Seasonal Variation in Transpiration and Stomatal Conductance of Three Savanna Tree Species in Ruma National Park, Kenya
Abstract
1. Introduction
2. Materials and Methods
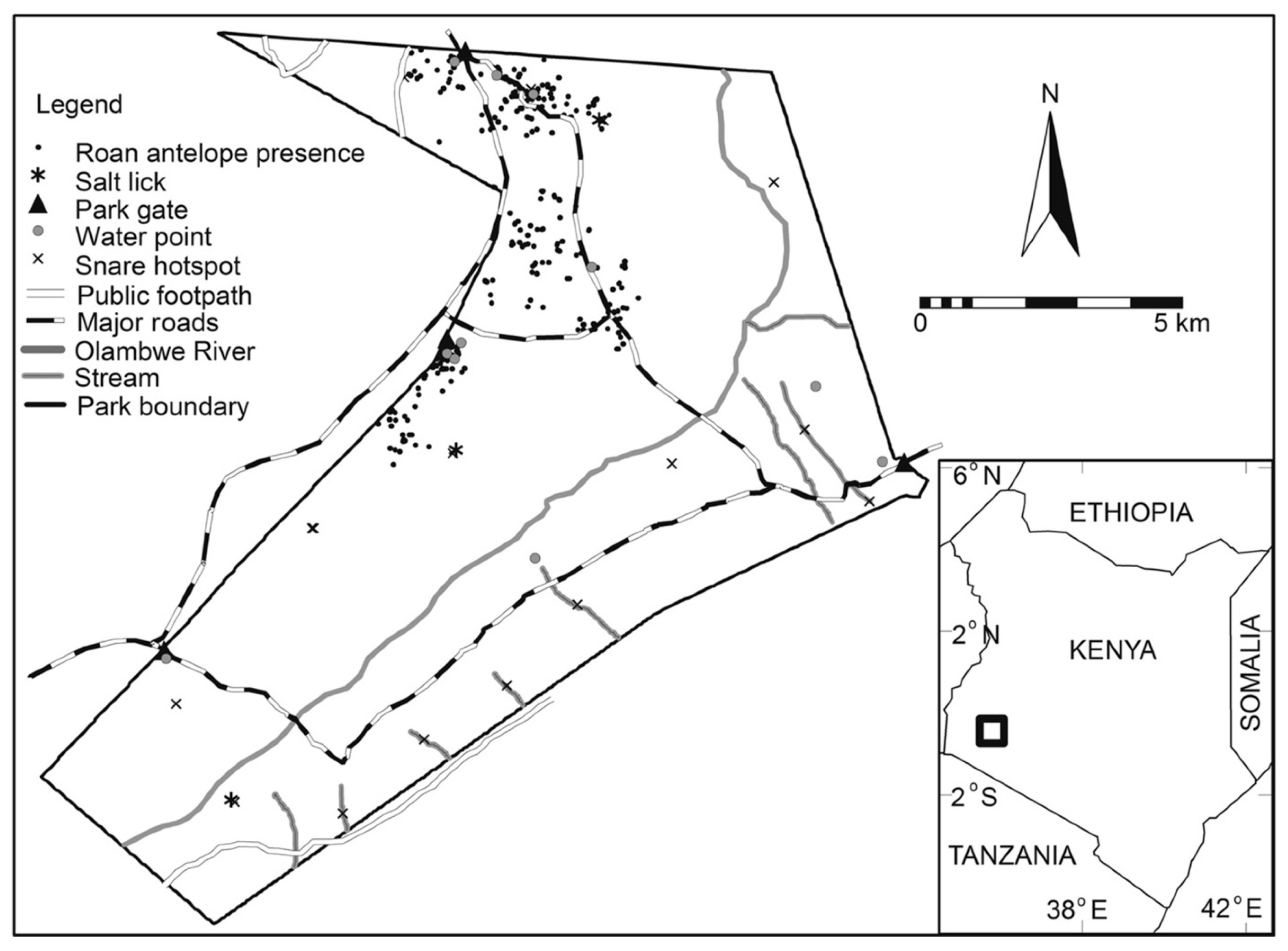
2.1. Study Site
2.2. Experimental Design and Vegetation Sampling
2.3. Data Collection
2.3.1. Microclimatic Measurements
2.3.2. Soil Moisture Monitoring
2.3.3. Leaf Gas Exchange Measurements
2.4. Data Analysis
3. Results
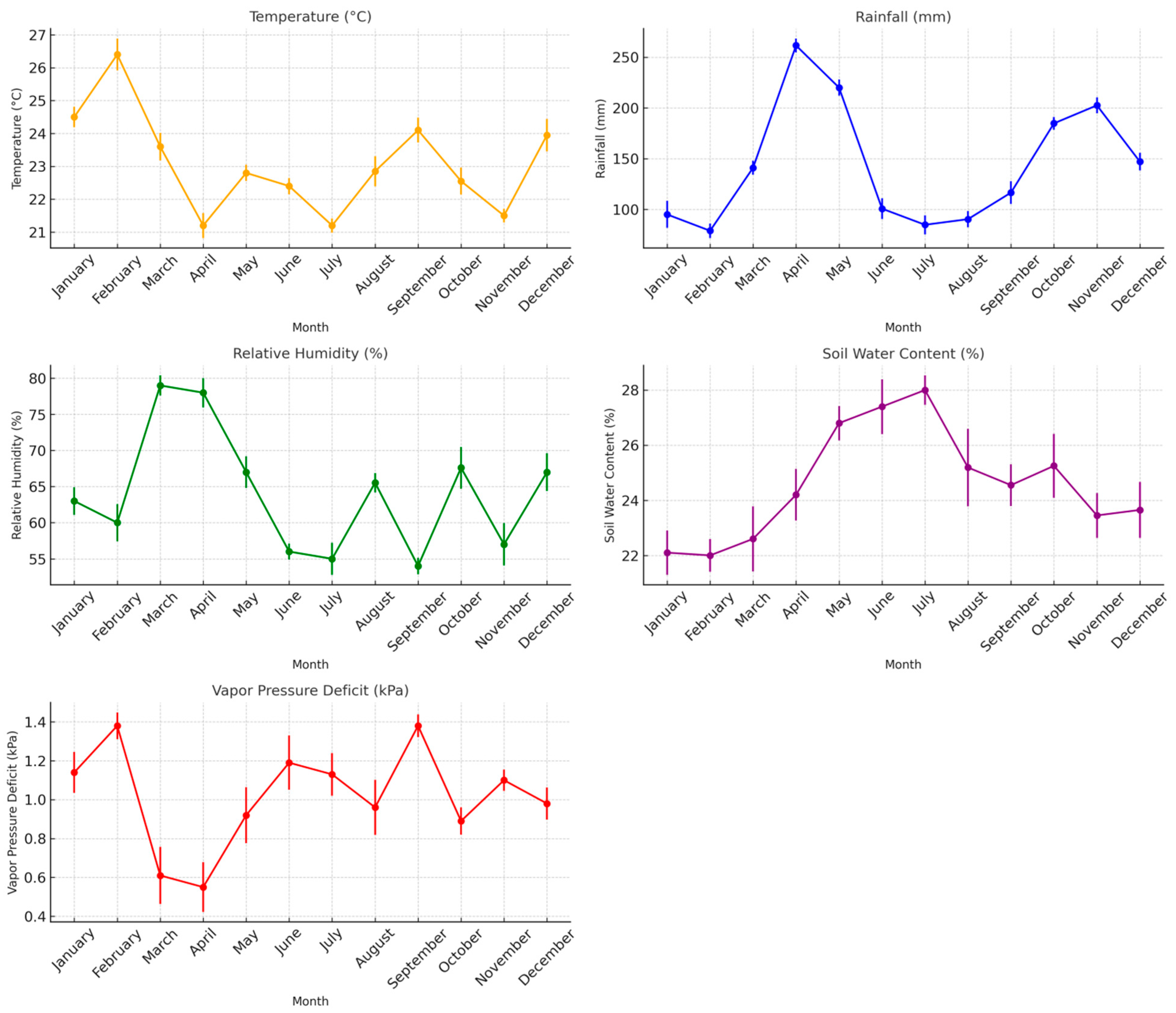
3.1. Climatic Conditions
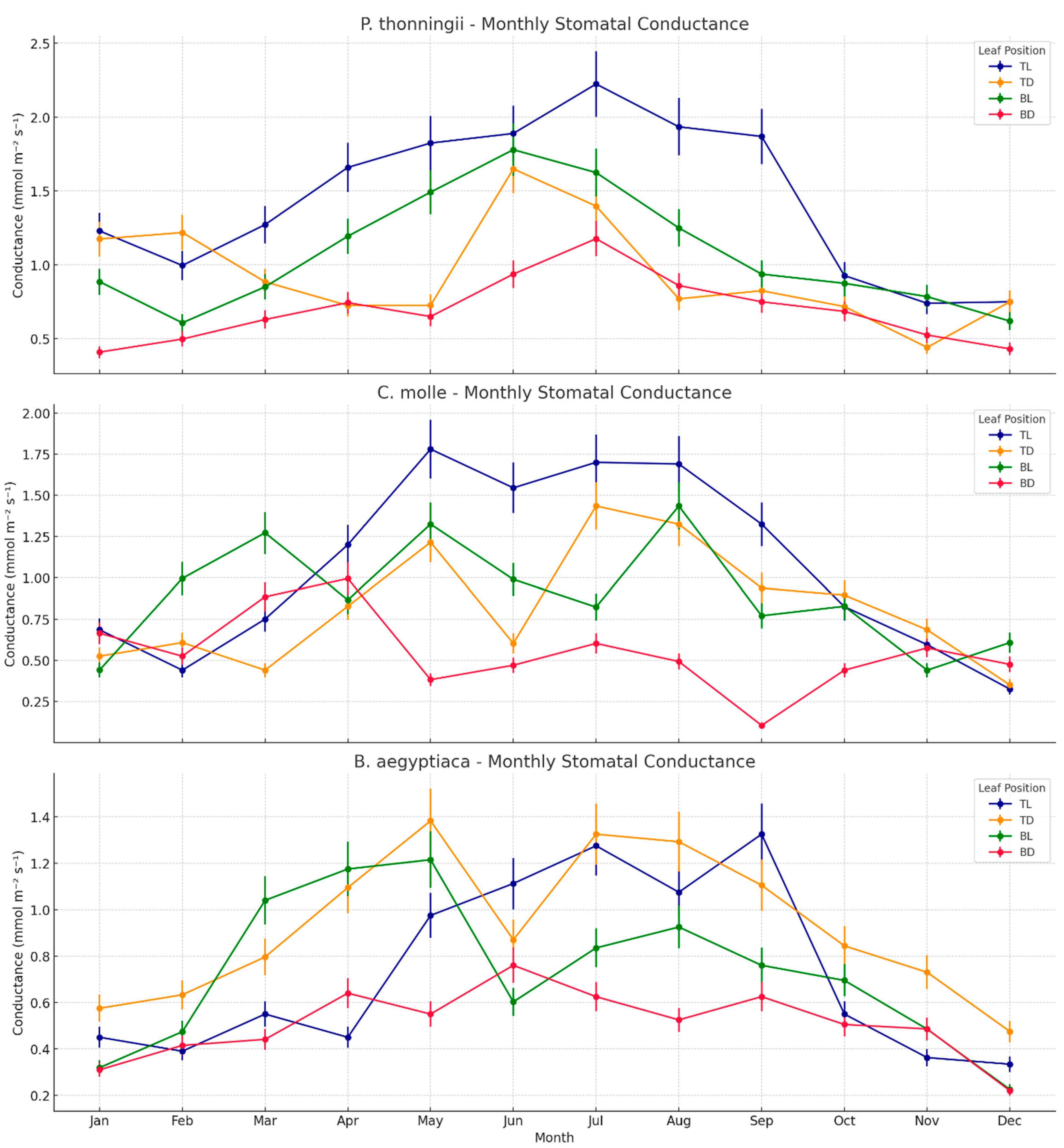
3.2. Stomatal Conductance in Light and Shaded Leaves
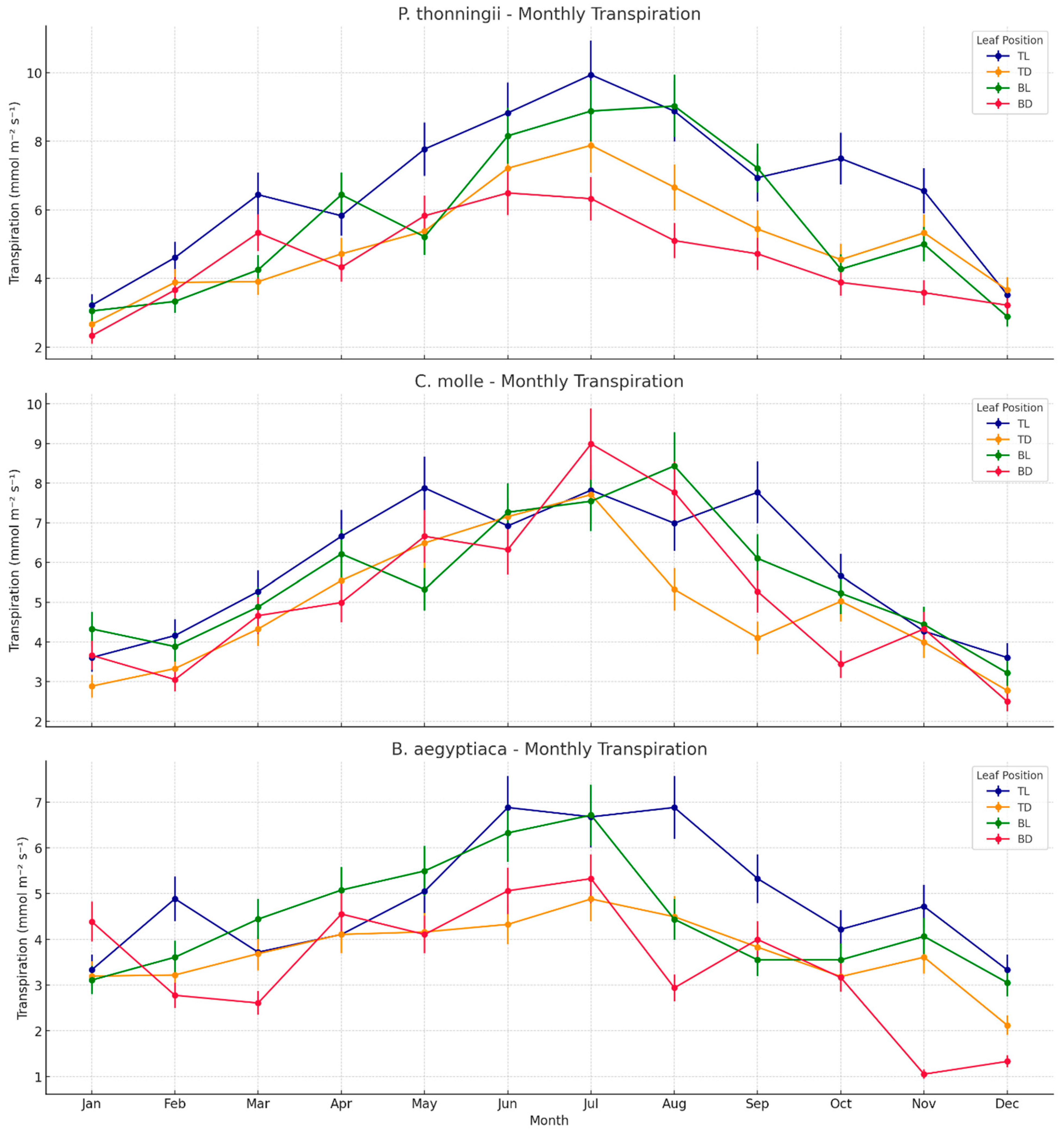
3.3. Transpiration Patterns in Light and Shaded Leaves
3.4. Relationships Between Stomatal Conductance, VPD, and Soil Moisture
3.5. Comparison of Tr and gs in Different Light Conditions
4. Discussion
4.1. Seasonal Responses and Species Adaptations
4.2. Influence of Light Conditions on Gas Exchange
4.3. Climatic Drivers of Gas Exchange
4.4. Interspecific Variation in Water-Use Strategies
4.5. Canopy Position and Physiological Plasticity
4.6. Ecological Implications and Broader Relevance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muthuri, C.W.; Ong, C.K.; Craigon, J.; Mati, B.M.; Ngumi, V.W.; Black, C.R. Gas exchange and water use efficiency of trees and maize in agroforestry systems in semi-arid Kenya. Agric. Ecosyst. Environ. 2009, 129, 497–507. [Google Scholar] [CrossRef]
- Ghimire, C.P.; Bruijnzeel, L.A.; Lubczynski, M.W.; Zwartendijk, B.W.; Odongo, V.O.; Ravelona, M.; van Meerveld, H.J. Transpiration and stomatal conductance in a top secondary tropical montane forest: Contrasts between native trees and invasive understorey shrubs. Tree Physiol. 2018, 38, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.; Marino, G.; Loreto, F.; Centritto, M. Integrating stomatal physiology and morphology: Evolution of stomatal control and development of future crops. Oecologia 2021, 197, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.N.; Quan, W. Variation of photosynthesis and pigment concentration relative to irradiance and nitrogen content for two coexisting desert shrubs. Ecol. Eng. 2013, 58, 238–248. [Google Scholar]
- Huo, H.; Wang, C.K. Effects of canopy position and leaf age on photosynthesis and transpiration of Pinus koraiensis. J. Appl. Ecol. 2007, 18, 1181–1186. [Google Scholar]
- Maina, J.N.; Quan, W.; Li, P. Effectiveness of photochemical reflectance index to trace vertical and seasonal chlorophyll a/b ratio in Haloxylon ammodendron. Acta Physiol. Plant. 2015, 37, 2. [Google Scholar]
- Quirk, J.; Bellasio, C.; Johnson, D.A.; Beerling, D.J. Response of photosynthesis, growth and water relations of a savannah-adapted tree and grass grown across high to low CO2. Ann. Bot. 2019, 124, 77–90. [Google Scholar] [CrossRef]
- Allen, S.J.; Grime, V.L. Measurements of transpiration from savannah shrubs using sap flow gauges. Agric. For. Meteorol. 1995, 75, 23–41. [Google Scholar] [CrossRef]
- Holdo, R.M.; Nippert, J.B. Transpiration dynamics support resource partitioning in African savanna trees and grasses. Ecology 2015, 96, 1466–1472. [Google Scholar] [CrossRef]
- Casaroli, D.; Sérvulo, A.C.O.; Vellame, L.M.; Alves Júnior, J.; Evangelista, A.W.P.; Mesquita, M.; Flores, R.A. Transpiration and growth of young African mahogany plants subject to different water regimes. Int. J. Biometeorol. 2020, 64, 1–13. [Google Scholar] [CrossRef]
- Dzikiti, S.; Ntuli, N.R.; Nkosi, N.N.; Ntshidi, Z.; Ncapai, L.; Gush, M.B.; Mostert, T.; Mpandeli, N.M.S.; Pienaar, H.H. Contrasting water use patterns of two drought-adapted native fruit tree species growing on nutrient-poor sandy soils in northern KwaZulu-Natal. S. Afr. J. Bot. 2022, 147, 197–207. [Google Scholar] [CrossRef]
- Mukhtar, R.B.; Aliero, M.M.; Abdullahi, S.; Bunza, M.R. The growth of Balanites aegyptiaca (L.) seedlings under varied watering intervals in the nursery. Agro-Science 2016, 15, 30–33. [Google Scholar] [CrossRef]
- Chidumayo, E. Dry season watering alters the significance of climate factors influencing phenology and growth of saplings of savanna woody species in central Zambia, southern Africa. Austral Ecol. 2015, 40, 794–805. [Google Scholar] [CrossRef]
- Otieno, D.O.; K’Otuto, G.O.; Jákli, B.; Schröttle, P.; Maina, J.N.; Jung, E.; Onyango, J.C. Spatial heterogeneity in ecosystem structure and productivity in a moist Kenyan savanna. Plant Ecol. 2011, 212, 769–783. [Google Scholar] [CrossRef]
- Dewar, R.C.; Tarvainen, L.; Parker, K.; Wallin, G.; McMurtrie, R.E. Why does leaf nitrogen decline within tree canopies less rapidly than light? An explanation from optimization subject to a lower bound on leaf mass per area. Tree Physiol. 2012, 32, 520–534. [Google Scholar] [CrossRef]
- Kimanzi, J.K.; Sanderson, R.A.; Rushton, S.P.; Mugo, M.J. Spatial distribution of snares in Ruma National Park, Kenya, with implications for management of the roan antelope Hippotragus equinus langheldi and other wildlife. Oryx 2015, 49, 295–302. [Google Scholar] [CrossRef]
- Maina, J.N.; Wang, Q. Seasonal response of chlorophyll a/b ratio to stress in a typical desert species: Haloxylon ammodendron. Arid Land Res. Manag. 2015, 29, 321–334. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs, and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Rodríguez, H.G.; Maiti, R.; Kumari, A. Research advances on leaf and wood anatomy of woody species of a Tamaulipan thorn scrub forest and its significance in taxonomy and drought resistance. For. Res. 2016, 5, 183–191. [Google Scholar]
- Chen, Z.; Li, S.; Wan, X.; Liu, S. Strategies of tree species to adapt to drought from leaf stomatal regulation and stem embolism resistance to root properties. Front. Plant Sci. 2022, 13, 926535. [Google Scholar] [CrossRef]
- Gupta, S.R.; Dagar, J.C.; Sileshi, G.W.; Chaturvedi, R.K. Agroforestry for climate change resilience in degraded landscapes. In Agroforestry for Sustainable Intensification of Agriculture in Asia and Africa; Springer: Berlin/Heidelberg, Germany, 2023; pp. 121–174. [Google Scholar]
- Querejeta, J.I.; Prieto, I.; Armas, C.; Casanoves, F.; Diémé, J.S.; Diouf, M.; Kaya, B.; Yossi, H.; Pugnaire, F.I.; Rusch, G.M. Higher leaf nitrogen content is linked to tighter stomatal regulation of transpiration and more efficient water use across dryland trees. New Phytol. 2022, 235, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Douthe, C.; Flexas, J. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant Cell Environ. 2018, 41, 436–450. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | P. thonningii | B. aegyptiaca | C. mole |
|---|---|---|---|
| Distribution | Woodland and wooded grasslands of sub-humid Africa | Tropical, dry-land African savanna | Throughout tropical Africa |
| Life form | Deciduous tree, 4–15 m in height | Spiny shrub or tree, up to 10 m in height | Variable shrub or small semi-deciduous tree, 3–13 m. |
| Leaf type | Broad-leaved | Compound leaves | Opposite and simple |
| Leaf Characteristics | Large, simple, two-lobed, leathery leaves (camel foot-like) | Reduced and spirally arranged leaves | Leathery leaves |
| Stem | Single-stem tree | Multi-branched | Crooked or leaning trunk |
| Root system | Deep roots | Complex system of strong, diagonally radiating roots | Does not have an aggressive root system |
| Species | Condition | Climate Variable | Transpiration | Stomata Conductance | ||
|---|---|---|---|---|---|---|
| R2 | p-Value | R2 | p-Value | |||
| P. thonningii | Light | Temperature (°C) | 0.353 | 0.026 | 0.020 | 0.820 |
| P. thonningii | Light | Rainfall (mm) | 0.026 | 0.542 | 0.137 | 0.633 |
| P. thonningii | Light | Relative Humidity (%) | 0.188 | 0.158 | 0.056 | 0.966 |
| P. thonningii | Light | Soil Water Content (%) | 0.285 | 0.167 | 0.010 | 0.966 |
| P. thonningii | Light | Vapor Pressure Deficit (kPa) | 0.019 | 0.729 | 0.014 | 0.957 |
| P. thonningii | Dark | Temperature (°C) | 0.040 | 0.527 | 0.026 | 0.778 |
| P. thonningii | Dark | Rainfall (mm) | 0.229 | 0.308 | 0.345 | 0.022 |
| P. thonningii | Dark | Relative Humidity (%) | 0.286 | 0.009 | 0.112 | 0.463 |
| P. thonningii | Dark | Soil Water Content (%) | 0.000 | 0.618 | 0.003 | 0.966 |
| P. thonningii | Dark | Vapor Pressure Deficit (kPa) | 0.295 | 0.013 | 0.129 | 0.230 |
| C. molle | Light | Temperature (°C) | 0.003 | 0.617 | 0.115 | 0.166 |
| C. molle | Light | Rainfall (mm) | 0.140 | 0.379 | 0.280 | 0.183 |
| C. molle | Light | Relative Humidity (%) | 0.000 | 0.564 | 0.421 | 0.027 |
| C. molle | Light | Soil Water Content (%) | 0.039 | 0.542 | 0.003 | 0.863 |
| C. molle | Light | Vapor Pressure Deficit (kPa) | 0.001 | 0.549 | 0.502 | 0.006 |
| C. molle | Dark | Temperature (Â °C) | 0.014 | 0.914 | 0.138 | 0.409 |
| C. molle | Dark | Rainfall (mm) | 0.002 | 0.471 | 0.000 | 0.618 |
| C. molle | Dark | Relative Humidity (%) | 0.241 | 0.051 | 0.236 | 0.264 |
| C. molle | Dark | Soil Water Content (%) | 0.014 | 0.880 | 0.322 | 0.112 |
| C. molle | Dark | Vapor Pressure Deficit (kPa) | 0.251 | 0.066 | 0.067 | 0.820 |
| B. aegyptiaca | Light | Temperature (°C) | 0.073 | 0.564 | 0.055 | 0.696 |
| B. aegyptiaca | Light | Rainfall (mm) | 0.001 | 0.863 | 0.417 | 0.006 |
| B. aegyptiaca | Light | Relative Humidity (%) | 0.227 | 0.111 | 0.076 | 0.534 |
| B. aegyptiaca | Light | Soil Water Content (%) | 0.127 | 0.331 | 0.003 | 0.880 |
| B. aegyptiaca | Light | Vapor Pressure Deficit (kPa) | 0.297 | 0.082 | 0.107 | 0.372 |
| B. aegyptiaca | Dark | Temperature (°C) | 0.056 | 0.571 | 0.000 | 0.914 |
| B. aegyptiaca | Dark | Rainfall (mm) | 0.030 | 0.499 | 0.042 | 0.649 |
| B. aegyptiaca | Dark | Relative Humidity (%) | 0.123 | 0.075 | 0.000 | 0.422 |
| B. aegyptiaca | Dark | Soil Water Content (%) | 0.015 | 0.795 | 0.022 | 0.391 |
| B. aegyptiaca | Dark | Vapor Pressure Deficit (kPa) | 0.182 | 0.077 | 0.000 | 0.778 |
| Species | Measure | Comparison | Season | p-Value | Significant |
|---|---|---|---|---|---|
| P. thonningii | Transpiration | TL vs. TD | Wet | 0.0528 | No |
| P. thonningii | Transpiration | TL vs.TD | Dry | 0.178 | No |
| P. thonningii | Transpiration | BL vs. BD | Wet | 0.8666 | No |
| P. thonningii | Transpiration | BL vs. BD | Dry | 0.179 | No |
| P. thonningii | Stomatal Conductance | TL vs. TD | Wet | 0.0292 | Yes |
| P. thonningii | Stomatal Conductance | TL vs. TD | Dry | 0.1062 | No |
| P. thonningii | Stomatal Conductance | BL vs. BD | Wet | 0.1078 | No |
| P. thonningii | Stomatal Conductance | BL vs. BD | Dry | 0.0574 | No |
| C. mole | Transpiration | TL vs. TD | Wet | 0.3086 | No |
| C. mole | Transpiration | TL vs. TD | Dry | 0.2755 | No |
| C. mole | Transpiration | BL vs. BD | Wet | 0.9645 | No |
| C. mole | Transpiration | BL vs. BD | Dry | 0.5638 | No |
| C. mole | Stomatal Conductance | TL vs. TD | Wet | 0.3316 | No |
| C. mole | Stomatal Conductance | TL vs. TD | Dry | 0.3883 | No |
| C. mole | Stomatal Conductance | BL vs. BD | Wet | 0.1723 | No |
| C. mole | Stomatal Conductance | BL vs. BD | Dry | 0.0149 | Yes |
| B. aegyptiaca | Transpiration | TL vs. TD | Wet | 0.5289 | No |
| B. aegyptiaca | Transpiration | TL vs. TD | Dry | 0.018 | Yes |
| B. aegyptiaca | Transpiration | BL vs. BD | Wet | 0.1559 | No |
| B. aegyptiaca | Transpiration | BL vs. BD | Dry | 0.1868 | No |
| B. aegyptiaca | Stomatal Conductance | TL vs. TD | Wet | 0.1377 | No |
| B. aegyptiaca | Stomatal Conductance | TL vs. TD | Dry | 0.5434 | No |
| B. aegyptiaca | Stomatal Conductance | BL vs. BD | Wet | 0.0016 | Yes |
| B. aegyptiaca | Stomatal Conductance | BL vs. BD | Dry | 0.3422 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyongesa, J.M.; Oronyi, W.; Lawrence, O.; Ronoh, E.K.; Mwalati, L.S.; Suba, V.; Gayo, L.; Nkengurutse, J.; Otieno, D.O.; Li, Y. Seasonal Variation in Transpiration and Stomatal Conductance of Three Savanna Tree Species in Ruma National Park, Kenya. Forests 2025, 16, 999. https://doi.org/10.3390/f16060999
Nyongesa JM, Oronyi W, Lawrence O, Ronoh EK, Mwalati LS, Suba V, Gayo L, Nkengurutse J, Otieno DO, Li Y. Seasonal Variation in Transpiration and Stomatal Conductance of Three Savanna Tree Species in Ruma National Park, Kenya. Forests. 2025; 16(6):999. https://doi.org/10.3390/f16060999
Chicago/Turabian StyleNyongesa, John Maina, Wycliff Oronyi, Oyoo Lawrence, Ernest Kiplangat Ronoh, Lindsay Sikuku Mwalati, Vincent Suba, Leopody Gayo, Jacques Nkengurutse, Denis Ochuodho Otieno, and Yuelin Li. 2025. "Seasonal Variation in Transpiration and Stomatal Conductance of Three Savanna Tree Species in Ruma National Park, Kenya" Forests 16, no. 6: 999. https://doi.org/10.3390/f16060999
APA StyleNyongesa, J. M., Oronyi, W., Lawrence, O., Ronoh, E. K., Mwalati, L. S., Suba, V., Gayo, L., Nkengurutse, J., Otieno, D. O., & Li, Y. (2025). Seasonal Variation in Transpiration and Stomatal Conductance of Three Savanna Tree Species in Ruma National Park, Kenya. Forests, 16(6), 999. https://doi.org/10.3390/f16060999






