Integrated Analysis of Phenotypic, Physiological, and Biochemical Traits in Betula platyphylla Sukaczev Under Cold Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrolyte Leakage
2.2. Chlorophyll Pigment Content
2.3. Antioxidant Enzymes
- A: control test tube value;
- B: real test tube value.
2.4. Determination of MDA and Proline Levels
2.5. Statistical Analysis
3. Results
3.1. Differential Impact of Cold Stress on B. platyphylla Phenotype
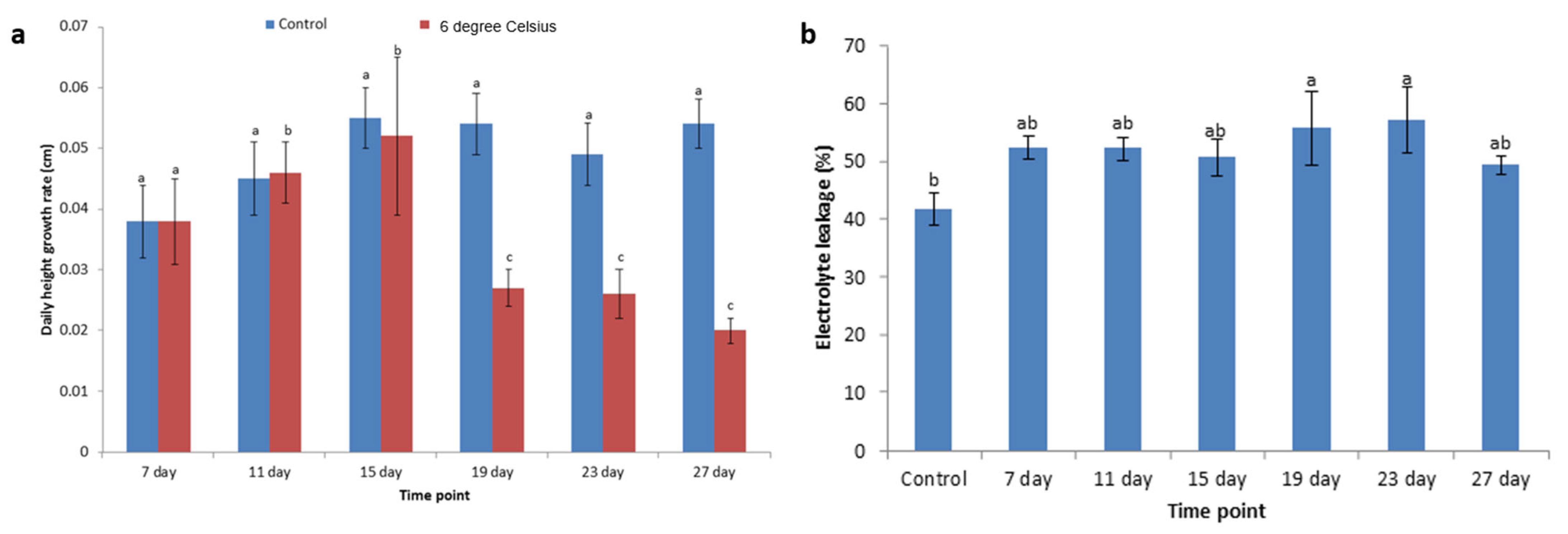
3.2. Changing the Height Growth Rate
3.3. Electrolyte Leakage Changes Under Cold Stress
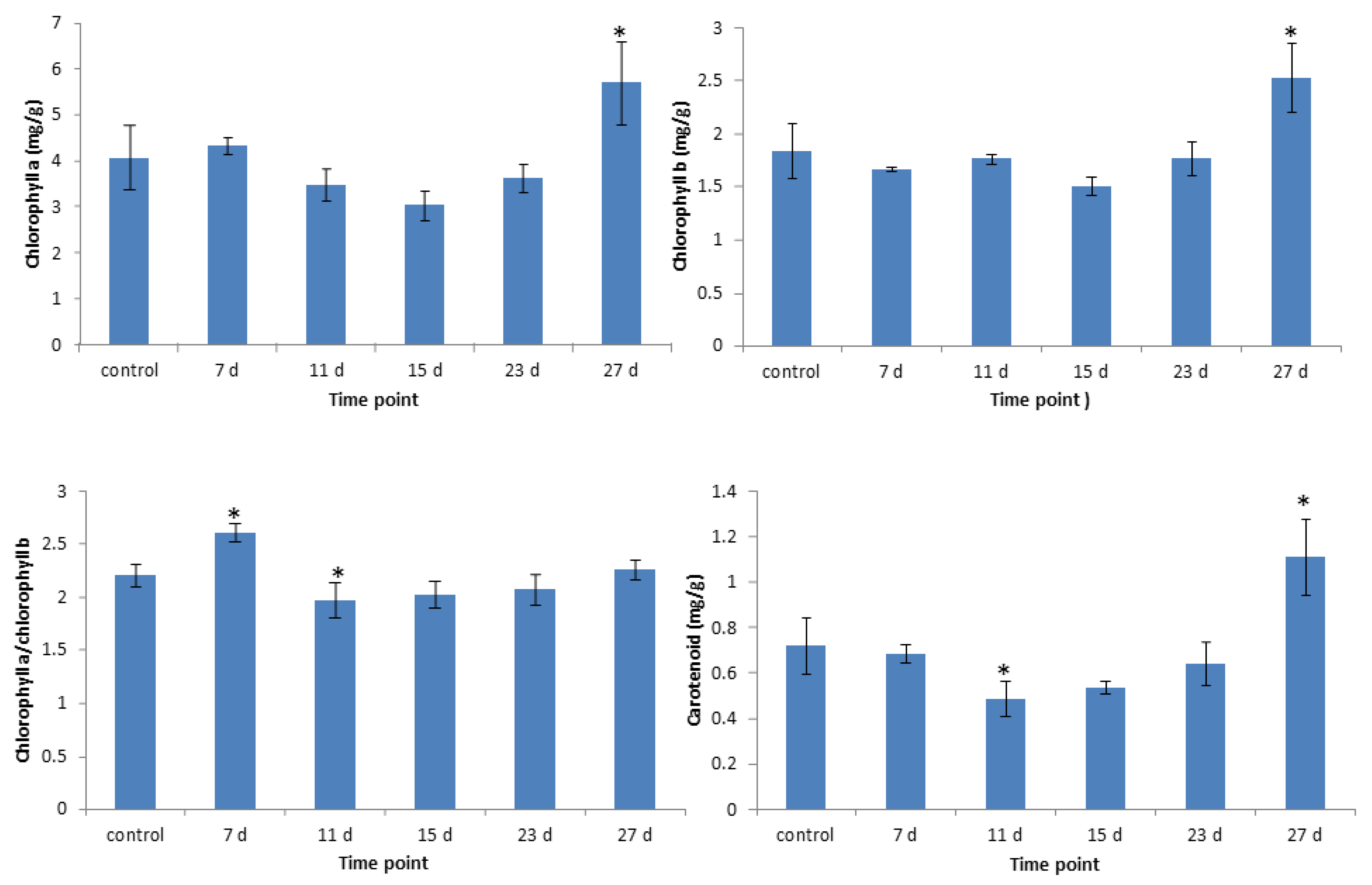
3.4. Changes in Chlorophyll Contents
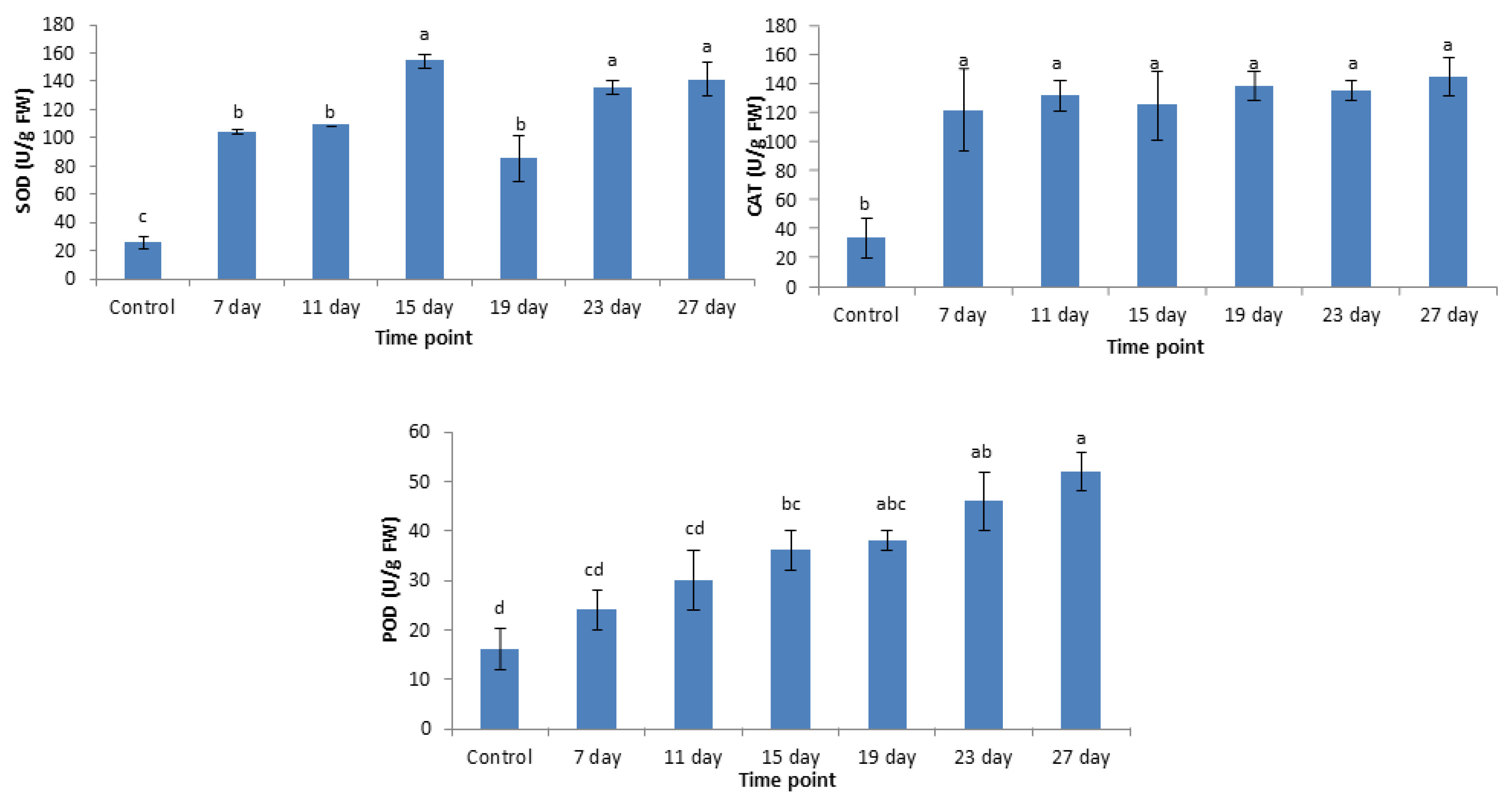
3.5. Antioxidant Enzyme Activities
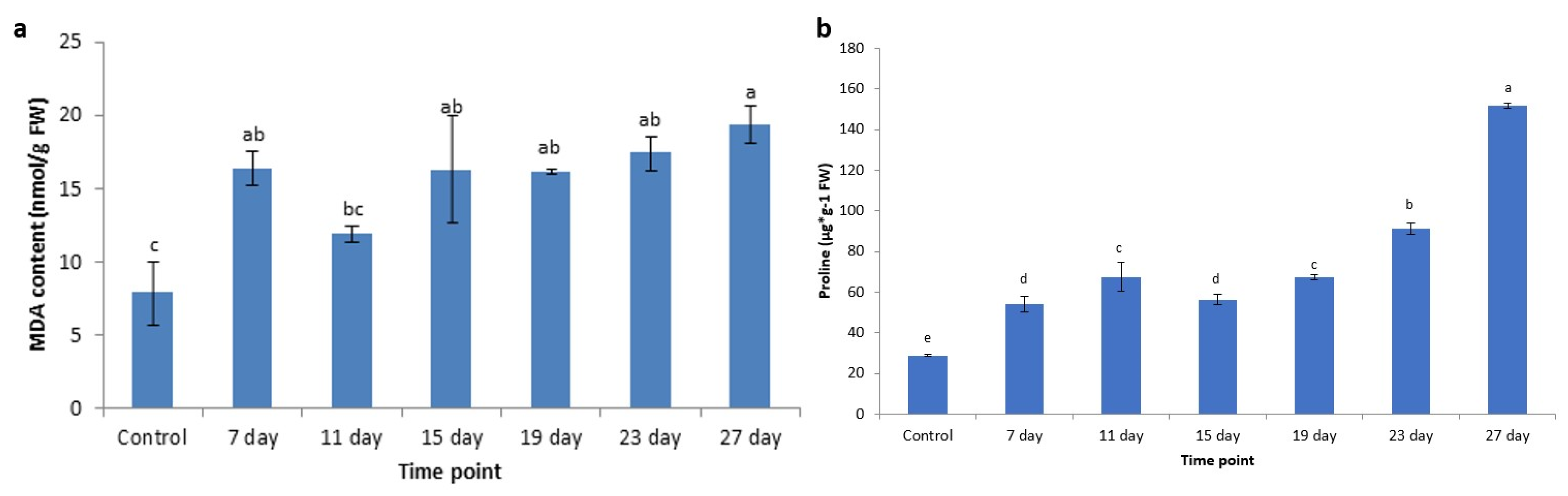
3.6. Changes of MDA Content Under Cold Stress
3.7. Changes of Proline Content Under Cold Stress
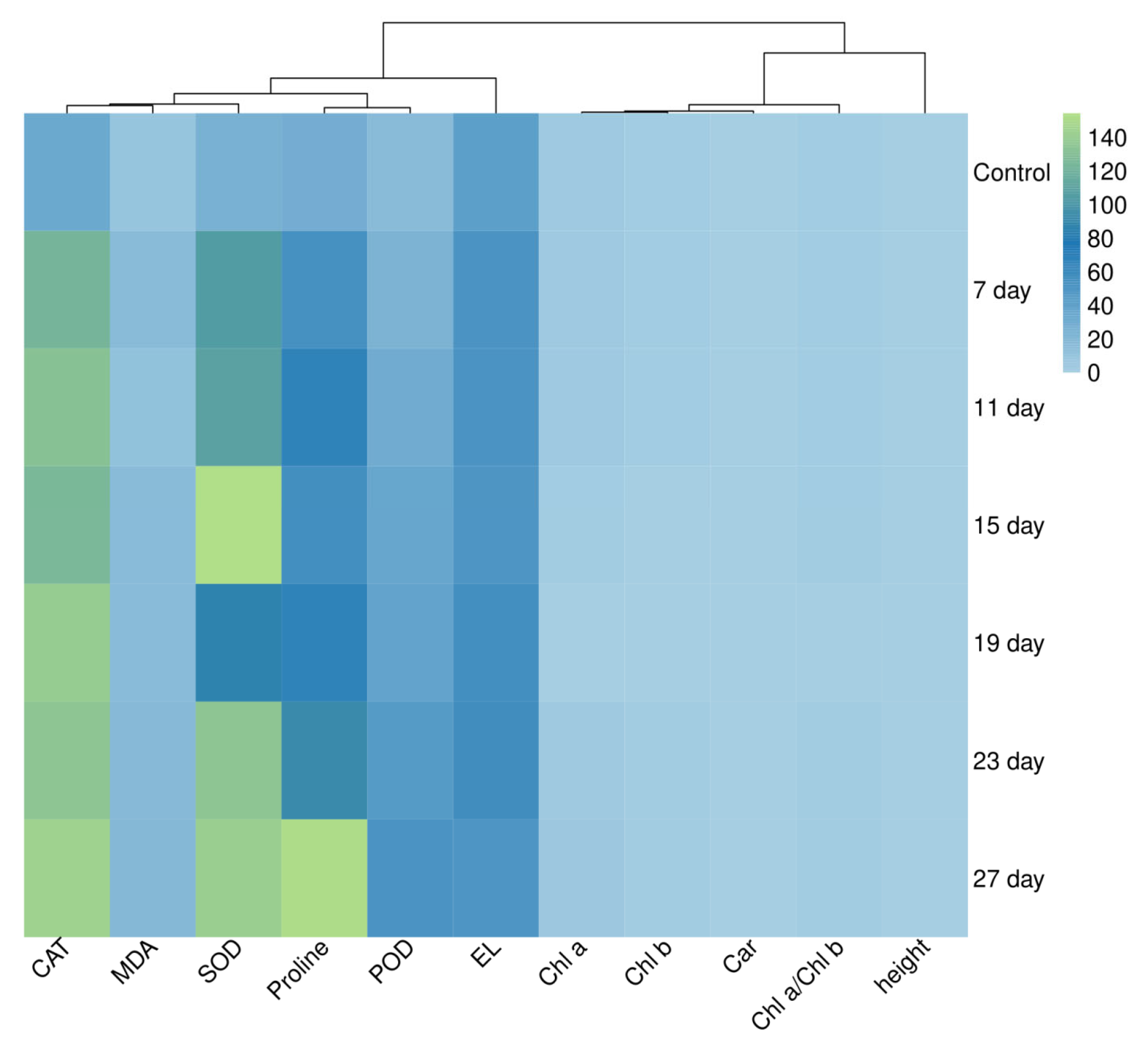
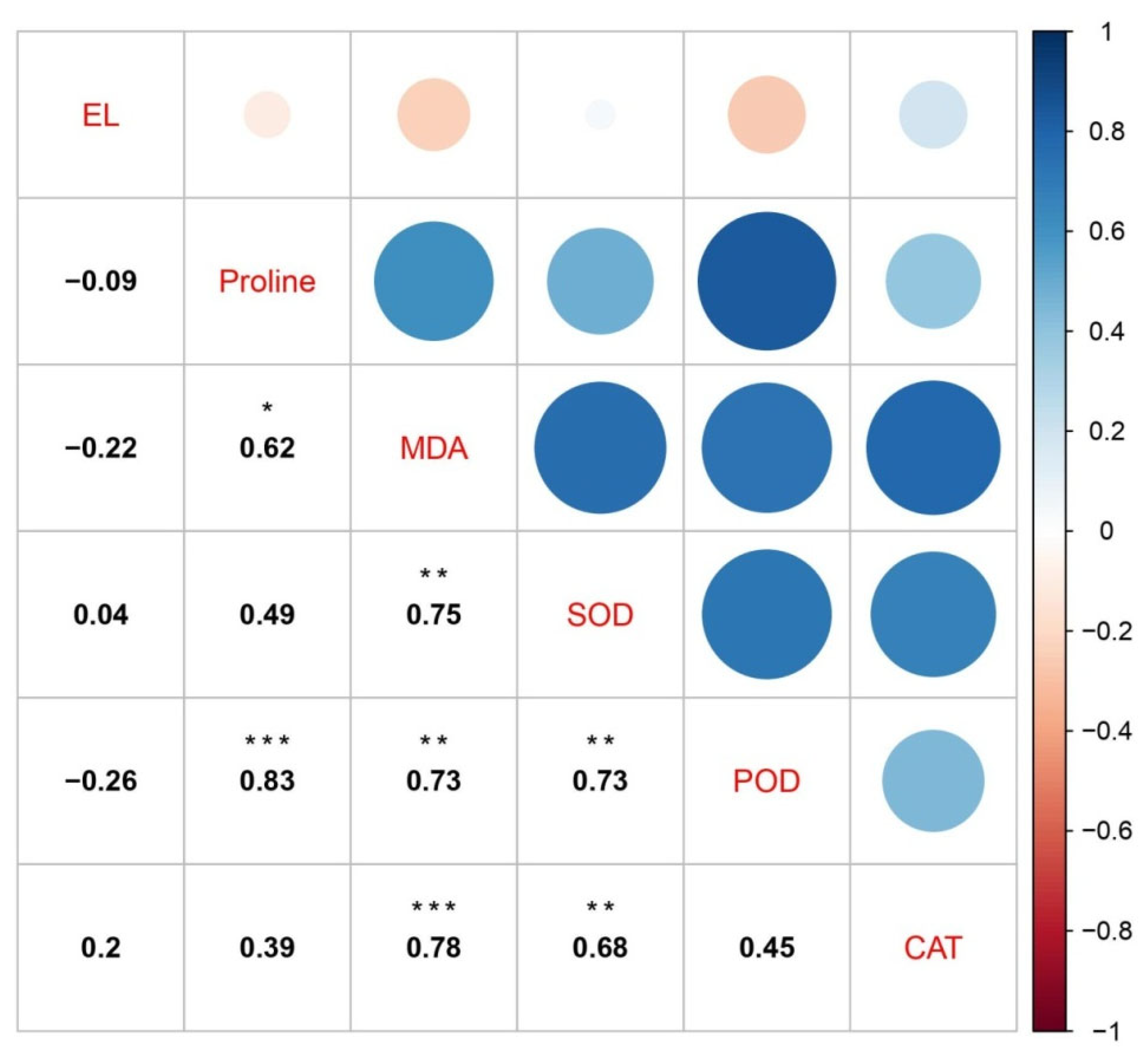
3.8. Plant Physiological and Biochemical Index Relationship
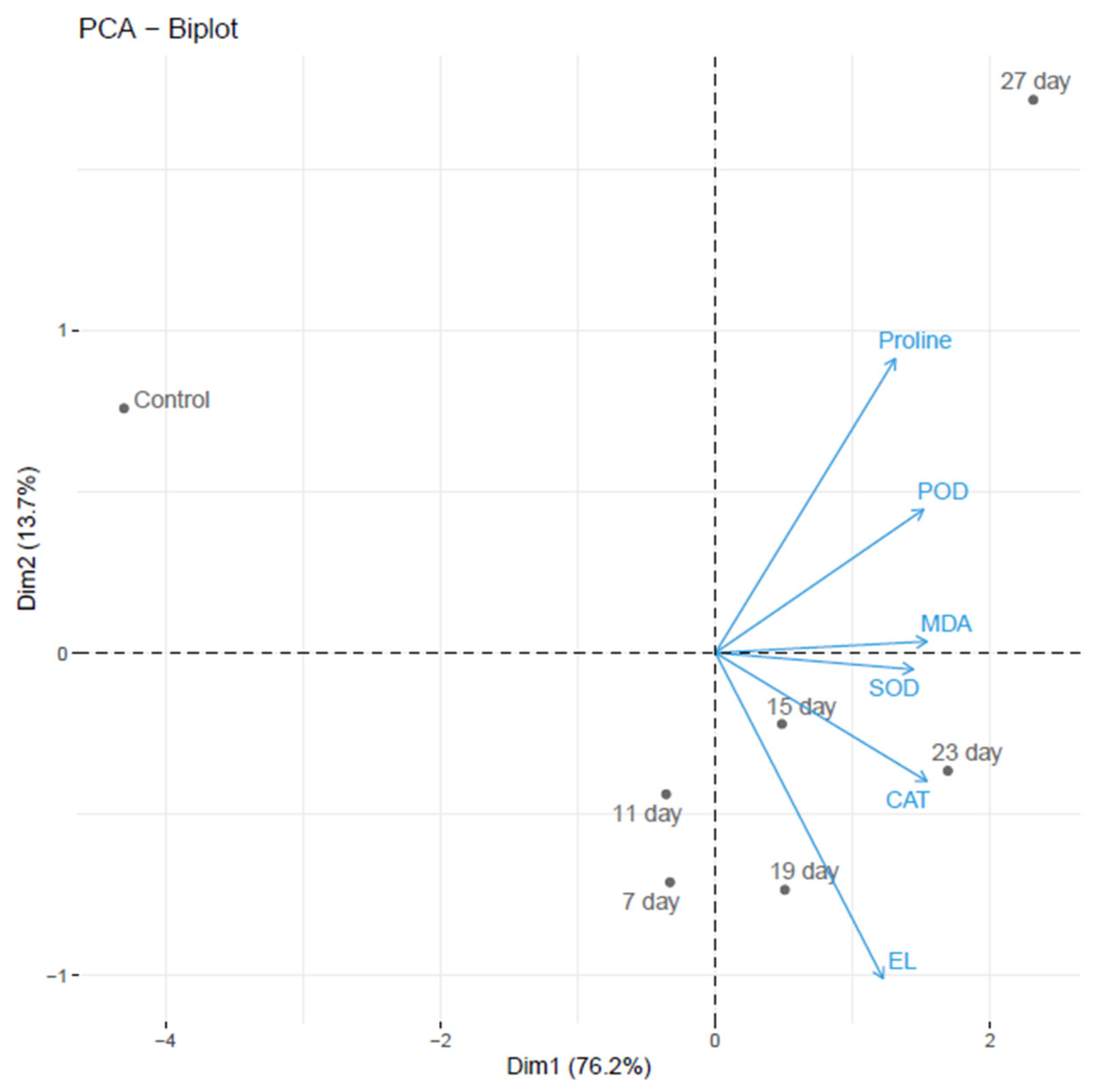
3.9. Bi-Plot Analysis of B. platyphylla Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, S.; Zhang, D.W.; Chen, S.; Chen, S. Transcriptome Sequencing Analysis of Birch (Betula platyphylla Sukaczev) under Low-Temperature Stress. Forests 2020, 11, 970. [Google Scholar] [CrossRef]
- WCSP World Checklist of Selected Plant Families. Available online: http://apps.kew.org/wcsp/ (accessed on 14 July 2025).
- Chen, T.Y.; Lou, A.R. Phylogeography and paleodistribution models of a widespread birch (Betula platyphylla Suk.) across East Asia: Multiple refugia, multidirectional expansion, and heterogeneous genetic pattern. Ecol. Evol. 2019, 9, 7792–7807. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, M.; Julkunen-Tiitto, R.; Mutikainen, P.; Walls, M.; Ovaska, J.; Vapaavuori, E. Trade-offs in phenolic metabolism of silver birch: Effects of fertilization, defoliation, and genotype. Ecology 1999, 80, 1970–1986. [Google Scholar] [CrossRef]
- Gradel, A.; Haensch, C.; Ganbaatar, B.; Dovdondemberel, B.; Nadaldorj, O.; Günther, B. Response of white birch (Betula platyphylla Sukaczev) to temperature and precipitation in the mountain forest steppe and taiga of northern Mongolia. Dendrochronologia 2017, 41, 24–33. [Google Scholar] [CrossRef]
- Wen, X.; Ding, Y.; Tan, Z.; Wang, J.; Zhang, D.; Wang, Y. Identification and characterization of cadmium stress-related LncRNAs from Betula platyphylla. Plant Sci. 2020, 299, 110601. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, P.; Zhang, T.; Li, Y.; Wang, Y.; Gao, C. Comprehensive analysis of BpHSP genes and their expression under heat stresses in Betula platyphylla. Environ. Exp. Bot. 2018, 152, 167–176. [Google Scholar] [CrossRef]
- Longo, V.; Janmohammadi, M.; Zolla, L.; Rinalducci, S. What Can Small Molecules Tell Us About Cold Stress Tolerance in Plants? In Cold Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 127–157. [Google Scholar]
- Ritonga, F.N.; Chen, S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Yan, S.; Chen, S.; Slamet, S.A.; Irmayanti, L.; Song, R.; Lin, X.; Jing, Y.; Farooq, U.; Khoso, M.A.; et al. Cold Acclimation Affects Physiological and Biochemical Characteristics of Betula platyphylla S. under Freezing Stress. Forests 2021, 12, 1777. [Google Scholar] [CrossRef]
- Renaut, J.; Lutts, S.; Hoffmann, L.; Hausman, J.-F. Responses of poplar to chilling temperatures: Proteomic and physiological aspects. Plant Biol. 2004, 7, 81–90. [Google Scholar] [CrossRef]
- Li, C.; Junttila, O.; Heino, P.; Palva, E.T. Low temperature sensing in silver birch (Betula pendula Roth) ecotypes. Plant Sci. 2004, 167, 165–171. [Google Scholar] [CrossRef]
- Oberschelp, G.P.J.; Morales, L.L.; Montecchiarini, M.L.; Harrand, L.; Podestá, F.E.; Margarit, E. Harder, better, faster, stronger: Frost tolerance of Eucalyptus benthamii under cold acclimation. Plant Physiol. Biochem. 2022, 186, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, T.; Rao, P.; Gao, K.; Yang, X.; Chen, Z.; An, X. Transcriptome profiling of Populus tomentosa under cold stress. Ind. Crops Prod. 2019, 135, 283–293. [Google Scholar] [CrossRef]
- Liu, X.; Lan, J.; Huang, Y.; Cao, P.; Zhou, C.; Ren, Y.; He, N.; Liu, S.; Tian, Y.; Nguyen, T. WSL5, a pentatricopeptide repeat protein, is essential for chloroplast biogenesis in rice under cold stress. J. Exp. Bot. 2018, 69, 3949–3961. [Google Scholar] [CrossRef]
- Çetinbaş-Genç, A.; Cai, G.; Del Duca, S. Treatment with spermidine alleviates the effects of concomitantly applied cold stress by modulating Ca2+, pH and ROS homeostasis, actin filament organization and cell wall deposition in pollen tubes of Camellia sinensis. Plant Physiol. Biochem. 2020, 156, 578–590. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Zhang, J.; Yang, H.; Xu, L.; Wang, Q.; Sack, L.; Wu, X.; Hou, J.; He, N. Variation in leaf chlorophyll concentration from tropical to cold-temperate forests: Association with gross primary productivity. Ecol. Indic. 2018, 85, 383–389. [Google Scholar] [CrossRef]
- Nischal, P.; Sharma, A.D. Stomatal and pollen dependant metabolic changes as a metric of stress tolerance and invasive potential of invasive plant—Lantana camara (L.) growing under abiotic stress like conditions. S. Afr. J. Bot. 2020, 131, 406–420. [Google Scholar] [CrossRef]
- Jamshidi Goharrizi, K.; Meru, G.; Ghotbzadeh Kermani, S.; Heidarinezhad, A.; Salehi, F. Short-term cold stress affects physiological and biochemical traits of pistachio rootstocks. S. Afr. J. Bot. 2021, 141, 90–98. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Ngatia, J.N.; Song, R.X.; Farooq, U.; Somadona, S.; Andi, T.L.; Chen, S. Abiotic stresses induced physiological, biochemical, and molecular changes in Betula platyphylla: A review. Silva Fenn. 2021, 55, 10516. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Yue, Y.; Jiang, H.; Du, J.; Shi, L.; Bin, Q.; Yang, X.; Wang, L. Variations in physiological response and expression profiles of proline metabolism-related genes and heat shock transcription factor genes in petunia subjected to heat stress. Sci. Hortic. 2019, 258, 108811. [Google Scholar] [CrossRef]
- Lv, J.; Li, Y.; Liu, Z.; Li, X.; Lei, X.; Gao, C. Response of BpBZR genes to abiotic stress and hormone treatment in Betula platyphylla. Plant Physiol. Biochem. 2020, 151, 157–165. [Google Scholar] [CrossRef]
- Cai, X.; Magwanga, R.O.; Xu, Y.; Zhou, Z.; Wang, X.; Hou, Y.; Wang, Y.; Zhang, Y.; Liu, F.; Wang, K. Comparative transcriptome, physiological and biochemical analyses reveal response mechanism mediated by CBF4 and ICE2 in enhancing cold stress tolerance in Gossypium thurberi. AoB Plants 2019, 11, plz045. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Martínez-Cuenca, M.-R.; Forner-Giner, M.Á. Cold Stress in Citrus: A Molecular, Physiological and Biochemical Perspective. Horticulturae 2021, 7, 340. [Google Scholar] [CrossRef]
- Mesa, T.; Polo, J.; Arabia, A.; Caselles, V.; Munné-Bosch, S. Differential physiological response to heat and cold stress of tomato plants and its implication on fruit quality. J. Plant Physiol. 2022, 268, 153581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Da Silva, J.A.T.; Niu, M.; Li, M.; He, C.; Zhao, J.; Zeng, S.; Duan, J.; Ma, G. Physiological and transcriptomic analyses reveal a response mechanism to cold stress in Santalum album L. leaves. Sci. Rep. 2017, 7, 42165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, K.; He, T.; Li, F.; Zhang, D.; Liu, J. The low temperature induced physiological responses of Avena nuda L., a cold-tolerant plant species. Sci. World J. 2013, 2013, 658793. [Google Scholar] [CrossRef]
- Ommen, O.E.; Donnelly, A.; Vanhoutvin, S.; Van Oijen, M.; Manderscheid, R. Chlorophyll content of spring wheat flag leaves grown under elevated CO2 concentrations and other environmental stresses within the ‘ESPACE-wheat’project. Eur. J. Agron. 1999, 10, 197–203. [Google Scholar] [CrossRef]
- Bocian, A.; Kosmala, A.; Rapacz, M.; Jurczyk, B.; Marczak, Ł.; Zwierzykowski, Z. Differences in leaf proteome response to cold acclimation between Lolium perenne plants with distinct levels of frost tolerance. J. Plant Physiol. 2011, 168, 1271–1279. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Larson, R.A. The antioxidants of higher plants. Phytochemistry 1988, 27, 969–978. [Google Scholar] [CrossRef]
- Amini, S.; Maali-Amiri, R.; Kazemi-Shahandashti, S.S.; López-Gómez, M.; Sadeghzadeh, B.; Sobhani-Najafabadi, A.; Kariman, K. Effect of cold stress on polyamine metabolism and antioxidant responses in chickpea. J. Plant Physiol. 2021, 258–259, 153387. [Google Scholar] [CrossRef]
- Demecsová, L.; Bočová, B.; Zelinová, V.; Tamás, L. Enhanced nitric oxide generation mitigates cadmium toxicity via superoxide scavenging leading to the formation of peroxynitrite in barley root tip. J. Plant Physiol. 2019, 238, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Lee, J.W.; Seo, J.S.; Shin, K.H.; Rhee, J.S.; Lee, J.S. Modulated expression and enzymatic activity of the monogonont rotifer Brachionus koreanus Cu/Zn-and Mn-superoxide dismutase (SOD) in response to environmental biocides. Chemosphere 2015, 120, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Kaouthar, F.; Ameny, F.K.; Yosra, K.; Walid, S.; Ali, G.; Faical, B. Responses of transgenic Arabidopsis plants and recombinant yeast cells expressing a novel durum wheat manganese superoxide dismutase TdMnSOD to various abiotic stresses. J. Plant Physiol. 2016, 198, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Uehara, N.; Sasaki, H.; Kobayashi, K.; Yamakawa, T. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiol. Biochem. 2013, 70, 396–402. [Google Scholar] [CrossRef]
- Dreyer, A.; Dietz, K.-J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef]
- Omara, R.I.; El-Kot, G.A.; Fadel, F.M.; Abdelaal, K.A.; Saleh, E.M. Efficacy of certain bioagents on patho-physiological characters of wheat plants under wheat leaf rust stress. Physiol. Mol. Plant Pathol. 2019, 106, 102–108. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hu, Y.; Chen, B.H.; Zhu, Y.F.; Mohammed, M.D.; Sofkova, S. Physiological mechanisms of resistance to cold stress associated with 10 elite apple rootstocks. J. Integr. Agric. 2018, 17, 857–866. [Google Scholar] [CrossRef]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Toscano, S.; Farieri, E.; Ferrante, A.; Romano, D. Physiological and Biochemical Responses in Two Ornamental Shrubs to Drought Stress. Front. Plant Sci. 2016, 7, 645. [Google Scholar] [CrossRef]
- Distelbarth, H.; Nägele, T.; Heyer, A.G. Responses of antioxidant enzymes to cold and high light are not correlated to freezing tolerance in natural accessions of Arabidopsis thaliana. Plant Biol. 2013, 15, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, Y.; Yan, J.; Jiang, J.; Cao, X.; Gao, J. Molecular characterization of elongase of very long-chain fatty acids 6 (elovl6) genes in Misgurnus anguillicaudatus and their potential roles in adaptation to cold temperature. Gene 2018, 666, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Demirel, U.; Morris, W.L.; Ducreux, L.J.M.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; et al. Physiological, Biochemical, and Transcriptional Responses to Single and Combined Abiotic Stress in Stress-Tolerant and Stress-Sensitive Potato Genotypes. Front. Plant Sci. 2020, 11, 169. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.-Y.; Wang, L.-C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Singh, R.; Rathore, D. Oxidative stress defence responses of wheat (Triticum aestivum L.) and chilli (Capsicum annum L.) cultivars grown under textile effluent fertilization. Plant Physiol. Biochem. 2018, 123, 342–358. [Google Scholar] [CrossRef]
- Abid, M.; Zhang, Y.J.; Li, Z.; Bai, D.F.; Zhong, Y.P.; Fang, J.B. Effect of salt stress on growth, physiological and biochemical characters of four kiwifruit genotypes. Sci. Hortic. 2020, 271, 109473. [Google Scholar] [CrossRef]
- Dai, F.; Huang, Y.; Zhou, M.; Zhang, G. The influence of cold acclimation on antioxidative enzymes and antioxidants in sensitive and tolerant barley cultivars. Biol. Plant. 2009, 53, 257–262. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritonga, F.N.; Slamet, S.A.; Irmayanti, L.; Anna, N.; Pebriandi; Chen, S. Integrated Analysis of Phenotypic, Physiological, and Biochemical Traits in Betula platyphylla Sukaczev Under Cold Stress Conditions. Forests 2025, 16, 1176. https://doi.org/10.3390/f16071176
Ritonga FN, Slamet SA, Irmayanti L, Anna N, Pebriandi, Chen S. Integrated Analysis of Phenotypic, Physiological, and Biochemical Traits in Betula platyphylla Sukaczev Under Cold Stress Conditions. Forests. 2025; 16(7):1176. https://doi.org/10.3390/f16071176
Chicago/Turabian StyleRitonga, Faujiah Nurhasanah, Syamsudin Ahmad Slamet, Laswi Irmayanti, Nelly Anna, Pebriandi, and Su Chen. 2025. "Integrated Analysis of Phenotypic, Physiological, and Biochemical Traits in Betula platyphylla Sukaczev Under Cold Stress Conditions" Forests 16, no. 7: 1176. https://doi.org/10.3390/f16071176
APA StyleRitonga, F. N., Slamet, S. A., Irmayanti, L., Anna, N., Pebriandi, & Chen, S. (2025). Integrated Analysis of Phenotypic, Physiological, and Biochemical Traits in Betula platyphylla Sukaczev Under Cold Stress Conditions. Forests, 16(7), 1176. https://doi.org/10.3390/f16071176






