Abstract
Climate change poses a significant threat to the persistence of rear-edge populations, which are located at the margins of a species’ distribution range and are particularly vulnerable to environmental shifts. This study focuses on Yew (Taxus baccata L.) in the Iberian Peninsula, representing the southernmost extent of its range, where warming temperatures and decreasing moisture may compromise its survival. Our research aims to assess the climate sensitivity and habitat variability of Yew, addressing the hypothesis that future climate scenarios will significantly reduce the species’ climatic suitability, particularly in southern and low-altitude regions, and that this reduction will negatively impact individual growth performance. We used species distribution models (SDMs) based on ecological niche modeling (ENM) to project the current and future distribution of suitable habitats for Yew under two climate scenarios (SSP126 and SSP585). The models were calibrated using bioclimatic variables, and the resulting suitability maps were integrated with field data on individual growth performance, measured as basal area increment over the last five years (BAI5). The ensemble model showed high predictive performance, highlighting precipitation seasonality and annual mean temperature as the most influential variables explaining the climatic suitability distribution in the Iberian Peninsula. Our results indicate a substantial reduction in suitable habitats for Yew, especially under the high-emission scenario (SSP585), with southern populations experiencing the greatest losses. Furthermore, individual growth was positively correlated with climatic suitability, confirming that populations in favorable habitats exhibit better performance. These findings highlight the vulnerability of rear-edge populations of Yew to climate change and underscore the need for targeted conservation strategies, including the identification of climatic refugia and the potential use of assisted migration.
1. Introduction
As research on climate change progresses, the effects of climatic variations on the distribution of plant species are becoming increasingly evident [1]. Paleobotanical and genetic studies consistently demonstrate how species distributions respond to these changes, often accompanied by shifts in physiological and genetic traits [2,3]. Forest ecosystems are particularly vulnerable, as alterations in temperature and precipitation directly affect the growth, distribution, and composition of the species that compose them [4].
Species facing changing climatic conditions can respond in three ways: migrate, adapt to the new conditions, or face extinction [2]. Understanding which of these strategies a species is likely to follow requires an analysis of the bioclimatic variables that define its habitat, identifying its ecological centroid—the set of optimal environmental conditions for its survival. Populations further from this centroid are generally more exposed to adverse conditions and, consequently, are more vulnerable to climate change [5,6,7]. Such peripheral populations often inhabit environments with suboptimal conditions, making them particularly sensitive to climatic shifts, which may transform these habitats into increasingly inhospitable areas [8]. Such peripheral populations often inhabit environments with suboptimal conditions, making them particularly sensitive to climatic shifts, which may transform these habitats into increasingly inhospitable areas [5,7,9].
Rear-edge populations, those situated at the southern or lower latitudinal limits of a species’ range, are of particular interest because they often harbor unique genetic diversity and ecological adaptations [5,7,10]. These populations, including those of Yew (Taxus baccata L.), in the Iberian Peninsula, are more exposed to climatic stress and are therefore valuable for understanding species’ responses to environmental change. Yet, despite their ecological importance, the impacts of climate change on rear-edge populations of Yew remain understudied, especially in the Iberian Peninsula, where warming temperatures and decreasing moisture pose serious threats [5].
In the context of this study, Yew was selected as a model species for assessing climate vulnerability in rear-edge populations. This species is native to Europe and is the only representative of the Taxaceae family on the continent. Although the International Union for Conservation of Nature (IUCN) classifies it as “Least Concern” (LC) [11], Yew appears on several regional threatened species lists, including the Spanish Red List [12]. Historically, Yew populations have been subjected to anthropogenic pressures, particularly the exploitation of its valuable wood [13], and more recently, climate change has exacerbated population declines, especially in southern regions [14], due to its slow growth rate, limited dispersal capacity, and preference for moist microhabitats [15,16]. The ecological centroid of Yew is located in Central Europe and the British Isles, where it forms dense, pure stands. However, at the ecological margins of its distribution, such as in the Iberian Peninsula, populations are reduced to small, isolated groups or even solitary individuals, often restricted to moist microhabitats, north-facing slopes, or under the canopy of other tree species [17]. These rear-edge populations represent the southernmost extent of the species’ range, making them particularly vulnerable to warming temperatures and reduced precipitation.
To assess the climate vulnerability of Yew in the Iberian Peninsula, we employed the Ecological Niche Modeling (ENM) framework [18]. This approach is grounded in the concept of the ecological niche as defined by Hutchinson [5,19], where the species’ distribution is determined by a set of environmental conditions that ensure its survival and reproduction. We used bioclimatic variables to model the current and future habitat suitability for Yew, identifying areas of potential habitat loss, stability, or gain under two climate scenarios (SSP126 and SSP585) [18]. These models provide spatially explicit projections of climatic suitability, allowing us to identify critical areas where the species is most vulnerable [20]. Furthermore, we integrated these suitability models with data on individual growth performance, providing a direct measure of how climatic conditions influence the demographic dynamics of Yew.
By combining species distribution modeling with demographic performance data, our study offers a spatially explicit assessment of climate vulnerability, identifying potential climatic refugia, areas at risk of habitat loss, and the factors driving population performance. This integrated approach not only enhances our understanding of the ecological processes shaping the distribution of Yew but also provides practical insights for conservation planning, including the identification of priority areas for protection and the potential application of assisted migration strategies [20,21,22].
We hypothesize that rear-edge populations of Yew in the Iberian Peninsula will exhibit a decline in suitable habitats under future climate scenarios (SSP126 and SSP585), particularly in low-altitude and southern regions, where climatic conditions will become increasingly unfavorable. Furthermore, we expect that individual growth performance will be positively associated with climatic suitability, indicating that populations located in more suitable habitats will show higher growth rates.
2. Materials and Methods
2.1. Species and Study Area
The species studied in this work, Yew, a native tree of temperate and Mediterranean European forests, exhibits slow growth and great longevity, reaching heights between 10 and 20 m [23]. Its chorology is established from North Africa to Scandinavia, and from the Iberian Peninsula to the Caspian Sea [3]. Its historical distribution is mainly confined to Great Britain and Northern Europe, where its ecological centroid is located [2,24].
In the Iberian Peninsula (Figure 1), this species inhabits a wide range of environmental conditions. In the south, where the climate is warmer and drier, its populations are small (20–150 trees) and are found in shaded and moist areas, with limited regeneration due to the scarce availability of water, posing a high risk of extinction. In contrast, in the north, it can form larger stands (1000–3000 individuals), growing in mixed forests alongside beeches (Fagus sylvatica L.), various species of oaks (Quercus spp.), and pines (Pinus spp.) [25].
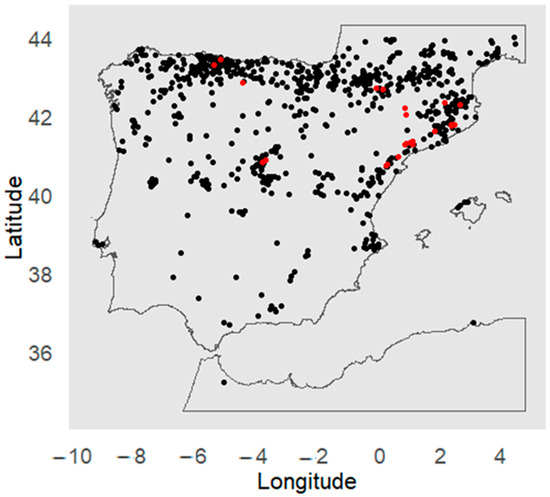
Figure 1.
Presence records (black) of Yew (Taxus baccata L.) used to model the distribution of its suitable habitats in the Iberian Peninsula. Red indicates the locations with records of individual growth (BAI5).
2.2. Distribution and Climatic Data
The GBIF (Global Biodiversity Information Facility) database was used to obtain presence records of Yew in the Iberian Peninsula (https://doi.org/10.15468/dl.5abefn, accessed on 25 April 2025). The dataset was manually cleaned to include only high-quality records for the analysis that met the following conditions: (i) georeferenced; (ii) with a recording year; (iii) coordinates within terrestrial ecosystems; (iv) and within the known native range of the species in the study area (Figure 1). A grid resolution of 5 × 5 km was used to eliminate duplicate records (i.e., only one occurrence record per 5 × 5 km grid), thus reducing spatial clustering (spatial bias) [19]. Of the total processed data, a subset was reserved as independent test data to evaluate the final model (5% of the presence). The remaining 95% of the presence data were used for calibration and internal validation processes of the candidate models (see section on climate suitability modeling).
Climatic variables to model the distribution of suitable climatic habitats for Yew were obtained from the CHELSA climate world database version 2.1 [26], with a 1 × 1 km resolution. The layers represent the annual climatic average for a time range between 1979 and 2019. Four bioclimatic variables were selected to represent a wide variety of seasonal and annual climatic patterns in the study area, minimizing redundancy (|r| ≤ 0.69; Table 1 and Figure S1). These variables were chosen for their close relationship with key ecological processes in plant species, such as distribution, reproduction, and phenology [27]. All spatial data processing was carried out using ArcGIS 10.3.

Table 1.
Estimate of the relative contribution of climatic variables in models predicting climatic habitat suitability for Taxus baccata. The values represent the percent contribution importance of each variable in the models. Values are the average and standard deviation over 10 replicate runs. bio01, annual mean temperature. Bio04, temperature seasonality. Bio12, annual precipitation. Bio15, precipitation seasonality. (Symbols in parentheses show the trend of the response curves for the variables: + increase, − decrease, Ω hump-shaped, = no trend.
For future climate (2081–2100), we used an ensemble method with two global climate models (GCMs) to address climate uncertainty. The selected models were the Community Climate System Model (CCSM4) [28] and the Hadley Global Environment Model (HadGEM2-ES) [29], both widely used to assess the effects of climate change on species distribution [30]. For future projections, we considered the Shared Socioeconomic Pathways (SSPs) defined in 2021 by the Sixth Assessment Report of the IPCC. These scenarios are divided into four projections, from which the most conservative (SSP126) and the most extreme (SSP585) were chosen. Future climatic data were obtained from the WorldClim dataset with a 5 × 5 km resolution.
2.3. Individual Growth Data of Natural Populations of Taxus baccata
To validate the results of the ENMs, we used individual growth data of Yew in field conditions, published in Sánchez-Martinez et al. (2021) [23], as an indicator of demographic performance (Figure 1). In summary, radial growth was estimated from increment cores extracted with a 5 mm borer at breast height from 235 trees distributed across 25 natural populations. The radial increment data were transformed into basal area increments accumulated over the last five years (BAI5, in cm2), allowing for a more accurate comparison of individual performance in relation to environmental conditions. This five-year window provides a balance between capturing recent growth trends while minimizing the influence of short-term fluctuations.
2.4. Ecological Niche and Climate Vulnerability Analysis
2.4.1. Climate Suitability Modeling
In this study, ENMs were used to evaluate the relationship between the geographic distribution of Yew and climatic variables, and to obtain measures of climate suitability throughout its distribution in the Iberian Peninsula. The assumption behind ENMs is that the distribution of species in geographic space (occurrence records) represents the range of environmental conditions under which species are able to reproduce and survive [21]. The model results can be interpreted as an index of the climatic suitability experienced by populations at a given site [20] and can be used to assess the relationship between habitat conditions and demographic processes related to the individual performance of natural populations of the species (e.g., growth).
To model the distribution of climatically suitable habitats, we used the Ensemble Modeling (EM) approach, designed to reduce biases and limitations inherent in the use of individual niche modeling techniques [31]. We used the BIOMOD2 [18] package in R [32] to fit four modeling methods: Generalized Linear Models (GLMs), Generalized Boosting Models (GBMs), Maximum Entropy (MaxEnt), and Random Forest (RF), which have demonstrated good performance in previous studies [33].
The models were calibrated with 642 presence records (95% of the total data) of the species and 10,000 pseudo-absences randomly generated, restricted to a minimum convex polygon produced by the full set of sampling points of the species. To evaluate the model’s performance, the ROC metric of the area under the curve (AUC) was used. This metric evaluates the model’s accuracy in correctly classifying presence and background. Values ranging from 0 to 0.5 indicate that the model performs no better than a null model, from 0.6 to 0.8 it classifies correctly, and from 0.9 to 1, the model is perfect. For each model, the AUC was calculated using a 15-partition validation sample, with 80% of the data used for training and 20% for testing.
The ensemble model was generated by weighting the projections of individual models with AUC >0.70. The EM evaluation was carried out using independent data (5% of the records were separated as independent data). The final result of the EM was converted into a binary map (0: unsuitable, 1: suitable), applying the Minimum Training Presence (MTP) threshold. Finally, the variable importance was calculated to assess the contribution of each predictor in the ensemble model, and response curves were generated to analyze the relationship between the species’ presence and each climatic variable.
To estimate the future climate suitability of Yew habitats, the ensemble model was projected onto future projections of climatic variables for the period (2081–2100) under different emission scenarios (SSP126 and SSP585). To distinguish suitable from unsuitable habitats, the model output was converted into binary maps using the MTP.
2.4.2. Using the Individual Performance of Natural Taxus baccata Populations to Validate the Output of the ENMs
To assess the quality of the predictions from the ensemble model and empirically analyze the effect of climate on the habitat suitability of Yew in the Iberian Peninsula, linear mixed-effects models (LMMs) were used with the function lmer from the lme4 package [34] in R version 4.3.2. The individual performance in terms of forest growth (BAI5) was considered as the response variable, while the climatic suitability of the habitat was included as a fixed explanatory variable. To account for structural and environmental variability between sites, the identity of the populations was included as a random factor, thus allowing the modeling of possible spatial differences not directly explained by climatic suitability. This approach enables the evaluation of the effect of climatic suitability on individual growth while considering spatial heterogeneity, thereby improving the inference about the ecological relationship between both variables. Finally, both BAI5 and climatic suitability were log-transformed.
2.5. Climate Vulnerability Analysis
The potential evolution of suitable habitats for Yew in the future in the Iberian Peninsula was evaluated. A range shift analysis was conducted using the BIOMOD2 package [18]. The process consists of comparing the binary climatic suitability maps of the present and future. Three range change metrics were then calculated: (i) loss = the value obtained from the suitable grid cells in the present model for the species that will no longer be suitable in the future; (ii) stability = the value obtained from the suitable grid cells in the present model that will remain suitable in the future; and (iii) gain = the value obtained from the grid cells in the present model that are not suitable now but will be suitable in the future.
To enhance figure clarity and visual consistency, including the Graphical Abstract (GA), image elements were edited using ChatGPT-4o (OpenAI, 2024, San Francisco, CA, USA), which assisted in adjusting layout and graphical components. All generated content was subsequently reviewed and manually refined by the authors.
3. Results
3.1. Climatic Suitability Analysis of Taxus baccata Habitat in the Iberian Peninsula
The average AUC values ranged between 0.82 ± 0.01 and 0.83 ± 0.01. These values indicated that the predictions of the individual models exhibited good discriminatory power compared to the expected value (0.5) of a random prediction. Despite the initial good performance of the models, the ensemble model outperformed these predictions with an AUC value of 0.97. The consensus threshold value used to produce the binary presence/absence map was 0.28. Among the four predictors considered for model execution, precipitation seasonality (bio15) and annual precipitation (bio12) consistently showed the highest contributions, followed by temperature seasonality (bio01) and lastly, the mean annual temperature (bio04) (Table 1).
Both precipitation seasonality and temperature seasonality (bio15 and bio04) were negatively associated with climatically suitable habitats for Yew in the study area. Thus, habitat preferences were located in regions with less variable climatic conditions throughout the year. Regarding annual precipitation (bio12), the data followed a typical Gaussian distribution (Table 1), with an optimal range between 500 and 1200 mm. In the case of annual temperature (bio01), it was negatively related to habitat suitability in the Iberian Peninsula; that is, suitable habitats for the analyzed species were located in areas with low annual temperatures.
The climatic suitability map for Yew in the Iberian Peninsula projected using the ensemble model (Figure 2) showed a spatial distribution of potentially favorable habitats for the species in the northern third of the peninsula, particularly in the Cantabrian Mountains and the Pyrenees, where humid conditions and stable, moderate temperatures prevail throughout the year. Additionally, areas of moderate suitability were identified in mountainous systems of the central and northeastern regions, where the topographic features of the terrain create microclimatic conditions favorable for the species. In contrast, climatic suitability decreased significantly toward the south of the Iberian Peninsula, where the climate is drier and warmer, likely limiting the species’ distribution. In the coastal areas of the eastern and southwestern regions, the low suitability also suggested that high temperatures and reduced water availability restricted the establishment of this tree. Only a few isolated areas in southern mountain systems, such as the Sierra Nevada, showed moderate suitability values.
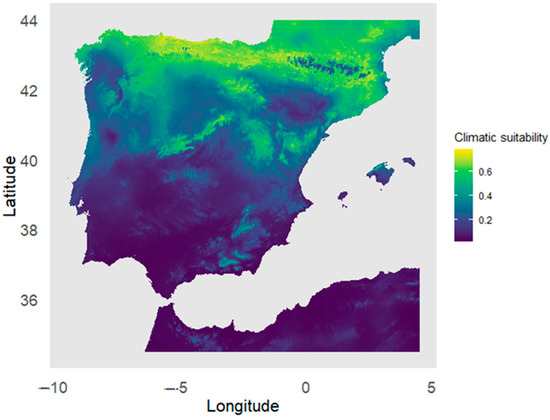
Figure 2.
Ensemble model projection of the potential distribution of climatic habitat suitability for Taxus baccata in the Iberian Peninsula.
3.2. Predicted Climatic Habitat Suitability and Individual Performance of Natural Populations of Taxus baccata
The LMM model showed a positive and significant relationship between climatic habitat suitability and individual growth of Yew, measured as basal increment over the past five years (Figure 3). The estimated coefficient for climatic suitability was 1.25 ± 1.35, with a p-value of 0.03, indicating that individuals located in habitats with higher climatic suitability tend to exhibit higher growth rates. These results support the usefulness of ENMs in predicting patterns of population performance in forest species, suggesting that climatic suitability not only determines the presence distribution of Yew, but also influences its growth and population dynamics.
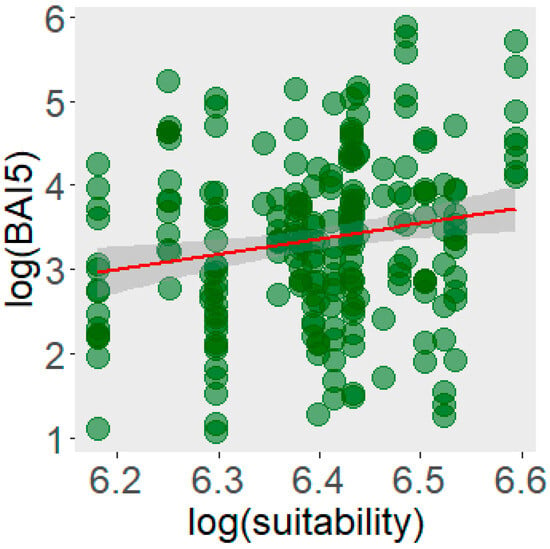
Figure 3.
Relationship between basal increment over the last five years and the climatic habitats suitable for Taxus baccata in the Iberian Peninsula.
3.3. Climatic Vulnerability Analysis of Taxus baccata in the Iberian Peninsula
The climate projections of the ensemble model under different future climate scenarios (Figure 4) reveal a significant loss of climatically suitable habitats for the species in the Iberian Peninsula (shown in red). Habitat loss is predicted to reach 43.1% under the ssp126 scenario and 68.4% under ssp585. Additionally, the percentage of habitat gain (shown in green) was 1.74% and 1.97 for ssp126 and ssp585, respectively, while stable habitats are projected to be 56.9% and 31.5% for both scenarios, respectively.
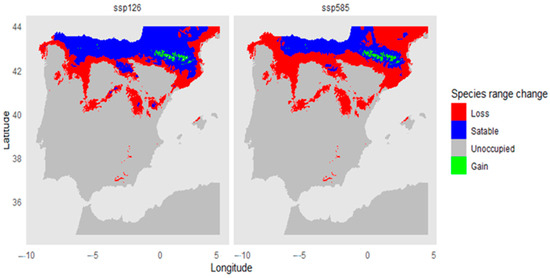
Figure 4.
Climatic vulnerability analysis of suitable Taxus baccata habitats for the 2060–2080 period under two climate change scenarios: ssp126 and ssp585.
Changes in the distribution of suitable habitat for Yew for the 2060–2080 period under both scenarios show a general trend of decreasing climatically adequate areas within the study region (Figure 4). A significant loss of suitable habitat (in red) is observed across large parts of northern Iberia, including the Cantabrian Mountains and the Pyrenees. This reduction is more pronounced under ssp585, indicating a more severe negative impact associated with higher emissions and more intense climate change.
Habitat stability areas (in blue) are mainly located in the northernmost part of the peninsula, including parts of the Pyrenees and some regions along the Cantabrian coast. However, the extent of these stable areas is smaller under ssp585 compared to ssp126, suggesting that climate scenarios with lower warming favor the persistence of the species in its current habitats. On the other hand, habitat gain (in green) is very limited in both scenarios, indicating that the species does not find new areas with favorable climatic conditions to offset the habitat losses within its current range.
These results point to a substantial reduction in suitable habitat for this tree species in the future, with greater impacts under high-emission scenarios. This underscores its vulnerability to climate change and highlights the importance of conservation strategies focused on areas identified as climatic refugia.
4. Discussion
This study develops a spatially explicit model to explore regional trends in the climatic habitat suitability of Yew in the Iberian Peninsula. The robustness of the modeling approach presented here is supported by the availability of high-quality occurrence records for the species within the study area, a suitable set of climatic variables to characterize the climatic species’ niche, and a strong ENM approach based on an ensemble of the most widely used modeling techniques [33]. It is important to acknowledge that this approach, by focusing on the species’ climatic niche, shares some common limitations of ENM models [35]. Non-climatic factors, such as biotic interactions, dispersal limitations, and land-use changes, can also significantly impact the distribution and regeneration of Yew populations [36,37,38]. For instance, herbivory by ungulates, competition with other tree species, and alterations in land use can modulate habitat quality and directly affect population dynamics [17,39]. Acknowledging these additional drivers provides a more comprehensive understanding of the species’ vulnerability. Nevertheless, we consider that this approach provides a useful initial assessment of the spatial vulnerability of Yew habitats to climate change, with the potential to guide conservation strategies in the most threatened areas.
4.1. Climatic Habitat Suitability and Regional Future Trends
The results of our modeling approach were satisfactory according to the evaluation metrics, allowing us to infer current and future patterns of climatic distribution on a solid basis [40]. As expected, in the southernmost regions of the species’ range (i.e., the Iberian Peninsula), areas with the highest values of climatic suitability were located in humid mountain zones. These patterns are consistent with those reported by Sanz et al. (2009) [39].
The analysis of the importance of climatic predictors revealed that the distribution patterns of the studied species appear to be limited by changes in precipitation across the study area. Despite being a species with a relatively broad tolerance range—between 500 and 1200 mm, according to our model—it is quite sensitive to drought [17]. Additionally, mean annual and seasonal temperatures seem to play a key role in its distribution patterns. The model suggests that high temperatures and temperature variability are limiting factors for the development of this tree in the southernmost regions, highlighting a clear vulnerability at the climatic extremes of peripheral populations within the analyzed area. Similar patterns have been reported in other rear-edge populations of Yew, such as those in the Hyrcanian forests of northern Iran, where increasing temperatures and decreasing moisture availability have been shown to constrain regeneration and threaten population persistence [41]. This reinforces the idea that marginal populations are particularly sensitive to climate warming across the species’ range.
Regarding regional trends in future habitat suitability, our results indicated a significant reduction in climatically suitable habitats in the Iberian Peninsula under both climate change scenarios for the 2060–2080 period. Overall, there is a clear trend toward habitat loss in currently favorable areas, especially in the mountainous regions of northern and northeastern Iberia. This trend is more pronounced under the high-emission scenario (SSP585), suggesting that a more drastic increase in temperature and changes in precipitation regimes could intensify the contraction of suitable areas for the species. Under the SSP126 scenario, which represents a future with effective climate change mitigation, the spatial patterns of habitat stability showed that certain areas in the north of the peninsula may remain suitable for the species, albeit with noticeable fragmentation, as previously observed in some mountainous regions of Spain [42]. Nonetheless, habitat loss remains dominant compared to potential gains. This result suggests that even under mitigation strategies, Yew could face constraints in its future distribution due to its sensitivity to climatic conditions [17]. Under the SSP585 scenario, the model projected a more severe reduction in suitable habitat, with greater losses in the Cantabrian and Pyrenean regions, where important populations of the species currently exist [43]. Moreover, the emergence of new climatically suitable areas was virtually nonexistent, indicating that future conditions will not only reduce current habitats but also fail to facilitate the colonization of new areas. This lack of expansion suggests that conservation strategies should focus on preserving relict populations and implementing adaptive management measures to mitigate the negative impacts of climate change [43]. These results are consistent with previous studies documenting the vulnerability of forest species to rising temperatures and declining moisture availability [44,45]. The projected habitat contraction reinforces the need to consider management actions aimed at conserving populations in climatic refugia, as well as restoration strategies in areas that may still retain favorable conditions in the future [46].
4.2. Relationship Between Individual Growth and Habitat Suitability, and Regional Trends Under Future Scenarios
The results of this study showed a positive and significant relationship between individual growth of Yew and the climatic suitability of its habitat, suggesting that individuals located in areas with more favorable climatic conditions exhibit higher growth rates. This finding supports the hypothesis that ENMs not only predict the spatial distribution of a species but can also reflect environmental conditions that influence its demographic performance [36]. This pattern is consistent with previous studies showing that Yew growth and regeneration rates are strongly limited by abiotic factors, particularly water availability [17,39].
Regarding future habitat trends in the Iberian Peninsula, our model predicts a significant reduction in currently favorable areas, especially in low-altitude regions and southern latitudes. This pattern suggests that Yew populations in these areas may experience increased climatic stress, potentially resulting in reduced individual growth and population declines, as has already been observed in other areas under suboptimal environmental conditions [47]. On the other hand, the models project some degree of stability and even slight expansion of suitable habitat in northern regions and at higher elevations, particularly in northern Iberia. This may indicate an altitudinal and latitudinal shift in populations in response to global warming—a phenomenon widely documented in climate-sensitive species [48,49]. However, the potential recolonization of cooler habitats will depend on the species’ dispersal capacity and the presence of suitable refugia in the areas projected to become favorable. Additionally, the interaction between climate change and other ecological factors could further exacerbate negative effects on Yew [50,51]. For instance, habitat fragmentation and reduced gene flow may limit the species’ adaptive capacity, increasing its vulnerability in the future [24,52]. Likewise, competition with more drought-tolerant species and the impact of disturbances such as overlogging and wildfires may accelerate the decline of populations at the southern limits of its distribution [3,14,15,23]. These results highlight the importance of integrating individual growth data with ENMs to evaluate not only the potential distribution of the species but also its long-term viability.
4.3. Management Implications
Our study highlights the important role of climatic variability, particularly drought, in shaping the distribution patterns of a relict species in the southernmost (peripheral) parts of its range [6]. Given the ecological and conservation value of the studied species, these findings may provide useful guidance for prioritizing management actions, especially in the current context of rapid climate change [53]. Our ENM suggests that the predicted reduction in climatic suitability may negatively affect the growth and regeneration of existing populations in the future. In this context, conservation strategies should prioritize the protection of climatic refugia in higher-altitude and northern regions of the Iberian Peninsula, where future conditions may remain favorable—aligning with findings from other regions. For example, in the Hyrcanian mountain forests, Yew is expected to experience an upward elevational shift in response to warming, highlighting the need to rethink current conservation and restoration strategies under future climate scenarios [54].
Given the species’ limited potential for natural expansion into new areas [50], we recommend implementing active management measures such as restoring degraded habitats, reducing competition with more drought-tolerant species [55], and mitigating stress factors such as overgrazing [53]. Additionally, assisted forest migration could be considered for highly vulnerable populations, promoting their establishment in areas projected to offer suitable climatic conditions in the long term [56]. However, the successful implementation of this strategy is not without challenges. Given the species’ slow growth rate and specific habitat requirements, several practical difficulties must be considered. For instance, firstly, the inherently slow growth rate of Yew can hinder the rapid establishment of translocated populations, rendering them susceptible to environmental stressors during the prolonged juvenile phase. Secondly, the successful regeneration of Yew often relies on the presence of nurse plants [57]. The absence of such facilitative vegetation can significantly impede seedling survival and establishment. Thirdly, there exists a considerable risk of maladaptation when introducing populations to novel environments. Translocated individuals may encounter soil conditions, biotic interactions, or disturbance regimes to which they are not locally adapted, potentially leading to reduced growth performance and survival [58]. Moreover, the ecological ramifications of introducing Yew beyond its historical range warrant careful consideration, as such actions could disrupt existing plant communities and alter ecosystem dynamics [22]. Despite these challenges, with meticulous planning and ecological foresight, assisted migration could serve as a valuable tool for the long-term conservation of Yew. Prioritizing areas identified by our models as having future climatic suitability, coupled with habitat restoration and the preservation of existing refugia, may enhance the resilience of yew populations in the face of climate change.
5. Conclusions
This study demonstrates that Taxus baccata faces high vulnerability to climate change in the Iberian Peninsula, particularly within rear-edge populations at the southern margin of its distribution range. Ecological niche models predict a significant loss of suitable habitat under future climate scenarios, with reductions exceeding 47% under the conservative SSP126 scenario and over 69% under the high-emission SSP585 scenario, with no substantial gains in newly suitable areas. These losses are concentrated in lowland and arid regions, where population viability is already constrained. The positive correlation between climatic suitability and individual growth (BAI5) validates the modeling approach, linking ecological projections with actual field-based demographic performance.
Although the study focuses primarily on climatic factors, we acknowledge that non-climatic variables such as biotic interactions (e.g., competition and herbivory) and land-use changes play a significant role in shaping species distribution, regeneration, and long-term persistence. These factors can modulate habitat quality and influence the effectiveness of conservation strategies. While assisted migration may represent a potential response to habitat loss, it also entails considerable risks, especially for a slow-growing species like T. baccata. Major challenges include low establishment success, limited seedling survival in the absence of nurse plants, the risk of maladaptation to new edaphic or biotic conditions, and potential ecological impacts on recipient communities. These limitations must be carefully weighed before adopting translocation strategies.
The results of this study provide a robust scientific foundation for guiding conservation policy and management. Integrating local environmental policies with European instruments such as the LIFE Programme of the European Union, which supports biodiversity and climate adaptation actions, could enhance efforts to protect climatic refugia, restore priority habitats, and implement adaptive management for relict species. Reinforcing the role of regional Red Lists and endangered species catalogs is also essential, as they help identify conservation priorities and justify urgent interventions. Future research should move toward a comprehensive approach that combines climate modeling with local ecological factors, population genetics, and socio-environmental data. Specifically, we recommend evaluating the functional connectivity between remnant habitats, assessing genetic diversity and gene flow among isolated populations, and exploring the resilience of natural regeneration in relation to understory structure and disturbance regimes. Moreover, investigating the effects of land-use changes, habitat fragmentation, and interspecific interactions on population dynamics is key. The development of integrated models that simulate management scenarios under varying climate and policy contexts will support the design of more effective, adaptive, and sustainable long-term conservation strategies for Taxus baccata and other similarly vulnerable species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16060931/s1, Figure S1: Correlation matrix of the variables used to calibrate the Ecological Niche Models of Taxus baccata in the Iberian Peninsula. Available at Zenodo: https://doi.org/10.5281/zenodo.15500994 (accessed on 29 May 2025).
Author Contributions
Conceptualization, R.E.H.-L. and J.Á.S.-A.; methodology, R.E.H.-L.; software, R.E.H.-L. and J.F.C.R.; validation, R.E.H.-L., D.R.-d.l.C. and J.Á.S.-A.; formal analysis, J.F.C.R.; investigation, J.F.C.R.; data curation, R.E.H.-L. and J.F.C.R.; writing—original draft preparation, J.F.C.R., R.E.H.-L., D.R.-d.l.C. and J.Á.S.-A.; writing—review and editing, J.F.C.R., R.E.H.-L., D.R.-d.l.C. and J.Á.S.-A.; visualization, R.E.H.-L.; supervision, R.E.H.-L., D.R.-d.l.C. and J.Á.S.-A. All authors have read and agreed to the published version of the manuscript.
Funding
R.E.H.-L. was supported by the Ministerio de Ciencia, Innovación y Universidades de España through a Juan de la Cierva Grant (JDC2022–050186-I) of the Programa Estatal para Desarrollar, Atraer y Retener Talento.
Data Availability Statement
All datasets were downloaded from public sources and previously published articles. See Section 2.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT-4o for the purposes of image editing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abeli, T.; Ghitti, M.; Sacchi, R. Does Ecological Marginality Reflect Physiological Marginality in Plants? Plant Biosyst. 2020, 154, 149–157. [Google Scholar] [CrossRef]
- Koç, D.; Svenning, J.-C.; Avci, M. Climate Change Impacts on the Potential Distribution of Taxus baccata L. in the Eastern Mediterranean and the Bolkar Mountains (Turkey) from Last Glacial Maximum to the Future. Eurasian J. For. Sci. 2018, 6, 69–82. [Google Scholar] [CrossRef]
- Iszkuło, G. Success and Failure of Endangered Tree Species: Low Temperatures and Low Light Availability Affect Survival and Growth of European Yew (Taxus baccata L.) Seedlings. Pol. J. Ecol. 2010, 58, 259–271. [Google Scholar]
- Alavi, S.J.; Ahmadi, K.; Hosseini, S.M.; Tabari, M.; Nouri, Z. The Response of English Yew (Taxus baccata L.) to Climate Change in the Caspian Hyrcanian Mixed Forest Ecoregion. Reg. Env. Change 2019, 19, 1495–1506. [Google Scholar] [CrossRef]
- Hernández-Lambraño, R.E.; de la Cruz, D.R.; Agudo, J.Á.S. Effects of the Climate Change on Peripheral Populations of Hydrophytes: A Sensitivity Analysis for European Plant Species Based on Climate Preferences. Sustainability 2021, 13, 3147. [Google Scholar] [CrossRef]
- Vilà-Cabrera, A.; Premoli, A.C.; Jump, A.S. Refining Predictions of Population Decline at Species’ Rear Edges. Glob. Change Biol. 2019, 25, 1549–1560. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving Biodiversity under Climate Change: The Rear Edge Matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction Risk from Climate Change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Accelerating Extinction Risk from Climate Change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Stanton, J.C.; Shoemaker, K.T.; Pearson, R.G.; Akçakaya, H.R. Warning Times for Species Extinctions Due to Climate Change. Glob. Change Biol. 2015, 21, 1066–1077. [Google Scholar] [CrossRef]
- IUCN Red List. IUCN Red List. IUCN Red List of Threatened Species: Taxus baccata. In IUCN Red List of Threatened Species; IUCN Red List: Cambridge, UK, 2010. [Google Scholar]
- Decreto 63/2007, de 14 de Junio, por el Que se Crean el Catálogo de Flora Protegida de Castilla y León y la Figura de Protección Denominada Microrreserva de Flora. Available online: https://bocyl.jcyl.es/boletines/2007/06/20/pdf/BOCYL-D-20062007-3.pdf (accessed on 29 May 2025).
- Uzquiano, P.; Allué, E.; Antolín, F.; Burjachs, F.; Picornel, L.; Piqué, R.; Zapata, L. All about Yew: On the Trail of Taxus baccata in Southwest Europe by Means of Integrated Palaeobotanical and Archaeobotanical Studies. Veget. Hist. Archaeobot. 2015, 24, 229–247. [Google Scholar] [CrossRef]
- Thomas, P.; Garcia-Marti, X. Response of European Yews to Climate Change: A Review. For. Syst. 2015, 24, eR01. [Google Scholar] [CrossRef]
- Iszkuło, G.; Didukh, Y.; Giertych, M.J.; Jasińska, A.K.; Sobierajska, K.; Szmyt, J. Weak Competitive Ability May Explain Decline of Taxus baccata. Ann. For. Sci. 2012, 69, 705–712. [Google Scholar] [CrossRef]
- Song, Y.; Sass-Klaassen, U.; Sterck, F.; Goudzwaard, L.; Akhmetzyanov, L.; Poorter, L. Growth of 19 Conifer Species Is Highly Sensitive to Winter Warming, Spring Frost and Summer Drought. Ann. Bot. 2021, 128, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.C. Shifting Limiting Factors for Population Dynamics and Conservation Status of the Endangered English Yew (Taxus baccata L., Taxaceae). For. Ecol. Manag. 2013, 291, 119–127. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R. Biomod2: Ensemble Platform for Species Distribution Modelling; The R Foundation: Vienna, Austria, 2014; Volume 2. [Google Scholar]
- Hernández-Lambraño, R.E.; González-Moreno, P.; Sánchez-Agudo, J.Á.; Hernández-Lambraño, R.E.; González-Moreno, P.; Sánchez-Agudo, J.Á. Towards the Top: Niche Expansion of Taraxacum Officinale and Ulex Europaeus in Mountain Regions of South America. Austral Ecol. 2017, 42, 577–589. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010; ISBN 1-139-48529-6. [Google Scholar]
- Soberon, J.; Peterson, A.T. Interpretation of Models of Fundamental Ecological Niches and Species’ Distributional Areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Peterson, K.; Bode, M. Using Ensemble Modeling to Predict the Impacts of Assisted Migration on Recipient Ecosystems. Conserv. Biol. 2021, 35, 678–687. [Google Scholar] [CrossRef]
- Sanchez-Martinez, P.; Marcer, A.; Mayol, M.; Riba, M. Shaping the Niche of Taxus baccata, a Modelling Exercise Using Biologically Meaningful Information. For. Ecol. Manag. 2021, 501, 119688. [Google Scholar] [CrossRef]
- Maroso, F.; Vera, M.; Ferreiro, J.; Mayol, M.; Riba, M.; Ramil-Rego, P.; Martínez, P.; Bouza, C. Genetic Diversity and Structure of Taxus baccata from the Cantabrian-Atlantic Area in Northern Spain: A Guide for Conservation and Management Actions. For. Ecol. Manag. 2021, 482, 118844. [Google Scholar] [CrossRef]
- Casals, P.; Camprodon, J.; Caritat, A.; Rios, A.I.; Guixé, D.; Garcia-Marti, X.; Martín-Alcón, S.; Coll, L. Forest Structure of Mediterranean Yew (Taxus baccata L.) Populations and Neighbor Effects on Juvenile Yew Performance in the NE Iberian Peninsula. For. Syst. 2015, 24, e042. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Hernández-Lambraño, R.E.; Parra, J.L.; Román, J.F.C.; Sánchez-Agudo, J.Á. Less Suitable Climatic Conditions and Pests Increase Tree Defoliation in Spanish Iberian Peninsula Forests. For. Ecol. Manag. 2024, 566, 122048. [Google Scholar] [CrossRef]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.R.; Lawrence, D.M.; Neale, R.B.; Rasch, P.J.; Vertenstein, M.; et al. The Community Climate System Model Version 4. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef]
- The HadGEM2 Development Team; Martin, G.M.; Bellouin, N.; Collins, W.J.; Culverwell, I.D.; Halloran, P.R.; Hardiman, S.C.; Hinton, T.J.; Jones, C.D.; McDonald, R.E.; et al. The HadGEM2 Family of Met Office Unified Model Climate Configurations. Geosci. Model. Dev. 2011, 4, 723–757. [Google Scholar] [CrossRef]
- Albuquerque, F.; Benito, B.; Rodriguez, M.Á.M.; Gray, C. Potential Changes in the Distribution of Carnegiea Gigantea under Future Scenarios. PeerJ 2018, 6, e5623. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; New, M. Ensemble Forecasting of Species Distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Hernández-Lambraño, R.E.; Carbonell, R.; Sánchez-Agudo, J. Making the Most of Scarce Data: Mapping Distribution Range and Variation in Population Abundance of a Threatened Narrow-Range Endemic Plant. J. Nat. Conserv. 2020, 57, 125889. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Package Lme4: Linear Mixed-Effects Models Using Eigen and S4; CRAN: Vienna, Austria, 2014; Volume 67. [Google Scholar]
- Jiménez-Valverde, A.; Lobo, J.M.; Hortal, J. Not as Good as They Seem: The Importance of Concepts in Species Distribution Modelling. Divers. Distrib. 2008, 14, 885–890. [Google Scholar] [CrossRef]
- Osorio-Olvera, L.; Soberón, J.; Falconi, M. On Population Abundance and Niche Structure. Ecography 2019, 42, 1415–1425. [Google Scholar] [CrossRef]
- Oldfather, M.F.; Kling, M.M.; Sheth, S.N.; Emery, N.C.; Ackerly, D.D. Range Edges in Heterogeneous Landscapes: Integrating Geographic Scale and Climate Complexity into Range Dynamics. Glob. Change Biol. 2020, 26, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- García, D.; Ramón Obeso, J. Facilitation by Herbivore-Mediated Nurse Plants in a Threatened Tree, Taxus baccata: Local Effects and Landscape Level Consistency. Ecography 2003, 26, 739–750. [Google Scholar] [CrossRef]
- Sanz, R.; Pulido, F.; Nogués-Bravo, D. Predicting Mechanisms across Scales: Amplified Effects of Abiotic Constraints on the Recruitment of Yew Taxus baccata. Ecography 2009, 32, 993–1000. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking Receiver Operating Characteristic Analysis Applications in Ecological Niche Modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Ahmadi, K.; Alavi, S.J.; Amiri, G.Z.; Hosseini, S.M.; Serra-Diaz, J.M.; Svenning, J.-C. The Potential Impact of Future Climate on the Distribution of European Yew (Taxus baccata L.) in the Hyrcanian Forest Region (Iran). Int. J. Biometeorol. 2020, 64, 1451–1462. [Google Scholar] [CrossRef]
- Dubreuil, M.; Riba, M.; González-Martínez, S.C.; Vendramin, G.G.; Sebastiani, F.; Mayol, M. Genetic Effects of Chronic Habitat Fragmentation Revisited: Strong Genetic Structure in a Temperate Tree, Taxus baccata (Taxaceae), with Great Dispersal Capability. Am. J. Bot. 2010, 97, 303–310. [Google Scholar] [CrossRef]
- González-Martínez, S.C.; Dubreuil, M.; Riba, M.; Vendramin, G.G.; Sebastiani, F.; Mayol, M. Spatial Genetic Structure of Taxus baccata L. in the Western Mediterranean Basin: Past and Present Limits to Gene Movement over a Broad Geographic Scale. Mol. Phylogenet. Evol. 2010, 55, 805–815. [Google Scholar] [CrossRef]
- Allen, C.; Macalady, A.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global Field Observations of Tree Die-off Reveal Hotter-Drought Fingerprint for Earth’s Forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef]
- Selwood, K.E.; Zimmer, H.C. Refuges for Biodiversity Conservation: A Review of the Evidence. Biol. Conserv. 2020, 245, 108502. [Google Scholar] [CrossRef]
- Ahmadi, K.; Jalil Alavi, S.; Zahedi Amiri, G.; Mohsen Hosseini, S.; Serra-Diaz, J.M.; Svenning, J.-C. Patterns of Density and Structure of Natural Populations of Taxus baccata in the Hyrcanian Forests of Iran. Nord. J. Bot. 2020, 38. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; Périé, C.; de Blois, S. Shifting with Climate? Evidence for Recent Changes in Tree Species Distribution at High Latitudes. Ecosphere 2014, 5, art83. [Google Scholar] [CrossRef]
- Rew, L.J.; McDougall, K.L.; Alexander, J.M.; Daehler, C.C.; Essl, F.; Haider, S.; Kueffer, C.; Lenoir, J.; Milbau, A.; Nuñez, M.A.; et al. Moving up and over: Redistribution of Plants in Alpine, Arctic, and Antarctic Ecosystems under Global Change. Arct. Antarct. Alp. Res. 2020, 52, 651–665. [Google Scholar] [CrossRef]
- Lavabre, J.E.; García, D. Geographic Consistency in the Seed Dispersal Patterns of Taxus baccata L. in the Iberian Peninsula. For. Syst. 2015, 24, e040. [Google Scholar] [CrossRef]
- Chybicki, I.J.; Oleksa, A. Seed and Pollen Gene Dispersal in Taxus baccata, a Dioecious Conifer in the Face of Strong Population Fragmentation. Ann. Bot. 2018, 122, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Pautasso, M. Geographical Genetics and the Conservation of Forest Trees. Perspect. Plant Ecol. Evol. Syst. 2009, 11, 157–189. [Google Scholar] [CrossRef]
- Smith, W.P.; Zollner, P.A. Sustainable Management of Wildlife Habitat and Risk of Extinction. Biol. Conserv. 2005, 125, 287–295. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Ahmadi, K.; Heydari, M.; Karami, O.; Esmailzadeh, O.; Heung, B. Elevational Shift of Endangered European Yew under Climate Change in Hyrcanian Mountain Forests: Rethinking Conservation-Restoration Strategies and Management. For. Ecol. Manag. 2023, 529, 120693. [Google Scholar] [CrossRef]
- Piovesan, G.; Presutti Saba, E.; Biondi, F.; Alessandrini, A.; Di Filippo, A.; Schirone, B. Population Ecology of Yew (Taxus baccata L.) in the Central Apennines: Spatial Patterns and Their Relevance for Conservation Strategies. Plant Ecol. 2009, 205, 23–46. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Ivetic, V.; Dumroese, R.K. Framing Recent Advances in Assisted Migration of Trees: A Special Issue. For. Ecol. Manag. 2024, 551, 121552. [Google Scholar] [CrossRef]
- Calvia, G.; Casula, P.; Farris, E.; Fenu, G.; Fantini, S.; Bacchetta, G. Shrub Cover and Soil Moisture Affect Taxus baccata L. Regeneration at Its Southern Range. Plants 2023, 12, 1819. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Aravanopoulos, F.A.; Budde, K.B.; Hansen, O.K.; Rellstab, C.; Schroeder, H.; Curtu, A.L. Resilient Forests for the Future. Tree Genet. Genomes 2024, 20, 17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).