Summer Drought Delays Leaf Senescence and Shifts Radial Growth Towards the Autumn in Corylus Taxa
Abstract
1. Introduction
2. Materials and Methods
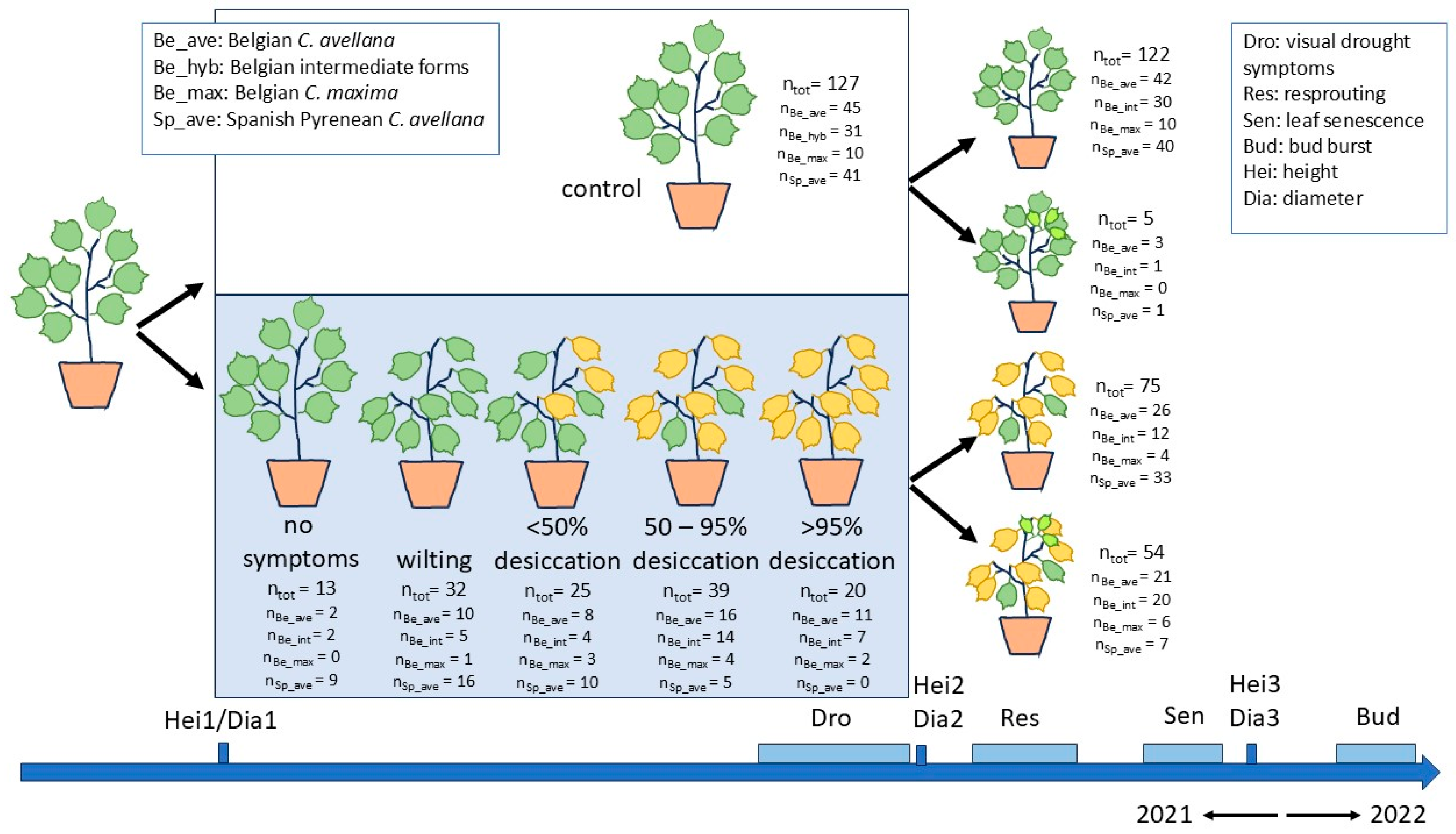
2.1. Plant Material
2.2. Drought Treatments
2.3. Observations and Measurements
2.4. Statistics
3. Results
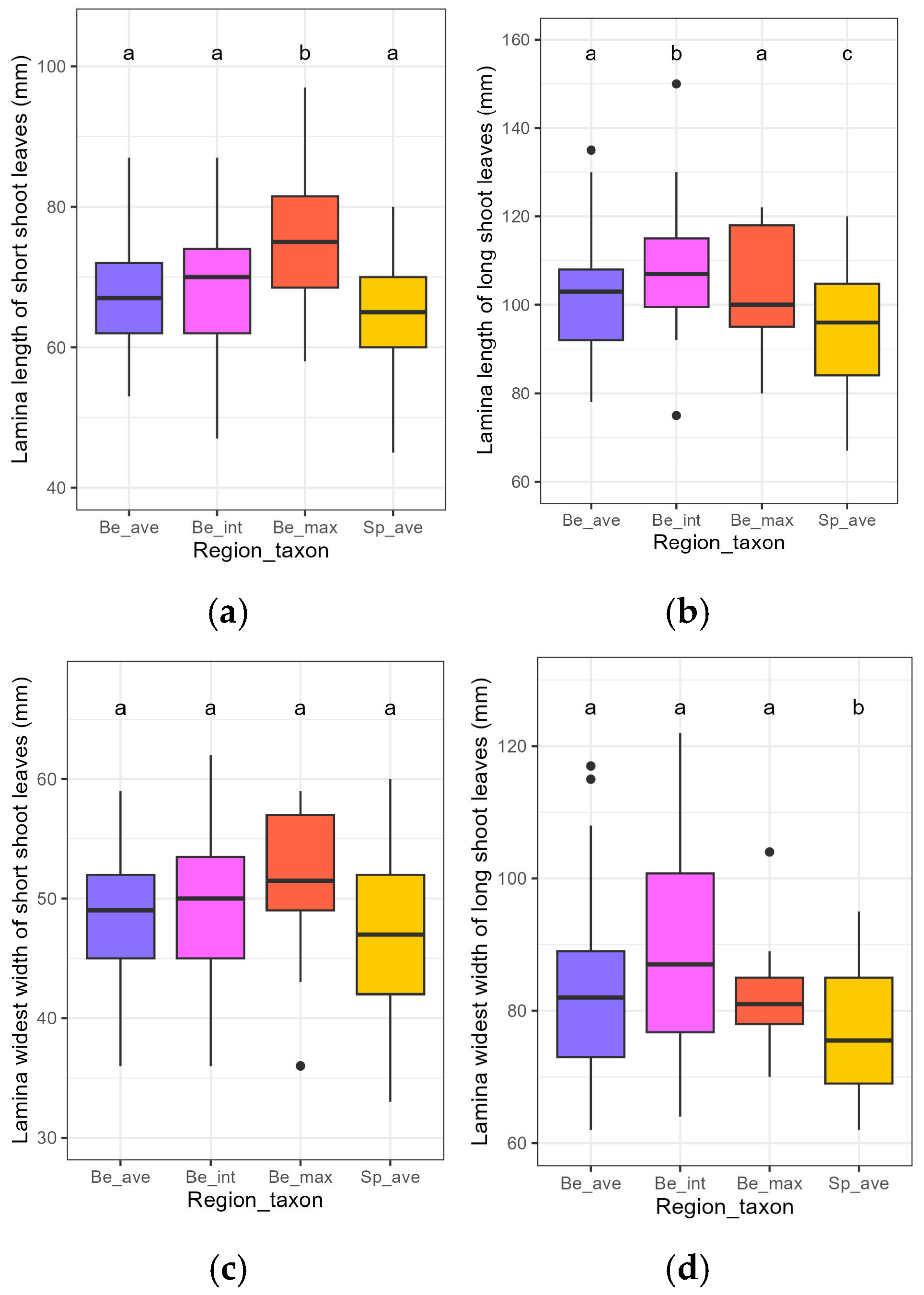
3.1. Initial Traits of the Plants
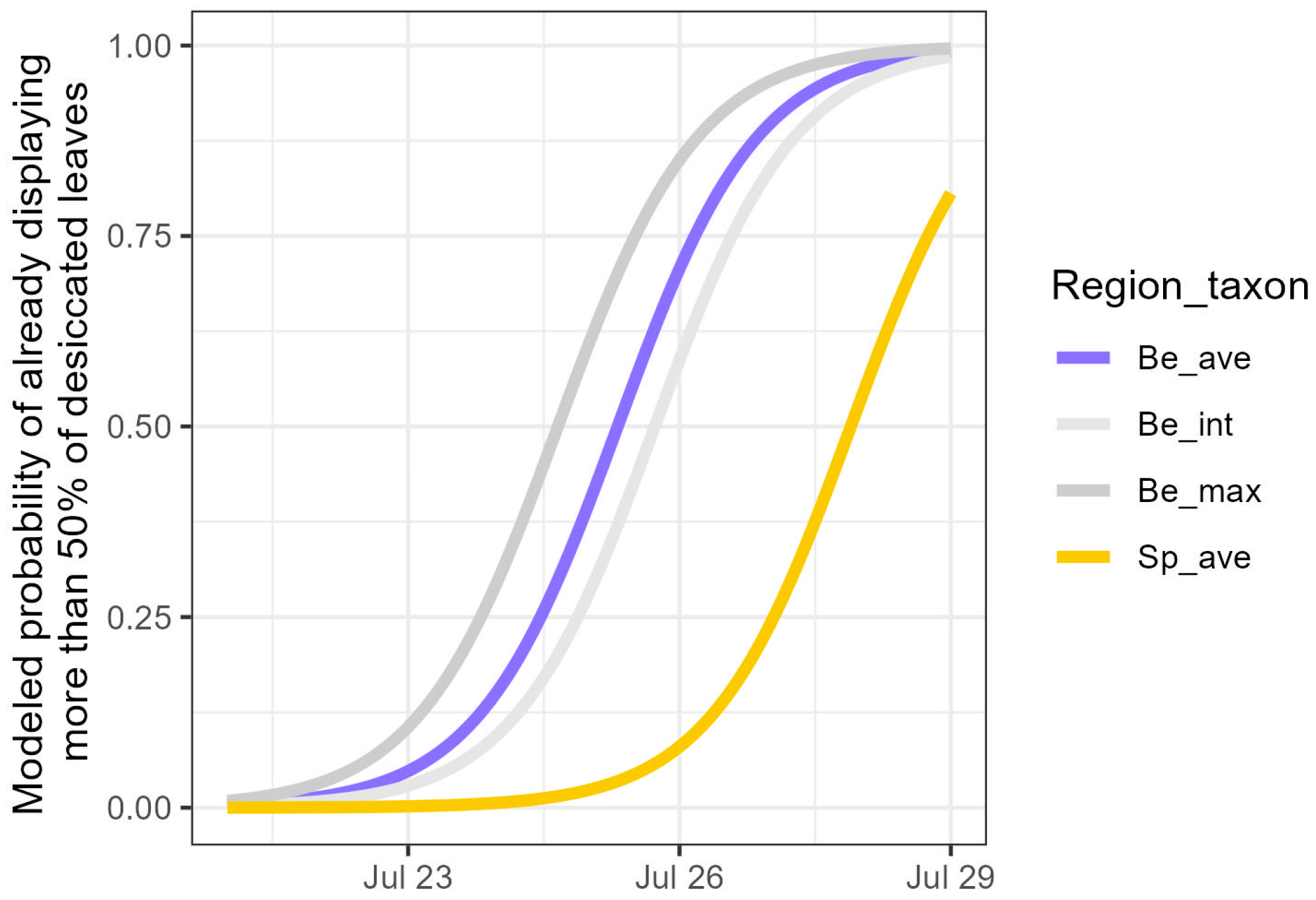
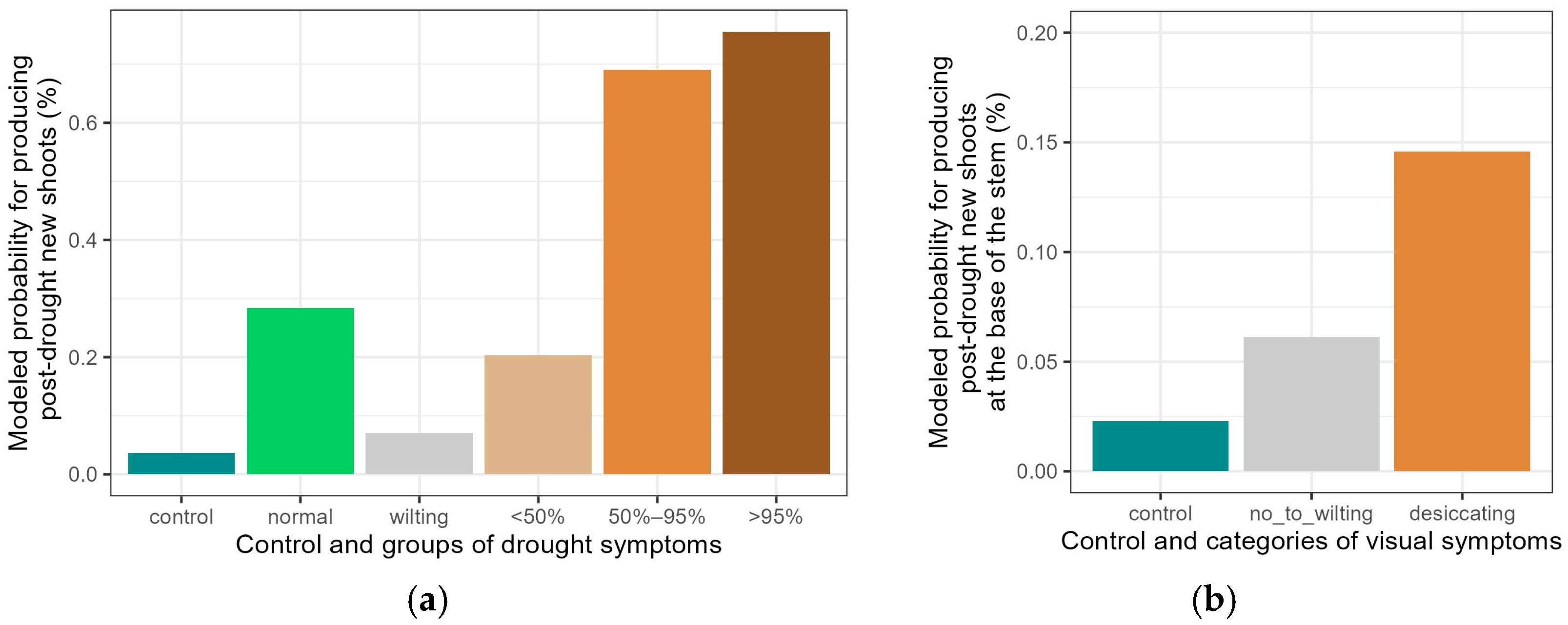
3.2. Development of Visual Drought Symptoms and Post-Drought Resprouting
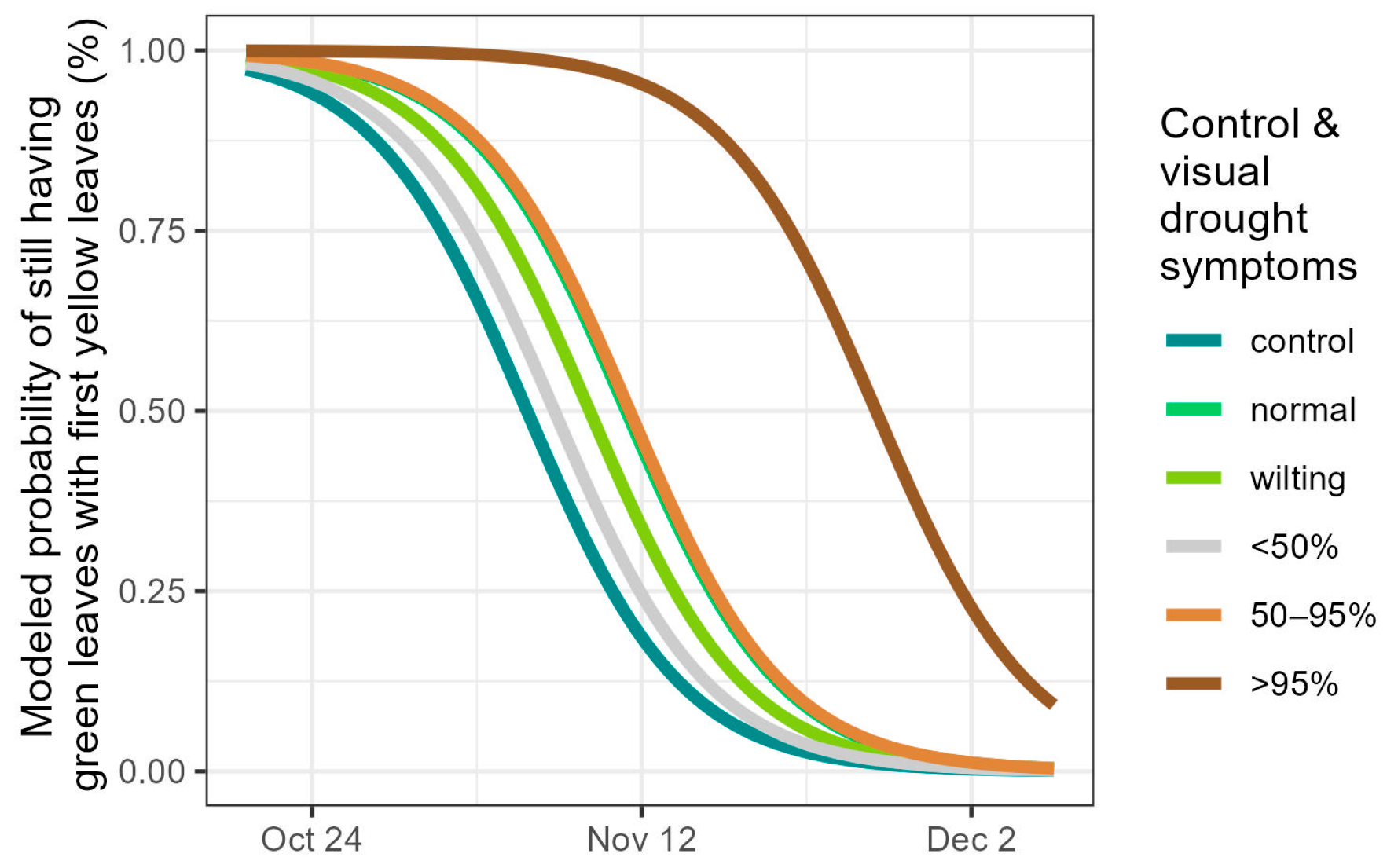
3.3. Autumn Leaf Senescence
3.4. Spring Bud Burst
3.5. Change in Height and Diameter
4. Discussion
4.1. Development of Visual Symptoms and Resprouting
4.2. Drought Affecting Sapling Size
4.3. Autumnal Leaf Senescence and Subsequent Spring Bud Burst
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef] [PubMed]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolstrom, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Müller, L.M.; Bahn, M. Drought legacies and ecosystem responses to subsequent drought. Glob. Change Biol. 2022, 28, 5086–5103. [Google Scholar] [CrossRef]
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; Van der Velde, M.; Vicca, S.; Babst, F. Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Change Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef]
- Vilonen, L.; Ross, M.; Smith, M.D. What happens after drought ends: Synthesizing terms and definitions. New Phytol. 2022, 235, 420–431. [Google Scholar] [CrossRef]
- Walthert, L.; Ganthaler, A.; Mayr, S.; Saurer, M.; Waldner, P.; Walser, M.; Zweifel, R.; von Arx, G. From the comfort zone to crown dieback: Sequence of physiological stress thresholds in mature European beech trees across progressive drought. Sci. Total Environ. 2021, 753, 141792. [Google Scholar] [CrossRef]
- Sterck, F.J.; Song, Y.; Poorter, L. Drought- and heat-induced mortality of conifer trees is explained by leaf and growth legacies. Sci. Adv. 2024, 10, eadl4800. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Marchin, R.; Zeng, H.; Hoffmann, W. Drought-deciduous behavior reduces nutrient losses from temperate deciduous trees under severe drought. Oecologia 2010, 163, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, U.; Windt, C.W.; Ponomarenko, A.; Zhang, Y.-J.; Gersony, J.; Rockwell, F.E.; Holbrook, N.M. Stomatal Closure, Basal Leaf Embolism, and Shedding Protect the Hydraulic Integrity of Grape Stems. Plant Physiol. 2017, 174, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.J.; Brodribb, T.J.; Jordan, G.J. Leaf hydraulics and drought stress: Response, recovery and survivorship in four woody temperate plant species. Plant Cell Environ. 2009, 32, 1584–1595. [Google Scholar] [CrossRef]
- Blackman, C.J.; Creek, D.; Maier, C.; Aspinwall, M.J.; Drake, J.E.; Pfautsch, S.; O’Grady, A.; Delzon, S.; Medlyn, B.E.; Tissue, D.T.; et al. Drought response strategies and hydraulic traits contribute to mechanistic understanding of plant dry-down to hydraulic failure. Tree Physiol. 2019, 39, 910–924. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Grote, R.; Mayr, S.; Arneth, A. Beyond the extreme: Recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol. 2019, 39, 1285–1299. [Google Scholar] [CrossRef]
- Arend, M.; Sever, K.; Pflug, E.; Gessler, A.; Schaub, M. Seasonal photosynthetic response of European beech to severe summer drought: Limitation, recovery and post-drought stimulation. Agric. For. Meteorol. 2016, 220, 83–89. [Google Scholar] [CrossRef]
- Spiess, N.; Oufir, M.; Matusikova, I.; Stierschneider, M.; Kopecky, D.; Homolka, A.; Burg, K.; Fluch, S.; Hausman, J.F.; Wilhelm, E. Ecophysiological and transcriptomic responses of oak (Quercus robur) to long-term drought exposure and rewatering. Environ. Exp. Bot. 2012, 77, 117–126. [Google Scholar] [CrossRef]
- Turcsán, A.; Steppe, K.; Sárközi, E.; Erdélyi, É.; Missoorten, M.; Mees, G.; Vander Mijnsbrugge, K. Early summer drought stress during the first growing year stimulates extra shoot growth in oak seedlings (Quercus petraea). Front. Plant Sci. 2016, 7, 193. [Google Scholar] [CrossRef]
- Kaya, Z.; Adams, W.T.; Campbell, R.K. Adaptive significance of intermittent shoot growth in Douglas-fir seedlings. Tree Physiol. 1994, 14, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Zohner, C.M.; Mo, L.D.; Pugh, T.A.M.; Bastin, J.F.; Crowther, T.W. Interactive climate factors restrict future increases in spring productivity of temperate and boreal trees. Glob. Change Biol. 2020, 26, 4042–4055. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; Wing, I.S.; et al. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Savolainen, O.; Pyhäjärvi, T.; Knürr, T. Gene Flow and Local Adaptation in Trees. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Alberto, F.J.; Aitken, S.N.; Alia, R.; Gonzalez-Martinez, S.C.; Hanninen, H.; Kremer, A.; Lefevre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change-evidence from tree populations. Glob. Change Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef]
- Zohner, C.M.; Benito, B.M.; Svenning, J.-C.; Renner, S.S. Day length unlikely to constrain climate-driven shifts in leaf-out times of northern woody plants. Nat. Clim. Chang. 2016, 6, 1120–1123. [Google Scholar] [CrossRef]
- Delpierre, N.; Dufrêne, E.; Soudani, K.; Ulrich, E.; Cecchini, S.; Boé, J.; François, C. Modelling interannual and spatial variability of leaf senescence for three deciduous tree species in France. Agric. For. Meteorol. 2009, 149, 938–948. [Google Scholar] [CrossRef]
- Rosenthal, S.I.; Camm, E.L. Photosynthetic decline and pigment loss during autumn foliar senescence in western larch (Larix occidentalis). Tree Physiol. 1997, 17, 767–775. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Moreels, S. Varying Levels of Genetic Control and Phenotypic Plasticity in Timing of Bud Burst, Flower Opening, Leaf Senescence and Leaf Fall in Two Common Gardens of Prunus padus L. Forests 2020, 11, 1070. [Google Scholar] [CrossRef]
- Gallinat, A.S.; Primack, R.B.; Wagner, D.L. Autumn, the neglected season in climate change research. Trends Ecol. Evol. 2015, 30, 169–176. [Google Scholar] [CrossRef]
- Enescu, C.; Durrant, T.; de Rigo, D.; Caudullo, G. Corylus avellana in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Ferreira, J.J.; Garcia-González, C.; Tous, J.; Rovira, M. Genetic diversity revealed by morphological traits and ISSR markers in hazelnut germplasm from northern Spain. Plant Breed. 2010, 129, 435–441. [Google Scholar] [CrossRef]
- Campa, A.; Trabanco, N.; Pérez-Vega, E.; Rovira, M.; Ferreira, J.J. Genetic relationship between cultivated and wild hazelnuts (Corylus avellana L.) collected in northern Spain. Plant Breed. 2011, 130, 360–366. [Google Scholar] [CrossRef]
- Čokeša, V.; Pavlović, B.; Stajic, S.; Poduška, Z.; Jović, Đ. Corylus, L.: Its diversity, geographical distribution and morpho-anatomical characteristics with special reference to the systematic classification and phylogenics of Turkish hazel (Corylus colurna L.). Sustain. For. Collect. 2020, 81–82, 1–17. [Google Scholar] [CrossRef]
- Palmé, A.E. Chloroplast DNA variation, postglacial recolonization and hybridization in hazel, Corylus avellana. Mol. Ecol. 2002, 11, 1769–1779. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Malanguis, J.M.; Moreels, S.; Turcsán, A.; Paino, E.N. Stimulation, Reduction and Compensation Growth, and Variable Phenological Responses to Spring and/or Summer & Autumn Warming in Corylus Taxa and Cornus sanguinea L. Forests 2022, 13, 654. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Christensen, R.H.B. Ordinal: Regression Models for Ordinal Data. R Package Version 2015.6-28. Available online: http://www.cran.r-project.org/package=ordinal/ (accessed on 15 February 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Zuur, A.; Ieno, E.; Walker, N.; Saveliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Vander Mijnsbrugge, K.; Moreels, S.; Moreels, S.; Buisset, D.; Vancampenhout, K.; Notivol Paino, E. Influence of Summer Drought on Post-Drought Resprouting and Leaf Senescence in Prunus spinosa L. Grow. A Common Garden. Plants 2025, 14, 1132. [Google Scholar]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Harrison, S.P.; Adams, H.D.; Kelley, D.I.; Li, G.; Tissue, D.T.; Dawson, T.E.; Fensham, R.; Medlyn, B.E.; Palmer, A.; et al. Drought and resprouting plants. New Phytol. 2015, 206, 583–589. [Google Scholar] [CrossRef]
- Gessler, A.; Bottero, A.; Marshall, J.; Arend, M. The way back: Recovery of trees from drought and its implication for acclimation. New Phytol. 2020, 228, 1704–1709. [Google Scholar] [CrossRef]
- Etzold, S.; Sterck, F.; Bose, A.K.; Braun, S.; Buchmann, N.; Eugster, W.; Gessler, A.; Kahmen, A.; Peters, R.L.; Vitasse, Y.; et al. Number of growth days and not length of the growth period determines radial stem growth of temperate trees. Ecol. Lett. 2022, 25, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Puchalka, R.; Prislan, P.; Klisz, M.; Koprowski, M.; Gricar, J. Tree-ring formation dynamics in Fagus sylvatica and Quercus petraea in a dry and a wet year. Dendrobiology 2024, 91, 1–15. [Google Scholar] [CrossRef]
- Trugman, A.T.; Detto, M.; Bartlett, M.K.; Medvigy, D.; Anderegg, W.R.L.; Schwalm, C.; Schaffer, B.; Pacala, S.W. Tree carbon allocation explains forest drought-kill and recovery patterns. Ecol. Lett. 2018, 21, 1552–1560. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Turcsan, A.; Maes, J.; Duchene, N.; Meeus, S.; Steppe, K.; Steenackers, M. Repeated Summer Drought and Re-watering during the First Growing Year of Oak (Quercus petraea) Delay Autumn Senescence and Bud Burst in the Following Spring. Front. Plant Sci. 2016, 7, 419. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Vandepitte, J.; Moreels, S.; Mihaila, V.-V.; De Ligne, L.; Notivol, E.; Van Acker, J.; Van den Bulcke, J. Timing of autumnal leaf senescence in a common shrub species depends on the level of preceding summer drought symptoms. Environ. Exp. Bot. 2023, 216, 105539. [Google Scholar] [CrossRef]
- Pflug, E.E.; Buchmann, N.; Siegwolf, R.T.W.; Schaub, M.; Rigling, A.; Arend, M. Resilient Leaf Physiological Response of European Beech (Fagus sylvatica L.) to Summer Drought Drought Release. Front. Plant Sci. 2018, 9, 187. [Google Scholar] [CrossRef]
- Estiarte, M.; Peñuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Change Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef]
- Blumstein, M.; Oseguera, M.; Caso-McHugh, T.; Des Marais, D.L. Nonstructural carbohydrate dynamics’ relationship to leaf development under varying environments. New Phytol. 2024, 241, 102–113. [Google Scholar] [CrossRef]
- Anonymous. Klimaatrapport 2020. Van Klimaatinformatie tot Klimaatdiensten; Koninklijk Meteorologisch Institutuut: Brussels, Belgium, 2020. [Google Scholar]

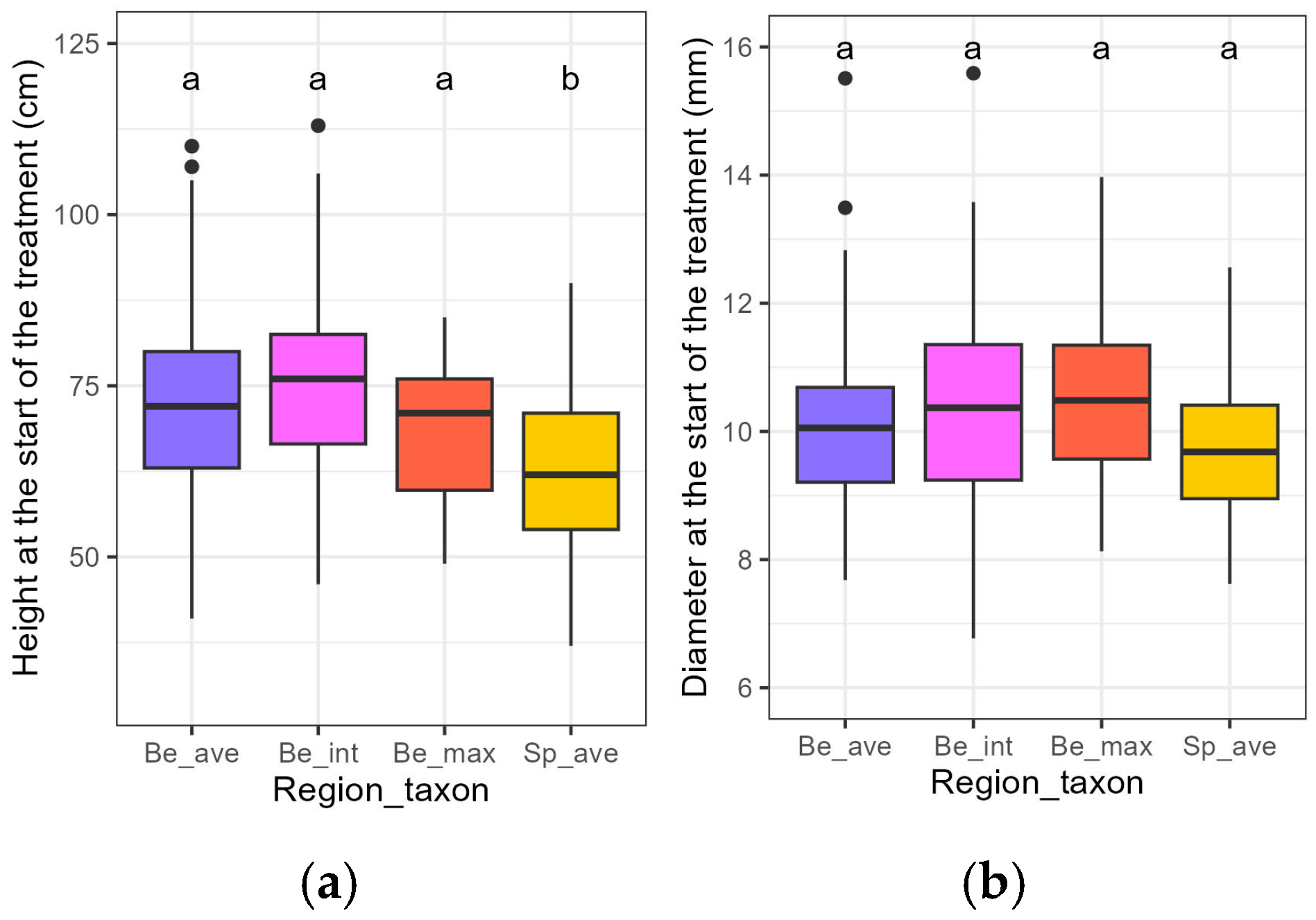


| Initial Height | Initial Diameter | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | Std. Error | t-Value | p-Value | Estimate | Std. Error | t-Value | p-Value |
| (Intercept) | 72.24 | 1.40 | 51.69 | <0.001 *** | 0.10 | 0.00 | 72.16 | <0.001 *** |
| Be_int | 2.29 | 2.19 | 1.04 | 0.298 | 0.00 | 0.00 | 1.20 | 0.233 |
| Be_max | −4.54 | 3.31 | −1.37 | 0.171 | 0.00 | 0.00 | 1.11 | 0.267 |
| Sp_ave | −10.52 | 2.04 | −5.15 | <0.001 *** | 0.00 | 0.00 | −1.87 | 0.062 |
| Lamina Length | Lamina Widest Width | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Location on the Plant | Variable | Estimate | Std. Error | t-Value | p-Value | Estimate | Std. Error | t-Value | p-Value |
| Short shoot | (Intercept) | 67.31 | 1.32 | 50.96 | <0.001 *** | 48.62 | 0.96 | 50.71 | <0.001 *** |
| Be_int | 0.72 | 2.07 | 0.35 | 0.728 | 0.51 | 1.50 | 0.34 | 0.736 | |
| Be_max | 8.89 | 3.10 | 2.87 | 0.005 ** | 2.38 | 2.25 | 1.06 | 0.292 | |
| Sp_ave | −2.68 | 1.91 | −1.40 | 0.164 | −2.28 | 1.39 | −1.64 | 0.103 | |
| Long shoot | (Intercept) | 102.16 | 2.06 | 49.64 | <0.001 *** | 82.91 | 1.95 | 42.42 | <0.001 *** |
| Be_int | 6.59 | 3.22 | 2.04 | 0.043 * | 5.22 | 3.06 | 1.71 | 0.091 | |
| Be_max | 0.96 | 5.04 | 0.19 | 0.850 | 0.31 | 4.79 | 0.07 | 0.948 | |
| Sp_ave | −6.91 | 3.00 | −2.30 | 0.023 * | −7.29 | 2.85 | −2.56 | 0.012 * | |
| Variable | Estimate | Std. Error | z-Value | p-Value |
|---|---|---|---|---|
| Day | −1.29 | 0.11 | −11.92 | <0.001 *** |
| Be_int | 0.53 | 1.02 | 0.52 | 0.602 |
| Be_max | −0.85 | 1.53 | −0.56 | 0.578 |
| Sp_ave | 3.32 | 1.04 | 3.19 | 0.001 ** |
| Hei1 | −0.26 | 0.04 | −6.67 | <0.001 *** |
| Location on the Plant | Variable | Estimate | Std. Error | z-Value | p-Value |
|---|---|---|---|---|---|
| Young crown | (Intercept) | −3.78 | 1.41 | −2.68 | 0.007 ** |
| Dro_normal | 2.36 | 0.91 | 2.60 | 0.009 ** | |
| Dro_wilting | 0.71 | 0.90 | 0.78 | 0.433 | |
| Dro_ < 50% | 1.92 | 0.69 | 2.77 | 0.006 ** | |
| Dro_50%–95% | 4.09 | 0.59 | 6.93 | <0.001 *** | |
| Dro_ > 95% | 4.42 | 0.74 | 5.95 | <0.001 *** | |
| Be_int | 0.44 | 0.51 | 0.87 | 0.387 | |
| Be_max | 0.31 | 0.76 | 0.41 | 0.681 | |
| Sp_ave | −0.36 | 0.58 | −0.62 | 0.533 | |
| Hei1 | 0.01 | 0.02 | 0.40 | 0.687 | |
| Base stem | (Intercept) | −4.44 | 1.65 | −2.70 | 0.007 ** |
| Dro_no-mild | 1.03 | 0.82 | 1.26 | 0.208 | |
| Dro_severe | 1.99 | 0.69 | 2.89 | 0.004 ** | |
| Hei1 | 0.01 | 0.02 | 0.48 | 0.635 |
| Autumn Leaf Senescence | Spring Bud Burst | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | Std. Error | z-Value | p-Value | Estimate | Std. Error | z-Value | p-Value |
| Day | 0.21 | 0.01 | 16.38 | <0.001 *** | −0.38 | 0.02 | −19.87 | <0.001 *** |
| Dro_normal | −1.28 | 0.46 | −2.77 | 0.006 ** | 1.37 | 0.70 | 1.95 | 0.051 |
| Dro_wilting | −0.81 | 0.33 | −2.48 | 0.013 * | 1.62 | 0.49 | 3.33 | <0.001 *** |
| Dro_ < 50% | −0.35 | 0.35 | −1.00 | 0.318 | 1.40 | 0.52 | 2.68 | 0.007 ** |
| Dro_50%–95% | −1.32 | 0.32 | −4.19 | <0.001 *** | 2.09 | 0.47 | 4.42 | <0.001 *** |
| Dro_ > 95% | −4.50 | 0.51 | −8.87 | <0.001 *** | 4.35 | 0.68 | 6.42 | <0.001 *** |
| Be_int | 0.46 | 0.26 | 1.78 | 0.075 | −1.67 | 0.38 | −4.37 | <0.001 *** |
| Be_max | −0.36 | 0.40 | −0.90 | 0.366 | −4.76 | 0.62 | −7.67 | <0.001 *** |
| Sp_ave | 0.44 | 0.26 | 1.68 | 0.093 | 0.29 | 0.38 | 0.75 | 0.453 |
| Hei3 | 0.04 | 0.01 | 7.25 | <0.001 *** | 0.04 | 0.01 | 5.03 | <0.001 *** |
| Trait | Variable | Value | Std. Error | DF | t-Value | p-Value |
|---|---|---|---|---|---|---|
| Change in height | (Intercept) | 26.61 | 3.04 | 250 | 8.76 | <0.001 *** |
| Tim | −18.57 | 1.12 | 250 | −16.64 | <0.001 *** | |
| Dro_normal | −6.45 | 2.97 | 246 | −2.17 | 0.031 * | |
| Dro_wilting | −9.42 | 2.00 | 246 | −4.72 | <0.001 *** | |
| Dro_ < 50% | −9.76 | 2.16 | 246 | −4.51 | <0.001 *** | |
| Dro_50%–95% | −15.12 | 1.82 | 246 | −8.32 | <0.001 *** | |
| Dro_ > 95% | −21.35 | 2.41 | 246 | −8.87 | <0.001 *** | |
| Be_int | −0.61 | 1.24 | 246 | −0.49 | 0.625 | |
| Be_max | −4.95 | 1.88 | 246 | −2.63 | 0.009 ** | |
| Sp_ave | −3.25 | 1.24 | 246 | −2.62 | 0.009 ** | |
| Hei1 | −0.03 | 0.04 | 246 | −0.68 | 0.500 | |
| Tim:Dro_normal | 6.73 | 3.66 | 250 | 1.84 | 0.067 | |
| Tim:Dro_wilting | 10.14 | 2.49 | 250 | 4.07 | <0.001 *** | |
| Tim:Dro_ < 50% | 7.65 | 2.75 | 250 | 2.78 | 0.006 ** | |
| Tim:Dro_50%–95% | 9.40 | 2.30 | 250 | 4.08 | <0.001 *** | |
| Tim:Dro_ > 95% | −3.58 | 3.03 | 250 | −1.18 | 0.239 | |
| Change in diameter | (Intercept) | 3.10 | 0.40 | 250 | 7.79 | <0.001 *** |
| Tim | −1.38 | 0.14 | 250 | −9.95 | <0.001 *** | |
| Dro_normal | −0.67 | 0.33 | 246 | −2.07 | 0.040* | |
| Dro_wilting | −0.80 | 0.22 | 246 | −3.62 | <0.001 *** | |
| Dro_ < 50% | −0.65 | 0.24 | 246 | −2.66 | 0.008 ** | |
| Dro_50%–95% | −1.00 | 0.20 | 246 | −4.91 | <0.001 *** | |
| Dro_ > 95% | −0.91 | 0.27 | 246 | −3.39 | 0.001 ** | |
| Be_int | 0.24 | 0.13 | 246 | 1.90 | 0.058 | |
| Be_max | 0.16 | 0.19 | 246 | 0.83 | 0.406 | |
| Sp_ave | −0.11 | 0.12 | 246 | −0.91 | 0.365 | |
| Dia1 | −0.17 | 0.04 | 246 | −4.67 | <0.001 *** | |
| Tim:Dro_normal | 1.05 | 0.46 | 250 | 2.30 | 0.022 * | |
| Tim:Dro_wilting | 1.11 | 0.31 | 250 | 3.60 | <0.001 *** | |
| Tim:Dro_ < 50% | 0.93 | 0.34 | 250 | 2.71 | 0.007 ** | |
| Tim:Dro_50%–95% | 1.34 | 0.29 | 250 | 4.68 | <0.001 *** | |
| Tim:Dro_ > 95% | 0.93 | 0.38 | 250 | 2.48 | 0.014* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vander Mijnsbrugge, K.; Pareijn, A.; Moreels, S.; Moreels, S.; Buisset, D.; Vancampenhout, K.; Notivol Paino, E. Summer Drought Delays Leaf Senescence and Shifts Radial Growth Towards the Autumn in Corylus Taxa. Forests 2025, 16, 907. https://doi.org/10.3390/f16060907
Vander Mijnsbrugge K, Pareijn A, Moreels S, Moreels S, Buisset D, Vancampenhout K, Notivol Paino E. Summer Drought Delays Leaf Senescence and Shifts Radial Growth Towards the Autumn in Corylus Taxa. Forests. 2025; 16(6):907. https://doi.org/10.3390/f16060907
Chicago/Turabian StyleVander Mijnsbrugge, Kristine, Art Pareijn, Stefaan Moreels, Sharon Moreels, Damien Buisset, Karen Vancampenhout, and Eduardo Notivol Paino. 2025. "Summer Drought Delays Leaf Senescence and Shifts Radial Growth Towards the Autumn in Corylus Taxa" Forests 16, no. 6: 907. https://doi.org/10.3390/f16060907
APA StyleVander Mijnsbrugge, K., Pareijn, A., Moreels, S., Moreels, S., Buisset, D., Vancampenhout, K., & Notivol Paino, E. (2025). Summer Drought Delays Leaf Senescence and Shifts Radial Growth Towards the Autumn in Corylus Taxa. Forests, 16(6), 907. https://doi.org/10.3390/f16060907






