Compaction of Iron Mining Tailings Impairs Seedling Emergence of Schinus terebinthifolia but Vigor Features Show Tolerance During Early Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Iron Mining Tailings Traits
2.2. Plant Material and Experimental Design
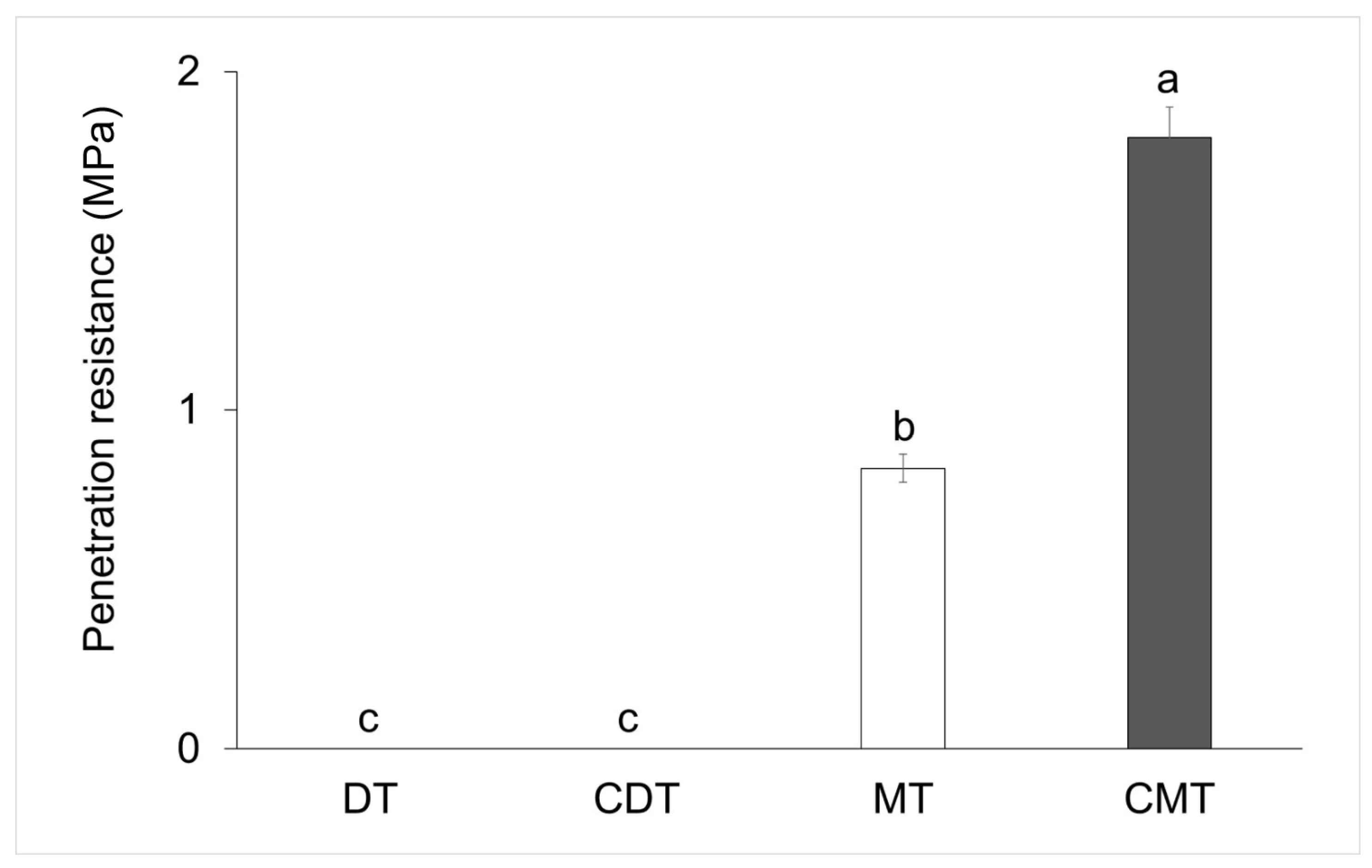
2.3. Penetration Resistance Analysis
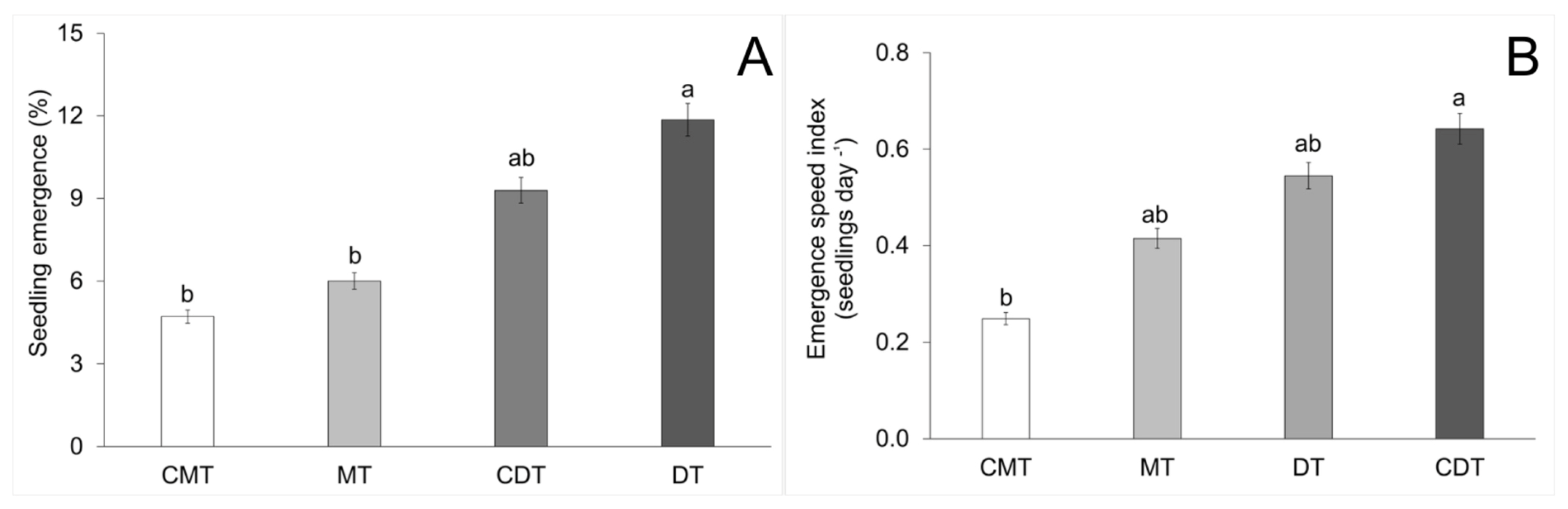
2.4. Emergence Analysis
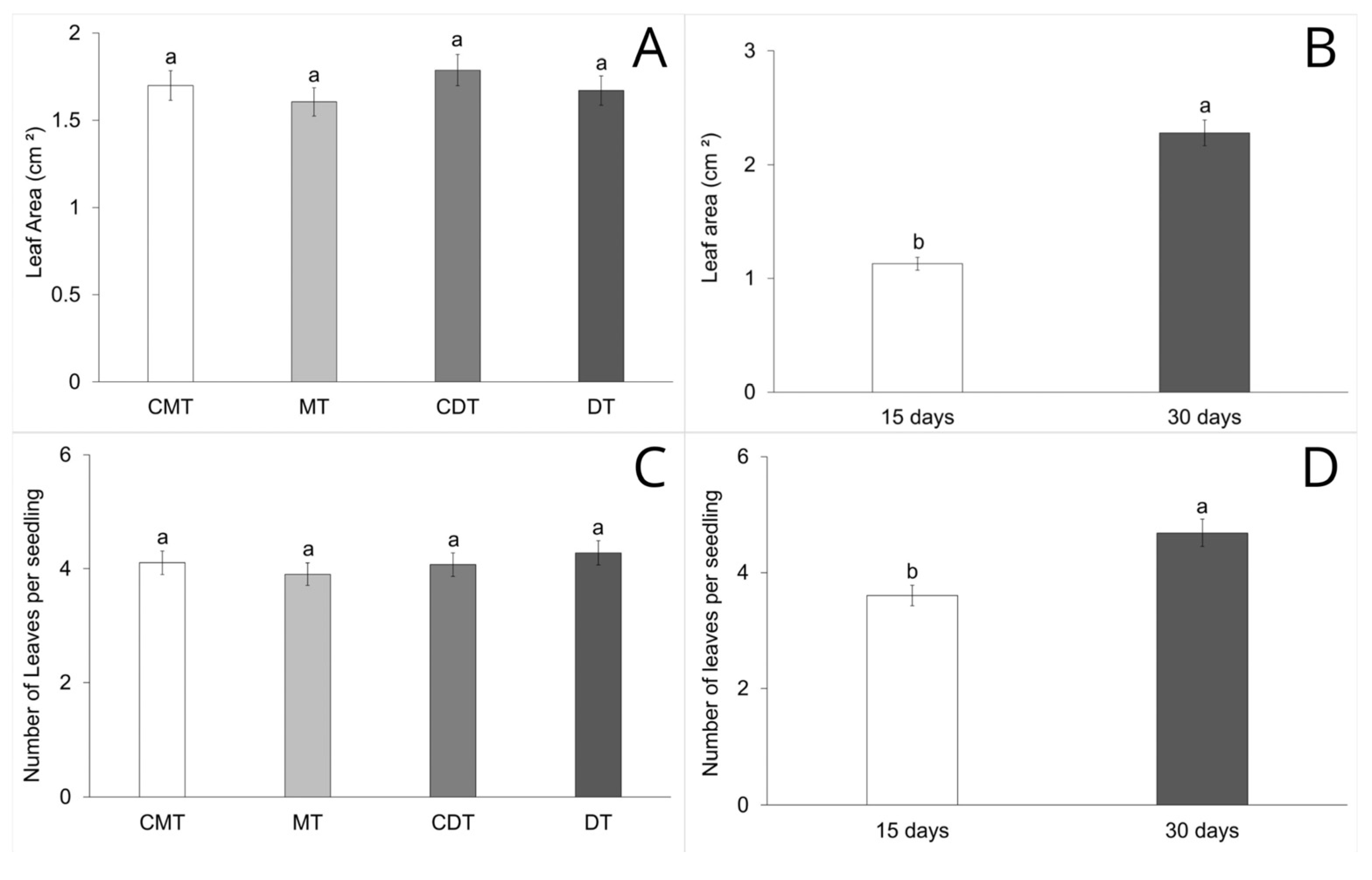
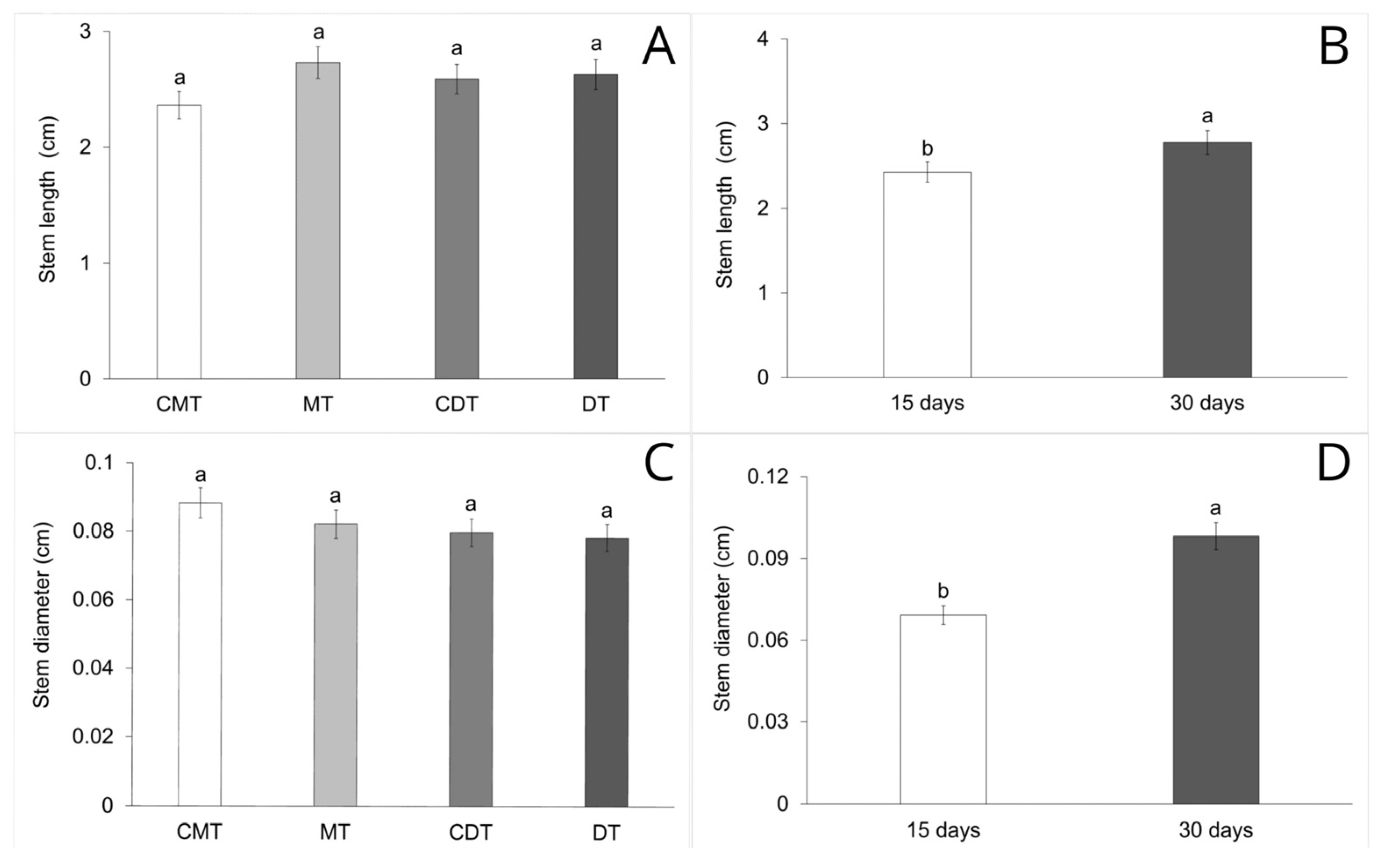
2.5. Seedling Biometry Analysis
2.6. Photochemical Analysis of Photosynthesis
2.7. Statistical Analyses
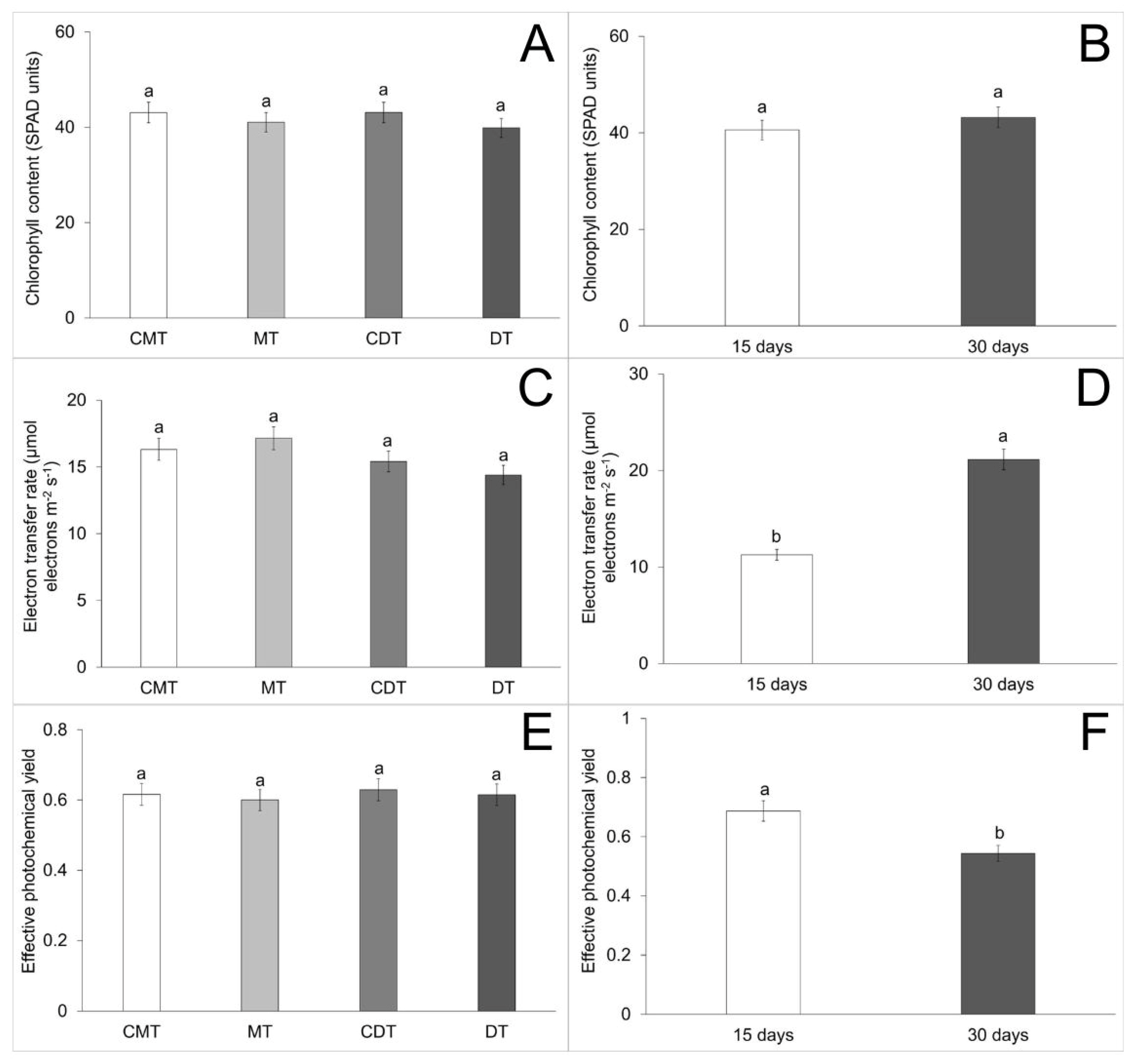
3. Results
4. Discussion
4.1. Compaction of Iron Mining Tailings and Its Effect on S. terebinthifolia Seedling Emergence
4.2. Tolerance of S. terebinthifolia Seedlings to Compaction of Iron Mining Tailings
4.3. Early Growth and Development of S. terebinthifolia Seedlings in Compacted Iron Mining Tailings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacaz, F.A.C.; Porto, M.F.S.; Pinheiro, T.M.M. Tragédias brasileiras contemporâneas: O caso do rompimento da barragem de rejeitos de Fundão/Samarco. Rev. Bras. Saúde Ocup. 2017, 42, 1–12. [Google Scholar] [CrossRef]
- Carmo, F.F.; Kamino, L.H.Y.; Junior, R.T.; Campos, I.C.; Carmo, F.F.; Silvino, G.; Pinto, C.E.F. Fundão tailings dam failures: The environment tragedy of the largest technological disaster of Brazilian mining in global context. Perspec. Ecol. Conserv. 2017, 15, 145–151. [Google Scholar] [CrossRef]
- Silva, C.A.; Coutinho, A.D.; Oliveira, J.F.; Teodoro, P.E. Analysis of the impact on vegetation caused by abrupt deforestation via orbital sensor in the environmental disaster of Mariana, Brazil. Land Use Policy 2018, 76, 10–20. [Google Scholar] [CrossRef]
- Pádua, M.P.; Caetano, A.L.; Polo, M.; Pasqual, M.; Pereira, F.J. Ecophysiological responses of Copaifera langsdorffii grown in mining tailings under lower water availability. Water Air Soil. Pollut. 2021, 232, 57. [Google Scholar] [CrossRef]
- Scarpa, A.L.M.; Cruz, Y.C.; Duarte, V.P.; Castro, E.M. Growth response, gas exchange, and leaf anatomy of Handroanthus spp. seedling in mine tailings enriched with nutrient solution. J. Soil. Sci. Plant Nutr. 2022, 22, 3774–3787. [Google Scholar] [CrossRef]
- Scarpa, A.L.M.; Rodrigues, F.A.; Cruz, Y.C.; Duarte, V.P.; Castro, E.M.; Pasqual, M.; Pereira, F.J. Seed germination, initial growth and leaf anatomy of seedlings of four tree species grown in mine tailings in Brazil. Seed Sci. Res. 2022, 32, 104–113. [Google Scholar] [CrossRef]
- Silva, P.N.; Reis, C.H.G.; Duarte, V.P.; Castro, E.M.; Pádua, M.P.; Pereira, F.J. Toxicity of iron mining tailings and potential for revegetation using Schinus terebinthifolia Raddi based on the emergence, growth, and anatomy of the species. Mining 2024, 4, 719–732. [Google Scholar] [CrossRef]
- Gomes, F.S.; Silva, P.N.; Reis, C.H.G.; Anchieta, M.R.; Santolino, A.C.; Nakamura, K.S.D.; Pereira, F.J. Iron mining tailing toxicity is increased by lower pH affecting lettuce seed germination, seedling early growth, and leaf anatomy. Environ. Sci. Pollut. Res. 2025, 32, 5788–5799. [Google Scholar] [CrossRef]
- Matos, L.P.; Andrade, H.; Marinato, C.S.; Prado, I.G.O. Limitations to use of Cassia grandis L. in the revegetation of the areas impacted with mining tailings from Fundão dam. Water Air Soil. Pollut. 2020, 231, 127. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrié, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef]
- Souza, E.V.; Andrade, G.C.; Araújo, H.H.; Dias-Pereira, J. Structural plasticity in leave of Schinus terebinthifolius (Anacardiaceae) populations from three contrasting tropical ecosystems. J. Torrey Bot. Soc. 2022, 149, 187–193. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na, NH4. North Carol. Soil. Test. Div. 1953, 2, 23–89. [Google Scholar]
- McLean, E.O.; Heddleson, M.R.; Bartlett, R.J.; Holowaychuk, N. Aluminum in soils: I. Extraction methods and magnitudes in clays and Ohio soils. Soil. Sci. Soc. Am. J. 1958, 22, 382–387. [Google Scholar] [CrossRef]
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, SP, Brazil, 2001. [Google Scholar]
- Bouyoucos, G.J. An improved type of soil hydrometer. Soil. Sci. 1950, 76, 377–378. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Cien. Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Peixoto, D.S.; Silva, B.M.; Oliveira, G.C.; Moreira, S.G.; Silva, F.; Curi, N. A soil compaction diagnosis method for occasional tillage recommendation under continuous no tillage system in Brazil. Soil Tillage Res. 2019, 194, 104307. [Google Scholar] [CrossRef]
- Assis, R.L.; Lazarini, G.D.; Lanças, K.P.; Cargnelutti, A. Avaliação da resistência do solo à penetração em diferentes solos com a variação do teor de água. Eng. Agric. 2009, 29, 558–568. [Google Scholar] [CrossRef]
- Shaheb, M.R.; Venkatesh, R.; Shearer, S.A. A review on the efect of soil compaction and its management for sustainable crop production. J. Biosyst. Eng. 2021, 46, 417–439. [Google Scholar] [CrossRef]
- Caetano, A.L.; Pádua, M.P.; Polo, M.; Pasqual, M.; Pereira, F.J. Growth, anatomy, and gas exchange of Cenostigma pluviosum cultivated under reduced water levels in iron mining tailings. J. Soils Sediments 2021, 22, 381–391. [Google Scholar] [CrossRef]
- Beutler, A.N.; Centurion, J.F.; Souza, Z.M.; Andrioli, I.; Roque, C.G. Retenção de água em dois tipos de Latossolo sob diferentes usos. Rev. Bras. Cien Solo 2002, 26, 829–834. [Google Scholar] [CrossRef]
- Silva, A.C.; Cavalcante, L.C.D.; Fabris, J.D.; Júnior, R.F.; Barral, M.; Laral, R.S.; Stumpf, H.O.; Barbosa, J.B.S. Chemical, mineralogical and physical characteristics of a material accumulated on the river margin from mud flowing from the collapse of the iron ore tailings dam in Bento Rodrigues, Minas Gerais, Brazil. Rev. Espinhaço 2016, 5, 44–53. [Google Scholar]
- Schaefer, C.E.G.R.; Santos, E.E.; Fernandes, E.I.; Assis, I.R. Paisagens de lama: Os tecnossolos para recuperação ambiental de áreas afetadas pelo desastre da Barragem de Fundão, em Mariana. Bol. Inf. SBCS 2016, 1, 18–23. [Google Scholar]
- Zanchi, C.S.; Silva, A.O.; Batista, E.R.; Peixoto, D.S.; Barbosa, M.V.; Santos, J.V.; Alvarenga, I.F.S.; Silva, B.M.; Carneiro, M.A.C. Pre-cultivation with herbaceous plants assists in the revegetation process of iron mining tailings with Enterolobium contortisiliquum. Water Air Soil. Pollut. 2022, 233, 231. [Google Scholar] [CrossRef]
- Montovani, E.C. Compactação do solo. Inf. Agropecuário 1987, 13, 52–55. [Google Scholar]
- Almeida, C.A.; Oliveira, A.F.; Pacheco, A.A.; Lopes, R.P.; Neves, A.A.; Queiroz, M.E.L.R. Characterization and evaluation of sorption potential of the iron mine waste after Samarco dam disaster in Doce River basin—Brazil. Chemosphere 2018, 209, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Richart, A.; Tavares, J.; Brito, O.R.; Llanillo, R.F.; Ferreira, R. Compactação do solo: Causas e efeitos. Semina 2005, 26, 321–344. [Google Scholar] [CrossRef]
- Bastos, R.S.; Mendonça, E.D.S.; Corrêa, M.M.; Costa, L.M. Formação e estabilização de Agregados do solo influenciados por ciclos de umedecimento e secagem após adição de compostos orgânicos com diferentes características hidrofóbicas. Rev. Bras. Cien Solo 2005, 29, 21–31. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Germination. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed Germination and Vigor. Ann. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Dürr, C.E.; Aubertot, J.N. Emergence of seedlings of sugar beet (Beta vulgaris L.) as affected by the size, roughness and position of aggregates in the seedbed. Plant Soil. 2000, 219, 211–220. [Google Scholar] [CrossRef]
- Johnson, M.; Khumbula, N. The Effect of Soil Compaction Levels on Germination and Biometric Characteristics of Coffee (Coffee arabica) Seedlings in the Nursery. Int. J. Agric. Res. 2007, 2, 581–589. [Google Scholar] [CrossRef]
- Tolon-Becerra, A.; Tourn, M.; Botta, G.F.; Latra-Bravo, X. Effects of different tillage regimes on soil compaction, maize (Zea mays L.) seedling emergence and yields in the eastern Argentinean Pampas region. Soil Tillage Res. 2011, 117, 184–190. [Google Scholar] [CrossRef]
- Pereira, E.B.; Nunes, E.M.; Souto, J.S.; Aguiar, P.; Rolim, H.O. Avaliação do crescimento de raízes e parte aérea da moringueira (Moringa oleifera) sob condições de solo compactado. Rev. Verde Agroecol. Desenvol. Sus. 2012, 7, 96–101. [Google Scholar]
- Dedecek, R.A.; Curcio, G.R.; Rachwal, M.F.G.; Simon, A.A. Effects of soil tillage systems on soil erosion and on black (Acacia mearnsii De Wild.) productivity. Cien For. 2007, 17, 205–215. [Google Scholar] [CrossRef]
- Araújo, L.H.B.; Silva, G.G.C.; Nóbrega, C.C.; Oliveira, E.M.M.; Pimenta, A.S. Effect of Soil Compaction on Aerial and Root Growth of Tabebuia caraiba Mart. Bur. Int. J. Agric. Sci. 2018, 10, 5589–5592. [Google Scholar]
- Goss, M.J. Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.). J. Exp. Bot. 1977, 28, 96–111. [Google Scholar] [CrossRef]
- Busse, M.D.; Fiddler, G.O.; Shestak, C.J. Conifer Root Proliferation after 20 Years of Soil Compaction. For. Sci. 2017, 63, 147–150. [Google Scholar] [CrossRef]
- Colombi, T.; Walter, A. Genetic Diversity under Soil Compaction in Wheat: Root Number as a Promising Trait for Early Plant Vigor. Front. Plant Sci. 2017, 8, 420. [Google Scholar] [CrossRef]
- Bengough, A.G.; Bransby, M.F.; Hans, J.; McKenna, S.J.; Roberts, T.J.; Valentine, T.A. Root responses to soil physical conditions; growth dynamics from field to cell. J. Exp. Bot. 2005, 57, 437–447. [Google Scholar] [CrossRef]
- Tubeileh, A.; Groleau-Renaud, V.; Plantureus, S.; Guckert, A.C. Effect of soil compaction on photosynthesis and carbon partitioning within a maize-soil system. Soil Tillage Res. 2003, 71, 151–161. [Google Scholar] [CrossRef]
- Polanco, M.C.; Zwiazek, J.J.; Voicu, M.C. Responses of ectomycorrhizal American elm (Ulmus americana) seedlings to salinity and soil compaction. Plant Soil. 2008, 308, 189–200. [Google Scholar] [CrossRef]
- Morales, F.; Pavlovic, A.; Abadía, A.; Abadía, J. Photosynthesis in Poor Nutrient Soils, in Compacted Soils, and under Drought. In The Leaf: A Platform for Performing Photosynthesis; Advances in Photosynthesis and Respiration; Springer: Cham, Switzerland, 2018; Volume 44, pp. 371–399. [Google Scholar] [CrossRef]
- Souza, R.F.S.; Santos, D.; Pereira, W.E.; Macedo, F.L.; Vedruscolo, J. Gas exchange and photochemical efficiency in lima bean genotypes grown in compacted soils. Rev. Caatinga 2018, 31, 306–314. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Y.; Li, C.; McHugh, A.D.; Li, Z.; Wu, C. Individual and combined effects of soil waterlogging and compaction on physiological characteristics of wheat in south-western China. Field Crops Res. 2018, 215, 163–172. [Google Scholar] [CrossRef]
| Macronutrients | mg kg−1 | Maximum Concentration Values Permitted for Potentially Toxic Elements (PTE) (mg kg−1) * |
|---|---|---|
| Phosphorus (P) | 10.7 | - |
| Magnesium (Mg) | 18.2 | - |
| Potassium (K) | 41.6 | - |
| Calcium (Ca) | 226.4 | - |
| Micronutrients | mg kg−1 | |
| Manganese (Mn) | 200.4 | - |
| Iron (Fe) | 189.3 | - |
| Zinc (Zn) | 0.7 | 300.0 |
| Copper (Cu) | 1.1 | 63.0 |
| Sodium (Na) | 37.0 | - |
| Potentially toxic elements | (mg kg−1) | |
| Aluminum (Al) | 80.9 | - |
| Chromium (Cr) | 0.07 | 75.0 |
| Lead (Pb) | 0.02 | 72.0 |
| Other characteristics | ||
| pH | 5.7 | - |
| Organic matter (mg kg−1) | 4.3 | - |
| Granulometry | (%) | |
| Clay | 12 | - |
| Silt | 39 | - |
| Sand | 49 | - |
| Variable | CV% | Mean Square Value | F Test Value | p Value |
|---|---|---|---|---|
| Penetration resistance (C) | 50.43 | 5.894046 | 53.517 | <0.0001 |
| Germination percentage (C) | 44.37 | 73.083333 | 5.852 | 0.0038 |
| Germination speed index (C) | 43.33 | 0.186249 | 4.575 | 0.0118 |
| Number of leaves (C) | 15.74 | 0.454327 | 1.101 | 0.3539 |
| Number of leaves (A) | 15.74 | 24.740540 | 59.949 | <0.0001 |
| Number of leaves (C × A) | 15.74 | 0.712271 | 1.726 | 0.1685 |
| Leaf area (C) | 31.01 | 0.116012 | 0.422 | 0.7374 |
| Leaf area (A) | 31.01 | 25.731590 | 93.707 | <0.0001 |
| Leaf area (C × A) | 31.01 | 0.151009 | 0.550 | 0.6498 |
| Stem length (C) | 20.53 | 0.465704 | 1.658 | 0.1830 |
| Stem length (A) | 20.53 | 2.603987 | 9.269 | 0.0032 |
| Stem length (A × C) | 20.53 | 0.299690 | 1.067 | 0.3681 |
| Stem diameter (C) | 23.48 | 0.000381 | 1.029 | 0.3845 |
| Stem diameter (A) | 23.48 | 0.017878 | 48.292 | <0.0001 |
| Stem diameter (C × A) | 23.48 | 0.000250 | 0.674 | 0.5705 |
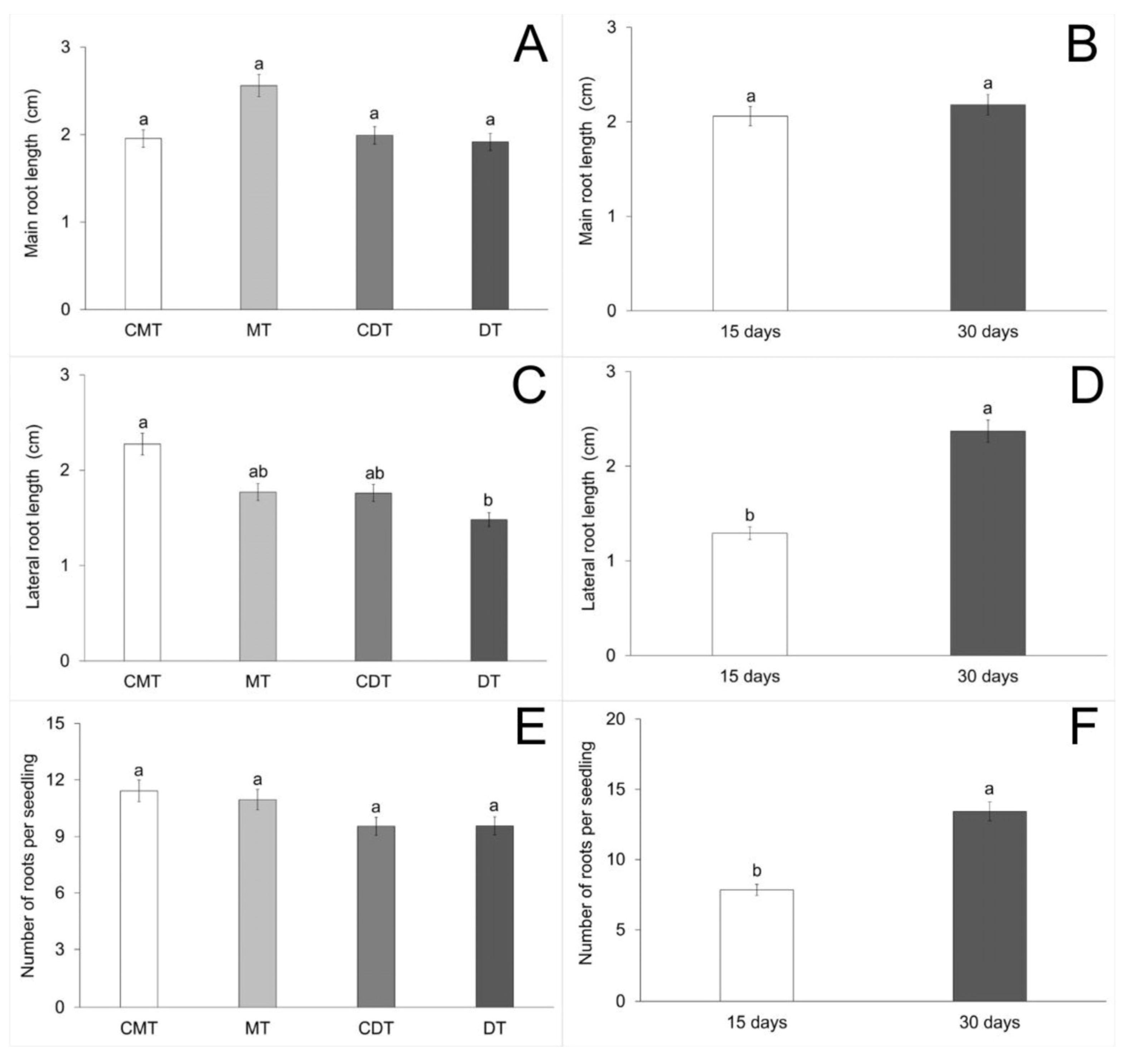
| Number of roots (C) | 49.67 | 19.715615 | 0.753 | 0.5239 |
| Number of roots (A) | 49.67 | 662.209710 | 25.290 | <0.0001 |
| Number of roots (C × A) | 49.67 | 7.467980 | 0.285 | 0.8361 |
| Main root length (C) | 46.81 | 1.888468 | 1.921 | 0.1341 |
| Main root length (A) | 46.81 | 0.280585 | 0.285 | 0.5949 |
| Main root length (C × A) | 46.81 | 0.454257 | 0.462 | 0.7096 |
| Lateral root length (C) | 47.12 | 2.029913 | 2.747 | 0.0494 |
| Lateral root length (A) | 47.12 | 22.311022 | 30.188 | <0.0001 |
| Lateral root length (C × A) | 47.12 | 0.766689 | 1.037 | 0.3816 |
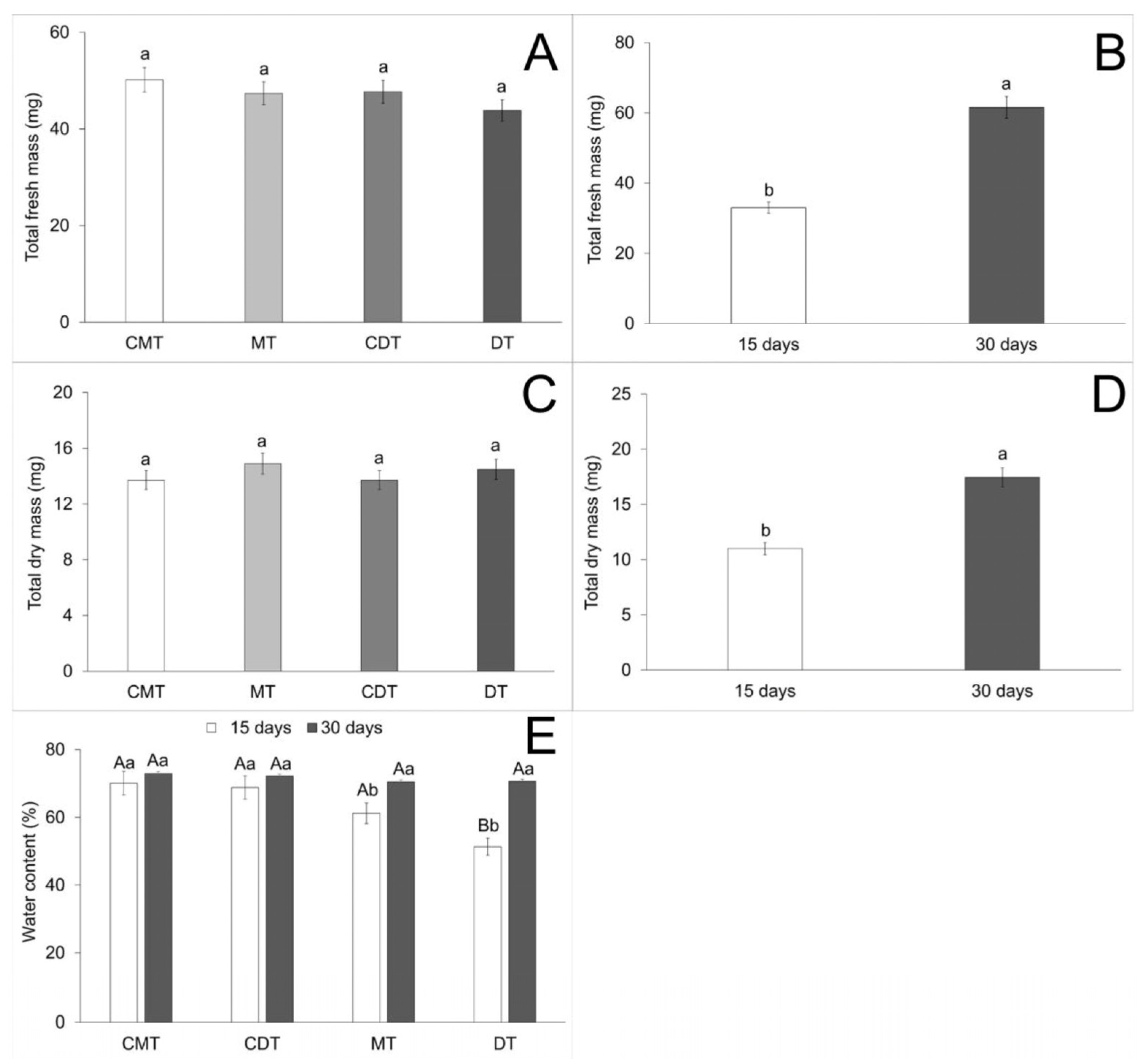
| Total fresh mass (C) | 33.95 | 135.832458 | 0.528 | 0.6646 |
| Total fresh mass (A) | 33.95 | 16,287.778125 | 63.278 | <0.0001 |
| Total fresh mass (C × A) | 33.95 | 190.291125 | 0.739 | 0.5320 |
| Total dry mass (C) | 30.95 | 6.427458 | 0.332 | 0.8024 |
| Total dry mass (A) | 30.95 | 831.405125 | 42.901 | <0.0001 |
| Total dry mass (C × A) | 30.95 | 11.767458 | 0.607 | 0.6124 |
| Water content (C) | 11.94 | 465.573548 | 7.232 | 0.0002 |
| Water content (A) | 11.94 | 1514.530888 | 23.526 | <0.0001 |
| Water content (C × A) * | 11.94 | 293.643410 | 4.561 | 0.0055 * |
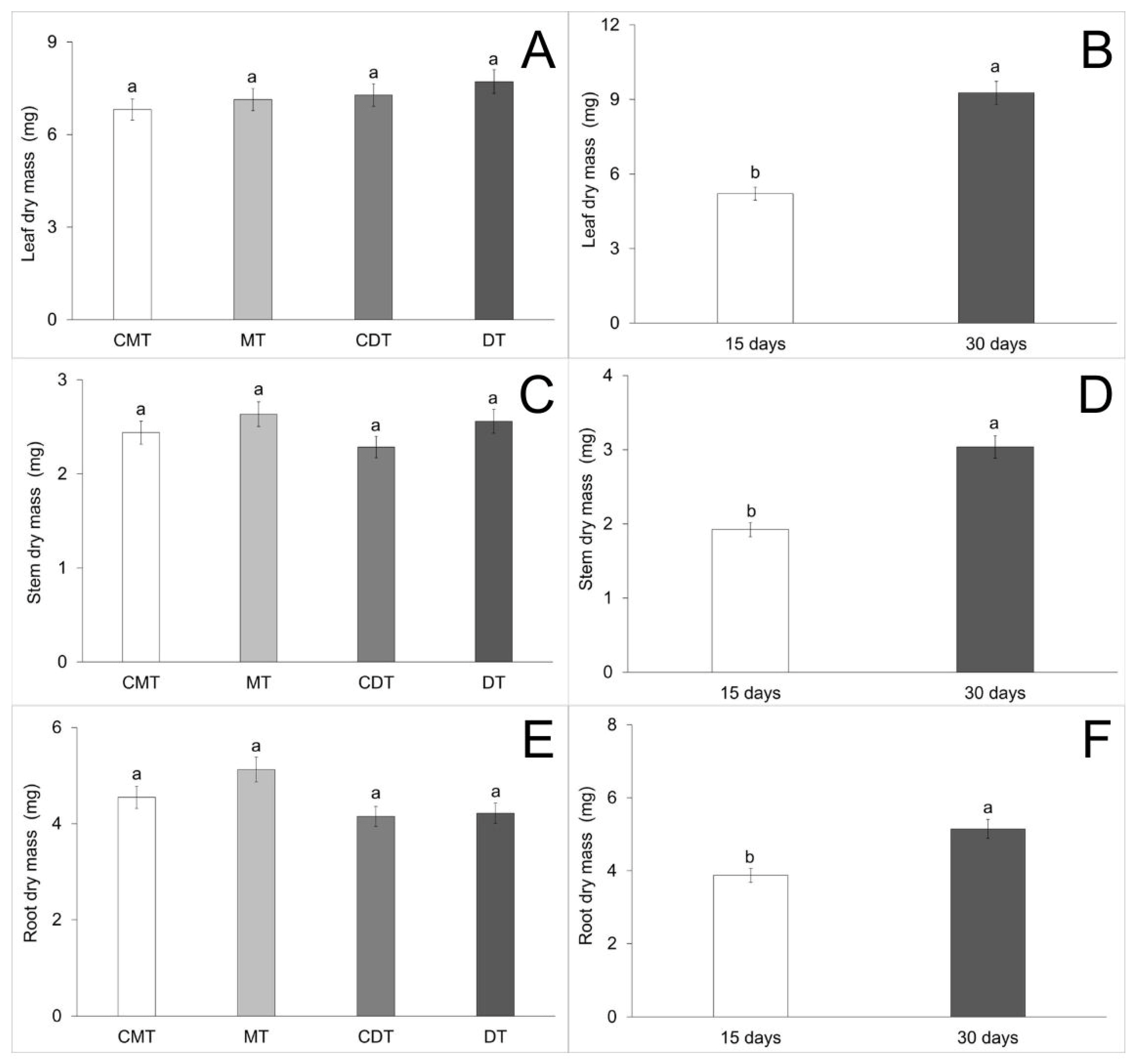
| Leaf dry mass (C) | 28.85 | 2.820333 | 0.647 | 0.5872 |
| Leaf dry mass (A) | 28.85 | 329.672000 | 75.648 | <0.0001 |
| Leaf dry mass (C × A) | 28.85 | 5.224333 | 1.199 | 0.3164 |
| Stem dry mass (C) | 34.36 | 0.467000 | 0.643 | 0.5897 |
| Stem dry mass (A) | 34.36 | 24.8645000 | 34.250 | <0.0001 |
| Stem dry mass (C × A) | 34.36 | 0.697500 | 0.961 | 0.4160 |
| Root dry mass (C) | 55.94 | 3.973792 | 0.625 | 0.6013 |
| Root dry mass (A) | 55.94 | 32.385125 | 5.091 | 0.0271 |
| Root dry mass (C × A) | 55.94 | 3.108125 | 0.489 | 0.6912 |
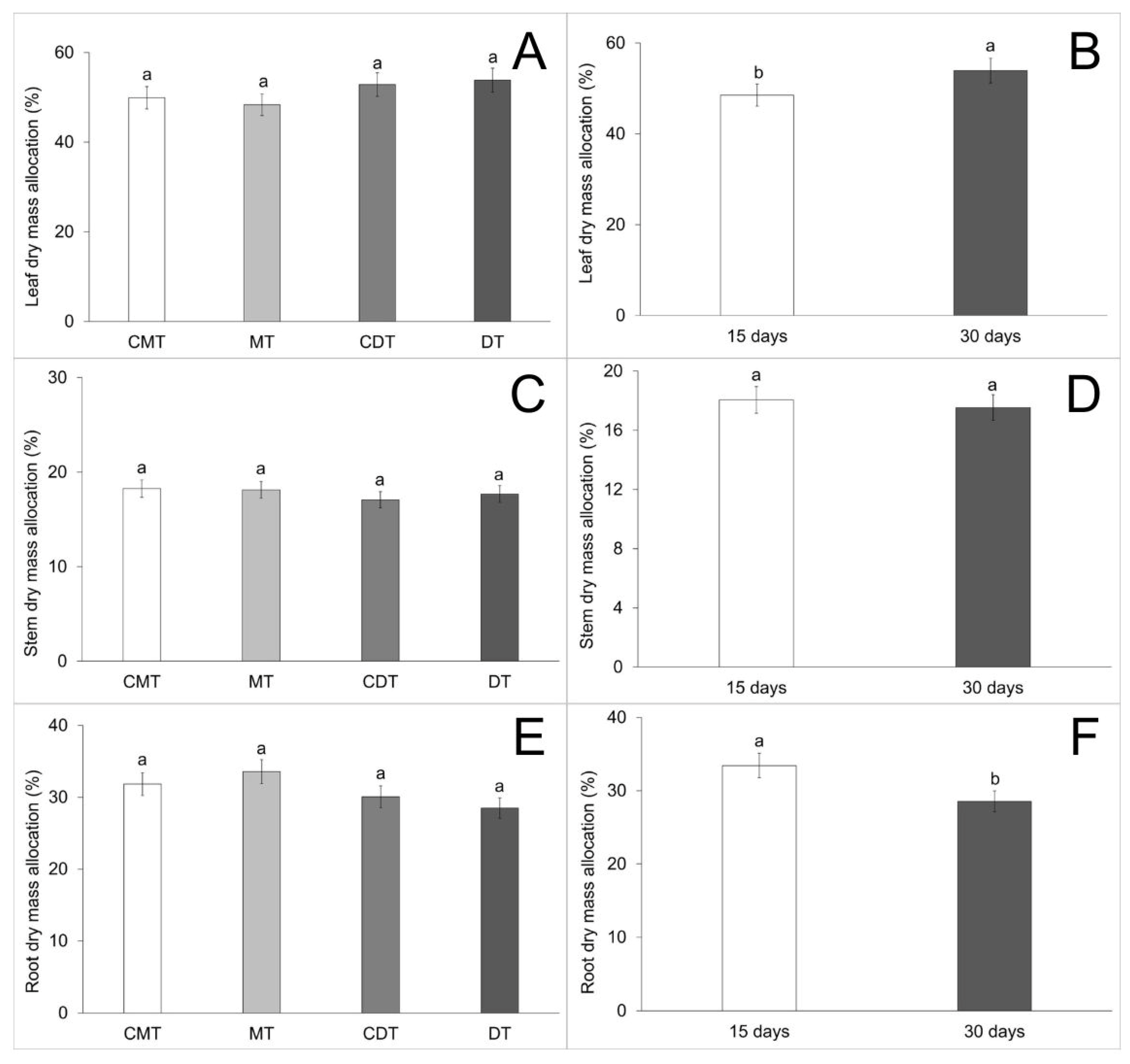
| Leaf dry mass allocation (C) | 18.52 | 130.353371 | 1.448 | 0.2361 |
| Leaf dry mass allocation (A) | 18.52 | 581.850781 | 6.462 | 0.0132 |
| Leaf dry mass allocation (C × A) | 18.52 | 77.094288 | 0.856 | 0.4679 |
| Stem dry mass allocation (C) | 25.43 | 5.500327 | 0.269 | 0.8480 |
| Stem dry mass allocation (A) | 25.43 | 5.325120 | 0.260 | 0.6116 |
| Stem dry mass allocation (C × A) | 25.43 | 12.748707 | 0.623 | 0.6026 |
| Root dry mass allocation (C) | 55.94 | 3.973792 | 0.625 | 0.6013 |
| Root dry mass allocation (A) | 55.94 | 32.385125 | 5.091 | 0.0271 |
| Root dry mass allocation (C × A) | 55.94 | 3.108125 | 0.489 | 0.6912 |
| Chlorophyll content (C) | 21.68 | 114.811673 | 1.396 | 0.2457 |
| Chlorophyll content (A) | 21.68 | 302.279391 | 3.676 | 0.0569 |
| Chlorophyll content (C × A) | 21.68 | 151.295337 | 1.840 | 0.1418 |
| Effective photochemical yield (C) | 12.21 | 0.005205 | 0.923 | 0.4316 |
| Effective photochemical yield (A) | 12.21 | 0.745344 | 132.175 | <0.0001 |
| Effective photochemical yield (C × A) | 12.21 | 0.003778 | 0.670 | 0.5718 |
| Electron transfer rate (C) | 42.83 | 56.578512 | 1.240 | 0.2969 |
| Electron transfer rate (A) | 42.83 | 4179.755792 | 91.616 | <0.0001 |
| Electron transfer rate (C × A) | 42.83 | 63.801778 | 1.398 | 0.2452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, P.N.; Duarte, V.P.; de Castro, E.M.; Silva, B.M.; Santos, J.d.J.; Pereira, F.J. Compaction of Iron Mining Tailings Impairs Seedling Emergence of Schinus terebinthifolia but Vigor Features Show Tolerance During Early Growth. Forests 2025, 16, 950. https://doi.org/10.3390/f16060950
da Silva PN, Duarte VP, de Castro EM, Silva BM, Santos JdJ, Pereira FJ. Compaction of Iron Mining Tailings Impairs Seedling Emergence of Schinus terebinthifolia but Vigor Features Show Tolerance During Early Growth. Forests. 2025; 16(6):950. https://doi.org/10.3390/f16060950
Chicago/Turabian Styleda Silva, Poliana Noemia, Vinícius Politi Duarte, Evaristo Mauro de Castro, Bruno Montoani Silva, Josiel de Jesus Santos, and Fabricio José Pereira. 2025. "Compaction of Iron Mining Tailings Impairs Seedling Emergence of Schinus terebinthifolia but Vigor Features Show Tolerance During Early Growth" Forests 16, no. 6: 950. https://doi.org/10.3390/f16060950
APA Styleda Silva, P. N., Duarte, V. P., de Castro, E. M., Silva, B. M., Santos, J. d. J., & Pereira, F. J. (2025). Compaction of Iron Mining Tailings Impairs Seedling Emergence of Schinus terebinthifolia but Vigor Features Show Tolerance During Early Growth. Forests, 16(6), 950. https://doi.org/10.3390/f16060950






