What Can Ground-Dwelling Ants Tell Us About Different Land-Use Systems in the Brazilian Amazon?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Design
2.3. Ant Sampling
2.4. Statistical Analyses
3. Results
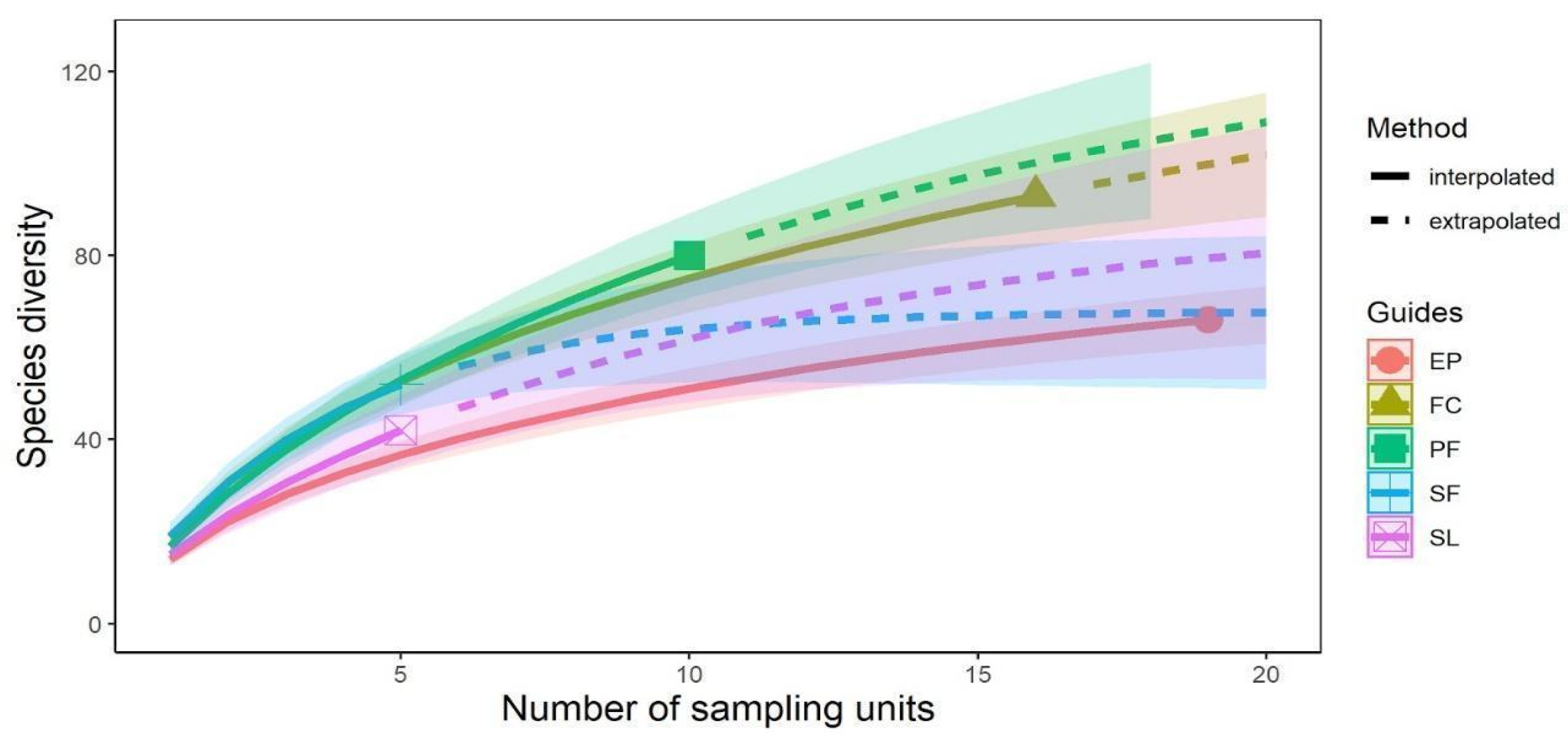
3.1. Species Richness vs. Different Land-Use Systems
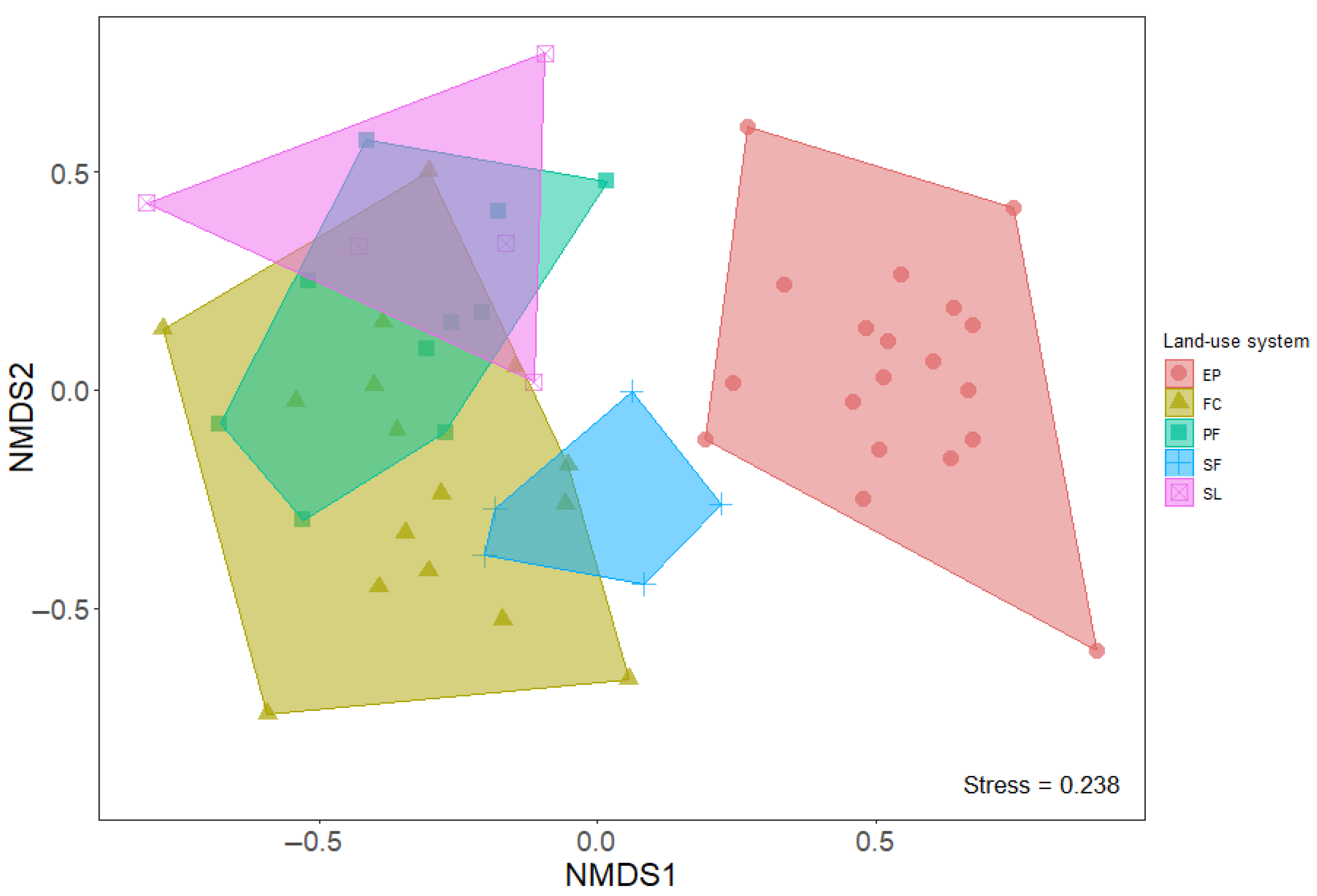
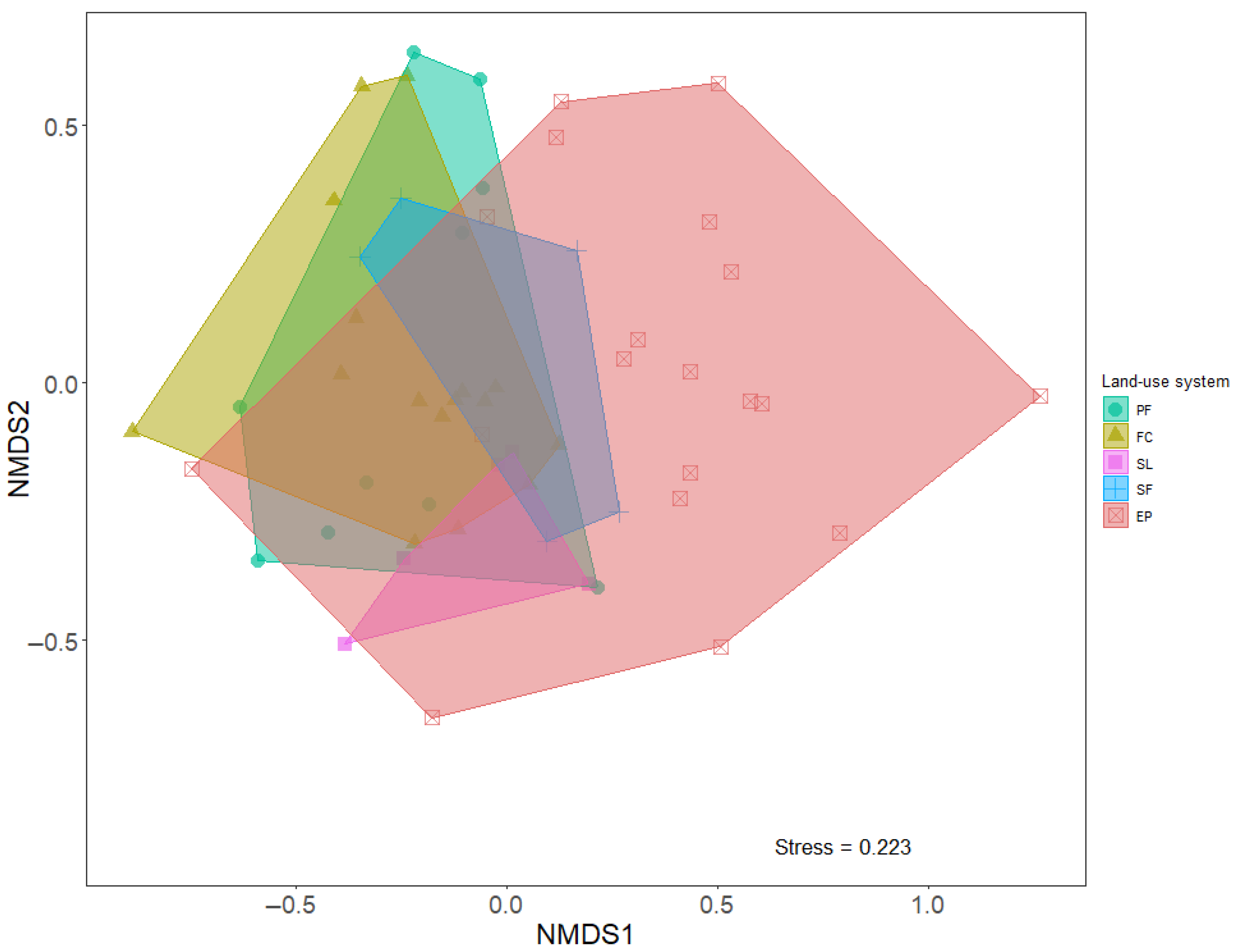
3.2. Species Composition vs. Different Land-Use Systems
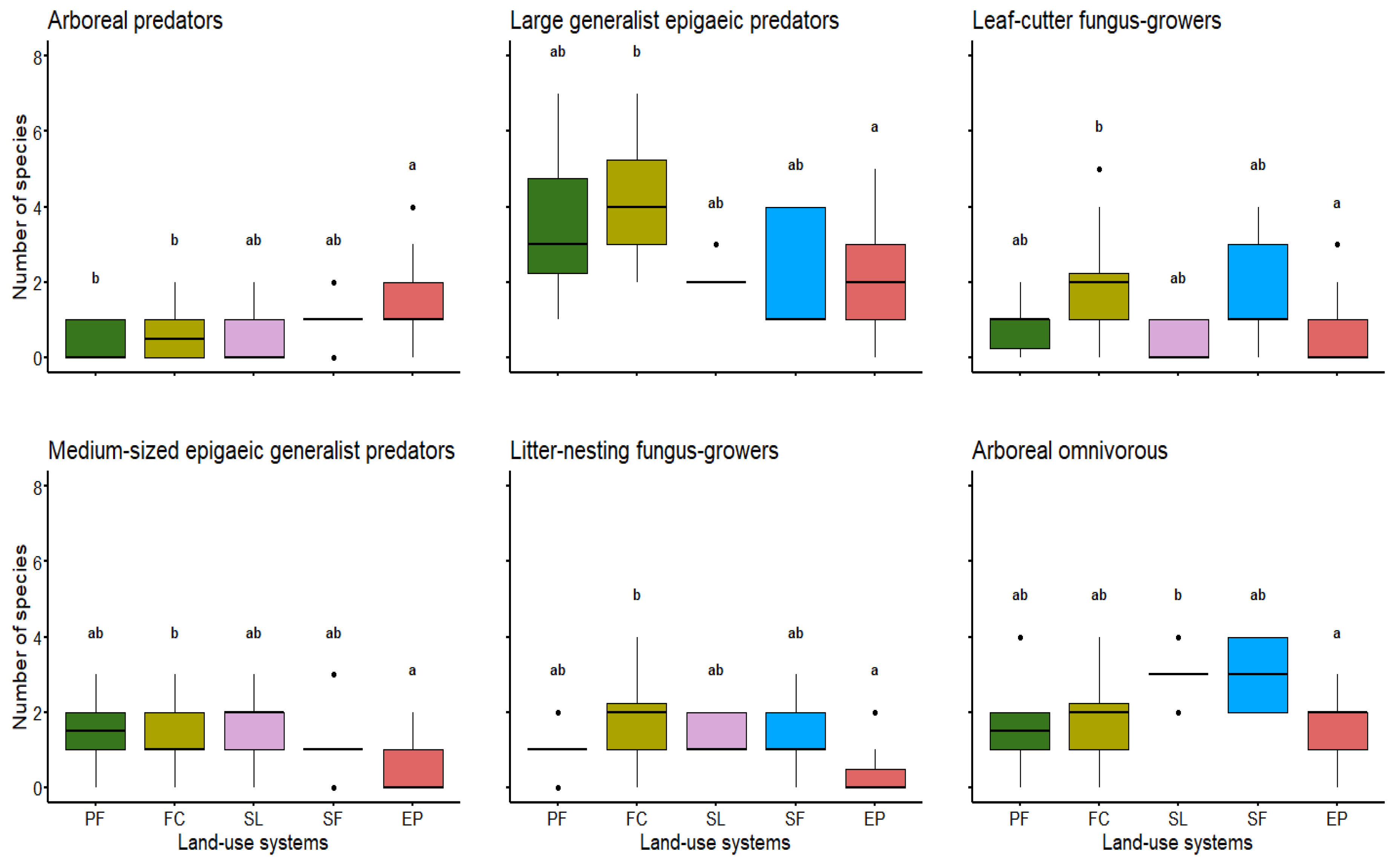
3.3. Guilds vs. Different Land-Use Systems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Mha | Mega hectare (ha × 106) |
| Aw | Tropical Monsoon Climate |
| °C | Degree Celsius |
| mm | Millimeter(s) |
| PF | Primary Forest |
| km | Kilometer(s) |
| SF | Secondary Forest |
| RIL | Reduced Impact Logging |
| FC | Forest Corridors |
| SL | Selective Logging |
| EP | Eucalyptus Plantation |
| ha | Hectare |
| h | Hour |
| UESB | Universidade Estadual do Sudoeste da Bahia |
| UFLA | Universidade Federal de Lavras |
| ANOVA | Analysis of Variance |
| PERMANOVA | Nonparametric Multivariate Analysis of Variance |
| NMDS | Non-Metric Multidimensional Scaling |
| PERMDISP | Homogeneity of Multivariate Dispersions Analysis |
| MaxT | Maximum Type Independence Test Statistic |
| Sobs | Richness Observed |
| Sest | Richness Estimated |
| SD | Standard Deviation |
| β | Beta Diversity |
References
- Barlow, J.; Ewers, R.M.; Anderson, L.; Aragao, L.E.O.C.; Baker, T.R.; Boyd, E.; Feldpausch, T.R.; Gloor, E.; Hall, A.; Malhi, Y.; et al. Using learning networks to understand complex systems: A case study of biological, geophysical and social research in the Amazon. Biol. Rev. 2011, 86, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Adams, G.L.; Albaladejo Robles, G.; Boakes, E.H.; Braga Ferreira, G.; Chapman, A.S.A.; Etard, A.; Gibb, R.; Millard, J.; Outhwaite, C.L.; et al. Climate and land-use change homogenise terrestrial biodiversity, with consequences for ecosystem functioning and human well-being. Emerg. Top. Life Sci. 2019, 3, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Teixeira, K.J.; Snyder, P.K.; Twine, T.E.; Cuadra, S.V.; Costa, M.H.; DeLucia, E.H. Climate-regulation services of natural and agricultural ecoregions of the Americas. Nat. Clim. Change 2012, 2, 177–181. [Google Scholar] [CrossRef]
- Berenguer, E.; Gardner, T.A.; Ferreira, J.; Aragão, L.E.O.C.; Camargo, P.B.; Cerri, C.E.; Durigan, M.; Oliveira Junior, R.C.; Vieira, I.C.G.; Barlow, J. Developing Cost-Effective Field Assessments of Carbon Stocks in Human-Modified Tropical Forests. PLoS ONE 2015, 10, e0133139. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.B.; Gangnon, R.E.; Barcellos, C.; Asner, G.P.; Patz, J.A. Influence of Deforestation, Logging, and Fire on Malaria in the Brazilian Amazon. PLoS ONE 2014, 9, e85725. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Berenguer, E.; França, F.; Ferreira, J.; Lees, A.C.; Louzada, J.; Sayer, E.J.; Solar, R.; Smith, C.C.; Aragão, L.E.O.C.; et al. Linking land-use and land-cover transitions to their ecological impact in the Amazon. Proc. Natl. Acad. Sci. USA 2022, 119, e2202310119. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Muscardi, D.; Almeida, S.S.P.; Schoereder, J.; Marques, T.; Sarcinelli, T.; Corrêa, A. Response of litter ants (Hymenoptera: Formicidae) to habitat heterogeneity and local resource availability in native and exotic forests. Sociobiology 2008, 52, 655–665. [Google Scholar]
- Ribas, C.R.; Campos, R.B.F.; Schmidt, F.A.; Solar, R.R.C. Ants as Indicators in Brazil: A Review with Suggestions to Improve the Use of Ants in Environmental Monitoring Programs. Psyche 2012, 2012, 636749. [Google Scholar] [CrossRef]
- Rizzotto, A.M.; Roani, A.H.; Guarda, C.; Giovenardi, R.; Lutinski, J.A. Mirmecofauna em áreas de preservação permanente e plantios florestais no noroeste do Rio Grande do Sul. Ciênc. Florest. 2019, 29, 1227–1240. [Google Scholar] [CrossRef]
- Solar, R.R.D.C.; Barlow, J.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Thomson, J.R.; Louzada, J.; Maués, M.; Moura, N.G.; Oliveira, V.H.F.; et al. How pervasive is biotic homogenization in human-modified tropical forest landscapes? Ecol. Lett. 2015, 18, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Ramírez, S.; Tscharntke, T.; Armbrecht, I.; Torres, W.; Grass, I. Decrease in β-diversity, but not in α-diversity, of ants in intensively managed coffee plantations. Insect Conserv. Diver. 2020, 13, 445–455. [Google Scholar] [CrossRef]
- Solar, R.R.D.C.; Barlow, J.; Andersen, A.N.; Schoereder, J.H.; Berenguer, E.; Ferreira, J.N.; Gardner, T.A. Biodiversity consequences of land-use change and forest disturbance in the Amazon: A multi-scale assessment using ant communities. Biol. Conserv. 2016, 197, 98–107. [Google Scholar] [CrossRef]
- Murray, B.R.; Baker, A.C.; Robson, T.C. Impacts of the Replacement of Native Woodland with Exotic Pine Plantations on Leaf-Litter Invertebrate Assemblages: A Test of a Novel Framework. Int. J. Ecol. 2009, 2009, 490395. [Google Scholar] [CrossRef]
- Azevedo, T.L.; Mello, A.A.; Ferreira, R.A.; Sanquetta, C.R.; Nakajima, N.Y. Equações hipsométricas e volumétricas para um povoamento de Eucalyptus sp. localizado na FLONA do Ibura, Sergipe. Rev. Ciênc. Agron. 2011, 6, 105–112. [Google Scholar] [CrossRef][Green Version]
- Beiroz, W.; Slade, E.M.; Barlow, J.; Silveira, J.M.; Louzada, J.; Sayer, E. Dung beetle community dynamics in undisturbed tropical forests: Implications for ecological evaluations of land-use change. Insect Conserv. Diver. 2016, 10, 94–106. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.A.; Araujo, I.S.; Ávila-Pires, T.C.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; Hernandez, M.I.M.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA 2007, 104, 18555–18560. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, P.L.B.; Viana, B.F.; Cardoso, M.Z.; De Melo, A.M.C.; Costa, M.G.C.; De Vasconcelos, R.N.; Dantas, T.B. What is the value of eucalyptus monocultures for the biodiversity of the Atlantic forest? A multitaxa study in southern Bahia, Brazil. J. For. Res. 2013, 24, 263–272. [Google Scholar] [CrossRef]
- Hawes, J.; Da Silva Motta, C.; Overal, W.L.; Barlow, J.; Gardner, T.A.; Peres, C.A. Diversity and composition of Amazonian moths in primary, secondary and plantation forests. J. Trop. Ecol. 2009, 25, 281–300. [Google Scholar] [CrossRef]
- Junqueira, L.K.; Florencio, D.F. Termite Damage in Agriculture Areas and Implanted Forests: An Ecological Approach. In Termites and Sustainable Management; Khan, M., Ahmad, W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 37–50. [Google Scholar] [CrossRef]
- Marsden, S.J.; Whiffin, M.; Galetti, M. Bird diversity and abundance in forest fragments and Eucalyptus plantations around an Atlantic forest reserve, Brazil. Biodivers. Conserv. 2001, 10, 737–751. [Google Scholar] [CrossRef]
- Wilker, I.; Queiroz, A.C.M.; Ribas, C.R.; Morini, M.S.C.; Lasmar, C.J.; Schmidt, F.A.; Feitosa, R.M.; Nogueira, A.; Baccaro, F.B.; Ulysséa, M.A.; et al. A systematic review of the land use change effects on ant diversity in Neotropics. Biol. Conserv. 2024, 299, 110778. [Google Scholar] [CrossRef]
- Paknia, O.; Pfeiffer, M. Hierarchical partitioning of ant diversity: Implications for conservation of biogeographical diversity in arid and semi-arid areas. Divers. Distrib. 2011, 17, 122–131. [Google Scholar] [CrossRef]
- Andersen, A.N. Not enough niches: Non-equilibrial processes promoting species coexistence in diverse ant communities. Austral Ecol. 2008, 33, 211–220. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Vilhena, J.M.S.; Facure, K.G.; Albernaz, A.L.K.M. Patterns of ant species diversity and turnover across 2000 km of Amazonian floodplain forest. J. Biogeogr. 2010, 37, 432–440. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Schmidt, F.A. Ant assemblages of Brazil nut trees Bertholletia excelsa in forest and pasture habitats in the Southwestern Brazilian Amazon. Biodivers. Conserv. 2019, 28, 329–344. [Google Scholar] [CrossRef]
- Schmidt, F.A.; Costa, M.M.S.D.; Martello, F.; De Oliveira, A.B.; Menezes, A.S.; Fontenele, L.K.; Morato, E.F.; Oliveira, M.A. Ant diversity studies in Acre: What we know and what we could do to know more? Bol. Mus. Para. Emílio Goeldi Ciênc. Nat. 2020, 15, 113–134. [Google Scholar] [CrossRef]
- Andersen, A.N. Responses of ant communities to disturbance: Five principles for understanding the disturbance dynamics of a globally dominant faunal group. J. Anim. Ecol. 2019, 88, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.R.; Brandão, C.R.F. Morphological patterns and community organization in leaf-litter ant assemblages. Ecol. Monogr. 2010, 80, 107–124. [Google Scholar] [CrossRef]
- Andersen, A. Functional groups and patterns of organization in North American ant communities: A comparison with Australia. J. Biogeogr. 1997, 24, 433–460. [Google Scholar] [CrossRef]
- Kwon, T.-S.; Lee, C.M.; Sung, J.H. Diversity decrease of ant (Formicidae, Hymenoptera) after a forest disturbance: Different responses among functional guilds. Zool. Stud. 2014, 53, 37. [Google Scholar] [CrossRef]
- Waltert, M.; Bobo, K.S.; Kaupa, S.; Montoya, M.L.; Nsanyi, M.S.; Fermon, H. Assessing Conservation Values: Biodiversity and Endemicity in Tropical Land Use Systems. PLoS ONE 2011, 6, e16238. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, A.C.M.; Rabello, A.M.; Braga, D.L.; Santiago, G.S.; Zurlo, L.F.; Philpott, S.M.; Ribas, C.R. Cerrado vegetation types determine how land use impacts ant biodiversity. Biodivers. Conserv. 2017, 29, 2017–2034. [Google Scholar] [CrossRef]
- Martello, F.; De Bello, F.; Morini, M.S.D.C.; Silva, R.R.; Souza-Campana, D.R.D.; Ribeiro, M.C.; Carmona, C.P. Homogenization and impoverishment of taxonomic and functional diversity of ants in Eucalyptus plantations. Sci. Rep. 2018, 8, 3266. [Google Scholar] [CrossRef] [PubMed]
- Arnan, X.; Cerdá, X.; Rodrigo, A.; Retana, J. Response of ant functional composition to fire. Ecography 2013, 36, 1182–1192. [Google Scholar] [CrossRef]
- Bihn, J.H.; Gebauer, G.; Brandl, R. Loss of functional diversity of ant assemblages in secondary tropical forests. Ecology 2010, 91, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Leal, I.R.; Filgueiras, B.K.C.; Gomes, J.P.; Iannuzzi, L.; Andersen, A.N. Effects of habitat fragmentation on ant richness and functional composition in Brazilian Atlantic forest. Int. J. Biodivers. Conserv. 2012, 21, 1687–1701. [Google Scholar] [CrossRef]
- Climate Data for Cities Worldwide. Available online: https://en.climate-data.org/ (accessed on 20 April 2021).
- Coutinho, S.d.C.; Pires, M.J.P. Jari um Banco Genético Para o Futuro; IMAGO: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- Barlow, J.; Louzada, J.; Parry, L.; Hernández, M.I.M.; Hawes, J.; Peres, C.A.; Vaz-de-Mello, F.Z.; Gardner, T.A. Improving the design and management of forest strips in human-dominated tropical landscapes: A field test on Amazonian dung beetles. J. Appl. Ecol. 2010, 47, 779–788. [Google Scholar] [CrossRef]
- Parry, L.; Barlow, J.; Peres, C.A. Allocation of hunting effort by Amazonian smallholders: Implications for conserving wildlife in mixed-use landscapes. Bio. Conserv. 2009, 142, 1777–1786. [Google Scholar] [CrossRef]
- Agosti, D.; Majer, J.D.; Alonso, L.E.; Schultz, T.R. Ants: Standard Methods for Measuring and Monitoring Biodiversity; Smithsonian Institution Press: London, UK; Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Bestelmeyer, B.; Agosti, D.; Alonso, L.; Brandao, C.R.; Brown, W.L.J.; Delabie, J.; Silvestre, R. Field techniques for the study of ground-dwelling ants: An overview, description and evaluation. In Ants: Standard Methods for Measuring and Monitoring Biodiversity; Smithsonian Institution Press: London, UK; Washington, DC, USA, 2000; pp. 122–144. [Google Scholar]
- Sheikh, A.H.; Ganaie, G.A.; Thomas, M.; Bhandari, R.; Rather, Y.A. Ant pitfall trap sampling: An overview. J. Entomol. Res. 2018, 42, 421–436. [Google Scholar] [CrossRef]
- Przybyszewski, K.R.; Silva, R.J.; Vicente, R.E.; Garcia Freitas, J.V.; Pereira, M.J.B.; Izzo, T.J.; Tonon, D.S. Can Baited Pitfall Traps for Sampling Dung Beetles Replace Conventional Traps for Sampling Ants? Sociobiology 2020, 67, 376. [Google Scholar] [CrossRef]
- Baccaro, F.B.; Feitosa, R.M.; Fernández, F.; Fernandes, I.O.; Izzo, T.J.; de Souza, J.L.P.; Solar, R.R.C. Guia Para os Gêneros de Formigas do Brasil; Instituto Nacional de Pesquisas da Amazônia: Manaus, Brazil, 2015. [Google Scholar] [CrossRef]
- Brandão, C.R.F.; Silva, R.R.; Delabie, J.H.C. Bioecologia e Nutrição de Insetos. Base para o Manejo Integrado de Pragas.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009. [Google Scholar]
- Gotelli, N.J.; Ellison, A.M.; Dunn, R.R.; Sanders, N.J. Counting ants (Hymenoptera: Formicidae): Biodiversity sampling and statistical analysis for myrmecologists. Myrmecol. News 2011, 15, 13–19. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.7. 2024. Available online: https://github.com/florianhartig/DHARMa (accessed on 15 July 2025).
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2025. Available online: https://rvlenth.github.io/emmeans/ (accessed on 15 July 2025).
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; Primer-E: Plymouth, UK, 2014. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Martinez Arbizu, P. PairwiseAdonis: Pairwise Multilevel Comparison Using adonis. R Package Version 0.4. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 15 July 2025).
- Anderson, M.J. Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Dinno, A.; dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums. R Package Version 1.3.6. Available online: https://cran.r-project.org/web/packages/dunn.test/index.html (accessed on 15 July 2025).
- R Core Team. R: The R Project for Statistical Computing. 2025. Available online: https://www.r-project.org/ (accessed on 15 July 2025).
- Albuquerque, E.Z.; Prado, L.P.D.; Andrade-Silva, J.; De Siqueira, E.L.S.; Da Silva Sampaio, K.L.; Alves, D.; Brandão, C.R.F.; Andrade, P.L.; Feitosa, R.M.; De Azevedo Koch, E.B.; et al. Ants of the State of Pará, Brazil: A historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 2021, 5001, 1–83. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.N.; Morato, E.F.; Oliveira, M.A.; Delabie, J.H.C. A riqueza e composição de formigas como indicadores dos efeitos do manejo florestal de baixo impacto em floresta tropical no estado do Acre. Rev. Árvore 2013, 37, 163–173. [Google Scholar] [CrossRef]
- Wilkie, K.T.; Mertl, A.L.; Traniello, J.F.A. Species Diversity and Distribution Patterns of the Ants of Amazonian Ecuador. PLoS ONE 2010, 5, e13146. [Google Scholar] [CrossRef]
- Chao, K.-J.; Phillips, O.L.; Baker, T.R.; Peacock, J.; Lopez-Gonzalez, G.; Vásquez Martínez, R.; Monteagudo, A.; Torres-Lezama, A. After trees die: Quantities and determinants of necromass across Amazonia. Biogeosciences 2009, 6, 1615–1626. [Google Scholar] [CrossRef]
- Frizzo, T.L.M.; Campos, R.I.; Vasconcelos, H.L. Contrasting Effects of Fire on Arboreal and Ground-Dwelling Ant Communities of a Neotropical Savanna. Biotropica 2012, 44, 254–261. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Bruna, E.M. Arthropod responses to the experimental isolation of Amazonian forest fragments. Zoologia 2012, 29, 515–530. [Google Scholar] [CrossRef]
- Azevedo-Ramos, C.; De Carvalho, O.; Do Amaral, B.D. Short-term effects of reduced-impact logging on eastern Amazon fauna. For. Ecol. Manag. 2006, 232, 26–35. [Google Scholar] [CrossRef]
- Gómez, C.; Abril, S. Selective logging in public pine forests of the central Iberian Peninsula: Effects of the recovery process on ant assemblages. For. Ecol. Manag. 2011, 262, 1061–1066. [Google Scholar] [CrossRef]
- Gunawardene, N.R.; Majer, J.D.; Edirisinghe, J.P. Investigating residual effects of selective logging on ant species assemblages in Sinharaja Forest Reserve, Sri Lanka. For. Ecol. Manag. 2010, 259, 555–562. [Google Scholar] [CrossRef]
- Chan, K.M.A.; Daily, G.C. The payoff of conservation investments in tropical countryside. Proc. Natl. Acad. Sci. USA 2008, 105, 19342–19347. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.A.; Barlow, J.; Chazdon, R.; Ewers, R.M.; Harvey, C.A.; Peres, C.A.; Sodhi, N.S. Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 2009, 12, 561–582. [Google Scholar] [CrossRef] [PubMed]
- King, J.R.; Tschinkel, W.R. Experimental evidence that human impacts drive fire ant invasions and ecological change. Proc. Natl. Acad. Sci. USA 2008, 105, 20339–20343. [Google Scholar] [CrossRef] [PubMed]
- Latreille, P.A. Histoire Naturelle des Fourmis, et Recueil de Mémoires et D’observations sur les Abeilles, les Araignées, les Faucheurs, et Autres Insectes; Impr. Crapelet (chez T. Barrois): Paris, France, 1802. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Morrison, A.J. Developing a global leadership model. Hum. Resour. Manag. 2000, 39, 117–131. [Google Scholar] [CrossRef]
- Beiroz, W.; Audino, L.; Queiroz, A.C.; Rabello, A.; Alves Boratto, I.; Silva, Z.; Ribas, C. Structure and composition of edaphic arthropod community and its use as bioindicators of environmental disturbance. Appl. Ecol. Environ. Res. 2014, 12, 481–491. [Google Scholar] [CrossRef]
- Schmidt, F.A.; Ribas, C.R.; Schoereder, J.H. How predictable is the response of ant assemblages to natural forest recovery? Implications for their use as bioindicators. Ecol. Indic. 2013, 24, 158–166. [Google Scholar] [CrossRef]
- Del Toro, I.; Ribbons, R.; Pelini, S. The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 2012, 17, 133–146. [Google Scholar]
- Loreau, M.; De Mazancourt, C. Biodiversity and ecosystem stability: A synthesis of underlying mechanisms. Ecol. Lett. 2013, 16, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.B. Impacts of Eucalyptus globulus plantations on Atlantic streams: Changes in invertebrate density and shredder traits. Fundam. Appl. Limnol. 2009, 175, 151–160. [Google Scholar] [CrossRef]
- Beier, P.; Noss, R.F. Do Habitat Corridors Provide Connectivity? Conserv. Biol. 1998, 12, 1241–1252. [Google Scholar] [CrossRef]
- Lees, A.C.; Peres, C.A. Conservation Value of Remnant Riparian Forest Corridors of Varying Quality for Amazonian Birds and Mammals. Conserv. Biol. 2008, 22, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Tewksbury, J.J.; Levey, D.J.; Haddad, N.M.; Sargent, S.; Orrock, J.L.; Weldon, A.; Danielson, B.J.; Brinkerhoff, J.; Damschen, E.I.; Townsend, P. Corridors affect plants, animals, and their interactions in fragmented landscapes. Proc. Natl. Acad. Sci. USA 2002, 99, 12923–12926. [Google Scholar] [CrossRef] [PubMed]
- Hilty, J.; Worboys, G.L.; Keeley, A.; Woodley, S.; Lausche, B.J.; Locke, H.; Carr, M.; Pulsford, I.; Pittock, J.; White, J.W.; et al. Guidelines for Conserving Connectivity Through Ecological Networks and Corridors; International Union for Conservation of Nature: Gland, Switzerland, 2020. [Google Scholar] [CrossRef]
- Philpott, S.M.; Arendt, W.J.; Armbrecht, I.; Bichier, P.; Diestch, T.V.; Gordon, C.; Greenberg, R.; Perfecto, I.; Reynoso-Santos, R.; Soto-Pinto, L.; et al. Biodiversity Loss in Latin American Coffee Landscapes: Review of the Evidence on Ants, Birds, and Trees. Conserv. Biol. 2008, 22, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.D.S.; Zanetti, R.; Santos, M.S.; Peñaflor, M.F.G.V.; Broglio, S.M.F.; Delabie, J.H.C. The Impact of Coffee and Pasture Agriculture on Predatory and Omnivorous Leaf-Litter Ants. J. Insect Sci. 2013, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Kassen, R. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 2002, 15, 173–190. [Google Scholar] [CrossRef]
- Wirth, R.; Meyer, S.; Leal, I.; Tabarelli, M. Plant Herbivore Interactions at the Forest Edge; In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2008; Volume 69, pp. 423–448. [Google Scholar] [CrossRef]
- Mayhé-Nunes, A.J. Sinopse do gênero Mycetarotes (Hym., Formicidae), com a descrição de duas especies novas. Bol. Entomol. Venez. 1995, 10, 197–205. [Google Scholar]
- Lucia, D.T.M.C. Formigas-Cortadeiras: Da Bioecologia ao Manejo; Editora UFV: Vicosa, Brazil, 2011. [Google Scholar]
- Lacau, L.; Villemant, C.; Bueno, O.C.; Delabie, J.H.C.; Lacau, S. Morphology of the eggs and larvae of Cyphomyrmex transversus Emery (Formicidae: Myrmicinae: Attini) and a note on the relationship with its symbiotic fungus. Zootaxa 2008, 1923, 37–54. [Google Scholar] [CrossRef]
- Silvestre, R.; Brandão, C.R.F.; Silva, R.R. Grupos funcionales de hormigas: El caso de los gremios del Cerrado. In Introducción a Las Hormigas de Las Región Neotropical; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2003; pp. 101–136. [Google Scholar]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lach, L.; Hooper-Bu`i, L.M. Consequences of ant inva-sions. In Ant Ecology; Lach, L., Parr, C.L., Abbott, K.L., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 261–286. [Google Scholar]
- Lindenmayer, D.; Pierson, J.; Barton, P.; Beger, M.; Branquinho, C.; Calhoun, A.; Caro, T.; Greig, H.; Gross, J.; Heino, J.; et al. A new framework for selecting environmental surrogates. Sci. Total Environ. 2015, 538, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Arcoverde, G.B.; Andersen, A.N.; Setterfield, S.A. Is livestock grazing compatible with biodiversity conservation? Impacts on savanna ant communities in the Australian seasonal tropics. Biodivers. Conserv. 2017, 26, 883–897. [Google Scholar] [CrossRef]


| Land-Use System | PF | SF | FC | SL | EP |
|---|---|---|---|---|---|
| PF | - | - | - | - | - |
| SF | 0.09 | - | - | - | - |
| FC | 0.13 | 0.22 | - | - | - |
| SL | 1.00 | 0.12 | 0.06 | - | - |
| EP | 0.01 | 0.01 | 0.01 | 0.01 | - |
| Land-Use System | PF | SF | FC | SL | EP |
|---|---|---|---|---|---|
| PF | - | - | - | - | - |
| SF | 1.00 | - | - | - | - |
| FC | 1.00 | 1.00 | - | - | - |
| SL | 1.00 | 1.00 | 0.48 | - | - |
| EP | 0.01 | 1.00 | 0.01 | 0.01 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, E.; Borges, C.M.; Albuquerque, E.Z.; Florencio, D.F.; Fernandes, I.; Tolentino, M.; Korasaki, V.; Louzada, J.; Zanetti, R. What Can Ground-Dwelling Ants Tell Us About Different Land-Use Systems in the Brazilian Amazon? Forests 2025, 16, 1190. https://doi.org/10.3390/f16071190
Silva E, Borges CM, Albuquerque EZ, Florencio DF, Fernandes I, Tolentino M, Korasaki V, Louzada J, Zanetti R. What Can Ground-Dwelling Ants Tell Us About Different Land-Use Systems in the Brazilian Amazon? Forests. 2025; 16(7):1190. https://doi.org/10.3390/f16071190
Chicago/Turabian StyleSilva, Elisangela, Cristina Machado Borges, Emília Zoppas Albuquerque, Daniela Faria Florencio, Izaias Fernandes, Mariana Tolentino, Vanesca Korasaki, Júlio Louzada, and Ronald Zanetti. 2025. "What Can Ground-Dwelling Ants Tell Us About Different Land-Use Systems in the Brazilian Amazon?" Forests 16, no. 7: 1190. https://doi.org/10.3390/f16071190
APA StyleSilva, E., Borges, C. M., Albuquerque, E. Z., Florencio, D. F., Fernandes, I., Tolentino, M., Korasaki, V., Louzada, J., & Zanetti, R. (2025). What Can Ground-Dwelling Ants Tell Us About Different Land-Use Systems in the Brazilian Amazon? Forests, 16(7), 1190. https://doi.org/10.3390/f16071190








