remote Sensing Data. Ocean. Coast. Manag. 2019, 169, 58–67. [Google Scholar] [CrossRef]
- Comisión Nacional de Áreas Naturales Protegidas; The Nature Conservancy y Conselva, Costas y Comunidades AC. Programa de Adaptación al Cambio Climático del Complejo Marismas Nacionales, Nayarit y Sinaloa; Secretaría de Medio Ambiente y Recursos Naturales: Ciudad de México, Mexico, 2016. [Google Scholar]
- CONANP. Programa de Manejo Reserva de La Biosfera Marismas Nacionales Nayarit; CONANP: Mexico City, Mexico, 2013. [Google Scholar]
- Aburto-Oropeza, O.; Ezcurra, E.; Danemann, G.; Valdez, V.; Murray, J.; Sala, E. Mangroves in the Gulf of California Increase Fishery Yields. Proc. Natl. Acad. Sci. USA 2008, 105, 10456–10459. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Adame, M.F.; Arifanti, V.B.; Schile-Beers, L.M.; Bernardino, A.F.; Bhomia, R.K.; Donato, D.C.; Feller, I.C.; Ferreira, T.O.; Jesus Garcia, M.d.C.; et al. Total Ecosystem Carbon Stocks of Mangroves across Broad Global Environmental and Physical Gradients. Ecol. Monogr. 2020, 90, e01405. [Google Scholar] [CrossRef]
- Chen, C.; Wu, W.; Fan, X.; Zhou, C.; Cui, T.; Wu, T.; Zhao, Q.; Jia, M. Linkage Analysis Between Coastline Change and Both Sides of Coastal Ecological Spaces. Water 2025, 17, 1505. [Google Scholar] [CrossRef]
- Peñaranda, P.M.L.; Cantera Kintz, J.R.; Peña Salamanca, E.J. Carbon Stocks in Mangrove Forests of the Colombian Pacific. Estuar. Coast. Shelf Sci. 2019, 227, 106299. [Google Scholar] [CrossRef]
- Howard, J.; Sutton-Grier, A.; Herr, D.; Kleypas, J.; Landis, E.; Mcleod, E.; Pidgeon, E.; Simpson, S. Clarifying the Role of Coastal and Marine Systems in Climate Mitigation. Front. Ecol. Environ. 2017, 15, 42–50. [Google Scholar] [CrossRef]
- Fan, J.; Kuang, C.; Cong, X.; Gong, L.; Wang, G.; Xing, R. Hydrodynamic Influences on Water Exchange Capacity of a Coastal Lagoon after Phasic Restoration Projects. Estuar. Coast. Shelf Sci. 2024, 299, 108671. [Google Scholar] [CrossRef]
- Kovacs, J.M.; Lu, X.X.; Flores-Verdugo, F.; Zhang, C.; Flores de Santiago, F.; Jiao, X. Applications of ALOS PALSAR for Monitoring Biophysical Parameters of a Degraded Black Mangrove (Avicennia germinans) Forest. ISPRS J. Photogramm. Remote Sens. 2013, 82, 102–111. [Google Scholar] [CrossRef]
- Valderrama-Landeros, L.; Blanco y Correa, M.; Flores-Verdugo, F.; Álvarez-Sánchez, L.F.; Flores-de-Santiago, F. Spatiotemporal Shoreline Dynamics of Marismas Nacionales, Pacific Coast of Mexico, Based on a Remote Sensing and GIS Mapping Approach. Environ. Monit. Assess. 2020, 192, 123. [Google Scholar] [CrossRef]
- García-Burgos, J.; Miquelajauregui, Y.; Vega, E.; Namdeo, A.; Ruíz-Olivares, A.; Mejía-Arangure, J.M.; Resendiz-Martinez, C.G.; Hayes, L.; Bramwell, L.; Jaimes-Palomera, M.; et al. Exploring the Spatial Distribution of Air Pollution and Its Association with Socioeconomic Status Indicators in Mexico City. Sustainability 2022, 14, 15320. [Google Scholar] [CrossRef]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural Climate Solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef]
- Veneranta, L.; Westerbom, M. Ecology and Evolution Variation in Community Structure and Abundance of Fish in Simple Structured Shallow Sandy Habitats. Ecol. Evol. 2024, 14, e70381. [Google Scholar] [CrossRef]
- Sutton-Grier, A.E.; Moore, A. Leveraging Carbon Services of Coastal Ecosystems for Habitat Protection and Restoration. Coast. Manag. 2016, 44, 259–277. [Google Scholar] [CrossRef]
- Danemann, G.; Cordero, G.; Cortés, M.; Torrescano, C.; Valdez, V. Valor Económico de Las Pesquerías Generadas Por El Ecosistema de Manglar en Marismas Nacionales, México; Resumen Técnico de Investigación, No Publicado; Pronatura Noroeste AC: Ensenada, México, 2010. [Google Scholar]
- Sullivan, J.C.; Wan, Y.; Willis, R.A. Modeling Floodplain Inundation, Circulation, and Residence Time Under Changing Tide and Sea Levels. Estuaries Coasts 2020, 43, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Ma, X.-M.; Yang, W.; Bai, J.; Yin, X.-A.; Zhao, Y.-W. The Connectivity Threshold of the Wetlands Based on Water Environment Response, and a Case Study of China’s Baiyangdian Wetland. J. Hydrol. 2024, 630, 130687. [Google Scholar] [CrossRef]
- Flores-de-Santiago, F.; Rodríguez-Sobreyra, R.; Álvarez-Sánchez, L.F.; Valderrama-Landeros, L.; Amezcua, F.; Flores-Verdugo, F. Understanding the Natural Expansion of White Mangrove (Laguncularia racemosa) in an Ephemeral Inlet Based on Geomorphological Analysis and Remote Sensing Data. J. Environ. Manag. 2023, 338, 117820. [Google Scholar] [CrossRef]
- Bardou, R.; Osland, M.J.; Scyphers, S.; Shepard, C.; Aerni, K.E.; Alemu I, J.B.; Crimian, R.; Day, R.H.; Enwright, N.M.; Feher, L.C.; et al. Rapidly Changing Range Limits in a Warming World: Critical Data Limitations and Knowledge Gaps for Advancing Understanding of Mangrove Range Dynamics in the Southeastern USA. Estuaries Coasts 2023, 46, 1123–1140. [Google Scholar] [CrossRef]


| Species | DBH Range | Allometric Equation | a | b | Reference Site | Reference |
|---|---|---|---|---|---|---|
| Avicennia germinans | 1–10 | 2.302 | −1.5852 | Terminos Lagoon, Mexico | [52] | |
| Avicennia germinans | >10 | 0.140 | 2.4 | Everglades USA | [53] | |
| Laguncularia racemosa | 1–10 | 2.192 | −1.5919 | Terminos Lagoon, Mexico | [52] | |
| Laguncularia racemosa | >10 | 1.930 | −0.441 | Everglades USA | [54] | |
| Rhizophora mangle | 1–10 | 2.507 | −1.5605 | Terminos Lagoon, Mexico | [52] | |
| Rhizophora mangle | >10 | 0.128 | 2.6 | French Guyana | [53] | |
| Conocarpus erectus | >1 | - | −1.349 | Asian Southeast | [55] | |
| Dead trees | - | - | - | Veracruz, México | [56] |
| LISA Cluster | Area (km2) | Carbon Metrics | Implications | ||
|---|---|---|---|---|---|
| Study Area | Protected 1 (P%) 2 | Mean ± SD (Mg C ha−1) | Total (Tg C) | ||
| High-High | 251.7 | 217.0 (28.7%) | 392 ± 56.2 | 9.9 | Priority for C projects |
| Low-Low | 201.3 | 132.0 (17.5%) | 198 ± 56.2 | 4.0 | Restoration potential |
| High-Low/Low-High | 201.3 | 148.7 (19.7%) | 281 ± 38.9 | 5.0 | Buffer zone investments |
| Not Significant | 100.7 | 65.3 (8.7%) | 262 ± 82.7 | 2.6 | Limited climate leverage |
| TOTAL | 755 | 563 (74.6%) | - | 21.5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troche-Souza, C.; Villeda-Chávez, E.; Vázquez-Balderas, B.; Velázquez-Salazar, S.; Vázquez-Morán, V.H.; Rosas-Aceves, O.G.; Flores-de-Santiago, F. Spatial Distribution of Mangrove Forest Carbon Stocks in Marismas Nacionales, Mexico: Contributions to Climate Change Adaptation and Mitigation. Forests 2025, 16, 1224. https://doi.org/10.3390/f16081224
Troche-Souza C, Villeda-Chávez E, Vázquez-Balderas B, Velázquez-Salazar S, Vázquez-Morán VH, Rosas-Aceves OG, Flores-de-Santiago F. Spatial Distribution of Mangrove Forest Carbon Stocks in Marismas Nacionales, Mexico: Contributions to Climate Change Adaptation and Mitigation. Forests. 2025; 16(8):1224. https://doi.org/10.3390/f16081224
Chicago/Turabian StyleTroche-Souza, Carlos, Edgar Villeda-Chávez, Berenice Vázquez-Balderas, Samuel Velázquez-Salazar, Víctor Hugo Vázquez-Morán, Oscar Gerardo Rosas-Aceves, and Francisco Flores-de-Santiago. 2025. "Spatial Distribution of Mangrove Forest Carbon Stocks in Marismas Nacionales, Mexico: Contributions to Climate Change Adaptation and Mitigation" Forests 16, no. 8: 1224. https://doi.org/10.3390/f16081224
APA StyleTroche-Souza, C., Villeda-Chávez, E., Vázquez-Balderas, B., Velázquez-Salazar, S., Vázquez-Morán, V. H., Rosas-Aceves, O. G., & Flores-de-Santiago, F. (2025). Spatial Distribution of Mangrove Forest Carbon Stocks in Marismas Nacionales, Mexico: Contributions to Climate Change Adaptation and Mitigation. Forests, 16(8), 1224. https://doi.org/10.3390/f16081224