Simulation and Prediction of the Potential Distribution of Two Varieties of Dominant Subtropical Forest Oaks in Different Climate Scenarios
Abstract
1. Introduction
2. Materials and Methods
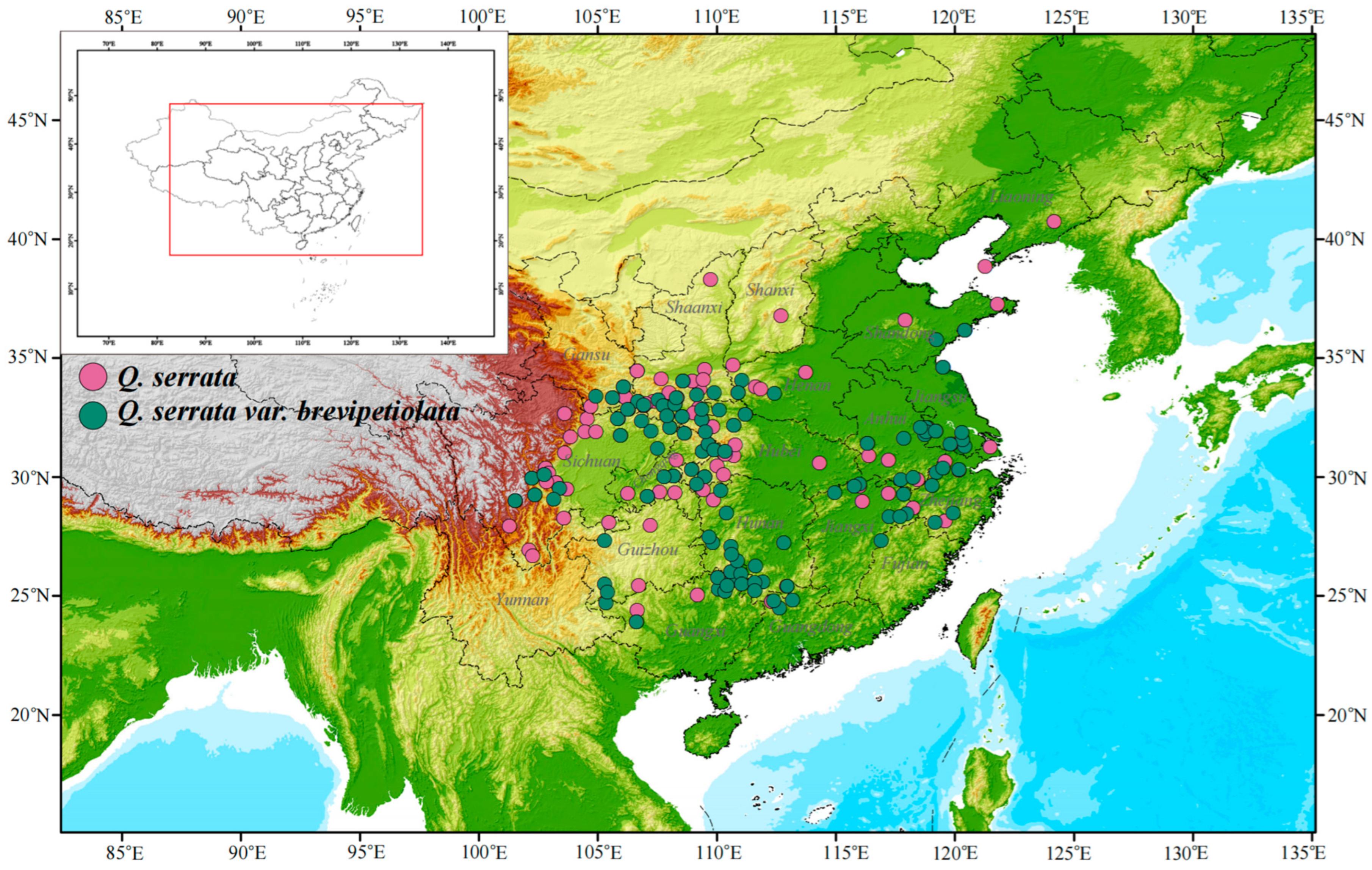
2.1. Species Occurrence Data
2.2. Environmental Variables
2.3. Niche Analyses in Geographical Space (G-Space)
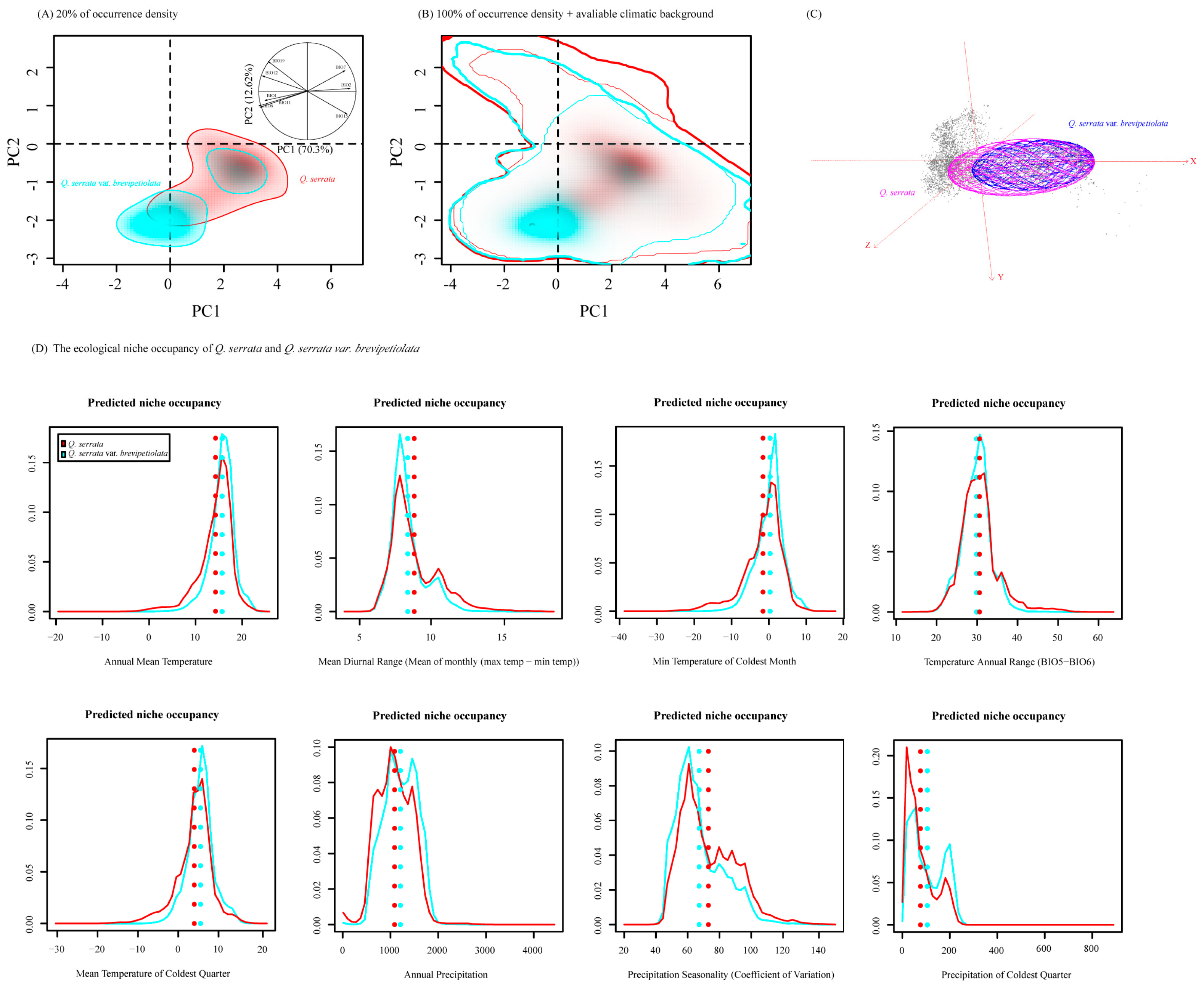
2.4. Niche Analyses in Environmental Space (E-Space)
2.5. Shifts in the Centroids of Suitable Distribution Areas
3. Results
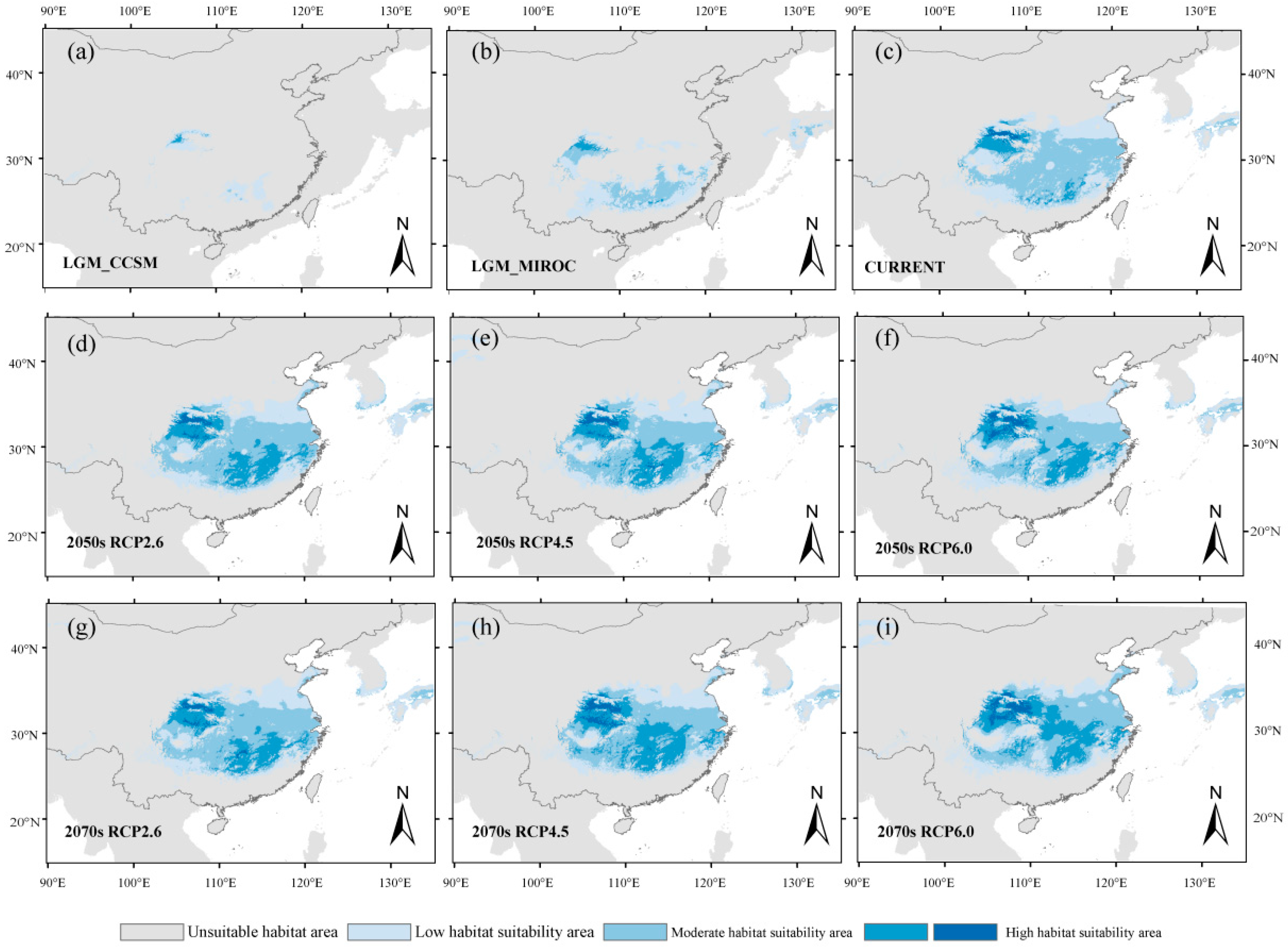
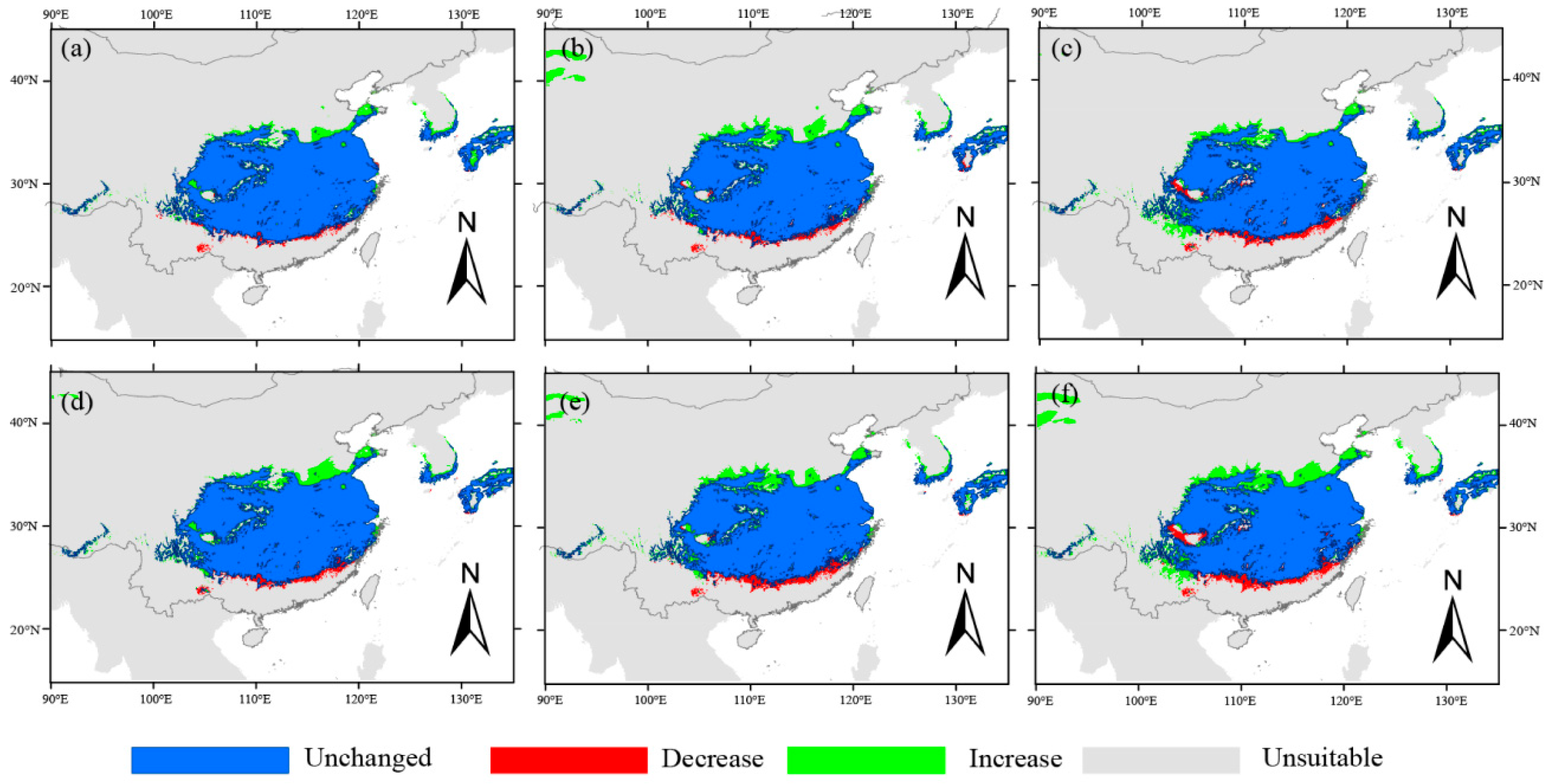
3.1. Potential Geographical Distribution of Q. serrata and Q. serrata var. brevipetiolata Under Different Climatic Scenarios
3.2. Niche Variation on E-Space
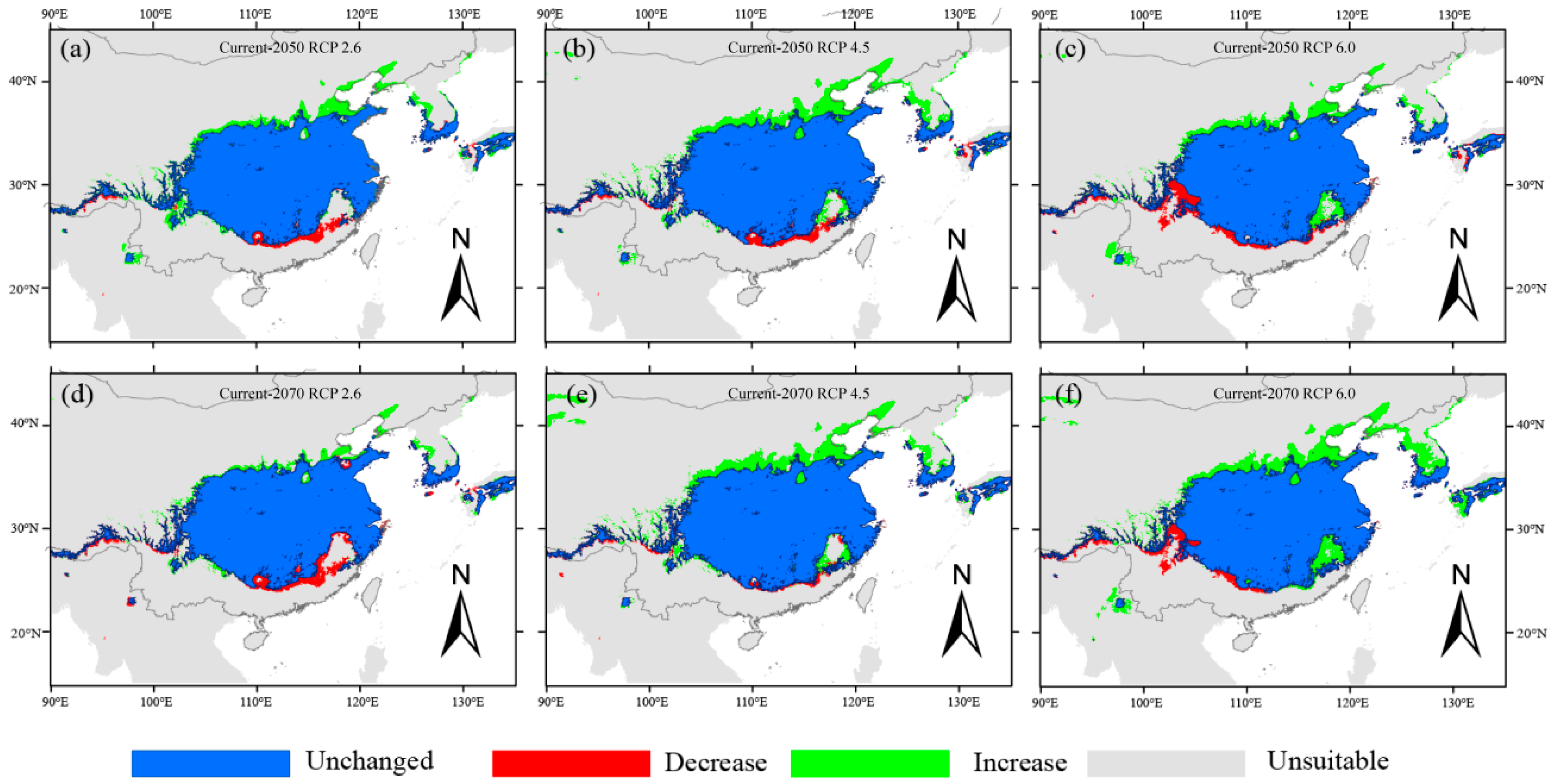
3.3. Changes in Habitat Suitability of Q. serrata and Q. serrata var. brevipetiolata
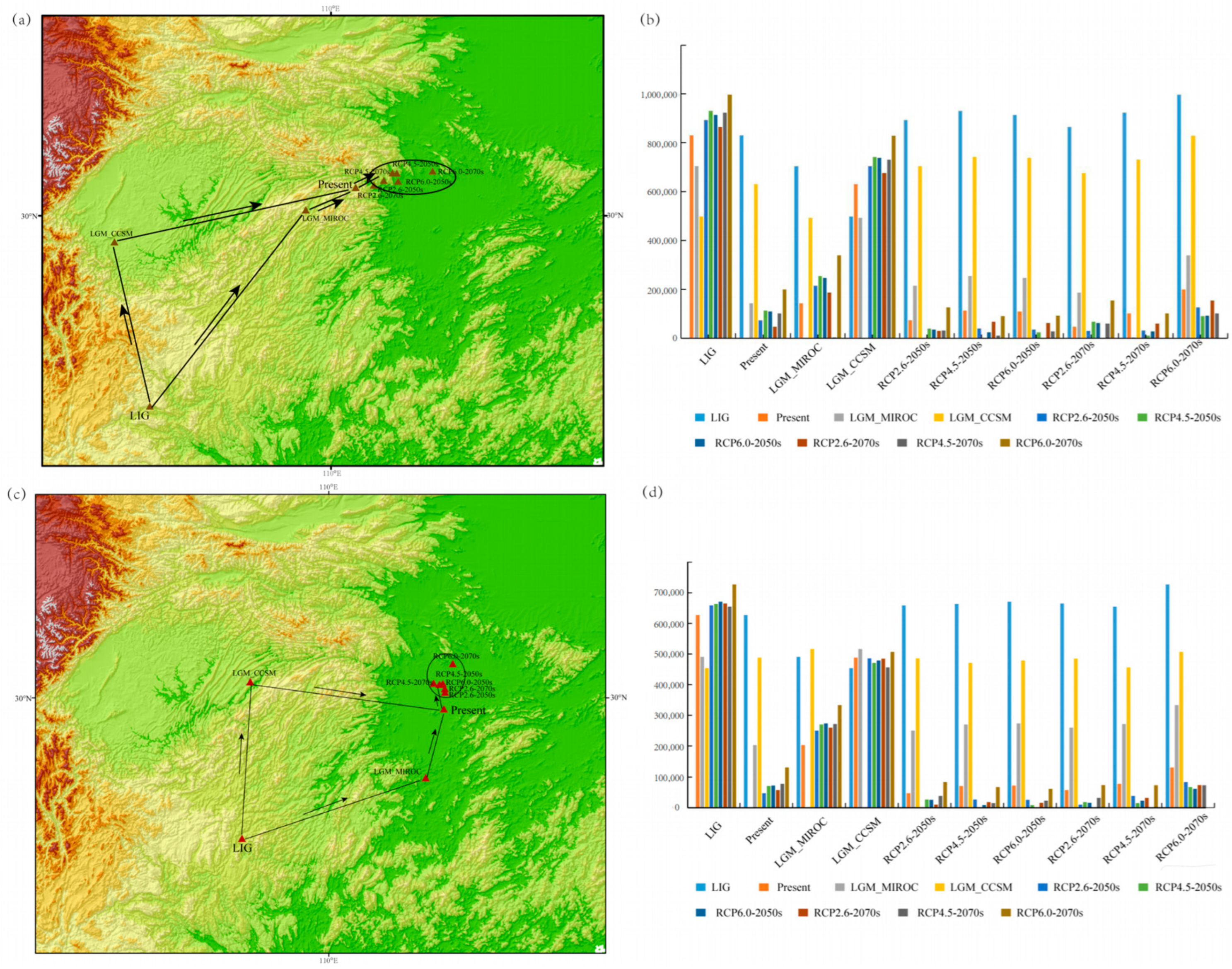
3.4. Displacement Trends Based on the Geometric Centers of Suitable Habitats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.L.; Li, X.; Zhao, Z.; Wei, H.; Gao, B.; Gu, W. Prediction of the potential geographic distribution of the ectomycorrhizal mushroom Tricholoma matsutake under multiple climate change scenarios. Sci. Rep. 2017, 7, 46221. [Google Scholar] [CrossRef] [PubMed]
- Euskirchen, E.S.; Carman, T.B.; McGuire, A.D. Changes in the structure and function of Northern Alaskan ecosystems when considering variable leaf-out times across groupings of species in a dynamic vegetation model. Glob. Change Biol. 2014, 20, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Rellstab, C.; Zoller, S.; Walthert, L.; Lesur, I.; Pluess, A.R.; Graf, R.; Bodénès, C.; Sperisen, C.; Kremer, A.; Gugerli, F. Signatures of local adaptation in candidate genes of oaks (Quercus spp.) with respect to present and future climatic conditions. Mol. Ecol. 2016, 25, 5907–5924. [Google Scholar] [CrossRef] [PubMed]
- Fordham, D.A.; Resit Akçakaya, H.; Araújo, M.B.; Elith, J.; Keith, D.A.; Pearson, R.; Auld, T.D.; Mellin, C.; Morgan, J.W.; Regan, T.J.; et al. Plant extinction risk under climate change: Are forecast range shifts alone a good indicator of species vulnerability to global warming? Glob. Change Biol. 2012, 18, 1357–1371. [Google Scholar] [CrossRef]
- Chen, X.D.; Yang, J.; Guo, Y.F.; Zhao, Y.M.; Zhou, T.; Zhang, X.; Ju, M.M.; Li, Z.H.; Zhao, G.F. Spatial genetic structure and demographic history of the dominant forest oak Quercus fabri hance in subtropical China. Front. Plant Sci. 2021, 11, 583284. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.G.; Jin, Y.; Wang, X.R.; Mao, J.F.; Li, Y. Predicting impacts of future climate change on the distribution of the widespread conifer Platycladus orientalis. PLoS ONE 2015, 10, e0132326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, H.; Zhang, X.; Liu, J.; Zhang, Q.; Gu, W. Non-pessimistic predictions of the distributions and suitability of Metasequoia glyptostroboides under climate change using a random forest model. Forests 2020, 11, 62. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Population studies-Animal ecology and demography-Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Bertrand, R.; Perez, V.; Gégout, J.C. Disregarding the edaphic dimension in species distribution models leads to the omission of crucial spatial information under climate change: The case of Quercus pubescens in France. Glob. Change Biol. 2012, 18, 2648–2660. [Google Scholar] [CrossRef]
- Faleiro, F.V.; Machado, R.B.; Loyola, R.D. Defining spatial conservation priorities in the face of land-use and climate change. Biol. Conserv. 2013, 158, 248–257. [Google Scholar] [CrossRef]
- Denk, T.; Grimm, G.W.; Manos, P.S.; Deng, M.; Hipp, A.L. An updated infrageneric classification of the oaks: Review of previous taxonomic schemes and synthesis of evolutionary patterns. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Springer: Cham, Switzerland, 2017; pp. 13–38. [Google Scholar]
- Wang, Y.; Lei, Y.; Huang, Y.; Wang, Z.; Xu, J.; He, X. Jasmonates from Chinese acorns (Quercus serrata var. brevipetiolata) exert pronounced anti-neuroinflammatory activities. Bioorg. Chem. 2020, 103, 104143. [Google Scholar] [CrossRef] [PubMed]
- Vila-Viçosa, C.; Arenas-Castro, S.; Marcos, B.; Honrado, J.; García, C.; Vázquez, F.M.; Almeida, R.; Gonçalves, J. Combining Satellite Remote Sensing and Climate Data in Species Distribution Models to Improve the Conservation of Iberian White Oaks (Quercus L.). ISPRS Int. J. Geo-Inf. 2020, 9, 735. [Google Scholar] [CrossRef]
- Mirhashemi, H.; Heydari, M.; Ahmadi, K.; Karami, O.; Kavgaci, A.; Matsui, T.; Heung, B. Species distribution models of Brant’s oak (Quercus brantii Lindl.): The impact of spatial database on predicting the impacts of climate change. Ecol. Eng. 2023, 194, 107038. [Google Scholar] [CrossRef]
- Dumolin-Lapègue, S.; Demesure, B.; Fineschi, S.; Le Corre, V.; Petit, R.J. Phylogeographic structure of white oaks throughout the European continent. Genetics 1997, 146, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.J.; Brewer, S.; Bordács, S.; Burg, K.; Cheddadi, R.; Coart, E.; Cottrell, J.; Csaikl, U.M.; van Dam, B.; Deans, J.D.; et al. Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For. Ecol. Manag. 2002, 156, 49–74. [Google Scholar] [CrossRef]
- Grivet, D.; Deguilloux, M.F.; Petit, R.J.; Sork, V.L. Contrasting patterns of historical colonization in white oaks (Quercus spp.) in California and Europe. Mol. Ecol. 2006, 15, 4085–4093. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, S.; Eastwood, A. The Red List of Oaks; Fauna and Flora International (FFI): Cambridge, UK, 2007. [Google Scholar]
- Xu, Y.C.; Ren, X.W. Flora of China; Science Press: Beijing, China, 1998; pp. 235–236. [Google Scholar]
- Huang, C.C.; Chang, Y.C. Fagaceae. In Flora Reipublicae Popularis Sinicae; Huang, C.C., Ed.; Science Press: Beijing, China, 1998; Volume 22. [Google Scholar]
- Huang, J.; Wang, Y.; Li, C.; Wang, X.; He, X. Triterpenes isolated from acorns of Quercus serrata var. brevipetiolata exert anti-inflammatory activity. Ind. Crops Prod. 2016, 91, 302–309. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, Y.; Wang, Y.; He, X. Potential anti-neuroinflammatory triterpenoids isolated from Chinese acorns (Quercus serrata var. brevipetiolata). J. Funct. Foods 2018, 50, 18–25. [Google Scholar] [CrossRef]
- Ju, M.; Yang, J.; Yue, M.; Zhao, G. Speciation patterns of related species under the hybrid zone: A case study of three sclerophyllous oaks in the east Himalaya-Hengduan Mountains. Mol. Ecol. 2023, 32, 4610–4626. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Chen, X.; Ni, J.; Cheddadi, R.; Guiot, J.; Han, H.; Harrison, S.P.; Huang, C.; Ke, M.; Kong, Z.; et al. Palaeovegetation of China: A pollen databased synthesis for the mid-Holocene and last glacial maximum. J. Biogeogr. 2000, 27, 635–664. [Google Scholar] [CrossRef]
- Harrison, S.P.; Yu, G.; Takahara, H.; Prentice, I.C. Palaeovegetation: Diversity of temperate plants in East Asia. Nature 2001, 413, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Shao, J.W.; Lu, C.; Zhang, X.P.; Qiu, Y.X. Chloroplast phylogeography of a temperate tree Pteroceltis tatarinowii (Ulmaceae) in China. J. Syst. Evol. 2012, l50, 325–333. [Google Scholar] [CrossRef]
- Collins, W.D.; Bitz, C.M.; Blackmon, M.L.; Bonan, G.B.; Bretherton, C.S.; Carton, J.A.; Chang, P.; Doney, S.C.; Hack, J.J.; Henderson, T.B.; et al. The community climate system model version 3 (CCSM3). J. Clim. 2006, 19, 2122–2143. [Google Scholar] [CrossRef]
- Hasumi, H.; Emori, S. K-1 Coupled GCM (MIROC) Description Center for Climate System Research. University of Tokyo: Tokyo, Japan, 2004. [Google Scholar]
- Rutledge, D. Estimating long-term world coal production with logit and probit transforms. Int. J. Coal Geol. 2011, 85, 23–33. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, X.D.; López-Pujol, J.; Bai, G.Q.; Herrando-Moraira, S.; Nualart, N.; Zhang, X.; Zhao, Y.M.; Zhao, G.F. Genetically-and environmentally-dependent processes drive interspecific and intraspecific divergence in the Chinese relict endemic genus Dipteronia. Plant Divers. 2024, 46, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Herrando-Moraira, S.; Nualart, N.; Herrando-Moraira, A.; Chung, M.Y.; López-Pujol, J. Climatic niche characteristics of native and invasive Lilium lancifolium. Sci. Rep. 2019, 9, 14334. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Zhang, X.; Zhang, H.; Zhou, T.; Zhao, Y.M.; Yang, J.; Zhao, G.F. Genetic Differentiation and Demographic History of Three Cerris Oak Species in China Based on Nuclear Microsatellite Makers. Forests 2021, 12, 1164. [Google Scholar] [CrossRef]
- Evans, M.E.; Smith, S.A.; Flynn, R.S.; Donoghue, M.J. Climate, niche evolution, and diversification of the “bird-cage” evening primroses (Oenothera, sections Anogra and Kleinia). Am. Nat. 2009, 173, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Yang, J.; Feng, L.I.; Zhou, T.; Zhang, H.; Li, H.M.; Zhao, G.F. Phylogeography and population dynamics of an endemic oak (Quercus fabri Hance) in subtropical China revealed by molecular data and ecological niche modeling. Tree Genet. Genomes 2020, 16, 2. [Google Scholar] [CrossRef]
- Tang, C.Q.; Dong, Y.F.; Herrando-Moraira, S.; Matsui, T.; Ohashi, H.; He, L.Y.; Nakao, K.; Tanaka, N.; Tomita, M.; Li, X.S.; et al. Potential effects of climate change on geographic distribution of the Tertiary relict tree species Davidia involucrata in China. Sci. Rep. 2017, 7, 43822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Li, Y.; Zhang, Q.; Fang, Y.M. Ancient east-west divergence, recent admixture, and multiple marginal refugia shape genetic structure of a widespread oak species (Quercus acutissima) in China. Tree Genet. Genomes 2018, 14, 88. [Google Scholar] [CrossRef]
- Xu, J.; Deng, M.; Jiang, X.L.; Westwood, M.; Song, Y.G.; Turkington, R. Phylogeography of Quercus glauca (Fagaceae), a dominant tree of East Asian subtropical evergreen forests, based on three chloroplast DNA interspace sequences. Tree Genet. Genomes 2015, 11, 805. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Kang, H.; Sun, X.; Yin, S.; Du, H.; Yamanaka, N.; Gapare, W.; Wu, H.X.; Liu, C.J. Phylogeography of Quercus variabilis based on chloroplast DNA sequence in East asia: Multiple glacial refugia and mainland-migrated island populations. PLoS ONE 2012, 7, e47268. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; Lister, A.M.; Barnes, I.; Dalen, L. Refugia revisited: Individualistic responses of species in space and time. Proc. R. Soc. B Biol. Sci. 2010, 277, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Jiang, D. Revisiting last glacial maximum climate over China and East Asian monsoon using PMIP3 simulations. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 453, 115–126. [Google Scholar] [CrossRef]
- Chabangborn, A.; Brandefelt, J.; Wohlfarth, B. Asian monsoon climate during the Last Glacial Maximum: Palaeo-data–model comparisons. Boreas 2014, 43, 220–242. [Google Scholar] [CrossRef]
- Alder, J.R.; Hostetler, S.W. Global climate simulations at 3000 year intervals for the last 21000 years with the GENMOM coupled atmosphere–ocean model. Clim. Past Discuss 2014, 10, 2925–2978. [Google Scholar]
- Birks, H.J.B.; Willis, K.J. Alpines, trees, and refugia in Europe. Plant Ecol. Divers 2008, 1, 147–160. [Google Scholar] [CrossRef]
- Fan, D.; Hu, W.; Li, B.; Morris, A.B.; Zheng, M.; Soltis, D.E.; Soltis, P.S.; Zhang, Z. Idiosyncratic responses of evergreen broad-leaved forest constituents in China to the late Quaternary climate changes. Sci. Rep. 2016, 6, 31044. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.F.; Cui, Z.J.; Su, Z. The Quaternary glaciations and environmental variations in China. Hebei Sci. Technol. Press Shijiazhuang 2006, 65, 101. [Google Scholar]
- Feng, L.; Zheng, Q.J.; Qian, Z.Q.; Yang, J.; Zhang, Y.P.; Li, Z.H.; Zhao, G.F. Genetic structure and evolutionary history of three Alpine Sclerophyllous oaks in East Himalaya-Hengduan Mountains and adjacent regions. Front. Plant Sci. 2016, 7, 1688. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to Quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Jansson, R.; Dynesius, M. The fate of clades in a world of recurrent climatic change: Milankovitch oscillations and evolution. Annu. Rev. Ecol. Syst. 2002, 33, 741–777. [Google Scholar] [CrossRef]
- Petit, R.J.; Hampe, A.; Cheddadi, R. Climate changes and tree phylogeography in the Mediterranean. Taxon 2005, 54, 877–885. [Google Scholar] [CrossRef]
- Fang, F.; Sun, H.; Zhao, Q.; Lin, C.; Sun, Y.F.; Gao, W.; Xu, J.J.; Zhou, J.Y.; Ge, F.; Liu, N.F. Patterns of diversity, areas ofendemism, andmultiple glacial refuges for freshwater crabs of the genus Sinopotamon in China Decapoda: Brachyura: Potamidae). PLoS ONE 2013, 8, e53143. [Google Scholar]
- Qu, Y.; Ericson, P.G.P.; Quan, Q.; Song, G.; Zhang, B.G.; Lei, F.M. Long-term isolation and stability explainhigh genetic diversity in the Eastern Himalaya. Mol. Ecol. 2014, 23, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Huang, M.; Wang, X.S.; Ji, L.Q.; Zhang, Y.L. Centers of endemism and diversitypatterns for typhlocybine leafhoppers (Hemiptera: Cicadellidae: Typhlocybinae) in China. Insect Sci. 2014, 21, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Ji, Y.K.; Zhao, Q.; Wang, Y.J.; Gao, W.; Chu, K.L.; Sun, H.Y. Phylogeography of the Chinese endemic freshwater crab Sinopotamon acutum (Brachyura, Potamidae). Zoo. Scr. 2015, 44, 653–666. [Google Scholar] [CrossRef]
- Li, J.; Wen, X. A study on the sources variation test of Platycladus orientalis seedling. Liaoning For. Sci. Technol. 1986, 6, 005. [Google Scholar]
- Liang, Y. Canonical correlation analysis of Platycladus orientalis between seedling growth and climatic factors. Sci. Silvae Sin. 1990, 26, 308–315. [Google Scholar]
- Yang, J.; Guo, Y.F.; Chen, X.D.; Zhang, X.; Ju, M.M.; Bai, G.Q.; Liu, Z.L.; Zhao, G.F. Framework phylogeny, evolution and complex diversification of Chinese oaks. Plants 2020, 9, 1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Song, J.; Zhang, R.; Yan, Y.; Wang, Y.; Du, F.K. Geometric morphometric analyses of leaf shapes in two sympatric Chinese oaks: Quercus dentata Thunberg and Quercus aliena Blume (Fagaceae). Ann. For. Sci. 2018, 75, 90. [Google Scholar] [CrossRef]
- Qi, M.; Wang, J.; Wang, R.; Song, Y.; Ueno, S.; Luo, Y.; Du, F.K. Intraspecific character displacement in oaks. Plant J. 2025, 122, e70165. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Riofrío-Dillon, G.; de Ruffray, P.; Vidal, C.; Pierrat, J.C.; Gégout, J.C. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.R.; Beißner, S.; Burga, C.A. Trends in the upward shift of alpine plants. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Massot, M.; Clobert, J.; Ferrière, R. Climate warming, dispersal inhibition and extinction risk. Glob. Change Biol. 2008, 14, 461–469. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Dai, L.M.; Pan, J. The trend of tree line on the northern slope of Changbai Mountain. J. For. Res. 2001, 12, 97–100. [Google Scholar]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-D.; Li, Y.; Guo, H.-Y.; Jia, L.-Q.; Yang, J.; Zhao, Y.-M.; Wei, Z.-F.; Zhang, L.-J. Simulation and Prediction of the Potential Distribution of Two Varieties of Dominant Subtropical Forest Oaks in Different Climate Scenarios. Forests 2025, 16, 1191. https://doi.org/10.3390/f16071191
Chen X-D, Li Y, Guo H-Y, Jia L-Q, Yang J, Zhao Y-M, Wei Z-F, Zhang L-J. Simulation and Prediction of the Potential Distribution of Two Varieties of Dominant Subtropical Forest Oaks in Different Climate Scenarios. Forests. 2025; 16(7):1191. https://doi.org/10.3390/f16071191
Chicago/Turabian StyleChen, Xiao-Dan, Yang Li, Hai-Yang Guo, Li-Qiang Jia, Jia Yang, Yue-Mei Zhao, Zuo-Fu Wei, and Lin-Jing Zhang. 2025. "Simulation and Prediction of the Potential Distribution of Two Varieties of Dominant Subtropical Forest Oaks in Different Climate Scenarios" Forests 16, no. 7: 1191. https://doi.org/10.3390/f16071191
APA StyleChen, X.-D., Li, Y., Guo, H.-Y., Jia, L.-Q., Yang, J., Zhao, Y.-M., Wei, Z.-F., & Zhang, L.-J. (2025). Simulation and Prediction of the Potential Distribution of Two Varieties of Dominant Subtropical Forest Oaks in Different Climate Scenarios. Forests, 16(7), 1191. https://doi.org/10.3390/f16071191





