Vegetation Structure and Habitat Characterization: An Ecological Basis for the Conservation of the Korean Endemic Plant, Taihyun’s Abelia (Zabelia tyaihyonii (Nakai) Hisauti & H.Hara, 1951; Caprifoliaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Survey Plot Placement and Survey Methods
2.3. Analysis
3. Results
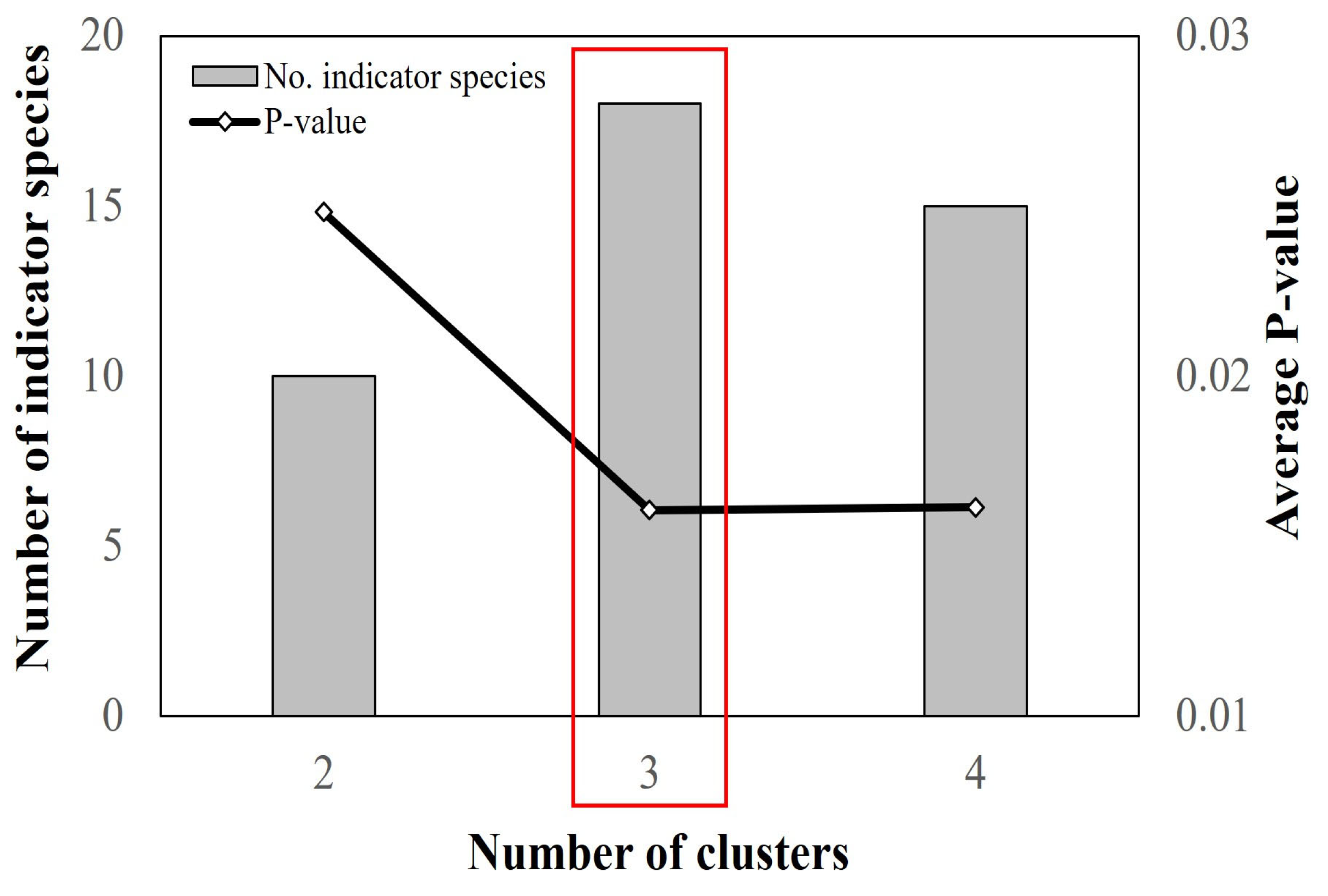
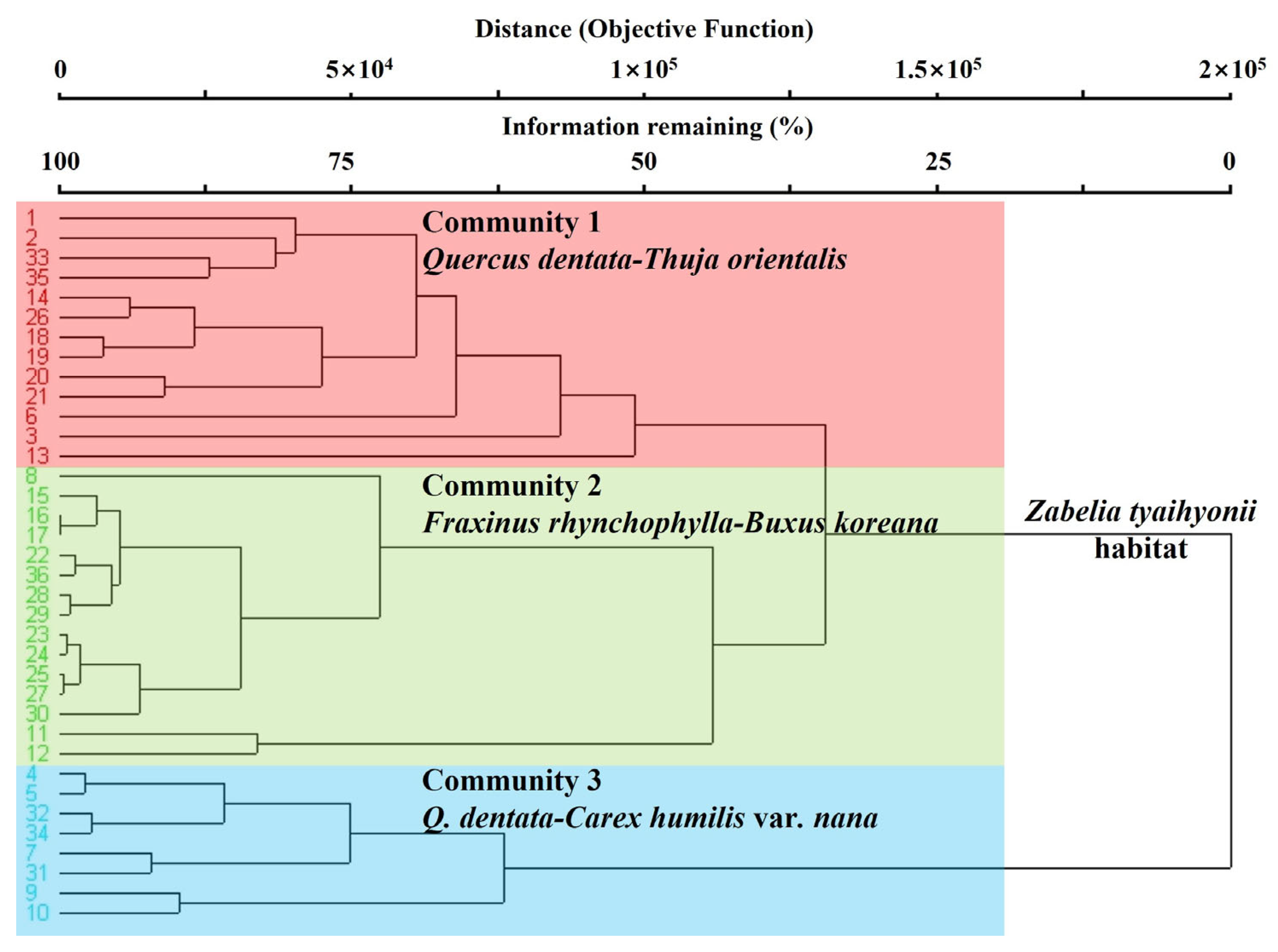
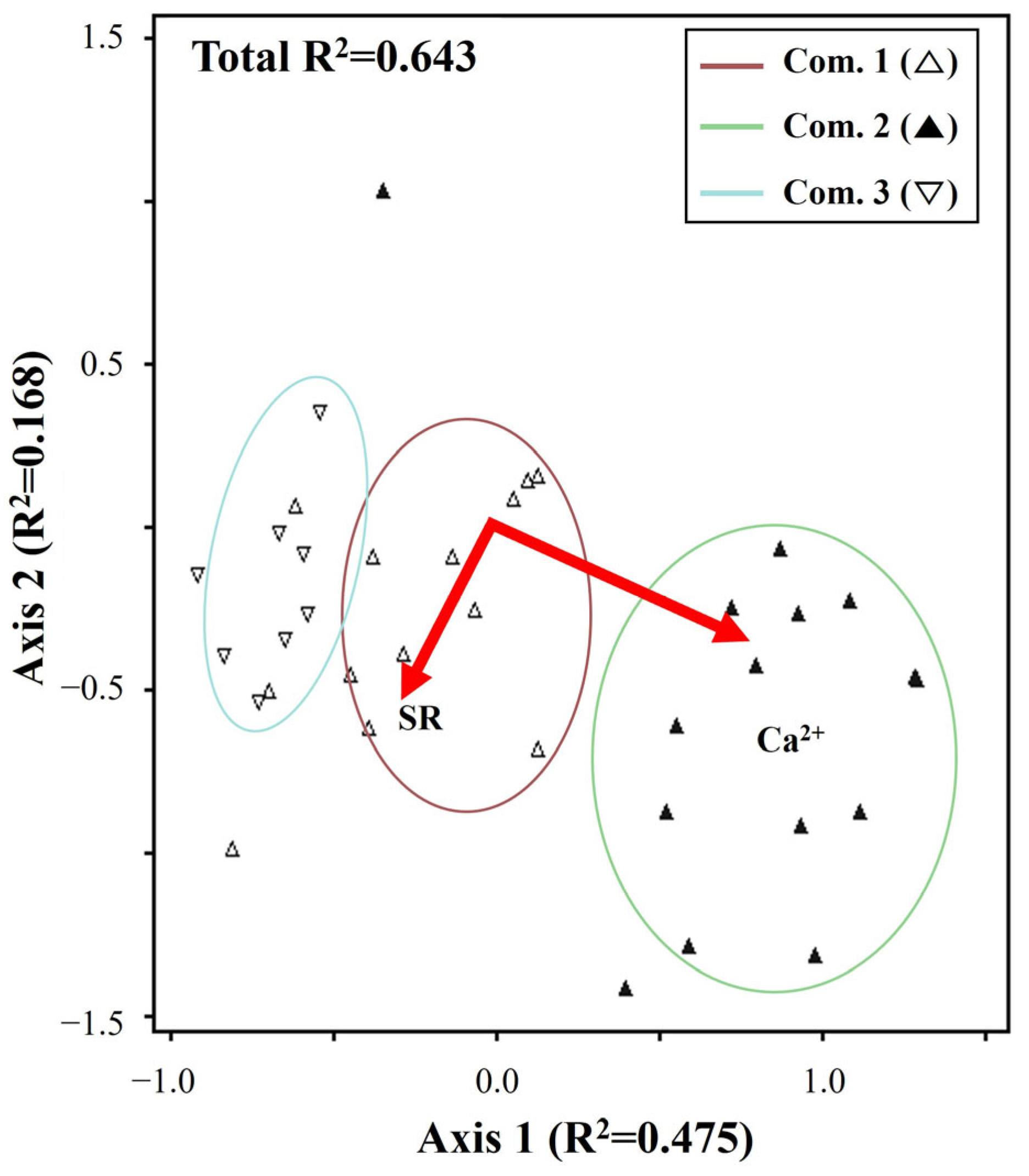
3.1. Community Identification and Habitat Characterization
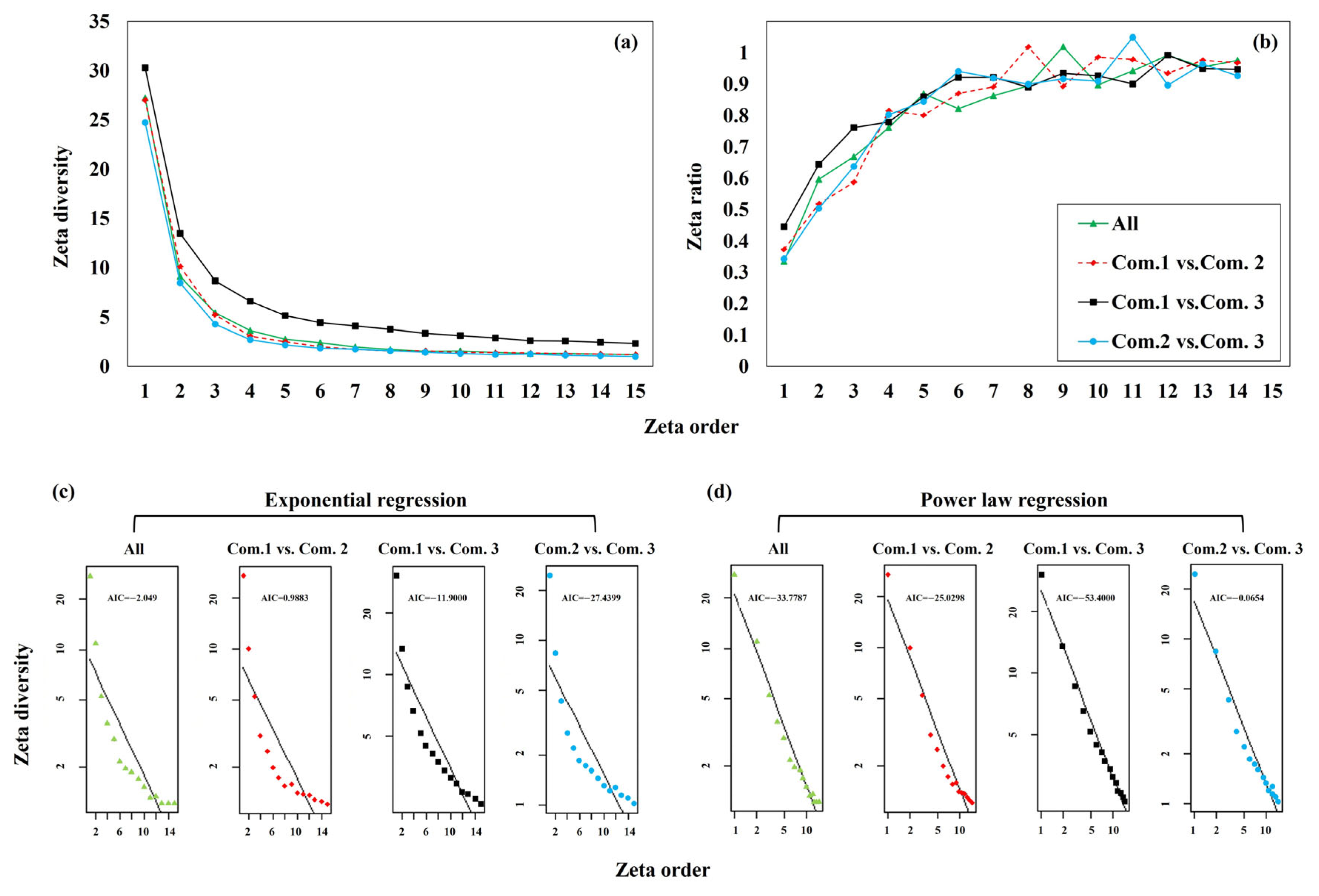
3.2. Zeta Diversity and Species Turnover
4. Discussion
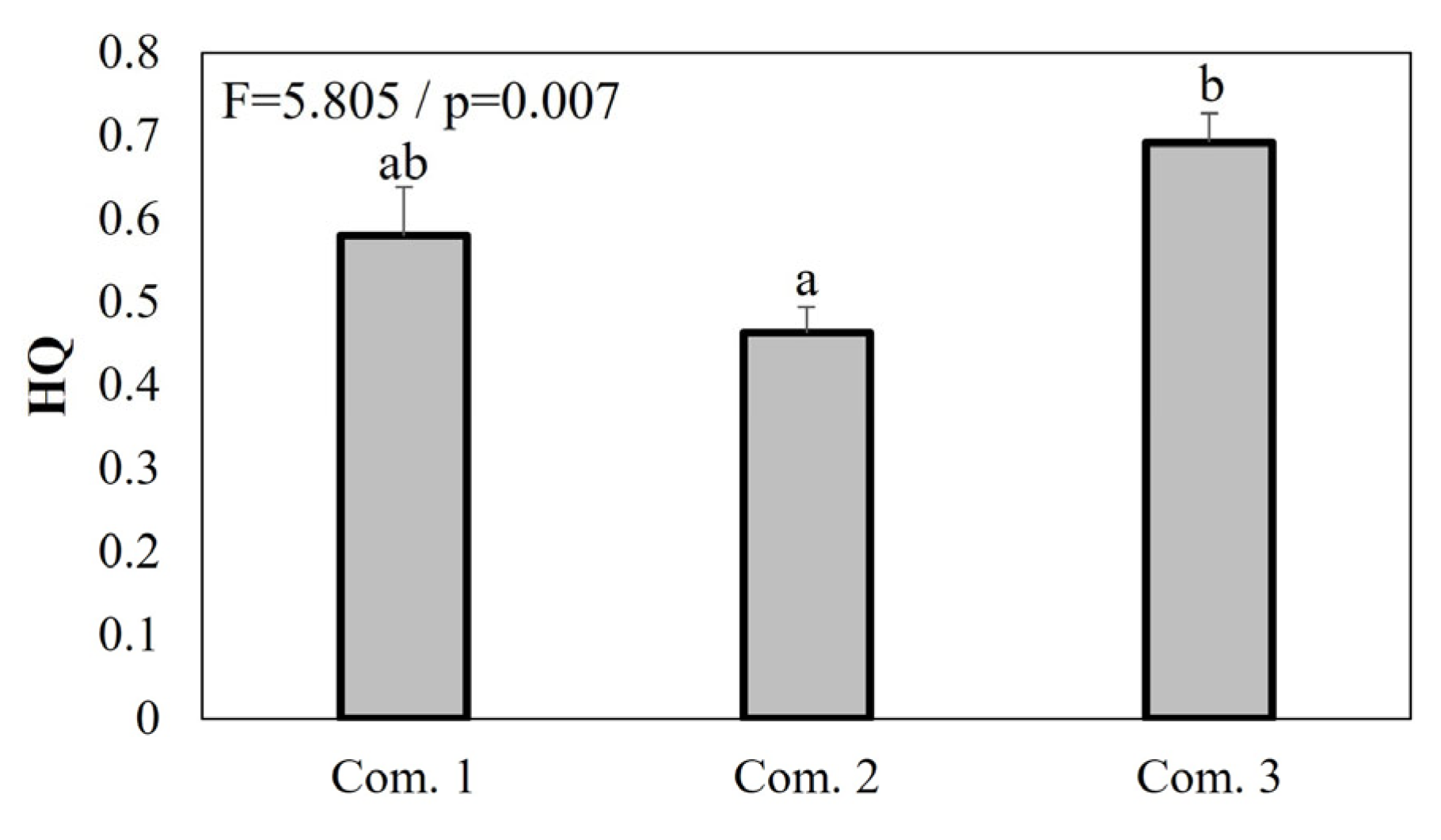
4.1. Community Identification and Habitat Characterization
4.2. Zeta Diversity and Species Turnover
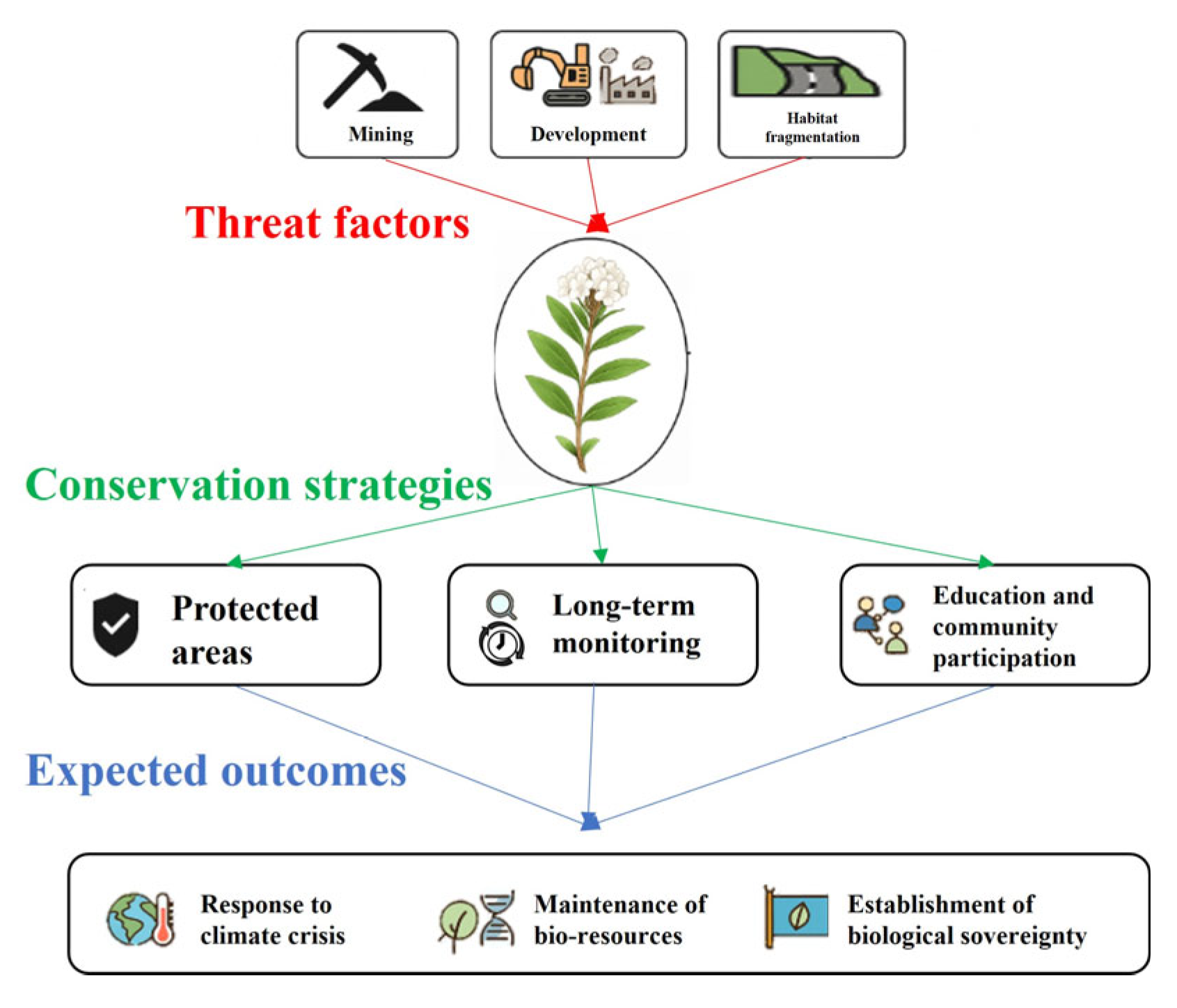
4.3. Conservation Strategy and Direction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Convention on Biological Diversity. Available online: https://www.cbd.int/gbf (accessed on 29 January 2024).
- Park, B.J.; Han, S.K.; Park, S.H.; Cheon, K.; Che, S.H.; Heo, T.I. Correlation between Vegetation Structure and Environmental Factors of Dracocephalum argunense Habitats in South Korea. J. Korean Soc. For. Sci. 2024, 113, 271–281. [Google Scholar]
- Koo, K.A.; Park, S.U. A Review of Ecological Niche Theory from the Early 1000s to the Present. Korean J. Environ. Ecol. 2021, 35, 316–335. [Google Scholar] [CrossRef]
- The Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 11 March 2025).
- Thompson, J.D.; Lavergne, S.; Affre, L.; Gaudeul, M.; Debussche, M. Ecological differentiation of Mediterranean endemic plants. Taxon 2005, 54, 967–976. [Google Scholar] [CrossRef]
- Aikens, M.L.; Roach, D.A. Population dynamics in central and edge populations of a narrowly endemic plant. Ecol. 2014, 95, 850–1860. [Google Scholar] [CrossRef]
- Chae, H.H.; Kim, Y.C.; Son, S.W. Korean and Worldwide Research Trends on Rare Plant and Endemic Plant in Korea. Korean J. Environ. Ecol. 2022, 36, 257–276. [Google Scholar] [CrossRef]
- Chung, G.Y.; Jang, H.D.; Chan, K.S.; Choi, H.J.; Kim, Y.S.; Kim, H.J.; Son, D.C. A checklist of endemic plants on the Korean Peninsula II. Korean J. Plant Taxon. 2023, 53, 79–101. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, K.S.; Lee, C.H.; Kim, Z.S. Genetic diversity and spatial structure in populations of Abelia tyaihyoni. J. Korean Soc. For. Sci. 2007, 96, 667–675. [Google Scholar]
- Chung, G.Y.; Chang, K.S.; Chung, J.M.; Choi, H.J.; Paik, W.G.; Hyun, J.O. checklist of endemic plants on the Korean Peninsula. Korean J. Plant Taxon. 2017, 47, 264–288. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, G.H.; Lee, S.B.; Shin, S.G.; Kim, J.S. A checklist of vascular plants in limestone areas on the Korean Peninsula. Korean J. Plant Taxon. 2021, 51, 250–293. [Google Scholar] [CrossRef]
- Kim, J.D.; Lee, H.J.; Lee, D.H.; Byeon, J.G.; Park, B.J.; Heo, T.I. Characteristics of environmental factors and vegetation Community of Zabelia tyaihyonii (Nakai) Hisauti & H.Hara among the target plant species for conservation in Baekdudaegan. J. Korean Soc. For. Sci. 2022, 111, 201–223. [Google Scholar]
- Korea National Arboretum. Rare plants in Korea; Sumeungil: Seoul, Republic of Korea, 2012; pp. 1–412. ISBN 978899745038196480. [Google Scholar]
- Chae, H.H.; Kim, Y.C.; Hong, B.R.; Lee, K.S.; Son, S. Distributional status and evaluation of species traits a Korean endemic plant of Zabelia tyaihyonii (Nakai) Hisauti and Hara (Caprifoliaceae). J. Asia-Pac. Biodivers 2023, 16, 372–383. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yim, G.J.; Lee, J.Y.; Ji, S.W. A Review of the Regeneration Models using a Closed Stone Quarry Area through Domestic and Overseas Cases. KSMER 2021, 58, 237–248. [Google Scholar] [CrossRef]
- Habel, J.C.; Assmann, T.; Schmitt, T.; Avise, J.C. Relict Species: From Past to Future; Springer: Berlin, Germany, 2010; pp. 1–5. ISBN 9783540921608. [Google Scholar]
- Grandcolas, P.; Nattier, R.; Trewick, S. Relict species: A relict concept? Trends Ecol. Evol. 2014, 29, 655–663. [Google Scholar] [CrossRef]
- Legg, S. IPCC, 2021: Climate Change 2021-The Physical Science Basis. Interaction 2021, 49, 44–45. [Google Scholar]
- Kim, K.A.; Jang, S.K.; Cheon, K.S.; Seo, W.B.; Yoo, K.O. Environmental and ecological characteristics of habitats of Abelia tyaihyoni Nakai. Korean J. Plant Taxon. 2010, 40, 135–144. [Google Scholar] [CrossRef]
- Ghimire, B.; Suh, G.U.; Lee, C.H.; Heo, K.; Jeong, M.J. Embryological studies on Abelia tyaihyoni Nakai (Caprifoliaceae). Flora 2018, 242, 79–88. [Google Scholar] [CrossRef]
- Lee, W.; Youn, J.S.; Kim, S.C.; Pak, J.H. The complete chloroplast genome sequence of limestone endemic, Zabelia tyaihyoni (Caprifoliaceae), in Korea. mtDNA Part B 2020, 5, 1947–1948. [Google Scholar] [CrossRef]
- Choi, I.S.; Han, E.K.; Wojciechowski, M.F.; Heo, T.I.; Park, J.S.; Yang, J.C.; Gantsetseg, A.; Cheon, K.S.; Tamaki, I.; Lee, J.H. The genetic structure and demographic history of Zabelia tyaihyonii, endemic to Korean limestone karst forests, based on genome-wide SNP markers. Ecol. Evol. 2023, 13, e10252. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.G. Classification and Assessment of Plant Communities; World Science: Seoul, Republic of Korea, 2006; pp. 33–60. ISBN 9788958810605. [Google Scholar]
- Korea National Arboretum. 2020. Forest of Korea(IV) Biogeography of Korea: Flora and Vegetation; Sumeungil: Seoul, Republic of Korea, 2020; pp. 42–79. ISBN 9791190509497. [Google Scholar]
- Nam, G.H.; Kim, J.H.; Kim, Y.C.; Kim, K.S.; Lee, B.Y. Floristic Study of County Pyeong-chang and Yeong-wol including Limestone Regions (Prov. Gangwon-do) from Korea. Korean J. Environ. Ecol. 2012, 26, 11–38. [Google Scholar]
- Climate Data in South Korea. Available online: http://www.kma.go.kr (accessed on 20 January 2025).
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge Der Vegetationskunde; Springer: Wien, Austria, 1964; pp. 17–205. ISBN 9783709181119. [Google Scholar]
- Ivanova, N. Global Overview of the Application of the Braun-Blanquet Approach in Research. Forests 2024, 15, 937. [Google Scholar] [CrossRef]
- Korea Fern Society. Ferns and Fern Allies of Korea; Geobook: Seoul, Republic of Korea, 2005; pp. 1–399. ISBN 9788995504925. [Google Scholar]
- Korea National Arboretum. Rare Plants Data Book in Korea; Geobook: Seoul, Republic of Korea, 2008; pp. 1–332. ISBN 9788991458352. [Google Scholar]
- Korea National Arboretum. Invasive Alien Plant Impact on Forest; Sumeungil: Seoul, Republic of Korea, 2015; pp. 1–280. ISBN 9791187031185. [Google Scholar]
- Cho, Y.H.; Kim, J.H.; Park, S.H. Grasses and Sedges in South Korea; Geobook: Seoul, Republic of Korea, 2016; pp. 1–528. ISBN 9788994242422. [Google Scholar]
- Korea National Arboretum. Checklist of Vascular Plants in Korea; Samsung edCOM: Seoul, Republic of Korea, 2017; pp. 1–1000. ISBN 9791188720125. [Google Scholar]
- Korea National Arboretum. Native & 100 Cultivar Caprifoliaceae; Blue Sensation: Seoul, Republic of Korea, 2022; pp. 1–147. ISBN 9791192743097. [Google Scholar]
- Knowledge system of National species in Korea. Available online: http://www.nature.go.kr/kpni (accessed on 3 January 2025).
- Kang, C. A study of the jackknife estimate. J. Ind. Sci. Technol. 2002, 34, 325–331. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecologcial Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; pp. 179–204. ISBN 9780972129008. [Google Scholar]
- Curtis, J.T.; McIntosh, R.P. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Park, B.J.; Heo, T.I.; Cheon, K.I. Analyzing Generalist Plant Species Using Topographic Characteristics of Picea jezoensis (Siebold & Zucc.) Carrière Forests in East Asia: From China (Mt. Changbai) to South Korea. Int. J. Plant Biol. 2024, 15, 320–339. [Google Scholar]
- Petroselli, A.; Vessella, F.; Lucia, C.; Piovesan, G.; Schirone, B. Ecological behavior of Quercus suber and Quercus ilex inferred by topographic wetness index (TWI). Trees 2013, 27, 1201–1215. [Google Scholar] [CrossRef]
- Zwolinski, Z.; Stefańska, E. Relevance of moving window size in landform classification by TPI. Geomorphometry Geosci. 2015, 2015, 273–277. [Google Scholar]
- McCune, B.; Keon, D. Equations for potential annual direct incident radiation and heat load. J. Veg. Sci. 2002, 13, 603–606. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Klute, A. Methods of Soil Analysis Part 1 Physical and Mineralogical Methods; American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. ISBN 9780891180883. [Google Scholar]
- Beckschäfer, P.; Seidel, D.; Kleinn, C.; Xu, J. On the exposure of hemispherical photographs in forests. Ifor.-Biogeosci. For. 2013, 6, 228. [Google Scholar] [CrossRef]
- Nelson, E.; Mendoza, G.; Regetz, J.; Polasky, S.; Tallis, H.; Cameron, D.; Chan, K.M.; Daily, G.C.; Goldstein, J.; Kareiva, P.M.; et al. Modeling multiple ecosystem services, biodiversity conservation, commodity production, and tradeoffs at landscape scales. Front. Ecol. Environ. 2009, 7, 4–11. [Google Scholar] [CrossRef]
- Gao, J.; Li, F.; Gao, H.; Zhou, C.; Zhang, X. The impact of land-use change on water-related ecosystem services: A study of the Guishui River Basin, Beijing, China. J. Clean. Prod. 2017, 163, 148–155. [Google Scholar] [CrossRef]
- Jang, J.E.; Kwon, H.Y.; Shing, H.S.; Lee, S.C.; Yu, B.H.; Jang, J.; Choi, S.H. Habitat quality analysis and evaluation of InVEST model using QGIS: Conducted in 21 national parks of Korea. Korean J. Environ. Ecol. 2022, 36, 102–111. [Google Scholar] [CrossRef]
- Nellemann, C.; Vistnes, I.; Jordhøy, P.; Strand, O. Winter distribution of wild reindeer in relation to power lines, roads and resorts. Biol. Conserv. 2001, 101, 351–360. [Google Scholar] [CrossRef]
- National Institute of Ecology. The Manual of Assessment Map of Ecosystem Service; Design Crepas: Seoul, Republic of Korea, 2022; pp. 54–61. ISBN 9791166981463. [Google Scholar]
- Kumar, P. Tools for Measuring modelling, and Valuing Ecosystem Services; IUCN: Gland, Switzerland, 2018; pp. 1–293. ISBN 9782831718965. [Google Scholar]
- National Institute of Biological Resources. Guidelines for Using the IUCN Red List Categories and Criteria (Korean Version); Doohyun Publishing: Seoul, Republic of Korea, 2015; pp. 57–71. ISBN 9788968112485. [Google Scholar]
- McGeoch, M.A.; Latombe, M.; Andrew, N.R.; Nakagawa, S.; Nipperess, D.A.; Roigé, M.; Marzinelli, E.M.; Campbell, A.H.; Vergés, A.; Thomas, T.; et al. Measuring continuous compositional change using decline and decay in zeta diversity. Ecology 2019, 100, e02832. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Gaston, K.J.; He, F. The distribution of species range size: A stochastic process. Proc. R. Soc. Lond. B 2002, 269, 1079–1086. [Google Scholar] [CrossRef]
- Zuppinger-Dingley, D.; Schmid, B.; Petermann, J.S.; Yadav, V.; De Deyn, G.B.; Flynn, D.F. Selection for niche differentiation in plant communities increases biodiversity effects. Nature 2014, 515, 108–111. [Google Scholar] [CrossRef]
- Latombe, G.; McGeoch, M.A.; Nipperess, D.A.; Hui, C. zetadiv: An R package for computing compositional change across multiple sites, assemblages or cases. bioRxiv. [CrossRef]
- Park, B.J.; Kim, J.J.; Byeon, J.G.; Cheon, K.; Joo, S.H.; Lee, Y.G. The classification of forest community and character of stand structure in Mt. Myeonbong: Focused on research forest in Kyungpook National University, Cheongsong. J. Korean Soc. For. Sci. 2016, 105, 391–400. [Google Scholar] [CrossRef]
- Weltzin, J.F.; Loik, M.E.; Schwinning, S.; Williams, D.G.; Fay, P.A.; Haddad, B.M.; Harte, J.; Huxman, T.E.; Knapp, A.K.; Lin, G.H.; et al. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience 2003, 53, 941–952. [Google Scholar] [CrossRef]
- Wentz, F.J.; Ricciardulli, L.; Hilburn, K.; Mears, C. How much more rain will global warming bring? Science 2007, 317, 233–235. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zwiers, F.W.; Hegerl, G.C.; Lambert, F.H.; Gillett, N.P.; Solomon, S.; Stott, P.A.; Nozawa, T. Detection of human influence on twentieth-century precipitation trends. Nature 2007, 448, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Romero, E.; Petrlic, K.; Verachtert, E.; Bochet, E.; Poesen, J. Effects of slope angle and aspect on plant cover and species richness in a humid Mediterranean badland. Earth Surf. Process. Landf. 2014, 39, 1705–1716. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology; Springer-Verlag: Berlin, Germany, 1975; pp. 1–252. ISBN 9783540068089. [Google Scholar]
- Barbour, M.G.; Burk, J.H.; Pitts, W.D. Terrestrial Plant Ecology; The Benjamin/Cummings: San Francisco, CA, USA, 1987; pp. 155–229. ISBN 9780805385745. [Google Scholar]
- Newton, A.C. Forest Ecology and Conservation: A handbook of Techniques; Oxford University Press: New York, NY, USA, 2007; pp. 85–146. ISBN 9780198567455. [Google Scholar]
- Percival, G.C.; Keary, I.P.; Sulaiman, A.H. An assessment of the drought tolerance of Fraxinus genotypes for urban landscape plantings. Urban For. Urban Green. 2006, 5, 17–27. [Google Scholar] [CrossRef]
- Asanok, L.; Marod, D. Environmental factors influencing tree species regeneration in different forest stands growing on a limestone hill in Phrae Province, Northern Thailand. J. For. Environ. Sci. 2016, 32, 237–252. [Google Scholar] [CrossRef]
- Lee, Y.N.; Oh, Y.J. Limestone flora of Todam, province Chung Buk in South Korea. J. Korean Res. Inst. Better Living 1970, 5, 101–115. [Google Scholar]
- Kim, J.H.; Mun, H.T.; Kwak, Y.S. Community structure and soil properties of the Pinus densiflora forests in limestone areas. Korean J. Ecol. 1990, 13, 285–295. (In Korean) [Google Scholar]
- Chytrý, M.; Danihelka, J.; Ermakov, N.; Hájek, M.; Hájková, P.; Kočí, M.; Kubešová, S.; Lustyk, P.; Otýpková, Z.; Popov, D.; et al. Plant species richness in continental southern Siberia: Effects of pH and climate in the context of the species pool hypothesis. Glob. Ecol. Biogeogr. 2007, 16, 668–678. [Google Scholar] [CrossRef]
- Gauld, J.H.; Robertson, J.S. Soils and their related plant communities of the Dalradian limestone of some sites in central Perthshire, Scotland. J. Ecol. 1985, 73, 91–112. [Google Scholar] [CrossRef]
- Jeffrey, D.W. Soil-Plant Relationships: An Ecological Approach; Timber Press: Portland, OR, USA, 1987; pp. 257–279. ISBN 9780881920630. [Google Scholar]
- DeJong, T.M. Comparison of three diversity indices based on their components of richness and evenness. Oikos 1975, 26, 222–227. [Google Scholar] [CrossRef]
- Norman, W.H.M.; David, M.; William, G.L.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Park, B.J.; Byeon, J.G.; Cheon, K. Study of ecological niche and indicator species by landforms and altitude of forest vegetation in Mt. Myeonbong. Korean J. Plant Res. 2019, 32, 325–337. [Google Scholar]
- Xu, L.; Chen, S.S.; Xu, Y.; Li, G.; Su, W. Impacts of land-use change on habitat quality during 1985–2015 in the Taihu Lake Basin. J. Sustain. 2019, 11, 1–21. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Alexander, L.E. Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 1998, 29, 207–231. [Google Scholar] [CrossRef]
- Turner II, B.L.; Lambin, E.F.; Reenberg, A. The emergence of land change science for global environmental change and sustainability. Proc. Natl. Acad. Sci. USA 2007, 104, 20666–20671. [Google Scholar] [CrossRef]
- Dukes, J.S. Biodiversity and invasibility in grassland microcosms. Oecologia 2001, 126, 563–568. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Carson, W.P. Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology 2001, 82, 913–919. [Google Scholar] [CrossRef]
- Czech, B.; Krausman, P.R.; Devers, P.K. Economic associations among causes of species endangerment in the United States. BioScience 2000, 50, 593–601. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, biodiversity, and conservation. BioScience 2002, 52, 883. [Google Scholar] [CrossRef]
- Dickman, C.R. Habitat fragmentation and vertebrate species richness in an urban environment. J. Appl. Ecol. 1987, 24, 337–351. [Google Scholar] [CrossRef]
- Hui, C.; McGeoch, M.A. Zeta diversity as a concept and metric that unifies incidence-based biodiversity patterns. Am. Nat. 2014, 184, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.J.; Archer, S.R. Niche differentiation and neutral theory: An integrated perspective on shrub assemblages in a parkland savanna. Ecology 2010, 91, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B. Joint consequences of dispersal and niche overlap on local diversity and resource use. J. Ecol. 2012, 100, 287–296. [Google Scholar] [CrossRef]
- Papuga, G.; Gauthier, P.; Pons, V.; Farris, E.; Thompson, J.D. Ecological niche differentiation in peripheral populations: A comparative analysis of eleven Mediterranean plant species. Ecography 2018, 41, 1650–1664. [Google Scholar] [CrossRef]
- Westman, W.E. How much are nature’s services worth? Science 1977, 197, 960–964. [Google Scholar] [CrossRef]
- Costanza, R.; d’Arge, R.; de Groot, S.; Farber, M.; Grasso, B.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Ecol. Econ. 1998, 25, 3–15. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; pp. 115–137. ISBN 9781559631496. [Google Scholar]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. The Methodological Assessment Report on Scenarios and Models of Biodiversity and Ecosystem Services; UN Campus: Bonn, Germany, 2016; p. 309. ISBN 9783981585207. [Google Scholar]

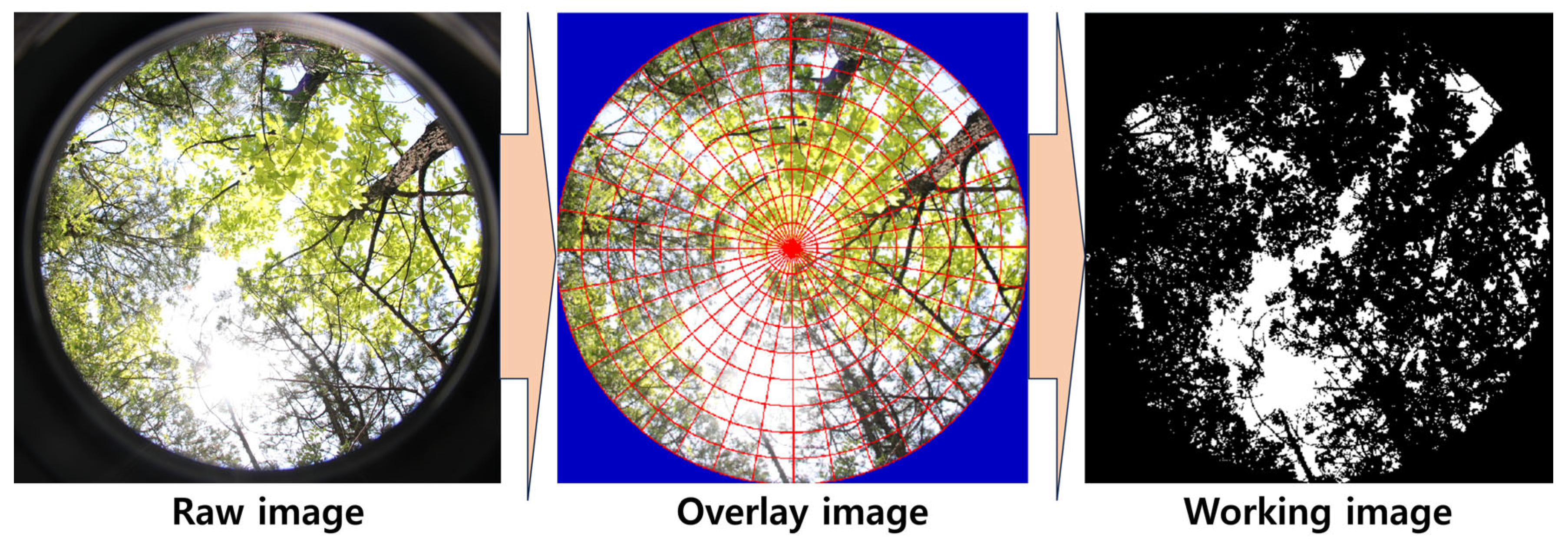
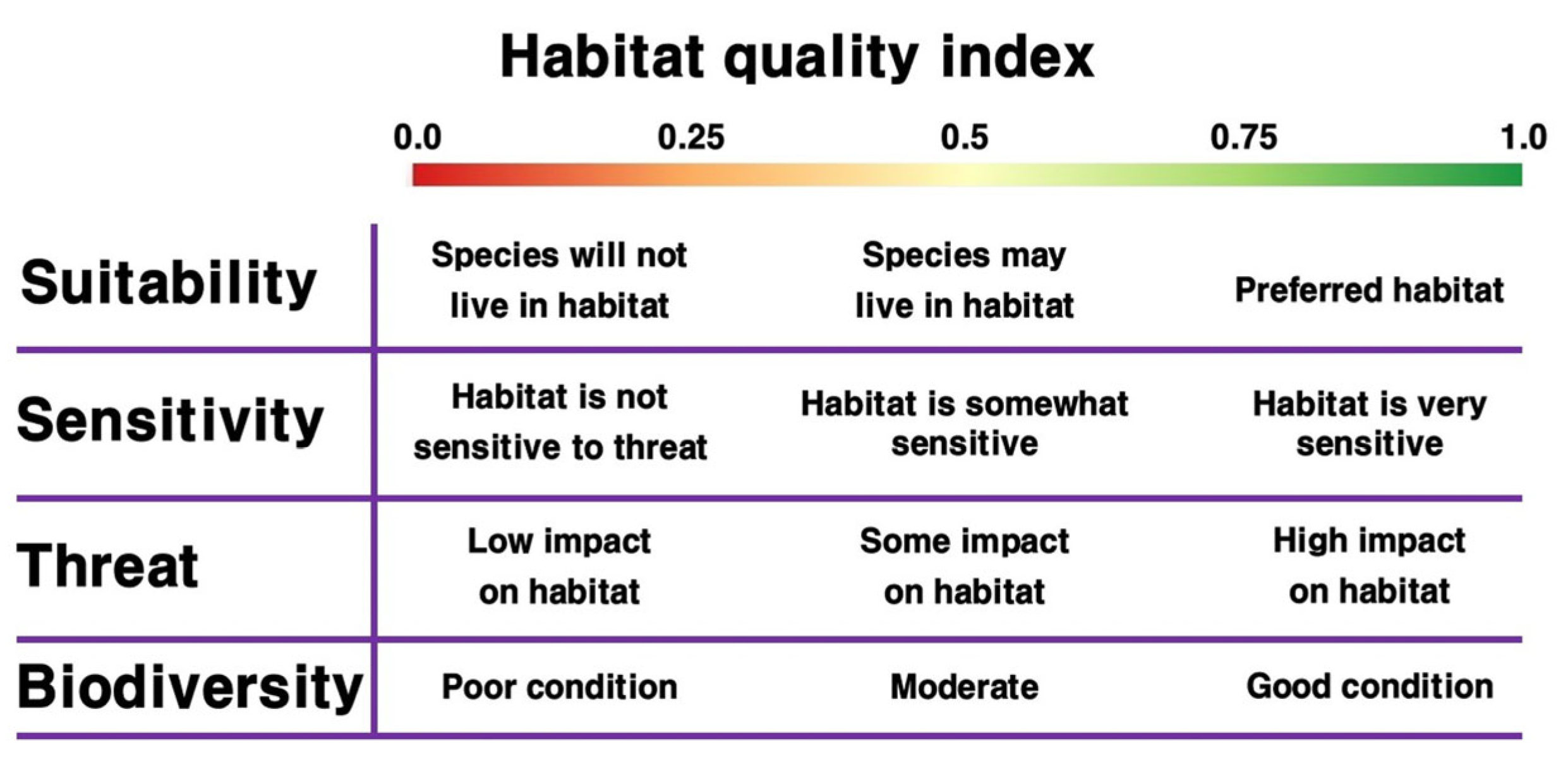
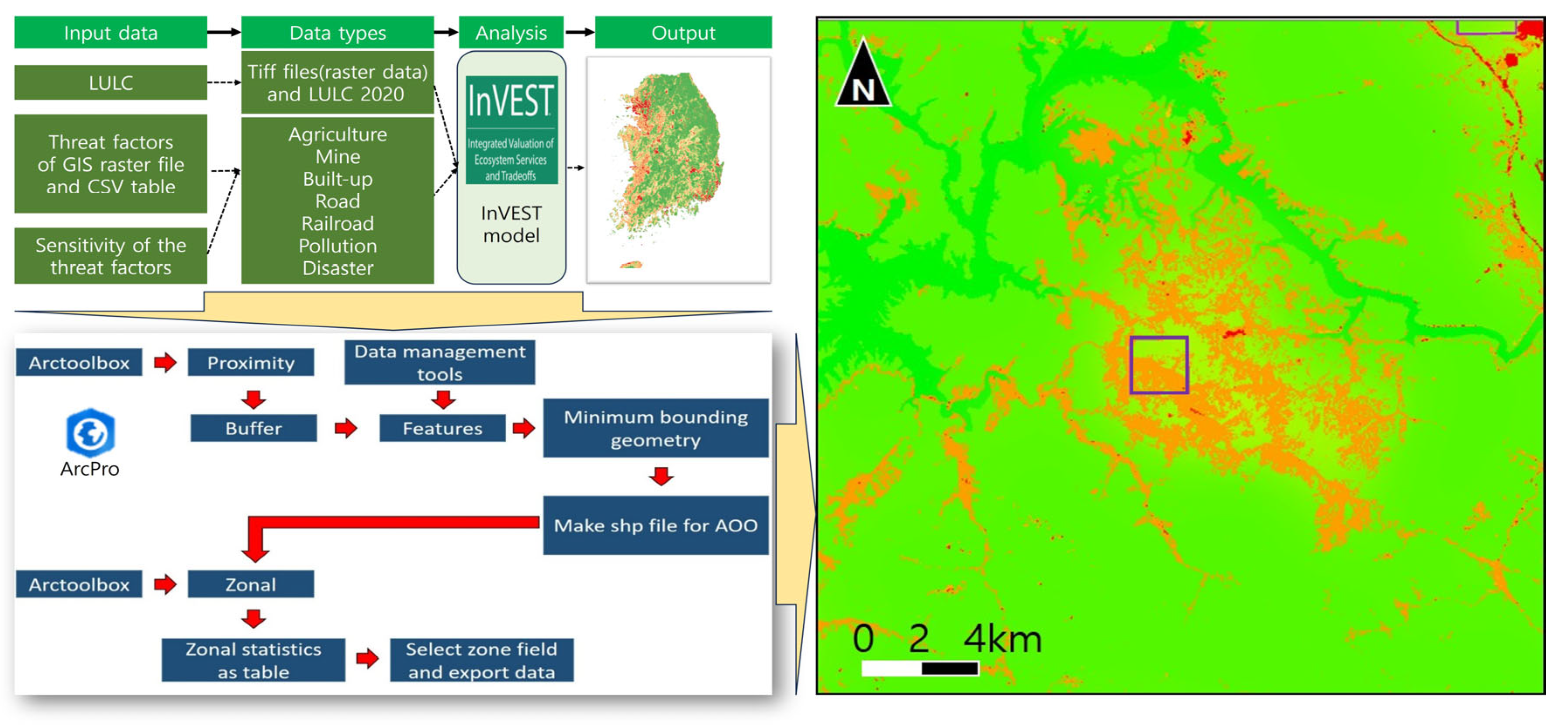
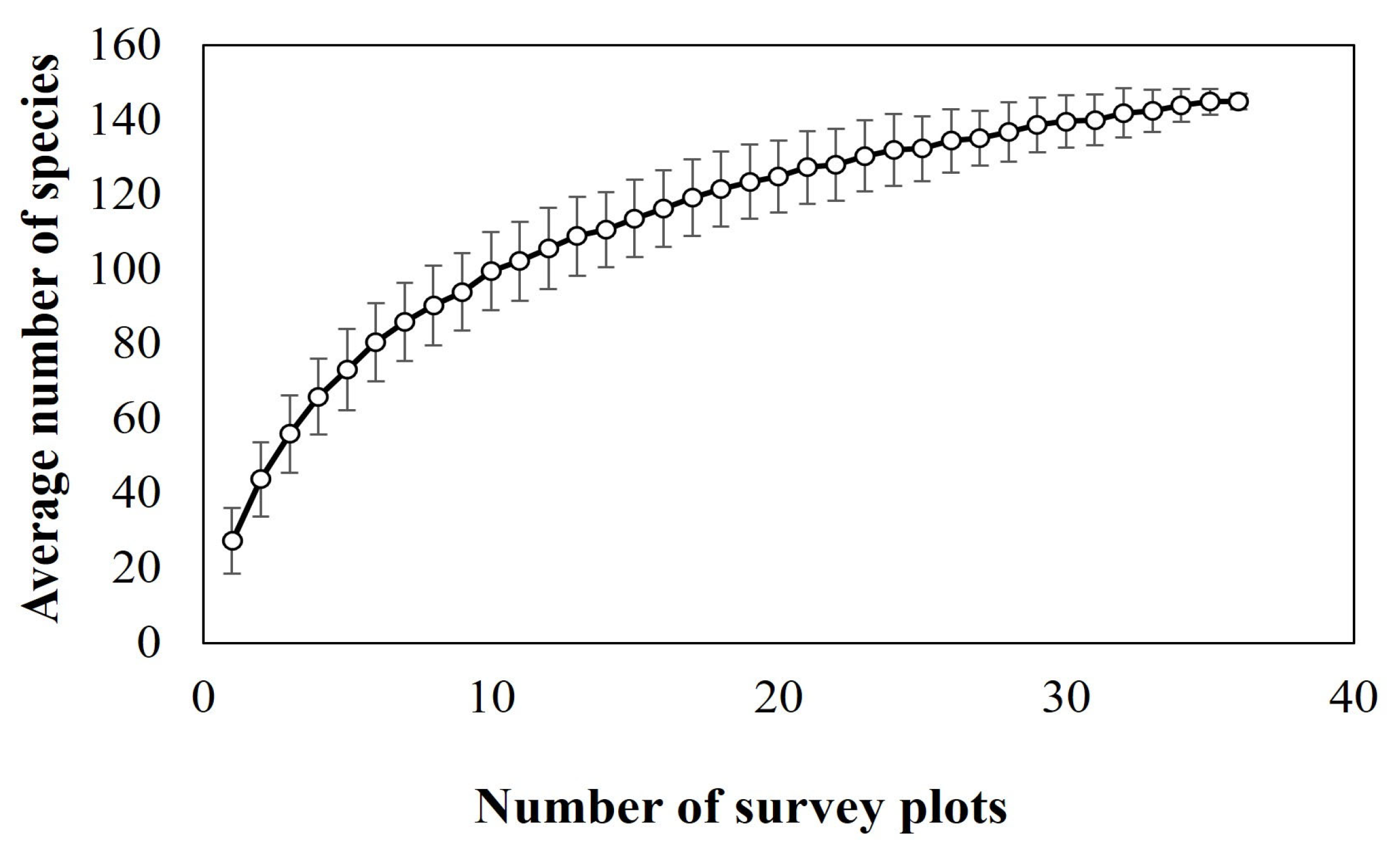

| Braun-Blanquet Scale | Range of Cover and Abundance (%) | Median Value |
|---|---|---|
| 5 | 75–100 | 87.5 |
| 4 | 50–75 | 62.5 |
| 3 | 25–50 | 37.5 |
| 2 | 12.5–25 | 18.75 |
| 1 | <12.5 numerous individuals | 9.375 |
| + | <5 few individuals | 4.69 |
| r | <2 few and unique individuals | 1.01 |
| Contents | Index | Methods |
|---|---|---|
| Physical | Organic matter | Loss on ignition (LECO Corp., LECO TGA801, St. Joseph, MI, USA) |
| Gravel fraction | Soil particle size analysis method [44] | |
| Chemical | pH | pH meter (Thermo Fisher Scientific, Orion Star™ A215 Benchtop Meter, Waltham, MA, USA) |
| Exchangeable cations (K+, Ca2+, Mg2+, Na+) | Atomic absorption spectroscopy (GBC Scientific Equipment Ltd., AVANTA, Dandenong, Victoria, Australia) | |
| Cation exchange capacity (CEC) | Ion exchange capacity determination and inductively coupled plasma atomic emission spectroscopy (Metrohm, 940 Professional IC vario, Herisau, Switzerland) | |
| Electrical conductivity (EC) | EC meter (Thermo Fisher Scientific, Orion Star™ A215 Benchtop Meter, Waltham, MA, USA) | |
| Exchangeable aluminum | Inductively coupled plasma atomic emission spectroscopy after extraction of 1 M KCl (GBC Scientific Equipment Ltd., AVANTA, Dandenong, Victoria, Australia) |
| Maximum Distance (km) | Weight | Threat Factor | Linear/Exponential | Description |
|---|---|---|---|---|
| 3 | 0.65 | Agriculture | linear | Agricultural lands |
| 6 | 0.48 | Mine | linear | Mine line |
| 8 | 0.90 | Built-up | exponential | Urban/developed |
| 3 | 0.75 | Road | linear | Road line |
| 2 | 0.52 | Railroad | linear | Railroad line |
| 5 | 0.63 | Pollution | exponential | Pollution lands |
| 2 | 0.40 | Disaster | linear | Disaster line |
| Species | Com. 1 | Com. 2 | Com. 3 |
|---|---|---|---|
| Zabelia tyaihyonii (Nakai) Hisauti & H.Hara | 9.91 | 12.82 | 24.86 |
| Quercus dentata Thunb. | 12.64 | - | 10.20 |
| Thuja orientalis L. | 5.94 | 2.80 | - |
| Fraxinus rhynchophylla Hance | 3.83 | 8.34 | 3.42 |
| Buxus koreana Nakai ex Chung & al. | 3.93 | 6.90 | - |
| Carex humilis var. nana (H.Lév. & Vaniot) Ohwi | 4.19 | - | 6.48 |
| Juniperus rigida Siebold & Zucc. | 3.99 | 2.43 | 3.15 |
| Spiraea chinensis Maxim. | 2.47 | . | 1.94 |
| Quercus variabilis Blume | 2.35 | - | - |
| Securinega suffruticosa (Pall.) Rehder | 2.25 | - | - |
| Carex lanceolata Boott | - | 3.83 | - |
| Thalictrum petaloideum L. | - | 3.03 | - |
| Euonymus alatus f. ciliatodentatus (Franch. & Sav.) Hiyama | - | 2.61 | - |
| Spodipogon sibiricus Trin. | - | 2.55 | - |
| Neillia uyekii Nakai | - | 2.07 | - |
| Ulmus macrocarpa Hance | - | - | 2.42 |
| Pinus densiflora Siebold & Zucc. | - | - | 2.30 |
| Rhamnus yoshinoi Makino | - | - | 1.99 |
| Rubia cordifolia var. pratensis Maxim. | - | - | 1.76 |
| Omitted Species (No.) | 48.51 (108) | 52.61 (109) | 41.48 (75) |
| Total | 100 | 100 | 100 |
| Contents | Species | IV | p |
|---|---|---|---|
| Com. 1 | Quercus dentata Thunb. | 53.9 | 0.0040 |
| Spiraea chinensis Maxim. | 47.6 | 0.0206 | |
| Quercus variabilis Blume | 46.2 | 0.0220 | |
| Miscanthus sinensis Andersson | 46.2 | 0.0022 | |
| Platycarya strobilacea Siebold & Zucc. | 38.5 | 0.0068 | |
| Thuja orientalis L. | 37.6 | 0.0490 | |
| Leibnitzia anandria (L.) Turcz. | 31.8 | 0.0480 | |
| Com. 2 | Buxus koreana Nakai ex Chung & al. | 48.0 | 0.0048 |
| Zabelia tyaihyonii (Nakai) Hisauti & H.Hara | 40.3 | 0.0002 | |
| Carex lanceolata Boott | 40.0 | 0.0096 | |
| Neillia uyekii Nakai | 40.0 | 0.0090 | |
| Com. 3 | Crepidiastrum sonchifolium (Bunge) Pak & Kawano | 62.5 | 0.0004 |
| Carex humilis var. nana(H.Lév. & Vaniot) Ohwi | 51.9 | 0.0082 | |
| Euonymus alatus (Thunb.) Siebold | 47.6 | 0.0276 | |
| Atractylodes ovata (Thunb.) DC. | 40.5 | 0.0146 | |
| Rhus javanica L. | 40.2 | 0.0176 | |
| Ulmus macrocarpa Hance | 39.9 | 0.0184 | |
| Pinus densiflora Siebold & Zucc. | 39.5 | 0.0260 |
| Contents | Com. 1 | Com. 2 | Com. 3 | F | p-Value | |
|---|---|---|---|---|---|---|
| Geography and vegetational Factors | Altitude (m) ns | 217.2 ± 7.7 | 221.2 ± 9.5 | 257.5 ± 25.8 | 2.302 | 0.116 |
| Slope (°) ns | 8.0 ± 1.1 | 5.8 ± 0.8 | 6.0 ± 1.4 | 1.517 | 0.234 | |
| TWI ns | 7.93 ± 0.43 | 9.16 ± 0.61 | 8.93 ± 1.11 | 1.048 | 0.362 | |
| TPI ns | −1.59 ± 2.45 | −3.22 ± 0.94 | −0.61 ± 2.20 | 0.472 | 0.628 | |
| Northness ns | −0.72 ± 0.10 | −0.63 ± 0.12 | −0.39 ± 0.20 | 1.443 | 0.251 | |
| Canopy openness (%) ns | 45.88 ± 5.64 | 47.41 ± 4.74 | 54.25 ± 4.31 | 0.569 | 0.572 | |
| Transmitted light (mole·m−2·d−1) ns | 14.87 ± 1.88 | 15.12 ± 1.66 | 18.29 ± 1.75 | 0.862 | 0.432 | |
| Soil properties | Organic matter (%) ns | 18.5 ± 1.0 | 16.9 ± 1.5 | 18.4 ± 0.6 | 0.539 | 0.588 |
| Gravel fraction (%) ns | 47.4 ± 3.5 | 43.6 ± 3.1 | 50.9 ± 4.8 | 0.913 | 0.411 | |
| pH ns | 7.77 ± 0.05 | 7.82 ± 0.09 | 7.68 ± 0.04 | 1.377 | 0.266 | |
| EC (ds/m) ns | 0.64 ± 0.07 | 0.76 ± 0.08 | 0.67 ± 0.08 | 0.669 | 0.519 | |
| K+ (cmol+/kg) ns | 0.327 ± 0.025 | 0.406 ± 0.055 | 0.343 ± 0.037 | 0.973 | 0.388 | |
| Ca2+ (cmol+/kg) * | 16.462 ± 1.398 a | 20.208 ± 1.564 b | 15.390 ± 1.396 a | 3.330 | 0.048 | |
| Mg2+ (cmol+/kg) ns | 5.208 ± 0.529 | 4.455 ± 0.468 | 6.238 ± 0.489 | 2.679 | 0.084 | |
| Na+ (cmol+/kg) ns | 0.038 ± 0.005 | 0.051 ± 0.006 | 0.044 ± 0.008 | 1.382 | 0.265 | |
| Al3+ (cmol+/kg) ns | 7.476 ± 1.813 | 6.898 ± 1.715 | 6.324 ± 1.732 | 0.086 | 0.916 | |
| CEC (cmol+/kg) ns | 0.038 ± 0.005 | 0.051 ± 0.006 | 0.044 ± 0.008 | 0.655 | 0.526 | |
| Species diversity | Species richness * | 31.77 ± 2.42 b | 23.00 ± 2.25 a | 28.00 ± 1.24 a | 4.328 | 0.021 |
| Shannon index * | 2.99 ± 0.11 b | 2.57 ± 0.08 a | 2.66 ± 0.10 a | 4.316 | 0.022 | |
| Evenness ** | 0.87 ± 0.01 b | 0.77 ± 0.02 a | 0.86 ± 0.01 b | 11.703 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.-J.; Heo, T.-I.; Cheon, K.-I. Vegetation Structure and Habitat Characterization: An Ecological Basis for the Conservation of the Korean Endemic Plant, Taihyun’s Abelia (Zabelia tyaihyonii (Nakai) Hisauti & H.Hara, 1951; Caprifoliaceae). Forests 2025, 16, 1042. https://doi.org/10.3390/f16071042
Park B-J, Heo T-I, Cheon K-I. Vegetation Structure and Habitat Characterization: An Ecological Basis for the Conservation of the Korean Endemic Plant, Taihyun’s Abelia (Zabelia tyaihyonii (Nakai) Hisauti & H.Hara, 1951; Caprifoliaceae). Forests. 2025; 16(7):1042. https://doi.org/10.3390/f16071042
Chicago/Turabian StylePark, Byeong-Joo, Tae-Im Heo, and Kwang-Il Cheon. 2025. "Vegetation Structure and Habitat Characterization: An Ecological Basis for the Conservation of the Korean Endemic Plant, Taihyun’s Abelia (Zabelia tyaihyonii (Nakai) Hisauti & H.Hara, 1951; Caprifoliaceae)" Forests 16, no. 7: 1042. https://doi.org/10.3390/f16071042
APA StylePark, B.-J., Heo, T.-I., & Cheon, K.-I. (2025). Vegetation Structure and Habitat Characterization: An Ecological Basis for the Conservation of the Korean Endemic Plant, Taihyun’s Abelia (Zabelia tyaihyonii (Nakai) Hisauti & H.Hara, 1951; Caprifoliaceae). Forests, 16(7), 1042. https://doi.org/10.3390/f16071042





