Seasonal and Edaphic Modulation Influences the Phenolic Contents and Antioxidant Activity in Cork Oak (Quercus suber L.): Evidence from the Algerian Mediterranean Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling
2.3. Soil Analysis
2.3.1. Physicochemical Characterization of Rhizospheric Soil
2.3.2. Total Polyphenol Content (TPC) Determination
2.4. Plant Material Analysis
2.4.1. Plant Extract Preparation
2.4.2. Quantitative Analyses
Total Polyphenol Content
Total Tannin Content
Total Flavonoid Content
2.5. Antioxidant Properties
2.5.1. DPPH Radical Scavenging Assay
2.5.2. Ferric Reducing Antioxidant Power (FRAP)
2.5.3. Total Antioxidant Capacity (TAC)
2.6. Statistical Analysis
3. Results
3.1. Rhizospheric Soil Analysis
3.2. Plant Material Analysis
3.2.1. Chemical Composition
Roots
Leaves
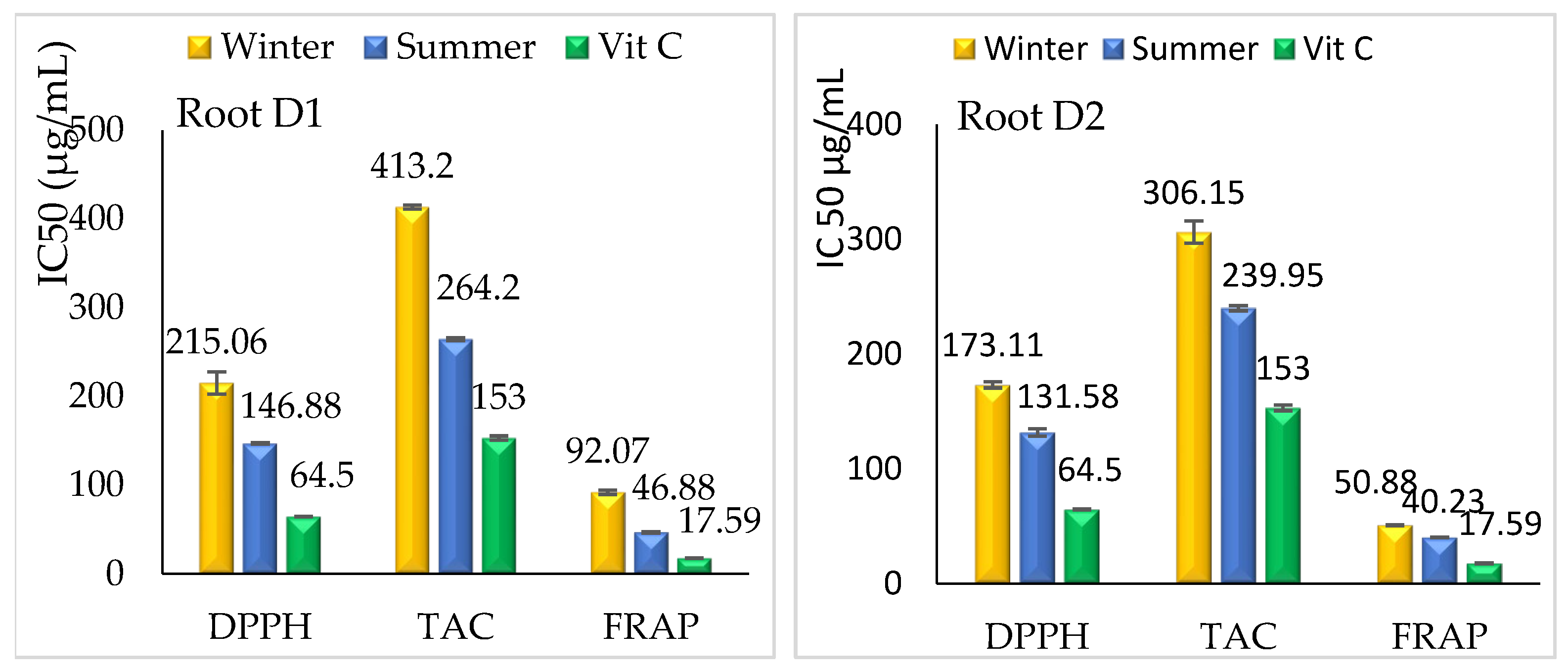
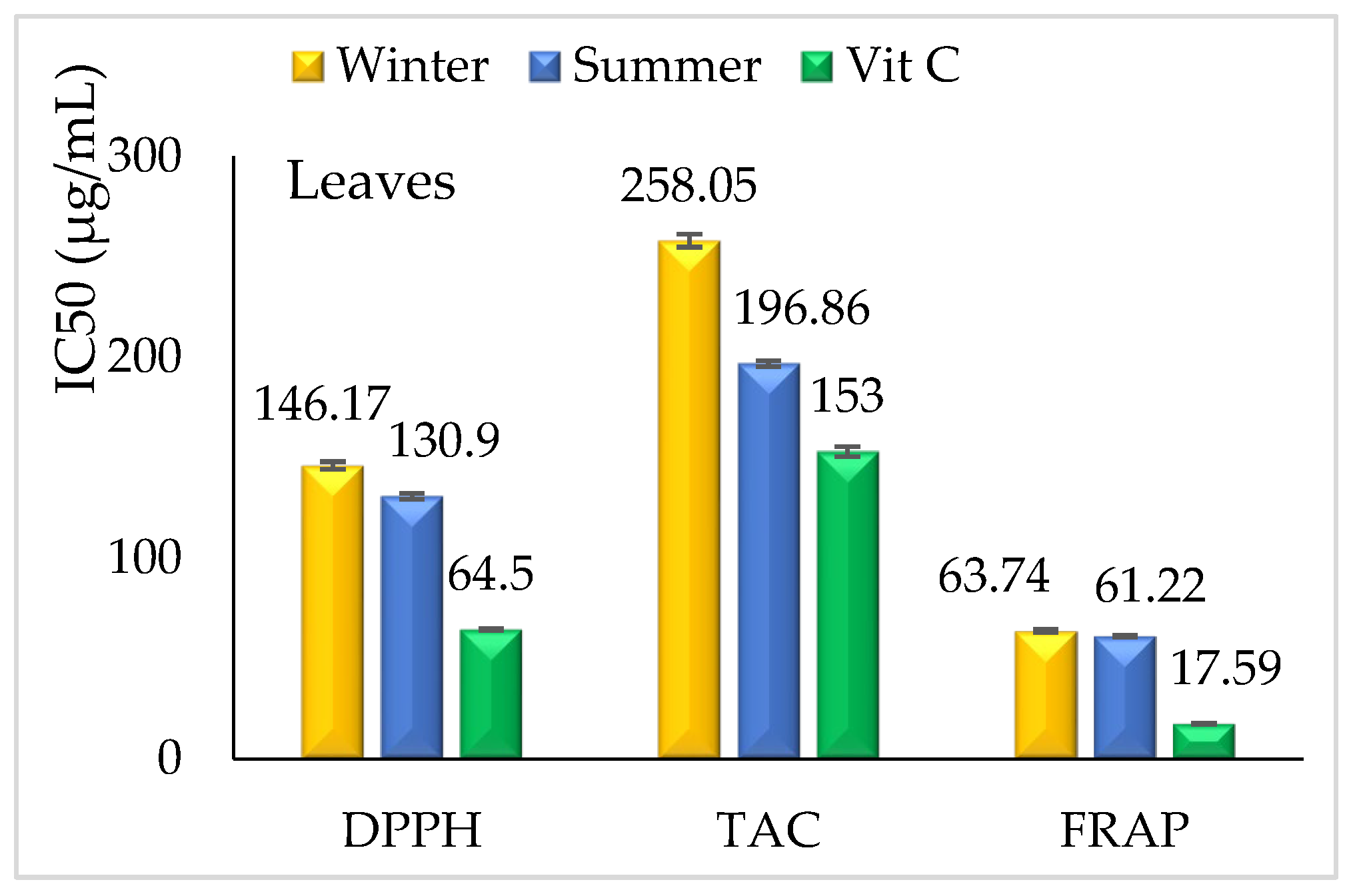
3.2.2. Antioxidant Activity
Roots
Leaves
3.3. Statistical Analysis
4. Discussion
4.1. Sol Analysis
4.2. Chemical Composition and Antioxidant Activities of Vegatal Material
4.3. Correlation Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Albassatneh, M.; Escudero, M.; Monnet, A.; Arroyo, J.; Bacchetta, G.; Bagnoli, F.; Dimopoulos, P.; Hampe, A.; Leriche, A.; Médail, F.; et al. Spatial patterns of genus-level phylogenetic endemism in the tree flora of Mediterranean Europe. Divers. Distrib. 2021, 27, 913–928. [Google Scholar] [CrossRef]
- Moreno, M.; Simioni, G.; Cailleret, M.; Ruffault, J.; Badel, É.; Carrière, S.; Davi, H.; Gavinet, J.; Huc, R.; Limousin, J.; et al. Consistently lower sap velocity and growth over nine years of rainfall exclusion in a Mediterranean mixed pine-oak forest. Agric. For. Meteorol. 2021, 308, 108472. [Google Scholar] [CrossRef]
- Almeida, T.; Pinto, G.; Correia, B.; Gonçalves, S.; Meijón, M.; Escandón, M. In-depth analysis of the Quercus suber metabolome under drought stress and recovery reveals potential key metabolic players. Plant Sci. 2020, 299, 110606. [Google Scholar] [CrossRef]
- Li, T.; Tiiva, P.; Rinnan, Å.; Julkunen-Tiitto, R.; Michelsen, A.; Rinnan, R. Long-term effects of elevated CO2, nighttime warming and drought on plant secondary metabolites in a temperate heath ecosystem. Ann. Bot. 2020, 125, 1065–1075. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Beran, F.; Köllner, T.G.; Gershenzon, J.; Tholl, D. Chemical convergence between plants and insects: Biosynthetic origins and functions of common secondary metabolites. New Phytol. 2018, 223, 52–67. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants: Chapter 8—An Overview. In Plant Metabolites and Regulation Under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Touhami, I.; Chirino, E.; Aouinti, H.; Khorchani, E.; Elaieb, M.; Khaldi, A.; Nasr, Z. Decline and dieback of cork oak (Quercus suber L.) forests in the Mediterranean basin: A case study of Kroumirie, Northwest Tunisia. J. For. Res. 2020, 31, 1461–1477. [Google Scholar] [CrossRef]
- Von Essen, M.; Rosário, I.; Santos-Reis, M.; Nicholas, K. Valuing and mapping cork and carbon across land use scenarios in a Portuguese montado landscape. PLoS ONE 2019, 14, e0212174. [Google Scholar] [CrossRef]
- Zhao, M.; Running, S.W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Natali, L.; Vangelisti, A.; Guidi, L.; Remorini, D.; Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, C.; Trivellini, C.; Vernieri, P.; et al. How Quercus ilex L. saplings face combined salt and ozone stress: A transcriptome analysis. BMC Genom. 2018, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Thyroff, E.; Burney, O.; Mickelbart, M.; Jacobs, D. Unraveling shade tolerance and plasticity of semi-evergreen oaks: Insights from maritime forest live oak restoration. Front. Plant Sci. 2019, 10, 1526. [Google Scholar] [CrossRef] [PubMed]
- Puyo, J.Y. Grandeurs et vicissitudes de l’aménagement des suberaies algériennes durant la période coloniale française (1830–1962). Forêt Méditerranéenne 2013, 34, 129–142. [Google Scholar]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nolã, A.; Ripullone, F.; Nole, A. Drought-induced oak decline in the western Mediterranean region: An overview on current evidences, mechanisms and management options to improve forest resilience. iForest 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Custódio, A.L.; Madeira, J.F.A. GLODS: Global and Local Optimization using Direct Search. J. Glob. Optim. 2015, 62, 1–28. [Google Scholar] [CrossRef]
- Santos, S.A.; Pinto, P.C.; Silvestre, A.J.; Neto, C.P. Chemical composition and antioxidant activity of phenolic extracts of cork from Quercus suber L. Ind. Crops Prod. 2010, 31, 521–526. [Google Scholar] [CrossRef]
- Touati, R.; Santos, S.A.; Rocha, S.M.; Belhamel, K.; Silvestre, A.J. The potential of cork from Quercus suber L. grown in Algeria as a source of bioactive lipophilic and phenolic compounds. Ind. Crops Prod. 2015, 76, 936–945. [Google Scholar] [CrossRef]
- Aronson, J. Current state of knowledge of wind energy impacts on bats in South Africa. Acta Chiropterologica 2022, 24, 221–238. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- UNDP. United Nations Development Programme Annual Report 2023; UNDP: New York, NY, USA, 2023. [Google Scholar]
- Ghefar, M.; Dehane, B. Etude des paramètres réglant la production du liège dans la subéraie de M’Sila (Oran, Algérie). Agric. For. J. 2018, 2, 48–58. [Google Scholar]
- Bouzid, A.; Bouzid, K.; Benabdeli, K. Contribution the Failure Mode Analysis and Criticality Evaluation Method to the Rehabilitation of Cork Oak (Quercus suber) Forests in Forest Massif of Tlemcen (Algeria). J. Manaj. Hutan Trop. 2022, 28, 191. [Google Scholar] [CrossRef]
- Orgeas, J.; Ourcival, J.M.; Bonin, G. Seasonal and spatial patterns of foliar nutrients in cork oak (Quercus suber L.) growing on siliceous soils in Provence (France). Plant Ecol. 2003, 164, 201–211. [Google Scholar] [CrossRef]
- Soil Science Society of America. Soil Analysis: A Comprehensive Guide; SSSA: Madison, WI, USA, 2020. [Google Scholar]
- Gee, G.; Bauder, J.W. Particule-size analysis. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- ISO 11277:2020; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. ISO: Geneva, Switzerland, 2020.
- Aubert, M.; Yéne’ Atangana, Q. Self-potential method in hydrogeological exploration of volcanic areas. Ground Water 1996, 34, 1010–1016. [Google Scholar] [CrossRef]
- Deng, J.; Camenen, B.; Legoût, C.; Nord, G. Estimation of fine sediment stocks in gravel bed rivers including the sand fraction. Sedimentology 2023, 71, 152–172. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular No. 939; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Mathieu, C.; Pieltain, F.; Jeanroy, E. Analyse Chimique des Sols: Méthodes Choisies; Tec & Doc: Paris, France, 2003; pp. 33–48. [Google Scholar]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Dallali, S.; Rouz, S.; Aichi, H.; Hassine, H.B. Phenolic content and allelopathic potential of leaves and rhizosphere soil aqueous extracts of white horehound (Marrubium vulgare L.). J. New Sci. 2017, 39, 2106–2120. [Google Scholar]
- Blum, K.; Noble, E.P.; Sheridan, P.J.; Finley, O.; Montgomery, A.; Ritchie, T.; Ozkaragoz, T.; Fitch, R.J.; Sadlack, F.; Sheffield, D. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol 1991, 8, 409–416. [Google Scholar] [CrossRef]
- Box, J.D. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Miftode, A.M.; Stefanache, A.; Şpac, A.F.; Miftode, R.S.; Miron, A.; Dorneanu, V. In vitro measurement of total antioxidant capacity of Crataegus macracantha Lodd leaves. Rev. Med.-Chir. Soc. Med. Nat. Iași 2016, 120, 2. [Google Scholar]
- Bourgou, S.; Beji, R.S.; Medini, F.; Ksouri, R. Effet du solvant et de la méthode d’extraction sur la teneur en composés phénoliques et les potentialités antioxydantes d’Euphorbia helioscopia. J. New Sci. 2016, 28, 1649–1655. [Google Scholar]
- Ouzid, Y.; Smail-Saadoun, N.; Houali, K. Comparative study of in vitro antioxidant activity of foliar endophytic fungi and leaves extracts of Peganum harmala of Dayateaiat (Laghouat, Algeria). J. Fundam. Appl. Sci. 2018, 10, 147–157. [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M. Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for food and non-food purposes. Food Chem. 2016, 213, 133–142. [Google Scholar]
- Naczk, M.; Oickle, D.; Pink, D.; Shahidi, F. Protein precipitating capacity of crude canola tannins: Effect of pH, tannin, and protein concentrations. J. Agric. Food Chem. 1996, 44, 2144–2148. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Determination of protein in tannin-protein precipitates. J. Agric. Food Chem. 1980, 28, 944–947. [Google Scholar] [CrossRef]
- Fattahi, S.; Zabihi, E.; Abedian, Z.; Pourbagher, R.; Motevalizadeh Ardekani, A.; Mostafazadeh, A.; Akhavan-Niaki, H. Total phenolic and flavonoid contents of aqueous extract of stinging nettle and in vitro antiproliferative effect on Hela and BT-474 cell lines. Int. J. Mol. Cell. Med. 2014, 3, 102–107. [Google Scholar]
- Smith, J.; Johnson, L.; Wang, R. Updated methods for measuring DPPH radical scavenging activity. J. Food Sci. 2020, 85, 1234–1240. [Google Scholar]
- Dupont, A. Vitamin C as a control in antioxidant assays. Food Chem. 2021, 345, 128–135. [Google Scholar]
- Fejes, S.; Blázovics, A.; Lugasi, A.; Lemberkovics, É.; Petri, G.; Kéry, Á. In vitro antioxidant activity of Anthriscus cerefolium L. (Hoffm.) extracts. J. Ethnopharmacol. 2000, 69, 259–265. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Mechergui, T.; Pardos, M.; Boussaidi, N.; Jacobs, D.F.; Catry, F.X. Problems and solutions to cork oak (Quercus suber L.) regeneration: A review. Inst. Cienc. For. (ICIFOR) 2023, 16, 10–22. [Google Scholar] [CrossRef]
- Kaci, M.B.N.; Ahmed, S.O.; Saad, L.; Halimi, R.; Khelfaoui, L.; Issaoun, D. Effect of fire recurrence on abundance and distribution of soil fauna under Quercus suber L. in Taksebt forest Zekri (Algeria). J. New Sci. 2018, 1, 3290–3298. [Google Scholar]
- Kalev, S.D.; Toor, G.S. The composition of soils and sediments. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 339–357. [Google Scholar]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin, Germany, 2003. [Google Scholar]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, H.; Bai, Y.; Dong, F.; Peng, C.; Yan, F.; Yang, G. Water table drawdown increases plant biodiversity and soil polyphenol in the Zoige Plateau. Ecol. Indic. 2021, 121, 107118. [Google Scholar] [CrossRef]
- Kainulainen, P.; Holopainen, J.K. Concentrations of secondary compounds in Scots pine needles at different stages of decomposition. Soil Biol. Biochem. 2002, 34, 37–42. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Berg, M.P.; Staudt, M.; Holmstrup, M.; Glasius, M.; Ellers, J.; Peñuelas, J. Plant secondary compounds in soil and their role in belowground species interactions. Trends Ecol. Evol. 2020, 35, 716–730. [Google Scholar] [CrossRef]
- Preston, C.; Bhatti, J.; Flanagan, L.; Norris, C. Stocks, chemistry and sensitivity to climate change of dead organic matter along the Canadian boreal forest transect case study. Clim. Change 2006, 74, 223–251. [Google Scholar] [CrossRef]
- Kanerva, S.; Kitunen, V.; Loponen, J.; Smolander, A. Phenolic compounds and terpenes in soil organic horizon layers under silver birch, Norway spruce and Scots pine. Biol. Fertil. Soils 2008, 44, 547–556. [Google Scholar] [CrossRef]
- Singh, A.; Bisht, M. Evaluation of in-vitro antioxidant potential and in-vivo hepatoprotective activity of root extract of Quercus oblongata D. DON. J. Drug Deliv. Ther. 2018, 8, 152–161. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [PubMed]
- Meziti, H.; Bouriche, H.; Kada, S.; Demirtas, I.; Kizil, M.; Senator, A.; Garrido, G. Phytochemical analysis, and antioxidant, anti-hemolytic and genoprotective effects of Quercus ilex L. and Pinus halepensis Mill. methanolic extracts. J. Pharm. Pharmacogn. Res. 2019, 7, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Solla, A.; Milanović, S.; Gallardo, A.; Bueno, A.; Corcobado, T.; Cáceres, Y.; Pulido, F. Genetic determination of tannins and herbivore resistance in Quercus ilex. Tree Genet. Genomes 2016, 12, 117. [Google Scholar] [CrossRef]
- Endo, I.; Kobatake, M.; Tanikawa, N.; Nakaji, T.; Ohashi, M.; Makita, N. Anatomical patterns of condensed tannin in fine roots of tree species from a cool-temperate forest. Ann. Bot. 2021, 128, 59–71. [Google Scholar] [CrossRef]
- Tattini, M.; Remorini, D.; Pinelli, P.; Agati, G.; Saracini, E.; Traversi, M.L.; Massai, R. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytol. 2006, 170, 779–794. [Google Scholar] [CrossRef]
- Peter Constabel, C.; Yoshida, K.; Walker, V. Diverse ecological roles of plant tannins: Plant defense and beyond. Recent Adv. Polyphen. Res. 2014, 4, 115–142. [Google Scholar]
- Yuan, Y.; Liu, Y.; Wu, C.; Chen, S.; Wang, Z.; Yang, Z.; Huang, L. Water deficit affected flavonoid accumulation by regulating hormone metabolism in Scutellaria baicalensis Georgi roots. PLoS ONE 2012, 7, e42907. [Google Scholar] [CrossRef]
- Mbinda, W.; Musangi, C. Antioxidant activity, total phenolic and total flavonoid. J. Phytopharm. 2019, 8, 161–166. [Google Scholar] [CrossRef]
- Tálos-Nebehaj, E.; Hofmann, T.; Albert, L. Seasonal changes of natural antioxidant content in the leaves of Hungarian forest trees. Ind. Crops Prod. 2017, 98, 53–59. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Díez-Marqués, C.; Tardío, J. Valorization of wild strawberry-tree fruits (Arbutus unedo L.) through nutritional assessment and natural production data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Lavado, G.; Ladero, L.; Cava, R. Cork oak (Quercus suber L.) leaf extracts potential use as natural antioxidants in cooked meat. Ind. Crops Prod. 2021, 160, 113086. [Google Scholar] [CrossRef]
- Elahi, M.Y.; Rouzbehan, Y. Characterization of Quercus persica, Quercus infectoria and Quercus libani as ruminant feeds. Anim. Feed Sci. Technol. 2008, 140, 78–89. [Google Scholar] [CrossRef]
- Ulusu, F.; Darıcı, C. The influence of tannins purified from Eastern Mediterranean Region plants (Pinus brutia Ten. and Quercus coccifera L.) on carbon mineralization: Antimicrobial and antimutagenic evaluation. Anatol. J. Bot. 2023, 7, 60–69. [Google Scholar] [CrossRef]
- Frouja, O.; Hammami, M.; Dakhlaoui, S.; Wannes, W.A.; Hessini, K.; Msaada, K.; Ahmed, H.B. Intra and interspecific variability of Quercus suber and Quercus canariensis, an intrinsic water-use efficiency approach for differentiation. Environ. Res. Commun. 2022, 4, 105002. [Google Scholar] [CrossRef]
- Yarnes, C.T.; Boecklen, W.J.; Tuominen, K.; Salminen, J.P. Defining phytochemical phenotypes: Size and shape analysis of phenolic compounds in oaks (Fagaceae, Quercus) of the Chihuahuan Desert. Botany 2006, 84, 1233–1248. [Google Scholar] [CrossRef]
- Magalhães, A.P.; Verde, N.; Reis, F.; Martins, I.; Costa, D.; Lino-Neto, T.; Azevedo, H. RNA-Seq and gene network analysis uncover activation of an ABA-dependent signalosome during the cork oak root response to drought. Front. Plant Sci. 2016, 7, 1195. [Google Scholar] [CrossRef]
- Toori, M.A.; Mirzaei, M.; Mirzaei, N.; Lamrood, P.; Mirzaei, A. Antioxidant and hepatoprotective effects of the internal layer of oak fruit (Jaft). J. Med. Plants Res. 2013, 7, 24–28. [Google Scholar]
- Hadidi, L.; Babou, L.; Zaidi, F.; Valentao, P.; Andrade, P.B.; Grosso, C. Quercus ilex L.: How season, plant organ and extraction procedure can influence chemistry and bioactivities. Chem. Biodivers. 2017, 14, e1600187. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef]
- Almeida, I.F.; Amaral, M.H.; Costa, P.C.; Bahia, M.F.; Valentao, P.; Andrade, P.B.; Pereira, T.M. Oak leaf extract as topical antioxidant: Free radical scavenging and iron chelating activities and in vivo skin irritation potential. Biofactors 2008, 33, 267–279. [Google Scholar] [CrossRef]
- Nikolaos, G.; Alexandras, P.; Evangelia, L.; Vassiliki, T.; Maria-Nektaria, N. Effect of ripening stage on the total phenolics content, lycopene and antioxidant activity of tomato fruits grown to a geothermal greenhouse. Biol. Hortic. Food Prod. Process. Technol. Environ. Eng. 2018, 23, 115–120. [Google Scholar]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Miani, A. SARS-CoV-2 RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ. Res. 2020, 188, 109754. [Google Scholar] [CrossRef] [PubMed]
- Gil-Pelegrín, E.; Peguero-Pina, J.J.; Sancho-Knapik, D. Oaks and people: A long journey together. In Oaks Physiological Ecology: Exploring the Functional Diversity of Genus Quercus L.; Springer: Cham, Switzerland, 2017; pp. 1–11. [Google Scholar]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Pérez-Girón, J.C.; Díaz-Varela, E.R.; Álvarez-Álvarez, P. Climate-driven variations in productivity reveal adaptive strategies in Iberian cork oak agroforestry systems. For. Ecosyst. 2022, 9, 100008. [Google Scholar] [CrossRef]
- Das, S.; Barman, S.; Teron, R.; Bhattacharya, S.S.; Kim, K.H. Secondary metabolites and anti-microbial/anti-oxidant profiles in Ocimum spp.: Role of soil physico-chemical characteristics as eliciting factors. Environ. Res. 2020, 188, 109749. [Google Scholar] [CrossRef]
- Duarte, A.R.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. Influence of spatial, edaphic and genetic factors on phenols and essential oils of Myrciaria cauliflora fruits. J. Braz. Chem. Soc. 2012, 23, 737–746. [Google Scholar] [CrossRef]
- Chludil, H.D.; Corbino, G.B.; Leicach, S.R. Soil quality effects on Chenopodium album flavonoid content and antioxidant potential. J. Agric. Food Chem. 2008, 56, 5050–5056. [Google Scholar] [CrossRef]
- Nait-Kaci, M.B.; Hedde, M.; Bourbia, S.M.; Derridj, A. Hierarchization of factors driving soil macrofauna in North Algeria groves. BASE Biotechnol. Agron. Soc. Environ. 2014, 18, 11–18. [Google Scholar]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in Northeast China: Jilin Province case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Roca-Fernández, A.I.; Dillard, S.L.; Soder, K.J. Ruminal fermentation and enteric methane production of legumes containing condensed tannins fed in continuous culture. J. Dairy Sci. 2020, 103, 7028–7038. [Google Scholar] [CrossRef]
- AL-Ghamdi, A.A.; Jais, H.M. Interaction between soil textural components, flavonoids in the roots and mycorrhizal colonization in Juniperus procera in Saudi Arabia. Afr. J. Microbiol. Res. 2013, 7, 996–1001. [Google Scholar]
- Vierheilig, H.; Bago, B.; Lerat, S.; Piché, Y. Shoot-produced, light-dependent factors are partially involved in the expression of the arbuscular mycorrhizal (AM) status of AM host and non-host plants. J. Plant Nutr. Soil Sci. 2002, 165, 21–25. [Google Scholar]
- Castells, E.; Peñuelas, J. Is there a feedback between N availability in siliceous and calcareous soils and Cistus albidus leaf chemical composition? Oecologia 2003, 136, 183–192. [Google Scholar] [CrossRef]
- Oliveira, J.S.D.; Ramos, N.P.; Júnior, J.L.; Xavier, L.P.; Andrade, E.H.; Mello, A.H.; Da Silva, J.K.R. Secondary metabolism and plant growth of Piper divaricatum (Piperaceae) inoculated with arbuscular mycorrhizal fungi and phosphorus supplementation. Agronomy 2022, 12, 596. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuoka, H.; Hayashi, H. Isolation and identification of a phosphate deficiency-induced C-glycosylflavonoid that stimulates arbuscular mycorrhiza formation in melon roots. Mol. Plant-Microbe Interact. 2002, 15, 334–340. [Google Scholar] [CrossRef]
- Tomasi, N.; Weisskopf, L.; Renella, G.; Landi, L.; Pinton, R.; Varanini, Z.; Cesco, S. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol. Biochem. 2008, 40, 1971–1974. [Google Scholar] [CrossRef]
- Watson, B.S.; Bedair, M.F.; Urbanczyk-Wochniak, E.; Huhman, D.V.; Yang, D.S.; Allen, S.N.; Sumner, L.W. Integrated metabolomics and transcriptomics reveal enhanced specialized metabolism in Medicago truncatula root border cells. Plant Physiol. 2015, 167, 1699–1716. [Google Scholar] [CrossRef]
- Malusà, E.; Russo, M.A.; Mozzetti, C.; Belligno, A. Modification of secondary metabolism and flavonoid biosynthesis under phosphate deficiency in bean roots. J. Plant Nutr. 2006, 29, 245–258. [Google Scholar] [CrossRef]
- Hawes, M.C.; Brigham, L.A.; Wen, F.; Woo, H.H.; Zhu, Y. Function of root border cells in plant health: Pioneers in the rhizosphere. Annu. Rev. Phytopathol. 1998, 36, 311–327. [Google Scholar]
- Zahedi, S.M.; Karimi, M.; Venditti, A. Plants adapted to arid areas: Specialized metabolites. Nat. Prod. Res. 2021, 35, 3314–3331. [Google Scholar]
- Bettaieb, I.; Zakhama, N.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 120, 271–275. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Química Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Urban, L.; Berti, L.; Bourgaud, F.; Gautier, H.; Léchaudel, M.; Joas, J.; Sallanon, H. The effect of environmental factors on biosynthesis of carotenoids and polyphenolics in fruits and vegetables: A review and prospects. In II International Symposium on Human Health Effects of Fruits and Vegetables: FAVHEALTH 2007; ISHS: Leuven, Belgium, 2007; pp. 339–344. [Google Scholar]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef]
- Brüggemann, W.; Bergmann, M.; Nierbauer, K.U.; Pflug, E.; Schmidt, C.; Weber, D. Photosynthesis studies on European evergreen and deciduous oaks grown under Central European climate conditions: II. Photoinhibitory and light-independent violaxanthin deepoxidation and downregulation of photosystem II in evergreen, winter-acclimated European Quercus taxa. Trees 2009, 23, 1091–1100. [Google Scholar]
- Korn, M.; Peterek, S.; Mock, H.P.; Heyer, A.G.; Hincha, D.K. Heterosis in the freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant Cell Environ. 2008, 31, 813–827. [Google Scholar] [CrossRef]
- Insam, H.; Seewald, M.S. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 2010, 46, 199–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Masante, T.; Cafà, S.; Di Iorio, A. Total polyphenol content and antioxidant activity of leaves and fine roots as indicators of drought resistance in the native Quercus robur and alien Quercus rubra. Forests 2024, 15, 1531. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; Ayadi, M.T. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2015, 5, 052–057. [Google Scholar]
- Chen, X.X.; Wu, X.B.; Chai, W.M.; Feng, H.L.; Shi, Y.; Zhou, H.T.; Chen, Q.X. Optimization of extraction of phenolics from leaves of Ficus virens. J. Zhejiang Univ. Sci. B 2013, 14, 903–915. [Google Scholar] [CrossRef]
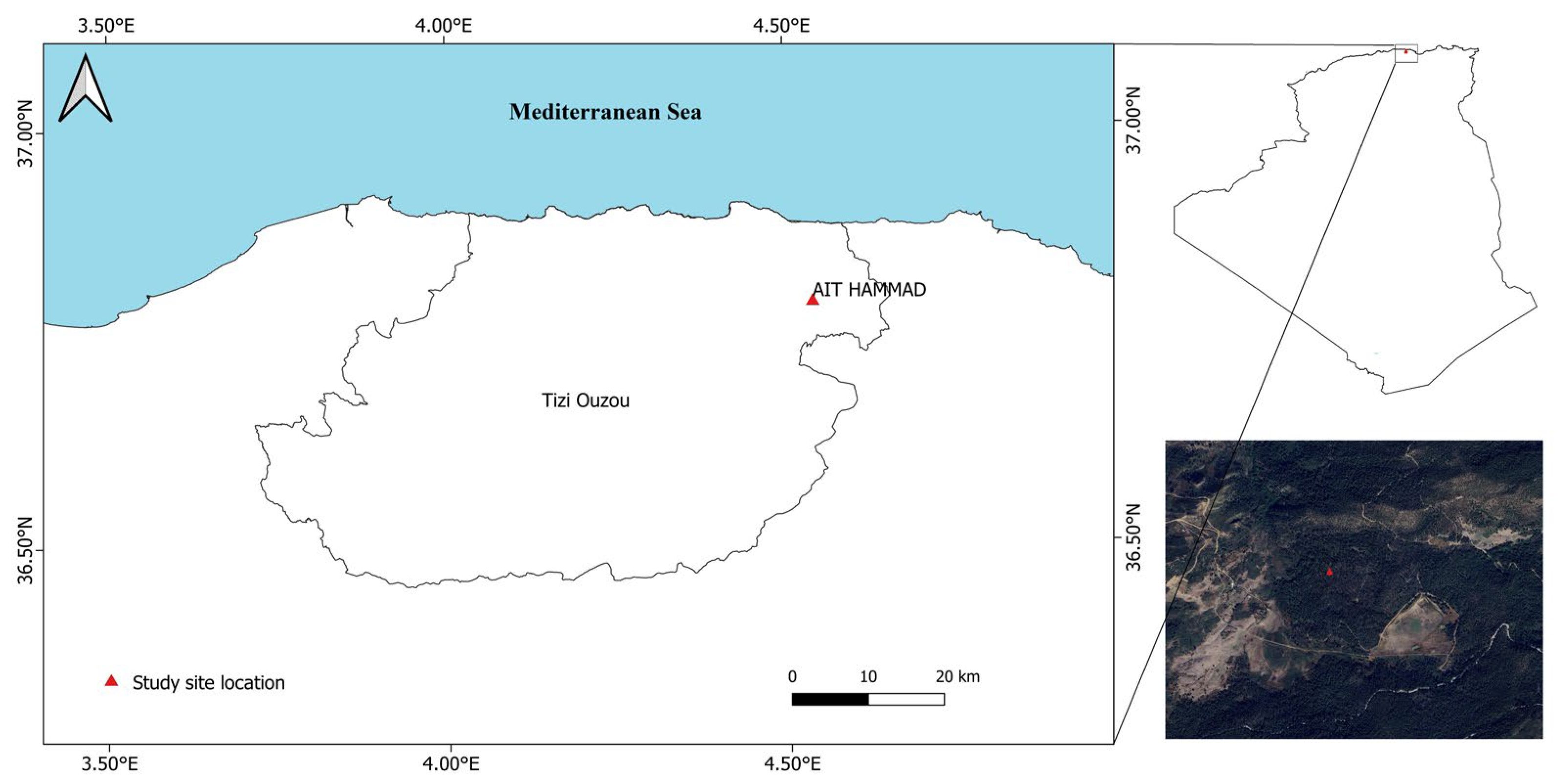





| Station Name | Altitude (m) | Latitude | Longitude | Slope (°) | Orientation |
|---|---|---|---|---|---|
| Ait Hammad | 800 | N36°47′24.8” | E004°32′40.1” | 0 | NE |
| Station Name | Mean Temperature (°C) | Precipitation (mm) | ||
|---|---|---|---|---|
| Winter (January) | Summer (July) | Winter (January) | Summer (July) | |
| Ait Hammad | 8.37 | 25.32 | 67.98 | 14.66 |
| Summer | Winter | p Value Soil Depth | |||
|---|---|---|---|---|---|
| Soil depth | D1 | D2 | D1 | D2 | |
| pH-water | 5.27± 0.43 ab | 4.93± 0.41 a | 5.36± 0.31 b | 5.25 ± 0.14 ab | 0.043 * |
| EC (ds/m) | 0.92 ± 0.34 b | 0.33 ± 0.07 a | 2.58± 0.78 c | 0.7 ± 0.31 ab | 0.000 *** |
| C % | 7.02 ± 1.83 | 2.59 ± 0.5 | 12.87 ± 2.49 | 3.39 ± 0.93 | 0.000 *** |
| N % | 0.43 ± 0.5 | 0.17 ± 0.09 | 0.38 ± 0.12 | 0.22 ± 0.11 | 0.000 *** |
| C/N | 16.33 ± 2.43 a | 15.22 ± 6.02 a | 33.88 ± 15.89 b | 15.39 ± 5.58 a | 0.000 *** |
| P (g/Kg) | 1.73 × 10−4 ± 0.14 b | 1.66 × 10−4 ± 0.167 b | 0.4 × 10−4 ± 0.033 a | 0.44 × 10−4 ± 0.035 a | 0.009 ** |
| Si % | 47.97 ± 14.61 a | 44.51 ± 14.73 a | 54.11 ± 18.4 a | 47.09 ± 12.81 a | 0.553 |
| S % | 51.91 ± 4.62 a | 55.37 ± 14.73 a | 45.76 ± 18.41 a | 52.77 ± 12.85 a | 0.553 |
| CL % | 0.12 ± 0.04 a | 0.12 ± 0.04 a | 0.13 ± 0.05 a | 0.14 ± 0.05 a | 0.734 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoceini-Bentaha, M.; Kadi-Bennane, S.; Boussoum, M.O.; Nabti, E.-H.; Kadir, N.; Mestar-Guechaoui, N.; Ibrahim, N.A.; Aleissa, M.S.; Basher, N.S.; Boudiaf, M.; et al. Seasonal and Edaphic Modulation Influences the Phenolic Contents and Antioxidant Activity in Cork Oak (Quercus suber L.): Evidence from the Algerian Mediterranean Forest. Forests 2025, 16, 906. https://doi.org/10.3390/f16060906
Hoceini-Bentaha M, Kadi-Bennane S, Boussoum MO, Nabti E-H, Kadir N, Mestar-Guechaoui N, Ibrahim NA, Aleissa MS, Basher NS, Boudiaf M, et al. Seasonal and Edaphic Modulation Influences the Phenolic Contents and Antioxidant Activity in Cork Oak (Quercus suber L.): Evidence from the Algerian Mediterranean Forest. Forests. 2025; 16(6):906. https://doi.org/10.3390/f16060906
Chicago/Turabian StyleHoceini-Bentaha, Melia, Saliha Kadi-Bennane, Mohand Ouidir Boussoum, El-Hafid Nabti, Nassima Kadir, Nadjet Mestar-Guechaoui, Nasir A. Ibrahim, Mohammed Saad Aleissa, Nosiba S. Basher, Malika Boudiaf, and et al. 2025. "Seasonal and Edaphic Modulation Influences the Phenolic Contents and Antioxidant Activity in Cork Oak (Quercus suber L.): Evidence from the Algerian Mediterranean Forest" Forests 16, no. 6: 906. https://doi.org/10.3390/f16060906
APA StyleHoceini-Bentaha, M., Kadi-Bennane, S., Boussoum, M. O., Nabti, E.-H., Kadir, N., Mestar-Guechaoui, N., Ibrahim, N. A., Aleissa, M. S., Basher, N. S., Boudiaf, M., Trabelsi, L., & Houali, K. (2025). Seasonal and Edaphic Modulation Influences the Phenolic Contents and Antioxidant Activity in Cork Oak (Quercus suber L.): Evidence from the Algerian Mediterranean Forest. Forests, 16(6), 906. https://doi.org/10.3390/f16060906





