Phytochemical Composition, Antioxidant, Anti-Inflammatory Activity, and DNA Protective Capacity of Moss Hypnum cupressiforme Hedw. from Bulgaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GC-MS Determination of Amino Acid Composition
2.3. HPLC Determination of Free Sugars
2.4. Sterol Composition
2.5. Total Chlorophyll and Carotenoids Content
2.6. Preparation of Ethanolic Extracts with Ultrasound-Assisted Extraction
2.7. Total Phenolic Content
2.8. HPLC Determination of Phenolic Compounds
2.9. Antioxidant Activity
2.9.1. DPPH Radical Scavenging Assay
2.9.2. ABTS Radical Scavenging Assay
2.10. In Vitro Albumin Denaturation Inhibition Assay
2.11. DNA Nicking Protection Assay
2.12. Statistics
3. Results and Discussion
3.1. Amino Acid Composition
3.2. Sugars
3.3. Sterols, Chlorophyll, and Carotenoids Content
3.4. Phenolic Compounds and Antioxidant Activities
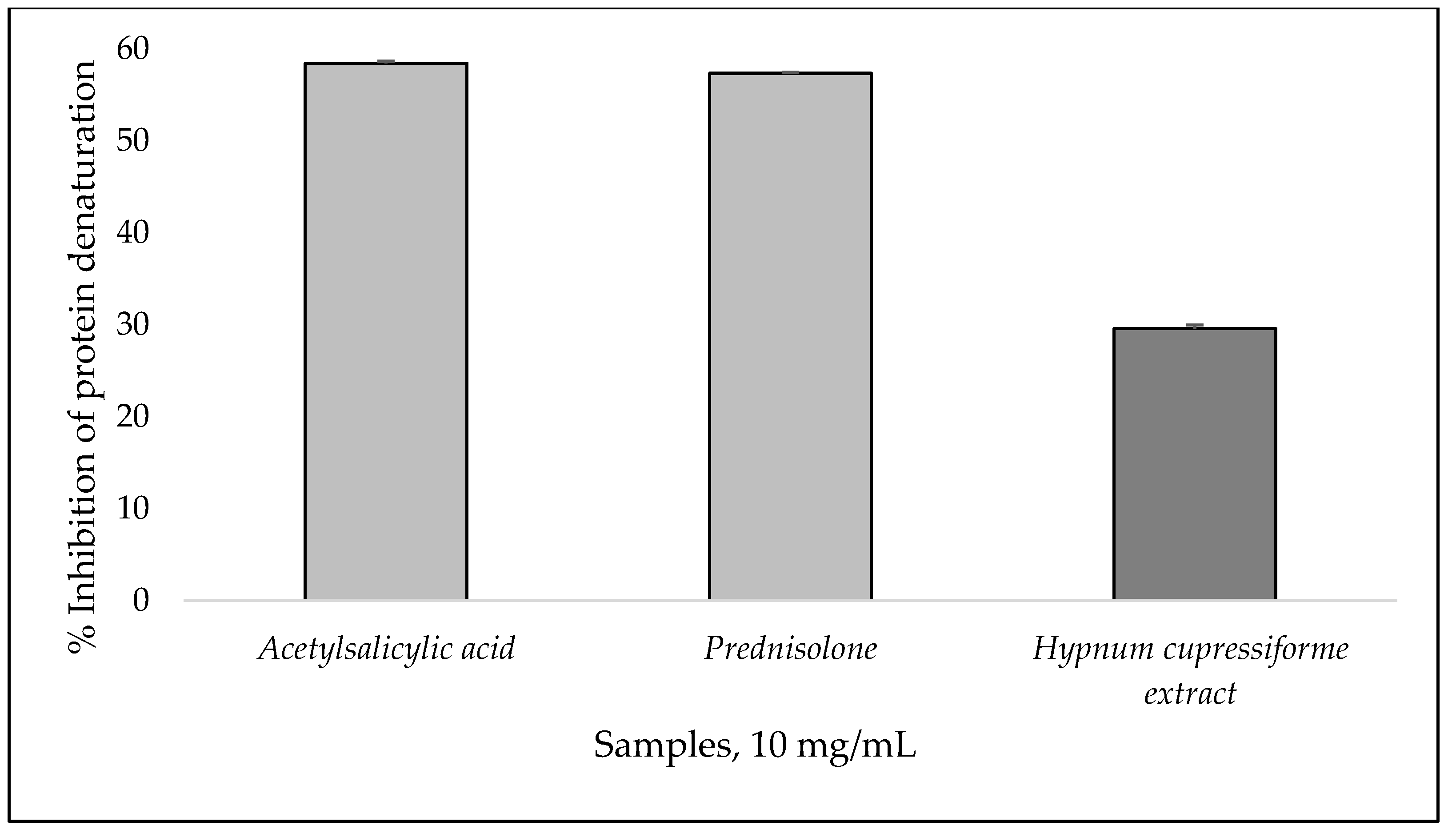
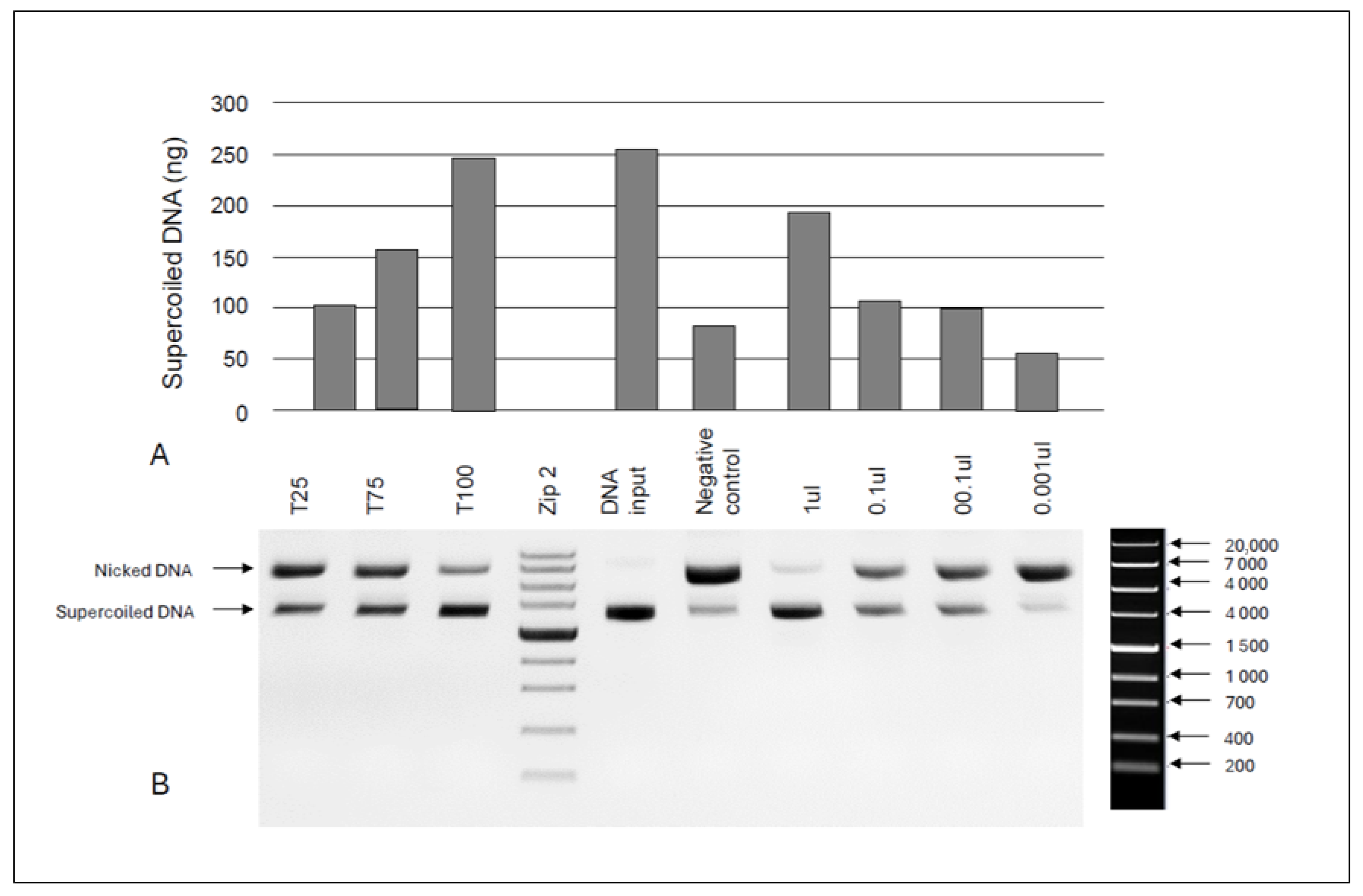
3.5. In Vitro Anti-Inflammatory Activity and DNA Protective Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lunić, T.M.; Oalđe, M.M.; Mandić, M.R.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Božić, B.D.J.; Božić Nedeljković, B.D. Extracts Characterization and In Vitro Evaluation of Potential Immunomodulatory Activities of the Moss Hypnum Cupressiforme Hedw. Molecules 2020, 25, 3343. [Google Scholar] [CrossRef] [PubMed]
- Klavina, L.; Springe, G.; Nikolajeva, V.; Martsinkevich, I.; Nakurte, I.; Dzabijeva, D.; Steinberga, I. Chemical Composition Analysis, Antimicrobial Activity and Cytotoxicity Screening of Moss Extracts (Moss Phytochemistry). Molecules 2015, 20, 17221–17243. [Google Scholar] [CrossRef]
- Vollár, M.; Gyovai, A.; Szűcs, P.; Zupkó, I.; Marschall, M.; Csupor-Löffler, B.; Bérdi, P.; Vecsernyés, A.; Csorba, A.; Liktor-Busa, E.; et al. Antiproliferative and Antimicrobial Activities of Selected Bryophytes. Molecules 2018, 23, 1520. [Google Scholar] [CrossRef]
- Cianciullo, P.; Maresca, V.; Sorbo, S.; Basile, A. Antioxidant and Antibacterial Properties of Extracts and Bioactive Compounds in Bryophytes. Appl. Sci. 2021, 12, 160. [Google Scholar] [CrossRef]
- Kirisanth, A.; Nafas, M.N.M.; Dissanayake, R.K.; Wijayabandara, J. Antimicrobial and Alpha-Amylase Inhibitory Activities of Organic Extracts of Selected Sri Lankan Bryophytes. Evid.-Based Complement. Altern. Med. 2020, 2020, 3479851. [Google Scholar] [CrossRef]
- Cheng, X.; Xiao, Y.; Wang, X.; Wang, P.; Li, H.; Yan, H.; Liu, Q. Anti-Tumor and Pro-Apoptotic Activity of Ethanolic Extract and Its Various Fractions from Polytrichum commune L. ex Hedw in L1210 Cells. J. Ethnopharmacol. 2012, 143, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Krzaczkowski, L.; Wright, M.; Reberioux, D.; Massiot, G.; Etievant, C.; Gairin, J.E. Pharmacological Screening of Bryophyte Extracts That Inhibit Growth and Induce Abnormal Phenotypes in Human HeLa Cancer Cells. Fundam. Clin. Pharmacol. 2009, 23, 473–482. [Google Scholar] [CrossRef]
- Wang, X.N.; Yu, W.T.; Lou, H.X. Antifungal Constituents from the Chinese Moss Homalia trichomanoides. Chem. Biodivers. 2005, 2, 139–145. [Google Scholar] [CrossRef]
- Singh, M.; Govindarajan, R.; Nath, V.; Rawat, A.K.S.; Mehrotra, S. Antimicrobial, Wound Healing and Antioxidant Activity of Plagiochasma appendiculatum Lehm. et Lind. J. Ethnopharmacol. 2006, 107, 67–72. [Google Scholar] [CrossRef]
- Mandić, M.R.; Oalđe, M.M.; Lunić, T.M.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Božić, B.D.; Nedeljković, B.D.B. Chemical Characterization and In Vitro Immunomodulatory Effects of Different Extracts of Moss Hedwigia ciliata (Hedw.) P. Beauv. from the Vršačke Planine Mts., Serbia. PLoS ONE 2021, 16, e0246810. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A. Chemical Constituents of Bryophytes: Structures and Biological Activity. J. Nat. Prod. 2017, 81, 641–660. [Google Scholar] [CrossRef]
- Klavina, L.; Ramawat, K.; Mérillon, J.M. Polysaccharides from Lower Plants: Bryophytes. In Polysaccharides; Springer: Cham, Switzerland, 2015; pp. 145–160. [Google Scholar]
- Lunić, T.M.; Mandić, M.R.; Oalđe Pavlović, M.M.; Sabovljević, A.D.; Sabovljević, M.S.; Božić Nedeljković, B.D.; Božić, B.D. The Influence of Seasonality on Secondary Metabolite Profiles and Neuroprotective Activities of Moss Hypnum cupressiforme Extracts: In Vitro and In Silico Study. Plants 2022, 11, 123. [Google Scholar] [CrossRef]
- Glime, J.M. Medical Uses: Medical Conditions. Chapter 2-1. In Bryophyte Ecology; Glime, J.M., Ed.; Michigan Technological University and the International Association of Bryologists: Houghton, MI, USA, 2017; Available online: http://digitalcommons.mtu.edu/bryophyte-ecology/ (accessed on 14 May 2025).
- Karataşa, H.; Yayintasb, O. Green Synthesis, Characterization and Biological Activity of Silver Nanoparticles of Hypnum cupressiforme Hedw Extract. J. Optoelectron. Biomed. Mater. 2024, 16, 63–72. [Google Scholar] [CrossRef]
- Lunić, T.; Božić, B.; Nedeljković, B.B. Immunomodulatory Potential of Hedwigia ciliata and Hypnum cupressiforme. In Bioactive Compounds in Bryophytes and Pteridophytes; Murthy, H.N., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Petkova, Z.; Teneva, O.; Antova, G.; Angelova-Romova, M.; Gecheva, G.; Dimitrova-Dyulgerova, I. Chemical Composition, Lipid-Soluble Bioactive Compounds and Potential Health Benefits of the Moss Hypnum cupressiforme Hedw. Plants 2023, 12, 4190. [Google Scholar] [CrossRef]
- Badridze, G.; Chkhubianishvili, E.; Rapava, L.; Kikvidze, M.; Chigladze, L.; Tsiklauri, N.; Tsilosani, K.; Chanishvili, S. Content of Active Metabolites in Some Species of Mosses of Georgia. Bull. Georg. Natl. Acad. Sci. 2020, 14, 127–131. [Google Scholar]
- Smolińska-Kondla, D.; Zych, M.; Ramos, P.; Wacławek, S.; Stebel, A. Antioxidant Potential of Various Extracts from 5 Common European Mosses and Its Correlation with Phenolic Compounds. Herba Pol. 2022, 68, 54–68. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization. Protein Quality Evaluation. In Proceedings of the Report of the Joint FAO/WHO Expert Consultation, Bethesda, MD, USA, 4–8 December 1989; FAO Food and Nutrition Paper No. 51. Food and Agriculture Organization: Rome, Italy, 1991. [Google Scholar]
- ISO 18609:2000; Animal and Vegetable Fats and Oils. Determination of Unsaponifiable Matter. Method Using Hexane Extraction. ISO: Geneva, Switzerland, 2000.
- ISO 12228-1:2014; Animal and Vegetable Fats and Oils. Determination of Individual and Total Sterols Contents. Gas Chromatographic Method. ISO: Geneva, Switzerland, 2014.
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of Total Carotenoids and Chlorophylls a and b of Leaf in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Ivanov, I.; Vrancheva, R.; Marchev, A.; Petkova, N.; Aneva, I.; Denev, P.; Georgiev, V.; Pavlov, A. Antioxidant Activities and Phenolic Compounds in Bulgarian Fumaria Species. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 296–306. [Google Scholar]
- Milusheva, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Mihaylova, R.; Nedialkov, P.; Cherneva, E.; Tumbarski, Y.; Tsoneva, S.; Todorova, M.; et al. Synthesis, molecular docking, and biological evaluation of novel anthranilic acid hybrid and its diamides as antispasmodics. Int. J. Mol. Sci. 2023, 24, 13855. [Google Scholar] [CrossRef]
- Andonova, T.; Petkova, Z.; Teneva, O.; Antova, G.; Apostolova, E.; Naimov, S.; Mladenova, T.; Slavov, I.; Dimitrova-Dyulgerova, I. Ailanthus altissima seed oil–A valuable source of lipid-soluble components with DNA protective and antiproliferative activities. Foods 2024, 13, 1268. [Google Scholar] [CrossRef]
- Rajiv, C.; Roy, S.S.; Tamreihao, K.; Kshetri, P.; Singh, T.S.; Sanjita Devi, H.; Sharma, S.K.; Ansari, M.A.; Devi, E.D.; Devi, A.K.; et al. Anticarcinogenic and Antioxidant Action of an Edible Aquatic Flora Jussiaea repens L. Using in vitro Bioassays and in vivo Zebrafish Model. Molecules 2021, 26, 2291. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.R.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Editorial: Amino Acids in Plants: Regulation and Functions in Development and Stress Defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections Between Amino Acid Metabolisms in Plants: Lysine as an Example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef]
- Nordin, A.; Gunnarsson, U. Amino acid accumulation and growth of Sphagnum under different levels of N deposition. Écoscience 2000, 7, 474–480. [Google Scholar] [CrossRef]
- Semerjyan, G.H.; Semerjyan, I.H. Investigation of amino acid composition of some mosses gathered from Armenia. Proc. YSU B Chem. Biol. Sci. 2021, 55, 290–295. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Falvo, M.J. Protein—Which is Best? J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; WHO Technical Report Series 935; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Wu, G. Amino Acids: Biochemistry and Nutrition; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Amino acid requirements in humans: An update. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 72–77. [Google Scholar]
- Pencharz, P.B. Protein and amino acids. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Jr., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Norton, L.E.; Layman, D.K. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 2006, 136, 533S–537S. [Google Scholar] [CrossRef]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef]
- Young, S.N.; Gauthier, S. Tryptophan Availability and the Control of 5-Hydroxytryptamine and Tryptamine Synthesis in Human CNS. In Serotonin; Haber, B., Gabay, S., Issidorides, M.R., Alivisatos, S.G.A., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1981; Volume 133, pp. 221–230. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [PubMed]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; Report of an FAO Expert Consultation; FAO: Rome, Italy, 2013. [Google Scholar]
- Schaafsma, G. The protein digestibility–corrected amino acid score. J. Nutr. 2000, 130, 1865S–1867S. [Google Scholar] [CrossRef]
- de Boer, J.; Aiking, H. On the merits of plant-based proteins for global food security: Marrying macro and micro perspectives. Ecol. Econ. 2011, 70, 1259–1265. [Google Scholar] [CrossRef]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Pejin, B.; Iodice, C.; Tommonaro, G.; Sabovljevic, M.; Bianco, A.; Tesevic, V.; Vajs, V.; De Rosa, S. Sugar composition of the moss Rhodobryum ontariense (Kindb.) Kindb. Nat. Prod. Res. 2012, 26, 209–215. [Google Scholar] [CrossRef]
- Rütten, D.; Santarius, K.A. Relationship between frost tolerance and sugar concentration of various bryophytes in summer and winter. Oecologia 1992, 91, 260–265. [Google Scholar] [CrossRef]
- Xinmei, X.; Ning, X.; Jianbin, H. Physiological function of phytosterol and its application. Anim. Husb. Feed Sci. 2015, 7, 67–69. [Google Scholar]
- Vecka, M.; Staňková, B.; Kutová, S.; Tomášová, P.; Tvrzická, E.; Žák, A. Comprehensive Sterol and Fatty Acid Analysis in Nineteen Nuts, Seeds, and Kernel. SN Appl. Sci. 2019, 1, 1531. [Google Scholar] [CrossRef]
- Othman, R.A.; Moghadasian, M.H. Beyond cholesterol-lowering effects of plant sterols: Clinical and experimental evidence of anti-inflammatory properties. Nutr. Rev. 2011, 69, 371–382. [Google Scholar] [CrossRef]
- Pareek, S.; Sagar, N.A.; Sharma, S.; Kumar, V.; Agarwal, T.; González-Aguilar, G.A.; Yahia, E.M. Chlorophylls: Chemistry and biological functions. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Yahia, E.M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 269–284. [Google Scholar]
- Sangeetha, R.K.; Baskaran, V. Carotenoid composition and retinol equivalent in plants of nutritional and medicinal importance: Efficacy of β-carotene from Chenopodium album in retinol-deficient rats. Food Chem. 2010, 119, 1584–1590. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Baqer, S.H.; Al-Younis, Z.K.; Al-Shawi, S.G. Extracting quercetin from different plant sources, purifying it using different extraction methods (chemical, physical, and enzymatic), and measuring its antioxidant activity. Front. Biosci. 2024, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C. Variation of Flavonoid Synthesis Induced by Ecological Factors. In Principles and Practices in Plant Ecology; CRC Press: Boca Raton, FL, USA, 2023; pp. 267–285. [Google Scholar]
- Sangeetha, G.; Vidhya, R. In vitro anti-inflammatory activity of different parts of Pedalium murex (L.). Int. J. Herb. Med. 2016, 4, 31–36. [Google Scholar]
- Okoli, C.O.; Akah, P.A.; Nwafor, S.V.; Anisiobi, A.I.; Ibegbunam, I.N.; Erojikwe, O. Anti-inflammatory activity of hexane leaf extract of Aspilia africana C.D. Adams. J. Ethnopharmacol. 2007, 109, 219–225. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Del Grossi Moura, M.; Cruz Lopes, L.; Silva, M.T.; Barberato-Filho, S.; Motta, R.H.L.; Bergamaschi, C.C. Use of steroid and nonsteroidal anti-inflammatories in the treatment of rheumatoid arthritis: Systematic review protocol. Medicine 2018, 97, e12658. [Google Scholar] [CrossRef] [PubMed]
- Madhuranga, H.D.T.; Samarakoon, D.N.A.W. In vitro anti-inflammatory egg albumin denaturation assay: An enhanced approach. Nat. Ayurvedic Med. 2023, 7, 1–6. [Google Scholar]
- Dharmadeva, S.; Galgamuwa, L.S.; Prasadinie, C.; Kumarasinghe, N. In vitro anti-inflammatory activity of Ficus racemosa L. bark using albumin denaturation method. AYU 2018, 39, 239–242. [Google Scholar] [CrossRef]
- Bailey-Shaw, Y.A.; Williams, L.A.; Green, C.E.; Rodney, S.; Smith, A.M. In-vitro evaluation of the anti-inflammatory potential of selected Jamaican plant extracts using the bovine serum albumin protein denaturation assay. Int. J. Pharm. Sci. Rev. Res. 2017, 47, 145–153. [Google Scholar]
- Yayintas, O.T.; Demir, N. Seasonal Changes of Antioxidant Activity and DNA Damage Protection Potential of Fontinalis antipyretica Hedw. and Hypnum cupressiforme Hedw. Fresenius Environ. Bull. 2019, 28, 6978–6987. [Google Scholar]
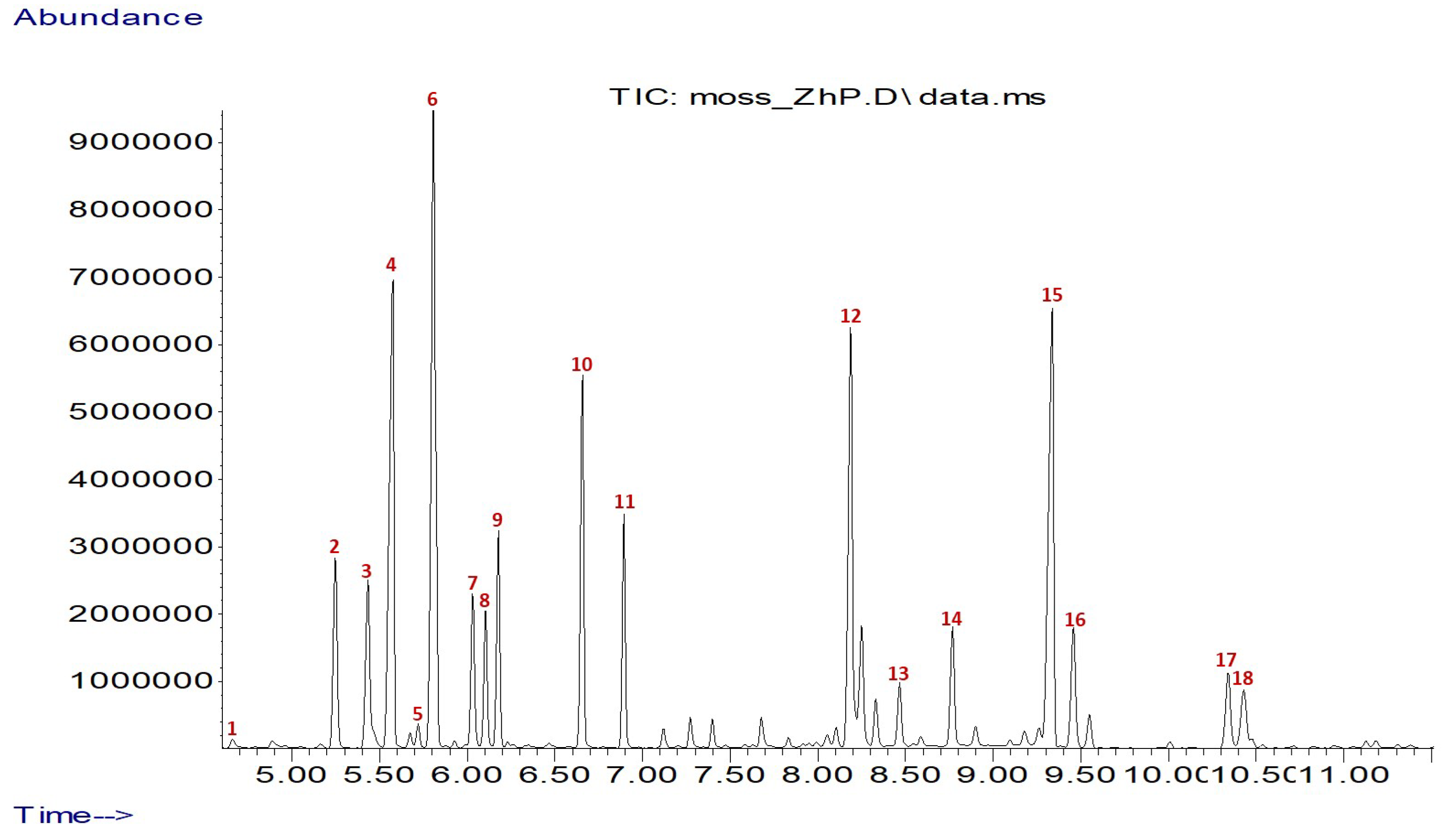
| No. | RT * (min) | Amino Acids | Mean ± SD |
|---|---|---|---|
| 1 | 4.66 | Alanine | 0.095 ± 0.03 |
| 2 | 5.25 | Valine 1 | 1.201 ± 0.31 |
| 3 | 5.43 | Leucine 1 | 1.463 ± 0.42 |
| 4 | 5.55 | Isoleucine 1 | 2.047 ± 0.73 |
| 5 | 5.71 | Glycine | 0.220 ± 0.06 |
| 6 | 5.82 | Proline | 2.282 ± 0.21 |
| 7 | 6.02 | Serine | 0.939 ± 0.24 |
| 8 | 6.09 | Threonine 1 | 1.015 ± 0.30 |
| 9 | 6.15 | Methionine 1 | 1.358 ± 0.41 |
| 10 | 6.66 | Aspartic acid | 1.132 ± 0.18 |
| 11 | 6.89 | Pyroglutamic acid | 0.738 ± 0.15 |
| 12 | 8.19 | Glutamic acid | 1.746 ± 0.12 |
| 13 | 8.47 | Phenylalanine 1 | 0.390 ± 0.09 |
| 14 | 8.78 | Arginine 3 | 0.354 ± 0.14 |
| 15 | 9.11 | Histidine 2 | 0.149 ± 0.03 |
| 16 | 9.38 | Lysine 1 | 1.175 ± 0.30 |
| 17 | 10.33 | Tryptophan 1 | 0.253 ± 0.07 |
| 18 | 10.46 | Tyrosine | 0.194 ± 0.04 |
| Total content of amino acids | 16.75 ± 3.83 | ||
| Amino Acids | Scoring Pattern, g/100 g * | Amino Acid Score |
|---|---|---|
| Histidine | 2.7 | 0.06 |
| Isoleucine | 4.7 | 0.44 |
| Leucine | 9.5 | 0.15 |
| Lysine | 7.8 | 0.15 |
| Methionine +cystine | 3.3 | 0.41 |
| Phenylalanine + Tyrosine | 10.2 | 0.06 |
| Threonine | 4.4 | 0.23 |
| Tryptophan | 1.4 | 0.18 |
| Valine | 6.4 | 0.19 |
| No. | RT (min) ** | Free Sugars | Mean ± SD |
|---|---|---|---|
| 1. | 7.550 | Fructose | 1.43 ± 0.1 |
| 2. | 8.145 | Mannose | 1.76 ± 0.1 |
| Total | 3.19 ± 0.2 | ||
| No. | RT (min) ** | Sterols | Mean ± SD |
|---|---|---|---|
| 1 | 13.702 | Cholesterol | 0.48 ± 0.02 |
| 2 | 14.508 | Brassicasterol | 0.05 ± 0.00 |
| 3 | 14.937 | Campesterol | 3.34 ± 0.08 |
| 4 | 15.352 | Stigmasterol | 4.37 ± 0.12 |
| 5 | 16.153 | β-Sitosterol | 4.29 ± 0.10 |
| 6 | 17.381 | ∆5-Avenasterol | 0.16 ± 0.03 |
| 7 | 17.482 | ∆7-Stigmasterol | 0.71 ± 0.04 |
| Total | 13.40 ± 0.39 | ||
| Components | Mean ± SD |
|---|---|
| Chl a | 237.37 ± 0.13 |
| Chl b | 117.39 ± 0.51 |
| Chl a + b | 354.77 ± 0.64 |
| Carotenoids | 91.53 ± 1.36 |
| Indicators | Mean ± SD | |
|---|---|---|
| Total phenols (mg GAE/g DW) | 0.98 ± 0.04 | |
| Content of quercetin, mg/g | 0.12 ± 0.01 | |
| Antioxidant activity | ||
| DPPH | I, % ** | 14.80 ± 0.08 |
| (mM TE/g DW) | 2.56 ± 0.02 | |
| ABTS | I, % | 33.28 ± 0.12 |
| (mM TE/g DW) | 4.15 ± 0.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova, Z.; Todorova, M.; Dincheva, I.; Ognyanov, M.; Naimov, S.; Apostolova, E.; Teneva, O.; Antova, G.; Gecheva, G. Phytochemical Composition, Antioxidant, Anti-Inflammatory Activity, and DNA Protective Capacity of Moss Hypnum cupressiforme Hedw. from Bulgaria. Forests 2025, 16, 951. https://doi.org/10.3390/f16060951
Petkova Z, Todorova M, Dincheva I, Ognyanov M, Naimov S, Apostolova E, Teneva O, Antova G, Gecheva G. Phytochemical Composition, Antioxidant, Anti-Inflammatory Activity, and DNA Protective Capacity of Moss Hypnum cupressiforme Hedw. from Bulgaria. Forests. 2025; 16(6):951. https://doi.org/10.3390/f16060951
Chicago/Turabian StylePetkova, Zhana, Mina Todorova, Ivayla Dincheva, Manol Ognyanov, Samir Naimov, Elena Apostolova, Olga Teneva, Ginka Antova, and Gana Gecheva. 2025. "Phytochemical Composition, Antioxidant, Anti-Inflammatory Activity, and DNA Protective Capacity of Moss Hypnum cupressiforme Hedw. from Bulgaria" Forests 16, no. 6: 951. https://doi.org/10.3390/f16060951
APA StylePetkova, Z., Todorova, M., Dincheva, I., Ognyanov, M., Naimov, S., Apostolova, E., Teneva, O., Antova, G., & Gecheva, G. (2025). Phytochemical Composition, Antioxidant, Anti-Inflammatory Activity, and DNA Protective Capacity of Moss Hypnum cupressiforme Hedw. from Bulgaria. Forests, 16(6), 951. https://doi.org/10.3390/f16060951








