Comparison Between Traditional Forest Inventory and Remote Sensing with Random Forest for Estimating the Periodic Annual Increment in a Dry Tropical Forest
Abstract
1. Introduction
2. Materials and Methods
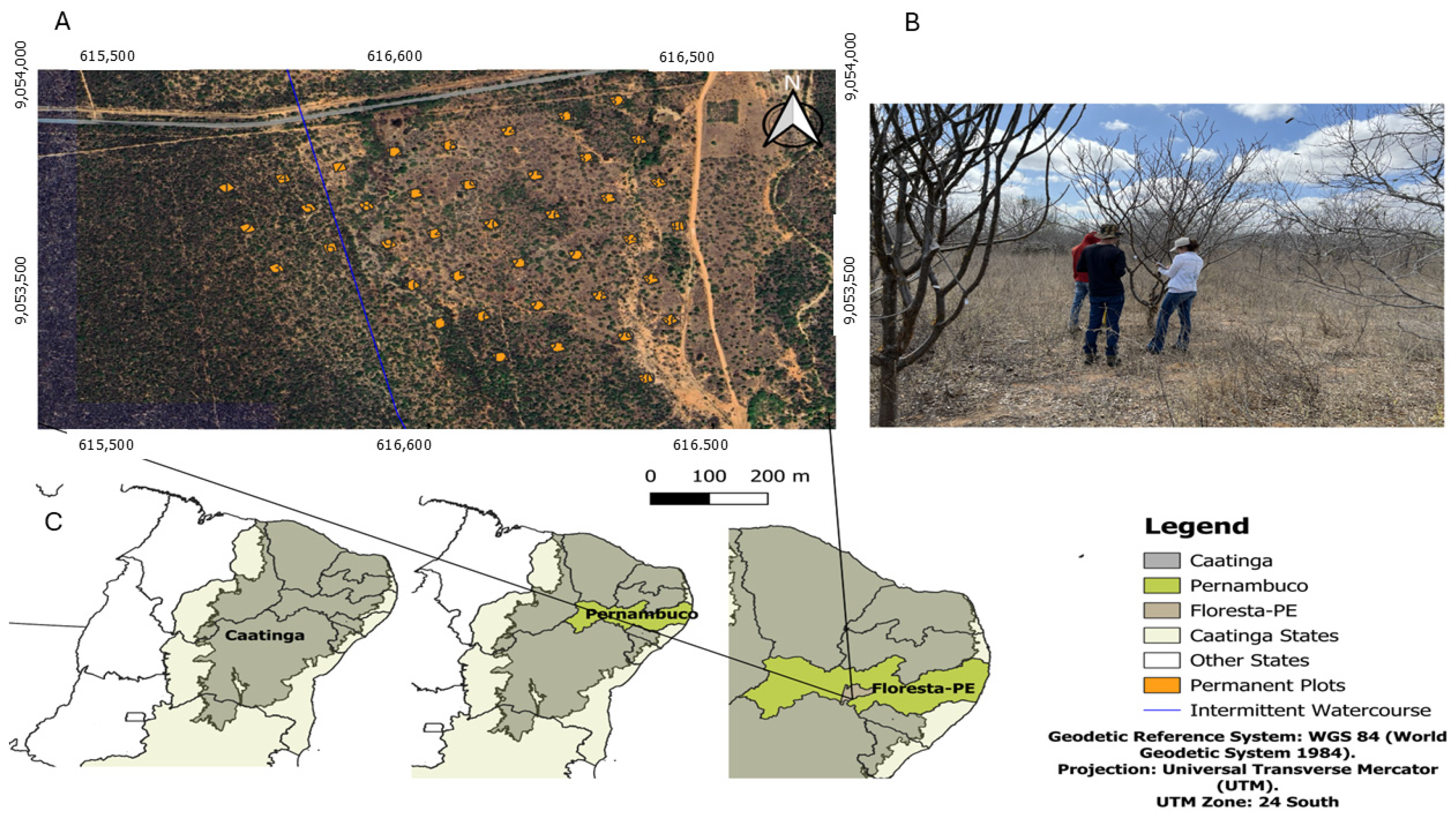
2.1. Study Area Location
2.2. Field Data Collection
2.3. Climatic Data
2.4. Remote Sensing Data
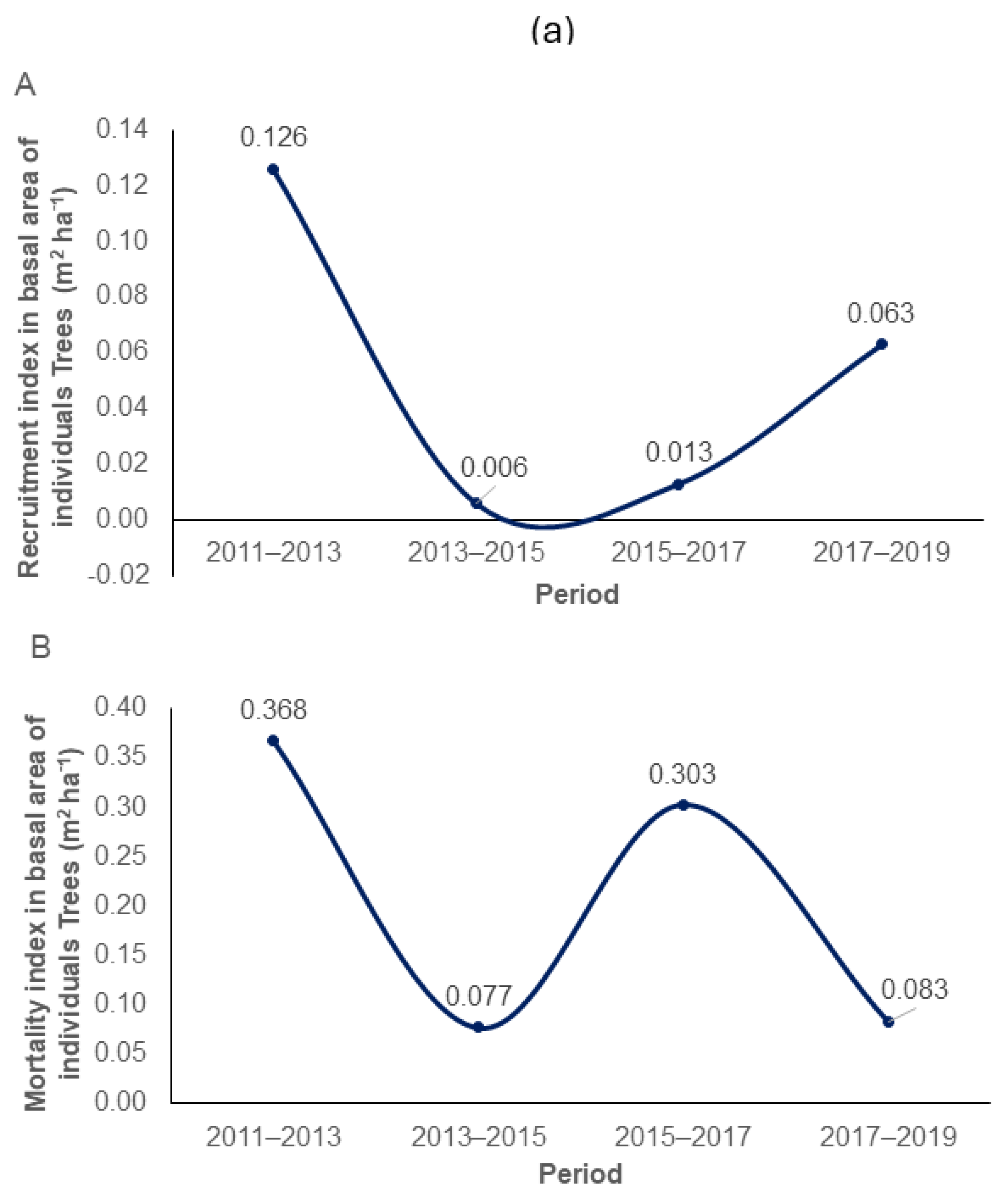
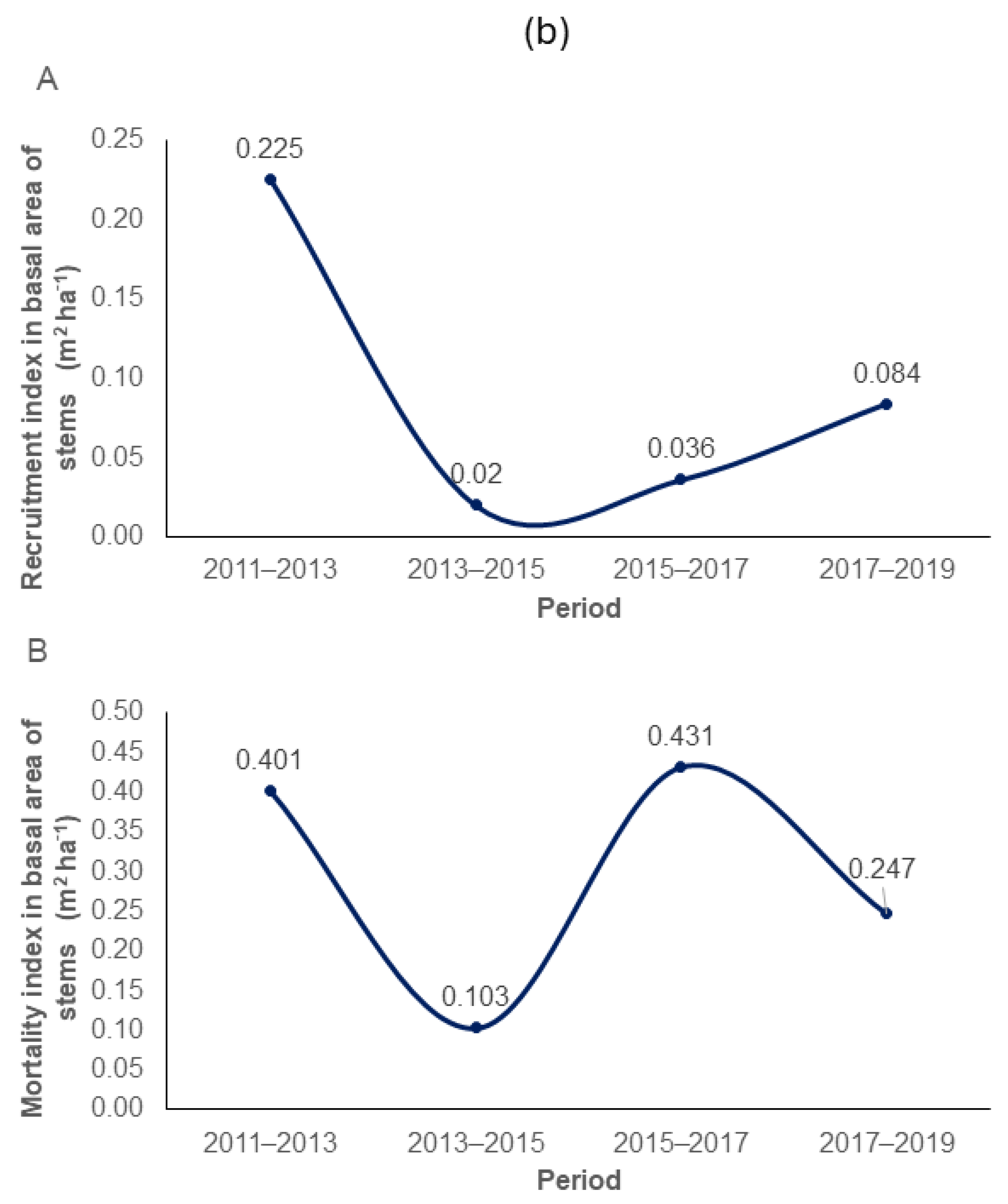
2.5. Recruitment and Mortality Dynamics
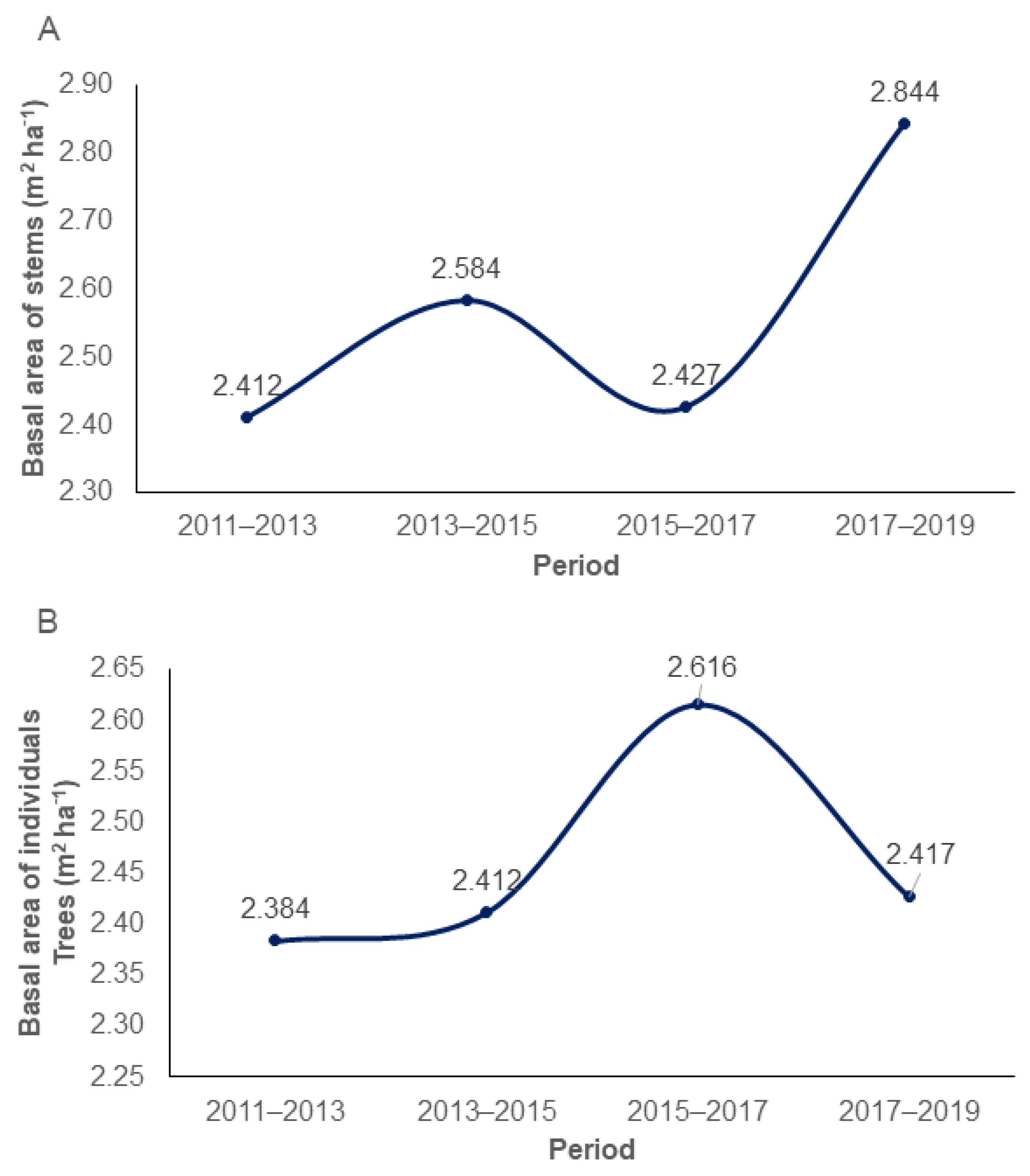
2.6. Periodic Annual Increments
2.7. Modeling with Random Forest
2.8. Bias Correction of Estimates
- (a)
- Estimation difference calculation: First, the absolute difference between the actual values and the predicted values was calculated and stored in a new column in the spreadsheet. This was carried out to quantify the estimation error for each observation (Equation (11)).
- (b)
- Data classification: Next, the actual data were classified into ranges using the Qcut function, which divides the data into quantiles. The data were segmented into six equally sized classes, with an additional unit added to each class to avoid zero-based indexing, resulting in classes numbered 1 to 6. These classes were stored in a new column (Equation (12)).
- (c)
- Correction factor calculation: For each previously defined class, a mean correction factor was calculated based on the estimation difference. This was carried out by grouping the data by class and obtaining the mean difference for each group. These factors represent the average adjustment required for each value class, considering both basal area and the Periodic Annual Increment (Equation (13)).
- (d)
- Application of the correction factor: The correction factor was then applied to adjust the original predictions. For each observation, the respective correction factor was added to the estimated value, resulting in a new column of corrected predictions (Equation (14)).
- (e)
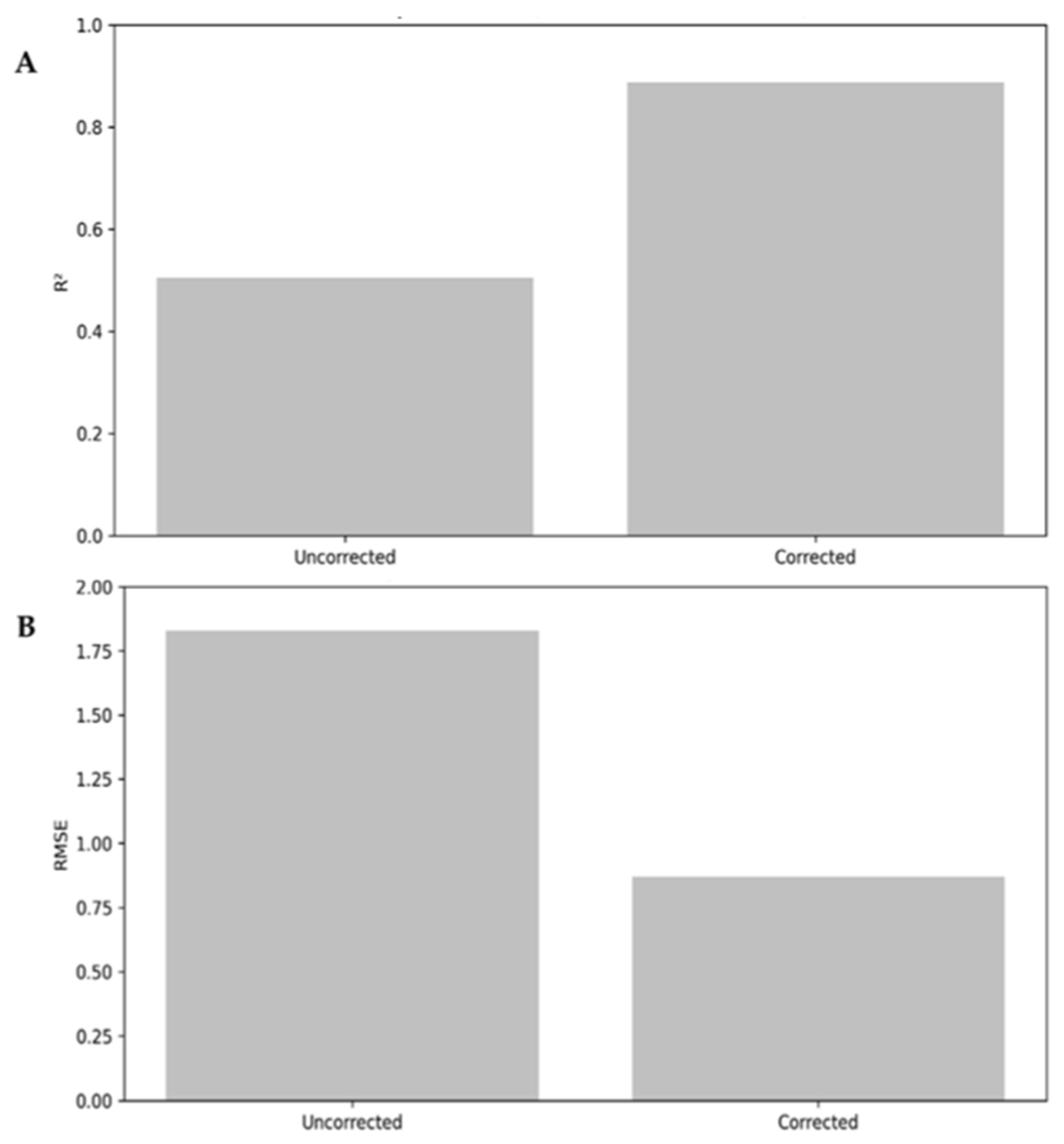
- Reevaluation of model metrics: After applying the correction factor, the model evaluation metrics were recalculated, including the coefficient of determination (R2) and the root mean square error (RMSE) (Equations (15) and (16))
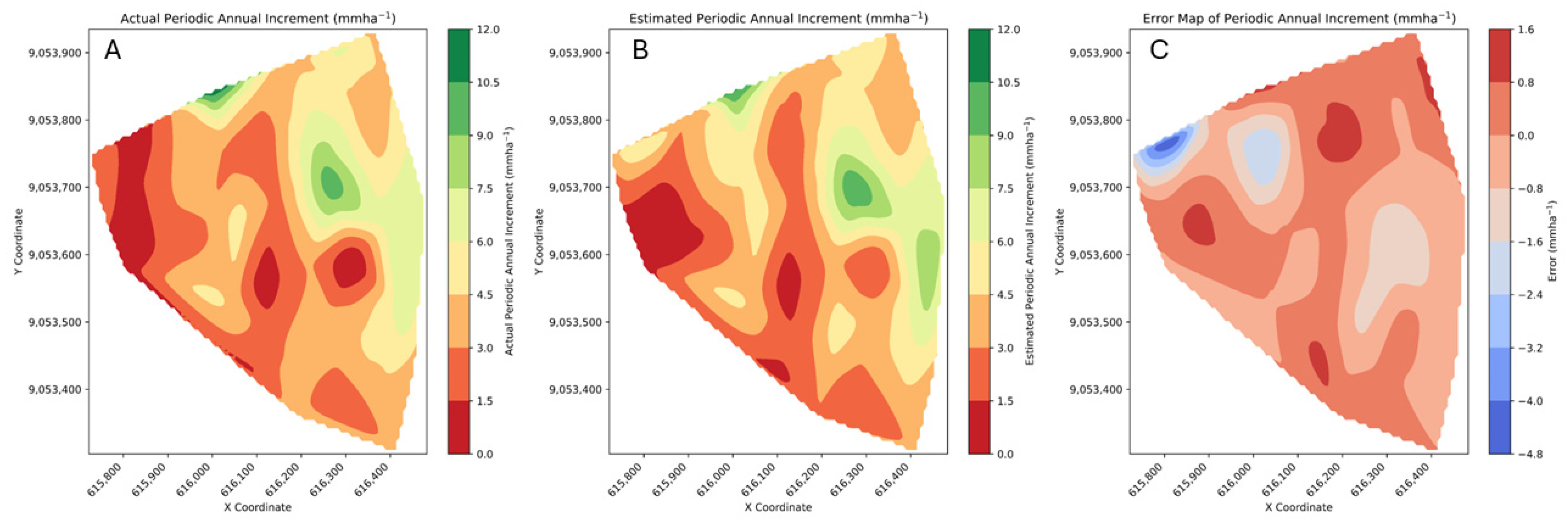
2.9. Generation of Spatial Maps
3. Results and Discussion
3.1. Growth Dynamics
3.2. Random Forest Model Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perrando, E.R.; Breunig, F.M.; Galvão, L.S.; Bostelmann, S.L.; Martarello, V.; Straube, J.; Conte, B.; Sestari, G.; Burgin, M.R.B.; De Marco, R. Evaluation of the Effects of Pine Management on the Water Yield and Quality in Southern Brazil. J. Sustain. For. 2021, 40, 217–233. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Agarwal, R. Sustainable Forest Land Management to Restore Degraded Lands; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Da Silva, A.F. Estrutura e Dinâmica Relacionadas A Fatores Ambientais em Floresta Seca Submetida ao Manejo Florestal. Ph.D. Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2023. [Google Scholar]
- Salazar, A.A.; Arellano, E.C.; Muñoz-sáez, A.; Miranda, M.D.; da Silva, F.O.; Zielonka, N.B.; Crowther, L.P.; Silva-ferreira, V.; Oliveira-reboucas, P.; Dicks, L.V. Restoration and Conservation of Priority Areas of Caatinga’s Semi-Arid Forest Remnants Can Support Connectivity within an Agricultural Landscape. Land 2021, 10, 550. [Google Scholar] [CrossRef]
- De Melo, C.L.S.M.S.; Ferreira, R.L.C.; da Silva, J.A.A.; Machuca, M.Á.H.; Cespedes, G.H.G. Dynamics of Dry Tropical Forest after Three Decades of Vegetation Suppression. Floresta Ambient. 2019, 26, e20171163. [Google Scholar]
- dos Santos, N.Á.T. Dinâmica da Floresta Seca SOB Diferentes Históricos de USO: Distribuição Diamétrica de Indivíduos e Fustes. Master’s Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2021. [Google Scholar]
- Dalla Lana, M.; Ferreira, R.L.C.; da Silva, J.A.A.; Duda, G.P.; Cespedes, G.H.G. Carbon Content in Shrub-Tree Species of the Caatinga. Floresta Ambient. 2019, 26, e20170617. [Google Scholar] [CrossRef]
- De Abreu, J.C.; da Silva, J.A.A.; Ferreira, R.L.C.; da Rocha, S.J.S.S.; Tavares, I.d.S.; Farias, A.A.; Villanova, P.H.; Viana, A.B.T.; Schettini, B.L.S.; Telles, L.A.d.A.; et al. Mixed Models for Nutrients Prediction in Species of the Brazilian Caatinga Biome. Árvore 2023, 47, e4712. [Google Scholar] [CrossRef]
- Salami, G.; Ferreira, R.L.C.; da Silva, J.A.A.; Freire, F.J.; Silva, E.A.; Diodato, M.A.; Pessi, D.D. Distribuição Espaço-Temporal Da Biomassa e Carbono Florestal Acima Do Solo Em Floresta Tropical Seca Sob Pressão Antrópica, Brasil. Terr@ Plur. 2024, 18, 1–27. [Google Scholar] [CrossRef]
- Moreira, G.L. Modelagem de Variáveis Biofísicas Em Floresta Tropical Seca Por Meio de Geotecnologias. Master’s Thesis, Universidade Federal Rural de Pernambuco, Recife, Brasil, 2021. [Google Scholar]
- de Oliveira, C.P.; Ferreira, R.L.C.; da Silva, J.A.A.; de Lima, R.B.; Silva, E.A.; da Silva, A.F.; de Lucena, J.D.S.; Dos Santos, N.A.T.; Lopes, I.J.C.; Pessoa, M.M.d.L.; et al. Modeling and Spatialization of Biomass and Carbon Stock Using LiDAR Metrics in Tropical Dry Forest, Brazil. Forests 2021, 12, 473. [Google Scholar] [CrossRef]
- Beck, H.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Lutsko, N.J.; Dufour, A.; Zeng, Z.; Jiang, X.; van Dijk, A.I.J.M.; Miralles, D.G. High-Resolution (1 Km) Köppen-Geiger Maps for 1901–2099 Based on Constrained CMIP6 Projections. Sci. Data 2023, 10, 724. [Google Scholar] [CrossRef]
- Ottoni, F.P.; Filgueira, C.T.S.; Lima, B.N.; Vieira, L.O.; Rangel-Pereira, F.; Oliveira, R.F. Extreme Drought Threatens the Amazon. Science 2023, 382, 1253–1255. [Google Scholar] [CrossRef]
- AR6 Synthesis Report: Climate Change 2023. Available online: https://www.ipcc.ch/report/ar6/syr/ (accessed on 20 March 2025).
- Santos, J.C.; Leal, I.R.; Almeida-Cortez, J.S.; Fernandes, G.W.; Tabarelli, M. Caatinga: The Scientific Negligence Experienced by a Dry Tropical Forest. Trop. Conserv. Sci. 2011, 4, 276–286. [Google Scholar] [CrossRef]
- Leal, I.R.; Da Silva, J.M.C.; Tabarelli, M.; Lacher, T.E. Changing the Course of Biodiversity Conservation in the Caatinga of Northeastern Brazil Cambiando El Curso de La Conservación de Biodiversidad En La Caatinga Del Noreste de Brasil. Conserv. Biol. 2005, 19, 701–706. [Google Scholar] [CrossRef]
- Pitman, N.C.A.; Silman, M.R.; Terborgh, J.W. Oligarchies in Amazonian Tree Communities: A Ten-Year Review. Ecography 2013, 36, 114–123. [Google Scholar] [CrossRef]
- Ter Steege, H.; Pitman, N.C.A.; Sabatier, D.; Baraloto, C.; Salomão, R.P.; Guevara, J.E.; Phillips, O.L.; Castilho, C.V.; Magnusson, W.E.; Molino, J.F.; et al. Hyperdominance in the Amazonian Tree Flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef] [PubMed]
- Bonal, D.; Burban, B.; Stahl, C.; Wagner, F.; Hérault, B. The Response of Tropical Rainforests to Drought—Lessons from Recent Research and Future Prospects. Ann. For. Sci. 2016, 73, 27–44. [Google Scholar] [CrossRef]
- Elias, F.; Marimon, B.S.; Marimon-Junior, B.H.; Budke, J.C.; Esquivel-Muelbert, A.; Morandi, P.S.; Reis, S.M.; Phillips, O.L. Idiosyncratic Soil-Tree Species Associations and Their Relationships with Drought in a Monodominant Amazon Forest. Acta Oecol. 2018, 91, 127–136. [Google Scholar] [CrossRef]
- Lloyd, J.; Domingues, T.F.; Schrodt, F.; Ishida, F.Y.; Feldpausch, T.R.; Saiz, G.; Quesada, C.A.; Schwarz, M.; Torello-Raventos, M.; Gilpin, M.; et al. Edaphic, Structural and Physiological Contrasts across Amazon Basin Forest-Savanna Ecotones Suggest a Role for Potassium as a Key Modulator of Tropical Woody Vegetation Structure and Function. Biogeosciences 2015, 12, 6529–6571. [Google Scholar] [CrossRef]
- Powers, J.S.; Feng, X.; Sánches-Azofeifa, A.; Medvigy, D. Focus on tropical dry forest ecosystems and ecosystem services in the face of global change. Environ. Res. Lett. 2018, 13, e090201. [Google Scholar] [CrossRef]
- Dickinson, M.B.; Hermann, S.M.; Whigham, D.F. Low Rates of Background Canopy-Gap Disturbance in a Seasonally Dry Forest in the Yucatan Peninsula with a History of Fires and Hurricanes. J. Trop. Ecol. 2001, 17, 895–902. [Google Scholar] [CrossRef]
- Doe Santos, J.R.; Narvaes, I.S.; Graca, P.M.L.A.; Goncalves, F.G.; dos Santos, J.R.; Narvaes, I.S.; Graca, P.M.L.A.; Goncalves, F.G. Polarimetric Responses and Scattering Mechanisms of Tropical Forests in the Brazilian Amazon. In Advances in Geoscience and Remote Sensing; Intechopen: Rijeka, Croatia, 2009. [Google Scholar] [CrossRef]
- da Conceição Bispo, P.; Picoli, M.C.A.; Marimon, B.S.; Marimon Junior, B.H.; Peres, C.A.; Menor, I.O.; Silva, D.E.; de Figueiredo Machado, F.; Alencar, A.A.C.; de Almeida, C.A.; et al. Overlooking Vegetation Loss Outside Forests Imperils the Brazilian Cerrado and Other Non-Forest Biomes. Nat. Ecol. Evol. 2023, 8, 12–13. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved Allometric Models to Estimate the Aboveground Biomass of Tropical Trees. Glob. Change Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Rosan, T.M.; Aragão, L.E.O.C.; Oliveras, I.; Phillips, O.L.; Malhi, Y.; Gloor, E.; Wagner, F.H. Extensive 21st-Century Woody Encroachment in South America’s Savanna. Geophys. Res. Lett. 2019, 46, 6594–6603. [Google Scholar] [CrossRef]
- Raymundo, D.; Oliveira-Neto, N.E.; Martini, V.; Araújo, T.N.; Calaça, D.; de Oliveira, D.C. Assessing Woody Plant Encroachment by Comparing Adult and Juvenile Tree Components in a Brazilian Savanna. Flora 2022, 291, 152060. [Google Scholar] [CrossRef]
- Bilar, A.B.C.; Pimentel, R.M.d.M.; Cerqueira, M.A. Monitoramento Da Cobertura Vegetal Através de Índices Biofísicos e Gestão de Áreas Protegidas. Geosul 2018, 33, 236–259. [Google Scholar] [CrossRef]
- García-Haro, F.J.; Campos-Taberner, M.; Muñoz-Marí, J.; Laparra, V.; Camacho, F.; Sánchez-Zapero, J.; Camps-Valls, G. Derivation of Global Vegetation Biophysical Parameters from EUMETSAT Polar System. ISPRS J. Photogramm. Remote Sens. 2018, 139, 57–74. [Google Scholar] [CrossRef]
- Piles, M.; Camps-Valls, G.; Chaparro, D.; Entekhabi, D.; Konings, A.G.; Jagdhuber, T. Remote Sensing of Vegetation Dynamics in Agro-Ecosystems Using Smap Vegetation Optical Depth and Optical Vegetation Indices. In Proceedings of the International Geoscience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017; pp. 4346–4349. [Google Scholar] [CrossRef]
- Verrelst, J.; Malenovský, Z.; Van der Tol, C.; Camps-Valls, G.; Gastellu-Etchegorry, J.P.; Lewis, P.; North, P.; Moreno, J. Quantifying Vegetation Biophysical Variables from Imaging Spectroscopy Data: A Review on Retrieval Methods. Surv. Geophys. 2019, 40, 589–629. [Google Scholar] [CrossRef]
- Gutiérrez-Castorena, E.V.; Silva-Núñez, J.A.; Gaytán-Martínez, F.D.; Encinia-Uribe, V.V.; Ramírez-Gómez, G.A.; Olivares-Sáenz, E. Evaluation of Statistical Models of NDVI and Agronomic Variables in a Protected Agriculture System. Horticulturae 2025, 11, 131. [Google Scholar] [CrossRef]
- Albuquerque, R.W.; Ferreira, M.E.; Olsen, S.I.; Tymus, J.R.C.; Balieiro, C.P.; Mansur, H.; Moura, C.J.R.; Costa, J.V.S.; Branco, M.R.C.; Grohmann, C.H. Forest Restoration Monitoring Protocol with a Low-Cost Remotely Piloted Aircraft: Lessons Learned from a Case Study in the Brazilian Atlantic Forest. Remote Sens. 2021, 13, 2401. [Google Scholar] [CrossRef]
- John, E.; Bunting, P.; Hardy, A.; Silayo, D.S.; Masunga, E. A Forest Monitoring System for Tanzania. Remote Sens. 2021, 13, 3081. [Google Scholar] [CrossRef]
- Wulder, M.A.; White, J.C.; Masek, J.G.; Dwyer, J.; Roy, D.P. Continuity of Landsat Observations: Short Term Considerations. Remote Sens. Environ. 2011, 115, 747–751. [Google Scholar] [CrossRef]
- Asner, G.P.; Powell, G.V.N.; Mascaro, J.; Knapp, D.E.; Clark, J.K.; Jacobson, J.; Kennedy-Bowdoin, T.; Balaji, A.; Paez-Acosta, G.; Victoria, E.; et al. High-Resolution Forest Carbon Stocks and Emissions in the Amazon. Proc. Natl. Acad. Sci. USA 2010, 107, 16738–16742. [Google Scholar] [CrossRef]
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.A.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S.; et al. Benchmark Map of Forest Carbon Stocks in Tropical Regions across Three Continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed]
- Mitchard, E.T.A.; Flintrop, C.M. Woody Encroachment and Forest Degradation in Sub-Saharan Africa’s Woodlands and Savannas 1982–2006. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120406. [Google Scholar] [CrossRef] [PubMed]
- Berra, E.F.; Fontana, D.C.; Yin, F.; Breunig, F.M. Harmonized Landsat and Sentinel-2 Data with Google Earth Engine. Remote Sens. 2024, 16, 2695. [Google Scholar] [CrossRef]
- Claverie, M.; Ju, J.; Masek, J.G.; Dungan, J.L.; Vermote, E.F.; Roger, J.C.; Skakun, S.V.; Justice, C. The Harmonized Landsat and Sentinel-2 Surface Reflectance Data Set. Remote Sens. Environ. 2018, 219, 145–161. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- da Silveira, H.L.F.; Galvão, L.S.; Sanches, I.D.A.; de Sá, I.B.; Taura, T.A. Use of MSI/Sentinel-2 and Airborne LiDAR Data for Mapping Vegetation and Studying the Relationships with Soil Attributes in the Brazilian Semi-Arid Region. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 179–190. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; The MIT Press: London, UK, 2016. [Google Scholar]
- Zhang, J.; Yin, K. Application of Gradient Boosting Model to Forecast Corporate Green Innovation Performance. Front. Environ. Sci. 2023, 11, 1252271. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.F.; Ghimire, B.; Rogan, J.; Chica-Olmo, M.; Rigol-Sanchez, J.P. An Assessment of the Effectiveness of a Random Forest Classifier for Land-Cover Classification. ISPRS J. Photogramm. Remote Sens. 2012, 67, 93–104. [Google Scholar] [CrossRef]
- Powell, S.L.; Cohen, W.B.; Healey, S.P.; Kennedy, R.E.; Moisen, G.G.; Pierce, K.B.; Ohmann, J.L. Quantification of Live Aboveground Forest Biomass Dynamics with Landsat Time-Series and Field Inventory Data: A Comparison of Empirical Modeling Approaches. Remote Sens. Environ. 2010, 114, 1053–1068. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random Forests for Classification in Ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2, 18–22. [Google Scholar]
- Machado, G.; Mendoza, M.R.; Corbellini, L.G. What Variables Are Important in Predicting Bovine Viral Diarrhea Virus? A Random Forest Approach. Vet. Res. 2015, 46, 85. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.C.; da Silva, J.A.A.; Alves, F.T., Jr.; de Lima, R.B.; Lana, M.D. Components of Growth for Tropical Dry Deciduous Forest, Brazil. In Proceedings of the ASA, CSSA & SSSA International Annual Meeting, Long Beach, CA, USA, 2–5 November 2014. [Google Scholar]
- Christina, R.; Silva, S.; Luiz, R.; Ferreira, C.; Antônio, J.; Da Silva, A.; Maria, I.; Meunier, J.; Berger, R. Aspectos Fitossociológicos e de Crescimento de Commiphora Leptophloeos No Semiárido Brasileiro. Pesqui. Florest. Bras. 2017, 37, 11–18. [Google Scholar] [CrossRef]
- Pimentel, D.J.O. Dinâmica da Vegetação Lenhosa em Área de Caatinga, Floresta. Master’s Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2012. [Google Scholar]
- Silva, G.F.; Mendonça, A.R.; Dias, A.N.; Nogueira, G.S.; Silva, J.A.A.; Oliveira, M.L.R.; Ferreira, R.L.C. Padronização da Simbologia em Mensuração e Manejo Florestal; Alegre: Espírito Santo, Brazil, 2022. [Google Scholar]
- Wan, Z. MODIS/Terra Land Surface Temperature/Emissivity Daily L3 Global 1 Km SIN Grid. Available online: https://lpdaac.usgs.gov/products/mod11a1v061/ (accessed on 21 March 2025).
- Funk, C.; Peterson, P.; Landsfeld, M.; Pedreros, D.; Verdin, J.; Shukla, S.; Husak, G.; Rowland, J.; Harrison, L.; Hoell, A.; et al. The Climate Hazards Infrared Precipitation with Stations—A New Environmental Record for Monitoring Extremes. Sci. Data 2015, 2, 150066. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Third Earth Resources Technology Satellite-1 Symposium. Volume I: Technical Presentations; NASA SP-351; NASA: Washington, DC, USA, 1973; pp. 309–317. [Google Scholar]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Huete, A.R. A Soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Use of a Green Channel in Remote Sensing of Global Vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A Normalized Difference Water Index for Remote Sensing of Vegetation Liquid Water from Space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Zha, Y.; Gao, J.; Ni, S. Use of Normalized Difference Built-up Index in Automatically Mapping Urban Areas from TM Imagery. Int. J. Remote Sens. 2003, 24, 583–594. [Google Scholar] [CrossRef]
- Rock, B.N.; Vogelmann, J.E.; Williams, D.L.; Vogelmann, A.F.; Hoshizaki, T. Remote Detection of Forest Damage. BioScience 1986, 36, 439–445. [Google Scholar] [CrossRef]
- Key, C.H.; Benson, N.C. Measuring and Remote Sensing of Burn Severity. In Proceedings of the Joint Fire Science Conference and Workshop, Boise, ID, USA, 12–16 June 2000; University of Idaho and International Association of Wildland Fire: Moscow, ID, USA, 2001; pp. 1–51. [Google Scholar]
- Hall, D.K.; Riggs, G.A. Normalized-Difference Snow Index (NDSI). In Encyclopedia of Snow, Ice and Glaciers; Singh, V.P., Singh, P., Haritashya, U.K., Eds.; Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2011; pp. 779–780. [Google Scholar] [CrossRef]
- Tucker, C.J.; Holben, B.N.; Elgin, J.H.; McMurtrey, J.E. Relationship of Spectral Data to Grain Yield Variation. Photogramm. Eng. Remote Sens. 1981, 47, 683–690. [Google Scholar]
- Buitinck, L.; Louppe, G.; Blondel, M.; Pedregosa, F.; Müller, A.C.; Grisel, O.; Niculae, V.; Prettenhofer, P.; Gramfort, A.; Grobler, J.; et al. API Design for Machine Learning Software: Experiences from the Scikit-Learn Project. arXiv 2013, arXiv:1309.0238. [Google Scholar]
- Pedregosa FABIANPEDREGOSA, F.; Michel, V.; Grisel Oliviergrisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Vanderplas, J.; Cournapeau, D.; Pedregosa, F.; Varoquaux, G.; et al. Scikit-Learn: Machine Learning in Python Gaël Varoquaux Bertrand Thirion Vincent Dubourg Alexandre Passos Pedregosa, Varoquaux, Gramfort Et Al. Matthieu Perrot. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 56–61. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. Bias-Variance Trade-Off for k-Fold Cross-Validation. Introd. Stat. Learn. Appl. R 2013, 7, 24. [Google Scholar]
- Spearman, C. The Proof and Measurement of Association between Two Things. Am. J. Psychol. 1904, 15, 101. [Google Scholar] [CrossRef]
- Cort, J. Willmott; Kenji Matsuura Advantages of the Mean Absolute Error (MAE) over the Root Mean Square Error (RMSE) in Assessing Average Model Performance. Clim. Res. 2005, 30, 79–82. [Google Scholar]
- Shepard, D. A Two-Dimensional Interpolation Function for Irregularly-Spaced Data. In Proceedings of the 1968 23rd ACM National Conference, New York, NY, USA, 27–29 August 1968; pp. 517–524. [Google Scholar] [CrossRef]
- Marengo, J.A.; Cunha, A.P.; Alves, L.M. A Seca de 2012-15 No Semiárido Do Nordeste Do Brasil No Contexto Histórico. Rev. Climanálise 2016, 4, 49–54. [Google Scholar]
- da Costa Júnior, D.S.; Ferreira, R.L.C.; da Silva, J.A.A.; da Silva, A.F.; Pessoa, M.M.D.L. Dinâmica de Crescimento de Uma Floresta Tropical Sazonalmente Seca No Semiárido Brasileiro. Ciênc. Florest. 2022, 32, 1594–1616. [Google Scholar] [CrossRef]
- Worbes, M. Annual Growth Rings, Rainfall-Dependent Growth and Long-Term Growth Patterns of Tropical Trees from the Caparo Forest Reserve in Venezuela. J. Ecol. 1999, 87, 391–403. [Google Scholar] [CrossRef]
- Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Scholz, F.G.; Franco, A.C.; Bustamante, M. Functional Convergence in Hydraulic Architecture and Water Relations of Tropical Savanna Trees: From Leaf to Whole Plant. Tree Physiol. 2004, 24, 891–899. [Google Scholar] [CrossRef]
- de la Cruz, D.B.C. Idade Relativa e Tempo de Passagem para Cenostigma Bracteosum (TUL.) E. Gagnon & G. P. Lewis NO Semiárido de Pernambuco; Universidade Federal Rural de Pernambuco: Recife, Brazil, 2024. [Google Scholar]
- Da Silva, J.L.B.; De Albuquerque Moura, G.B.; De França, E.; Silva, Ê.F.; Lopes, P.M.O.; Da Silva, T.T.F.; Lins, F.A.C.; De Oliveira Silva, D.A.; Ortiz, P.F.S. Dinâmica Espaço-Temporal Da Cobertura Vegetal de Caatinga Por Sensoriamento Remoto Em Município Do Semiárido Bras. Rev. Bras. De Ciênc. Agrár. 2019, 14, 7128. [Google Scholar] [CrossRef]
- Estrada Zúñiga, A.C.; Cárdenas Rodriguez, J.; Víctor, J.; Saya, B.; Ñaupari Vásquez, J.; Zúñiga, E.; Rodriguez, C.; Ñaupari Vásquez, J. Estimación de La Biomasa de Una Comunidad Vegetal Altoandina Utilizando Imágenes Multiespectrales Adquiridas Con Sensores Remotos UAV y Modelos de Regresión Lineal Múltiple, Máquina de Vectores Soporte y Bosques Aleatorios. Sci. Agropecu. 2022, 13, 301–310. [Google Scholar] [CrossRef]
- Ferreira, M.B.; Ferreira, R.L.C.; da Silva, J.A.A.; de Lima, R.B.; Silva, E.A.; de Sousa, A.N.; de La Cruz, D.B.C.; da Silva, M.V. Spatial-Temporal Dynamics of Water Resources in Seasonally Dry Tropical Forest: Causes and Vegetation Response. AgriEngineering 2024, 6, 2526–2552. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Forest Aboveground Biomass Estimation Using Landsat 8 and Sentinel-1A Data with Machine Learning Algorithms. Sci. Rep. 2020, 10, 9952. [Google Scholar] [CrossRef]
- Guzmán-Quesada, J.A. Remote Sensing Tools for Detecting and Quantifying Lianas and Trees at the Tropical Dry Forest. Ph.D. Thesis, University of Alberta, Edmonton, Alberta, Canada, 2021. [Google Scholar]
- Barbosa, H.A.; Huete, A.R.; Baethgen, W.E. A 20-Year Study of NDVI Variability over the Northeast Region of Brazil. J. Arid. Environ. 2006, 67, 288–307. [Google Scholar] [CrossRef]
- Miot, H.A. Análise de Correlação Em Estudos Clínicos e Experimentais. J. Vasc. Bras. 2018, 17, 275–279. [Google Scholar] [CrossRef]
- Suleymanov, A.; Gabbasova, I.; Komissarov, M.; Suleymanov, R.; Garipov, T.; Tuktarova, I.; Belan, L. Random Forest Modeling of Soil Properties in Saline Semi-Arid Areas. Agriculture 2023, 13, 976. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, L.; Wang, M.; Wu, J.; Zhou, W.; Zhang, Q.; Deng, M. A Random Forest Model for Drought: Monitoring and Validation for Grassland Drought Based on Multi-Source Remote Sensing Data. Remote Sens. 2022, 14, 4981. [Google Scholar] [CrossRef]
- Fremout, T.; Cobián-De Vinatea, J.; Thomas, E.; Huaman-Zambrano, W.; Salazar-Villegas, M.; Limache-de la Fuente, D.; Bernardino, P.N.; Atkinson, R.; Csaplovics, E.; Muys, B. Site-Specific Scaling of Remote Sensing-Based Estimates of Woody Cover and Aboveground Biomass for Mapping Long-Term Tropical Dry Forest Degradation Status. Remote Sens. Environ. 2022, 276, 113040. [Google Scholar] [CrossRef]
- Breidenbach, J.; McRoberts, R.E.; Astrup, R. Empirical Coverage of Model-Based Variance Estimators for Remote Sensing Assisted Estimation of Stand-Level Timber Volume. Remote Sens. Environ. 2016, 173, 274–281. [Google Scholar] [CrossRef]
- Stojanova, D.; Panov, P.; Kobler, A.; Džeroski, S.; Taškova, K. Learning to Predict Forest Fires with Different Data Mining Techniques. In Proceedings of the Conference on Data Mining and Data Warehouses, Las Vegas, NV, USA, 26–29 June 2006; pp. 255–258. [Google Scholar]
- Alberto, J.; Becerra, B.; De, S.; Jean, C.; Henry, P.; Ometto, B. Relação Das Sazonalidades Da Precipitação e Da Vegetação No Bioma Caatinga: Abordagem Multitemporal. In Proceedings of the XVII Simpósio Brasileiro de Sensoriamento Remoto—SBSR, São José dos Campos, Brazil, 25 April 2015; pp. 6668–6674. [Google Scholar]
- da Costa Júnior, D.S.; Ferreira, R.L.C.; da Silva, J.A.A.; Silva, J.W.L.; de Lima Pessoa, M.M. Influências Ambientais No Incremento Anual Da Área Basal, Em Ambiente de Caatinga. Ciênc. Florest. 2024, 34, e67699. [Google Scholar] [CrossRef]
- Andrade, W.M.; Lima, E.d.A.; Rodal, M.d.J.N.; Encarnação, C.R.F.; Pimentel, R.M.d.M. Revista de Geografia. RECIFE 2009, 26, 161–184. [Google Scholar]
- Galvíncio, J.D.; Mendes, S.M.; Morais, Y.C.B.; Souza, W.M.; De Moura, M.S.B.; Santos, W. Correlação Linear Entre a Precipitação e o Índice de Área Foliar Do Bioma Caatinga. Rev. Bras. Geogr. Fís. 2020, 13, 3304–3314. [Google Scholar] [CrossRef]
- Jardim, A.M.d.R.F.; Araújo Júnior, G.D.N.; da Silva, M.V.; Dos Santos, A.; da Silva, J.L.B.; Pandorfi, H.; de Oliveira-Júnior, J.F.; Teixeira, A.H.d.C.; Teodoro, P.E.; de Lima, J.L.M.P.; et al. Using Remote Sensing to Quantify the Joint Effects of Climate and Land Use/Land Cover Changes on the Caatinga Biome of Northeast Brazilian. Remote Sens. 2022, 14, 1911. [Google Scholar] [CrossRef]
- Ferreira, J.M.S.; Ferreira, H.d.S.; da Silva, H.A.; dos Santos, A.M.; Galvíncio, J.D. Análise Espaço-Temporal Da Dinâmica Da Vegetação de Caatinga No Município de Petrolina—PE (Analysis Space-Time from Dynamics of Caatinga Vegetation in the Municipality of Petrolina—PE). Rev. Bras. Geogr. Fís. 2012, 5, 904–922. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, T.; Zhang, X.; Wu, Y.; Xu, X.; Wang, Z.; Zou, F.; Zhang, C.; Xu, C.; Ou, G. Interacting Sentinel-2A, Sentinel 1A, and GF-2 Imagery to Improve the Accuracy of Forest Aboveground Biomass Estimation in a Dry-Hot Valley. Forests 2024, 15, 731. [Google Scholar] [CrossRef]
- Oliveira, S.N.; de Carvalho Júnior, O.A.; Gomes, R.A.T.; Guimarães, R.F.; McManus, C.M. Deforestation Analysis in Protected Areas and Scenario Simulation for Structural Corridors in the Agricultural Frontier of Western Bahia, Brazil. Land Use Policy 2017, 61, 40–52. [Google Scholar] [CrossRef]




| Vegetation Index | Formula | Reference Source |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | (NIR − RED)/(NIR + RED) | [58] |
| Enhanced Vegetation Index (EVI) | 2.5 × (NIR − RED)/(NIR + 6 × RED − 7.5 × BLUE + 1) | [59] |
| Soil-Adjusted Vegetation Index (SAVI) | (1 + L) × (NIR − RED)/(NIR + RED + L) | [60] |
| Green Normalized Difference Vegetation Index (GNDVI) | (NIR − GREEN)/(NIR + GREEN) | [61] |
| Normalized Difference Water Index (NDWI) | (NIR − SWIR)/(NIR + SWIR) or (GREEN − NIR)/(GREEN + NIR) | [62] |
| Normalized Difference Built-up Index (NDBI) | (SWIR − NIR)/(SWIR + NIR) | [63] |
| Moisture Stress Index (MSI) | SWIR/NIR | [64] |
| Normalized Burn Ratio (NBR) | (NIR − SWIR)/(NIR + SWIR) | [65] |
| Normalized Difference Snow Index (NDSI) | (GREEN − SWIR)/(GREEN + SWIR) | [66] |
| Leaf Area Index (LAI) | Ln((0.69 − SAVI)/0.59)/0.91 | [67] |
| Variable | Mean | Median | Min | Max | SD | CV (%) |
|---|---|---|---|---|---|---|
| PAI | 4.30 | 3.94 | 0.58 | 16.57 | 2.59 | 60.27 |
| Blue | 0.06 | 0.05 | 0.02 | 0.09 | 0.02 | 32.94 |
| Green | 0.09 | 0.09 | 0.04 | 0.14 | 0.02 | 25.30 |
| Red | 0.12 | 0.13 | 0.03 | 0.20 | 0.04 | 36.11 |
| NIR | 0.27 | 0.26 | 0.19 | 0.38 | 0.03 | 12.89 |
| SWIR1 | 0.33 | 0.34 | 0.18 | 0.45 | 0.07 | 21.48 |
| SWIR2 | 0.23 | 0.24 | 0.08 | 0.36 | 0.07 | 31.81 |
| NDVI | 0.38 | 0.29 | 0.17 | 0.82 | 0.19 | 50.36 |
| EVI | 0.24 | 0.17 | 0.10 | 0.56 | 0.12 | 51.61 |
| SAVI | 0.25 | 0.19 | 0.11 | 0.54 | 0.12 | 46.55 |
| GNDVI | 0.49 | 0.43 | 0.31 | 0.76 | 0.12 | 24.51 |
| NDWI | −0.10 | −0.16 | −0.29 | 0.31 | 0.15 | −160.12 |
| NDBI | 0.10 | 0.16 | −0.31 | 0.29 | 0.15 | 160.12 |
| MSI | 1.27 | 1.38 | 0.53 | 1.83 | 0.35 | 27.33 |
| NBR | 0.10 | 0.03 | −0.19 | 0.62 | 0.21 | 214.83 |
| NDSI | −0.57 | −0.56 | −0.64 | −0.51 | 0.02 | −4.36 |
| LAI | 0.95 | 0.93 | 0.67 | 1.35 | 0.13 | 13.31 |
| Variable | Correlation | p-Value |
|---|---|---|
| NIR | 0.254451 | 0.000004 |
| LAI | 0.244708 | 0.000010 |
| NDSI | 0.201745 | 0.000281 |
| NDVI | 0.118065 | 0.034761 |
| Class | Intervals (mm.ha−1) | Correction Factor (FC) |
|---|---|---|
| 1 | 0.5835–1.8916 | −0.8685 |
| 2 | 1.8916–2.9572 | −0.6340 |
| 3 | 2.9572–3.9390 | −0.4750 |
| 4 | 3.9390–4.8921 | −0.1901 |
| 5 | 4.8921–6.4431 | 0.2911 |
| 6 | 6.4431–16.5665 | 1.5334 |
| Period | Mean Precipitation (mm) | Mean PAI (mm) |
|---|---|---|
| 2011–2013 | 286.2 | 4.04 |
| 2013–2015 | 245.9 | 3.73 |
| 2015–2017 | 372.5 | 4.23 |
| 2017–2019 | 461.7 | 6.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finger, A.P.; Ferreira, R.L.C.; Lana, M.D.; Silva, J.A.A.d.; Silva, E.A.; Breunig, F.M.; Bispo, P.d.C.; Liesenberg, V.; Nogueira, S.S. Comparison Between Traditional Forest Inventory and Remote Sensing with Random Forest for Estimating the Periodic Annual Increment in a Dry Tropical Forest. Forests 2025, 16, 998. https://doi.org/10.3390/f16060998
Finger AP, Ferreira RLC, Lana MD, Silva JAAd, Silva EA, Breunig FM, Bispo PdC, Liesenberg V, Nogueira SS. Comparison Between Traditional Forest Inventory and Remote Sensing with Random Forest for Estimating the Periodic Annual Increment in a Dry Tropical Forest. Forests. 2025; 16(6):998. https://doi.org/10.3390/f16060998
Chicago/Turabian StyleFinger, Anelisa Pedroso, Rinaldo Luiz Caraciolo Ferreira, Mayara Dalla Lana, José Antônio Aleixo da Silva, Emanuel Araújo Silva, Fábio Marcelo Breunig, Polyanna da Conceição Bispo, Veraldo Liesenberg, and Sara Sebastiana Nogueira. 2025. "Comparison Between Traditional Forest Inventory and Remote Sensing with Random Forest for Estimating the Periodic Annual Increment in a Dry Tropical Forest" Forests 16, no. 6: 998. https://doi.org/10.3390/f16060998
APA StyleFinger, A. P., Ferreira, R. L. C., Lana, M. D., Silva, J. A. A. d., Silva, E. A., Breunig, F. M., Bispo, P. d. C., Liesenberg, V., & Nogueira, S. S. (2025). Comparison Between Traditional Forest Inventory and Remote Sensing with Random Forest for Estimating the Periodic Annual Increment in a Dry Tropical Forest. Forests, 16(6), 998. https://doi.org/10.3390/f16060998








