Electrophysiology and Behavior of Tomicus yunnanensis to Pinus yunnanensis Volatile Organic Compounds Across Infestation Stages in Southwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Species Identification
2.2. VOC Extraction and Characterization in Pinus yunnanensis Across Infestation Stages
2.3. Identification of Semiochemicals Mediating Behavioral Responses in Tomicus yunnanensis
2.4. Data Analyses
3. Results
3.1. VOC Profiling in Pinus yunnanensis Across Infestation Stages
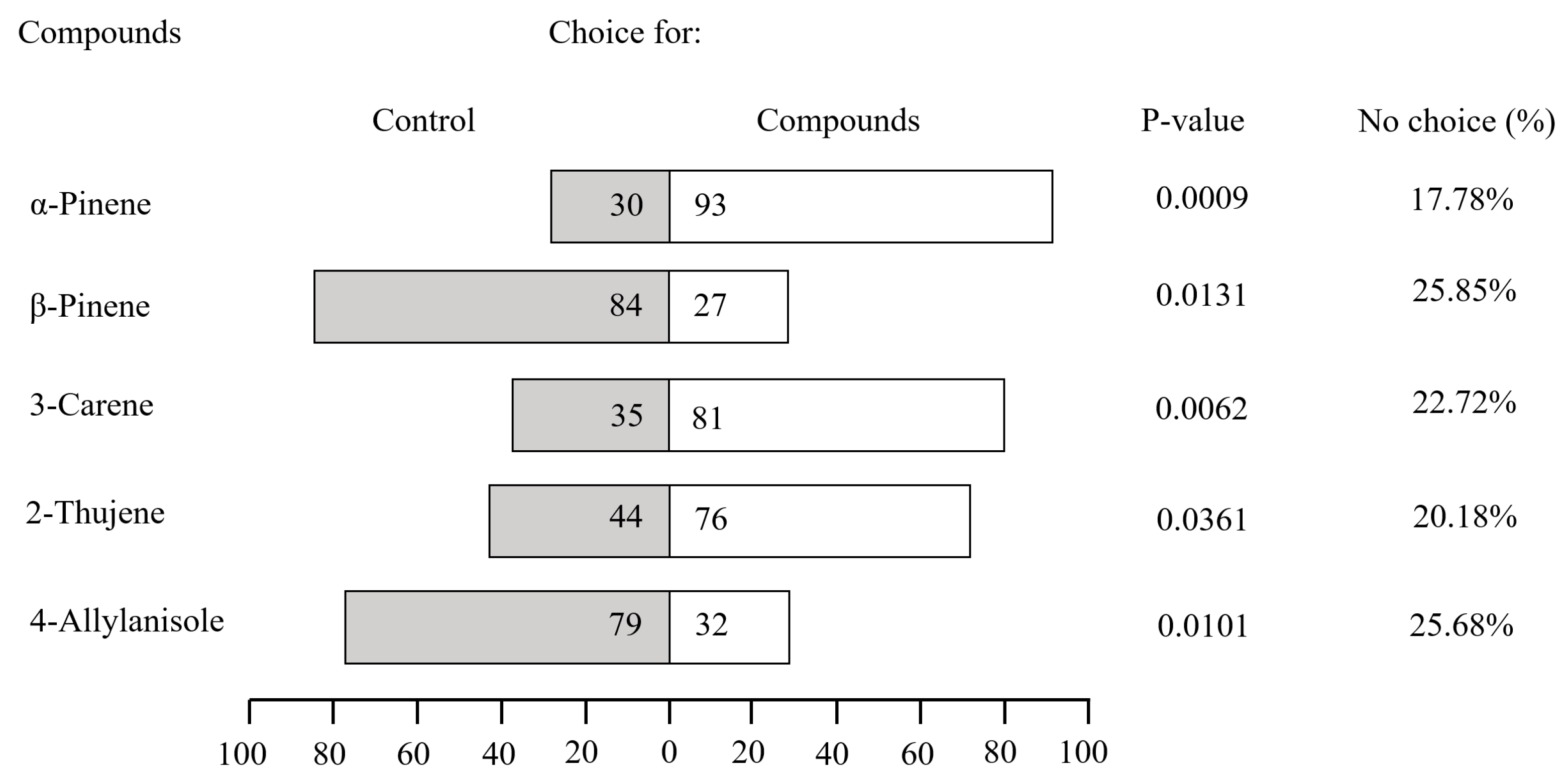
3.2. Behavioral Bioassays of Tomicus yunnanensis to Semiochemicals: Attraction and Repellency Mediated by Host-Derived Volatiles
4. Discussion
4.1. Volatile Compound Composition in Pinus yunnanensis
4.2. Functional Role of 4-Allylanisole in Mediating Host Selection of Tomicus yunnanensis
4.3. Ecological Roles of VOCs in Tomicus yunnanensis Chemical Ecology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pincebourde, S.; van Baaren, J.; Rasmann, S.; Rasmont, P.; Rodet, G.; Martinet, B.; Calatayud, P.A. Plant-Insect interactions in a changing world. Adv. Bot. Res. 2017, 81, 289–332. [Google Scholar]
- Aartsma, Y.; Bianchi, F.J.; vander Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef] [PubMed]
- Beyaert, I.; Hilker, M. Plant odour plumes as mediators of plant-insect interactions. Biol. Rev. 2014, 89, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.I.; Bohlmann, J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006, 170, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Krokene, P. Conifer defence and resistance to bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 177–207. [Google Scholar]
- Byers, J.A. Host-tree chemistry affecting colonization in bark beetles. In Chemical Ecology of Insects 2; Cardé, R.T., Bell, W.J., Eds.; Chapman & Hall.: Boca Raton, FL, USA, 1995. [Google Scholar]
- Raffa, K.F.; Erbilgin, N.; Klepzig, K.D.; Smalley, E.B. Interactions among conifer terpenoids and bark beetles across multiple levels of scale: An attempt to understand links between population patterns and physiological processes. Oecologia 2005, 146, 1–14. [Google Scholar]
- Chen, J.; Yuan, X.; Yan, W.; Zhang, L.; Liu, X. Composition and variation of volatile organic compounds emitted by Pinus yunnanensis under different health conditions. For. Res. 2020, 33, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Gao, W.Z.; Cai, H.Z.; Teng, J. Research progress on chemical ecological management of three Tomicus Species (Coleoptera: Scolytidae) in Yunnan province of China. J. Entomol. Sci. 2025, 60, 192–204. [Google Scholar]
- Byers, J.A.; Zhang, Q. Chemical ecology of bark beetles in regard to search and selection of host trees. In Recent Advances in Entomological Research; Springer: Berlin/Heidelberg, Germany, 2011; pp. 150–190. [Google Scholar]
- Liu, N.Y.; Li, Z.B.; Zhao, N.; Song, Q.S.; Zhu, J.Y.; Yang, B. Identification and characterization of chemosensory gene families in the bark beetle, Tomicus yunnanensis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 25, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Hu, S.J.; Ma, X.Y.; Chen, J.M.; Li, Q.Q.; Ye, H. Origin and expansion of the Yunnan Shoot Borer, Tomicus yunnanensis (coleoptera: Scolytinae): A mixture of historical natural expansion and contemporary human-mediated relocation. PLoS ONE 2014, 9, e111940. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Zhao, N.; Yang, B. Global transcriptome profiling of the pine shoot beetle, Tomicus yunnanensis (Coleoptera: Scolytinae). PLoS ONE 2012, 7, e32291. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Ye, H.; Wang, H.; Clarke, S.R.; Lu, J. Response of Tomicus yunnanensis (Coleoptera: Scolytinae) to infested and uninfested Pinus yunnanensis bolts. J. Econ. Entomol. 2010, 103, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.X.; Liu, F.; Zhang, S.F.; Kong, X.B.; Zhang, Z. Semiochemical regulation of the intraspecific and interspecific behavior of Tomicus yunnanensis and Tomicus minor during the shoot-feeding phase. J. Chem. Ecol. 2019, 45, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, L.R.; Faccoli, M.; Ye, H. Description of the Yunnan shoot borer, Tomicus yunnanensis Kirkendall & Faccoli sp. n. (Curculionidae, Scolytinae), an unusually aggressive pine shoot beetle from southern China, with a key to the species of Tomicus. Zootaxa 2008, 1819, 25–39. [Google Scholar] [CrossRef]
- Duan, Y.; Kerdelhué, C.; Ye, H.; Lieutier, F. Genetic study of the forest pest Tomicus piniperda (Col., Scolytinae) in Yunnan province (China) compared to Europe: New insights for the systematics and evolution of the genus Tomicus. Heredity 2004, 93, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhang, M.D.; Qian, L.B.; Ze, S.Z.; Yang, B.; Li, Z.B. Electrophysiological and behavioral responses of Tomicus yunnanensis to plant volatiles from primarily infected Pinus yunnanensis in Yunnan, southwest China. J. Environ. Entomol. 2021, 43, 1389–1397. [Google Scholar]
- Cui, Y.J.; Zhang, M.D.; Zhu, H.I.; Yang, P.; Yang, B.; Li, Z.B. Fine structure of the mouthparts of three Tomicus beetles co-infecting Pinus yunnanensis in southwestern China with some functional comments. Insects 2023, 14, 933. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.B.; Zhang, M.D.; Liu, J.J.; Li, Z.B. Effects of 4 kinds of odor-active compounds of non-host alnus ferdinandi-coburgii on the post-embryonic development of Tomicus yunnanensis(Coleoptera: Curculionidae: Scolytinae). J. Southwest For. Univ. 2023, 43, 96–102. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1; Allured Pub Corp: Carol Steam, IL, USA, 2017; pp. 9–53. [Google Scholar]
- Althoff, E.R.; Aukema, B.H.; Sullivan, B.T. Pheromone composition of the eastern larch beetle Dendroctonus simplex Leconte (Coleoptera: Curculionidae): Quantitative analyses and olfactory responses. J. Chem. Ecol. 2025, 51, 18. [Google Scholar] [CrossRef] [PubMed]
- Heng, G.X.; McMillin, J.; Wagner, M.; Zhou, J.; Zhou, Z.; Xu, X. Altitudinal variation in foliar chemistry and anatomy of yunnan pine, Pinus yunnanensis, and pine sawfly (Hym., Diprionidae) performance. J. Appl. Entomo. 1999, 123, 465–471. [Google Scholar]
- Yan, Z.; Ma, H.; Ze, S. Difference of taxis responses of Tomicus yunnanensis to volatile extracts from trunks and branches of Pinus yunnanensis. J. Envir. Entomol. 2011, 33, 191–194. [Google Scholar]
- Ding, J.K.; Ding, L.S. Chemical constituents of pine needle oil from Pinus yunnanensis and Pinus kesiya. Plant Diver. Resour. 1987, 9, 505–508. [Google Scholar]
- Yang, Y.; Yang, M.F.; Yang, Z.H.; Huang, J.Y.; Wang, C.Y. Chemical constituents of volatile from pine needles of Pinus yunnanensis. Sci. Silvae Sin. 2009, 45, 173–177. [Google Scholar]
- Yin, C.X.; Gao, Z.L.; Lv, J.; Ye, H. Test on the taxis responses of Tomicus piniperda to the volatiles of Yunnan pine shoot. Entomol. Knowl. 2002, 39, 384–386. [Google Scholar]
- Yin, X.B.; Geng, S.X.; Zheng, W. Differences of physical and chemical characteristics of oleoresin among provenances from Pinus yunnanensis. J. W. China For. Sci. 2007, 36, 34–41. [Google Scholar]
- Agelopoulos, N.G.; Pickett, J.A. Headspace analysis in chemical ecology: Effects of different sampling methods on ratios of volatile compounds present in headspace samples. J. Chem. Ecol. 1998, 24, 1161–1172. [Google Scholar] [CrossRef]
- Faccoli, M.; Anfora, G.; Tasin, M. Responses of the Mediterranean pine shoot beetle Tomicus destruens (Wollaston) to pine shoot and bark volatiles. J. Chem. Ecol. 2008, 34, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Munro, H.L.; Gandhi, K.J.; Barnes, B.F.; Montes, C.R.; Nowak, J.T.; Shepherd, W.P.; Villari, C.; Sullivan, B.T. Electrophysiological and behavioral responses Dendroctonus frontalis and D. terebrans (Coleoptera: Curculionidae) to resin odors of host pines (Pinus spp.). Chemoecology 2020, 30, 215–231. [Google Scholar] [CrossRef]
- Bo, J.; Li, W.; Li, X.; Li, Z.; Mao, X.; Yang, B.; Zhao, N. Mechanisms of impact of Alnus ferdinandi-coburgii odor substances on host location of Tomicus yunnanensis. Insects 2025, 16, 553. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L. Influence of Semiochemical Cues on Mountain Pine Beetle Flight and Subsequent Effect of Flight on Host Colonisation Processes. Master’s Thesis, Department of Biological Sciences University of Alberta, Edmonton, AB, Canada, 2019. [Google Scholar]
- Zhao, T.; Krokene, P.; Hu, J.; Christiansen, E.; Björklund, N.; Långström, B.; Borg-Karlson, A.K. Induced terpene accumulation in Norway spruce inhibits bark beetle colonization in a dose-dependent manner. PLoS ONE 2011, 6, e26649. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kalunke, R.M.; Giri, A.P. Towards comprehension of complex chemical evolution and diversification of terpene and phenylpropanoid pathways in Ocimum species. RSC Adv. 2015, 5, 106886–106904. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Seybold, S.J.; Bohlmann, J.; Raffa, K.F. Biosynthesis of coniferophagous bark beetle pheromones and conifer isoprenoids: Evolutionary perspective and synthesis. Can. Entomol. 2012, 132, 697–753. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lu, M.; Gillette, N.E.; Wingfield, M.J. Red turpentine beetle: Innocuous native becomes invasive tree killer in China. Annu. Rev. Entomol. 2013, 58, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.T. Composition of attractant semiochemicals of north American species of Dendroctonus bark beetles: A review. Forests 2024, 15, 642. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Zheng, Y. Feeding preferences and responses of Monochamus Saltuarius to volatile components of host pine trees. Insects 2022, 13, 888. [Google Scholar] [CrossRef] [PubMed]
- Manuel, C.F.; Leonardo, B.; Ivette, S.; Cristian, M.; Andrés, Q. Volatiles induction in response to mechanical damage is reduced by domestication in murtilla. Bol. Latinoam. Caribe Plant. Med. Aromat. 2019, 18, 435–443. [Google Scholar]
- Sullivan, B.T.; Munro, H.L.; Shepherd, W.P.; Gandhi, K.J. 4-allylanisole as a lure adjuvant for Dendroctonus frontalis (Coleoptera: Curculionidae: Scolytinae) and two associated beetles. J. Appl. Entomol. 2022, 146, 813–822. [Google Scholar] [CrossRef]
- Emerick, J.J.; Snyder, A.I.; Bower, N.W.; Snyder, M.A. Mountain pine beetle attack associated with low levels of 4-allylanisole in ponderosa pine. Environ. Entomol. 2008, 37, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Haack, R.A.; Lawrence, R.K.; Petrice, T.R.; Poland, T.M. Disruptant effects of 4-allylanisole and verbenone on Tomicus piniperda (Coleoptera: Scolytidae) response to baited traps and logs. Great Lakes Entomol. 2004, 37, 131–141. [Google Scholar] [CrossRef]
- Lehmanski, L.M.A.; Kandasamy, D.; Andersson, M.N.; Netherer, S.; Alves, E.G.; Huang, J.; Hartmann, H. Addressing a century-old hypothesis–do pioneer beetles of Ips typographus use volatile cues to find suitable host trees? New Phytol. 2023, 238, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, R.W.; Gaylord, M.L.; Martinson, S.; Wagner, M.R. Attraction to monoterpenes and beetle-produced compounds by syntopic Ips and Dendroctonus bark beetles and their predators. Agric. For. Entomol. 2012, 14, 207–215. [Google Scholar] [CrossRef]
- Li, L.S.; Shu, N.B.; Huai, K.Y. Trapping experiments of Tomicus piniperda to the chemical volatiles. Entomol. Knowl. 1993, 30, 159–161. [Google Scholar]
- Lieutier, F.; Långström, B. The genus Tomicus. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
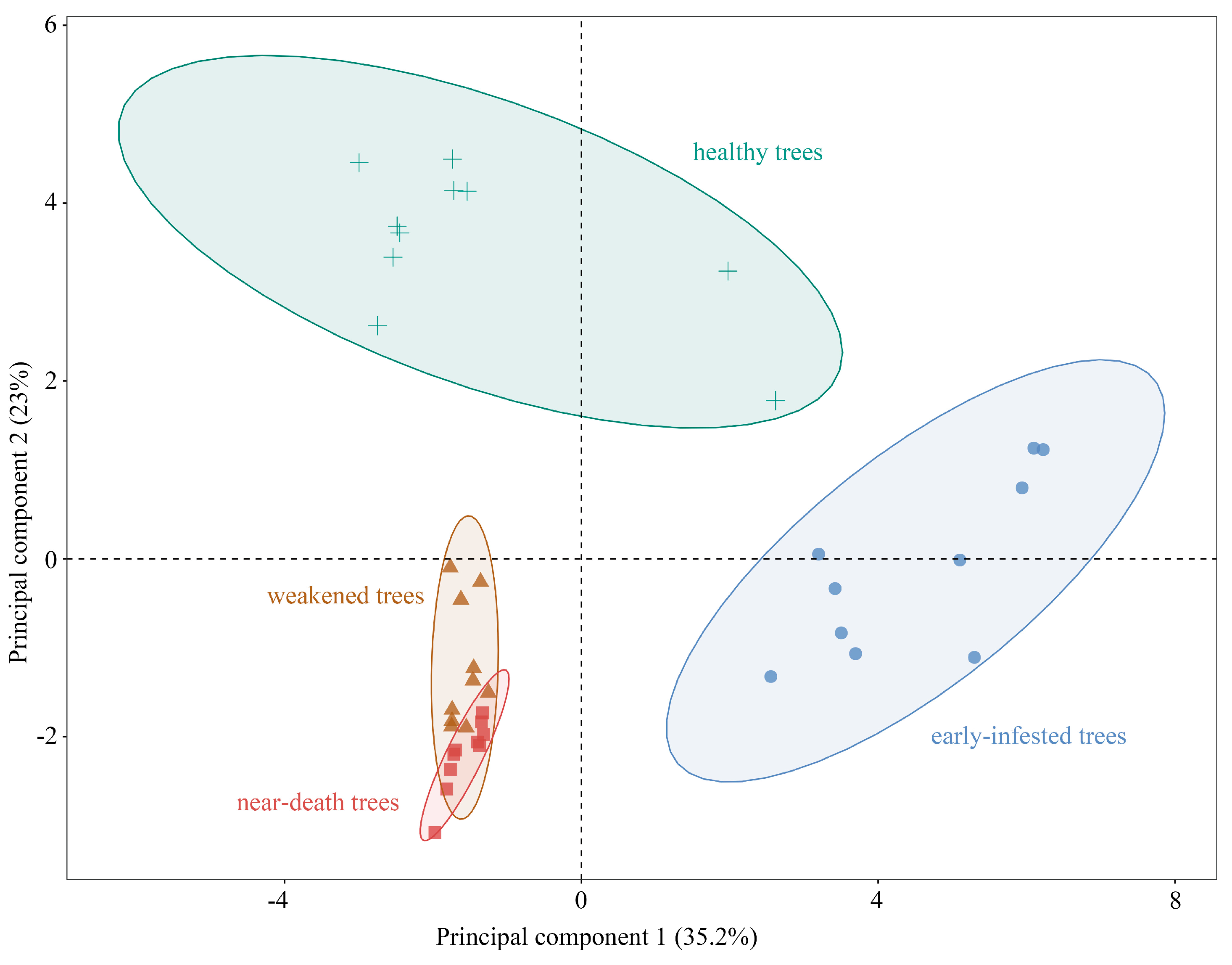
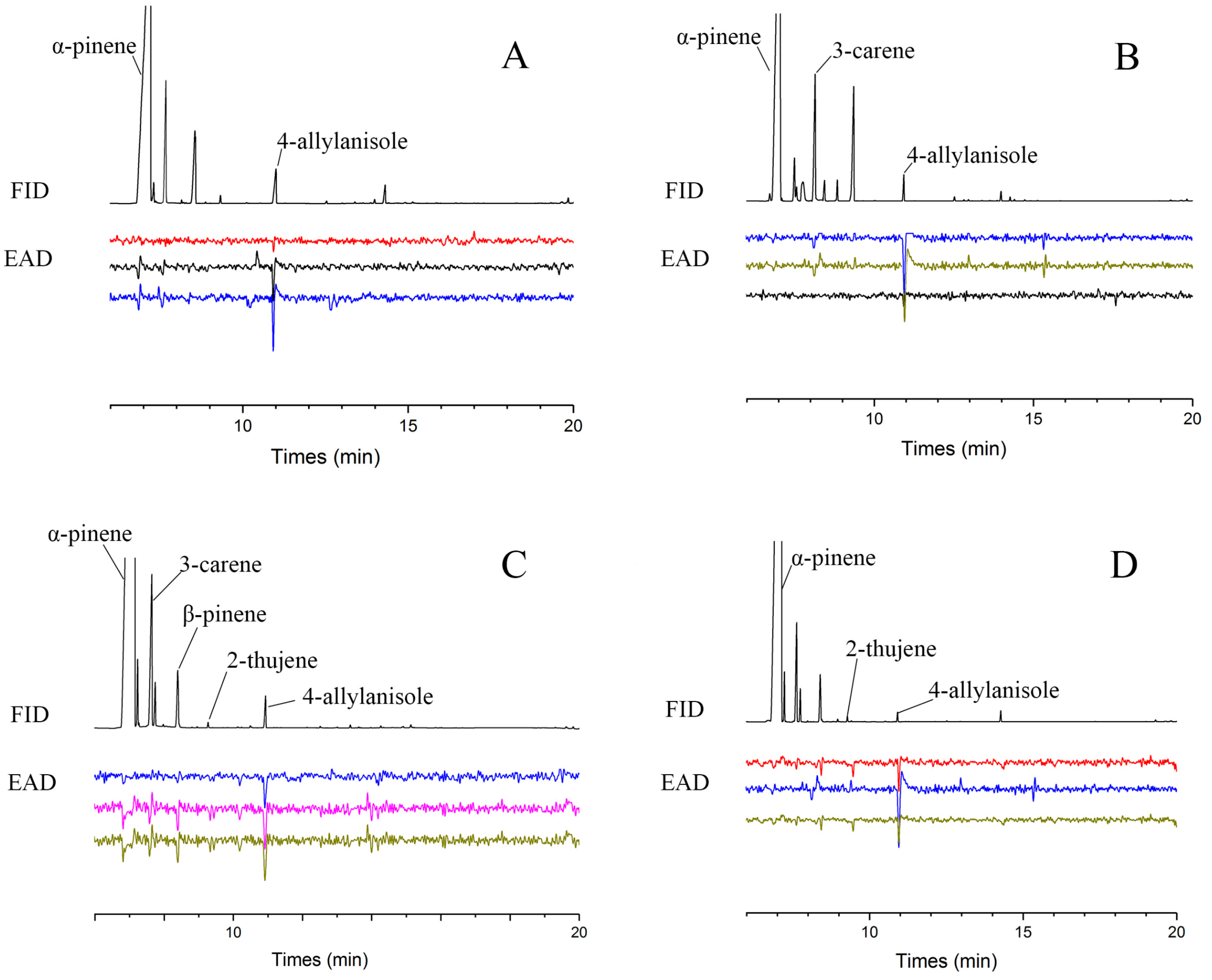
| Stage | Pine Needle Coloration | Shoot Infestation Rate (%) | Trunk and Crown Traits |
|---|---|---|---|
| Healthy | Green to light green | 1–20 | Shoots and primary branches exhibit boreholes and resin exudates |
| Early-infested | Gray-green | 21–50 | Shoots and trunks exhibit abundant boreholes and resin exudates, accompanied by crown dehydration and progressive wilting |
| Weakened | Grayish-yellow | 51–100 | The trunk exhibits numerous boreholes and resin exudates, while the crown shows complete dehydration, chlorosis, and wilting |
| Near-Dead | Reddish-yellow | - | The trunk exhibits numerous emergence holes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, M.; Qian, L.; Wang, Z.; Li, Z. Electrophysiology and Behavior of Tomicus yunnanensis to Pinus yunnanensis Volatile Organic Compounds Across Infestation Stages in Southwest China. Forests 2025, 16, 1178. https://doi.org/10.3390/f16071178
Liu J, Zhang M, Qian L, Wang Z, Li Z. Electrophysiology and Behavior of Tomicus yunnanensis to Pinus yunnanensis Volatile Organic Compounds Across Infestation Stages in Southwest China. Forests. 2025; 16(7):1178. https://doi.org/10.3390/f16071178
Chicago/Turabian StyleLiu, Jinlin, Mengdie Zhang, Lubing Qian, Zhenji Wang, and Zongbo Li. 2025. "Electrophysiology and Behavior of Tomicus yunnanensis to Pinus yunnanensis Volatile Organic Compounds Across Infestation Stages in Southwest China" Forests 16, no. 7: 1178. https://doi.org/10.3390/f16071178
APA StyleLiu, J., Zhang, M., Qian, L., Wang, Z., & Li, Z. (2025). Electrophysiology and Behavior of Tomicus yunnanensis to Pinus yunnanensis Volatile Organic Compounds Across Infestation Stages in Southwest China. Forests, 16(7), 1178. https://doi.org/10.3390/f16071178






