Metabolomic Investigations Reveal Properties of Natural Low-Temperature Adaptation Strategies in Five Evergreen Trees
Abstract
1. Introduction
2. Results
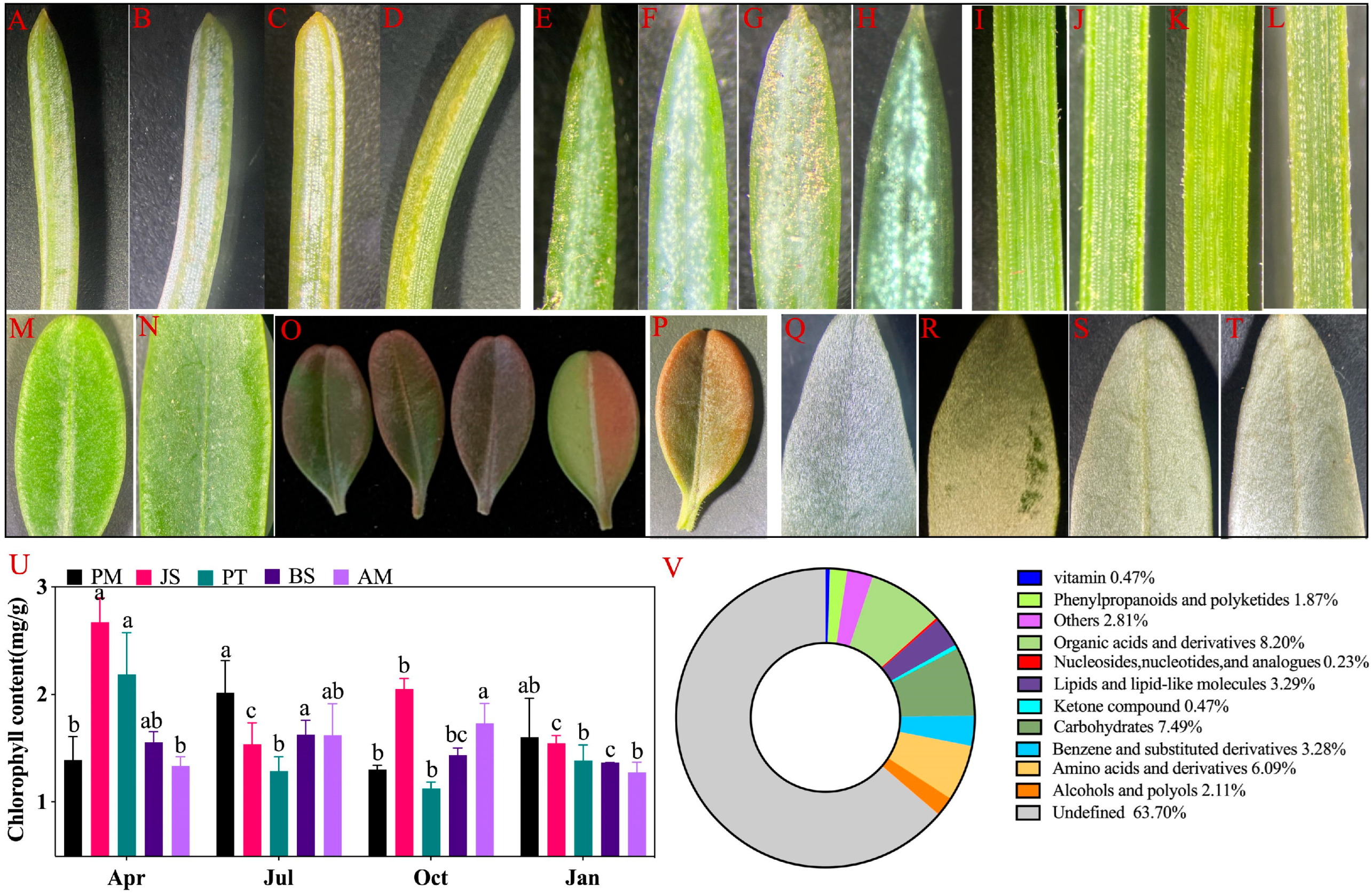
2.1. Seasonal Variation and Interspecific Differences in the Chlorophyll Content of Five Evergreens
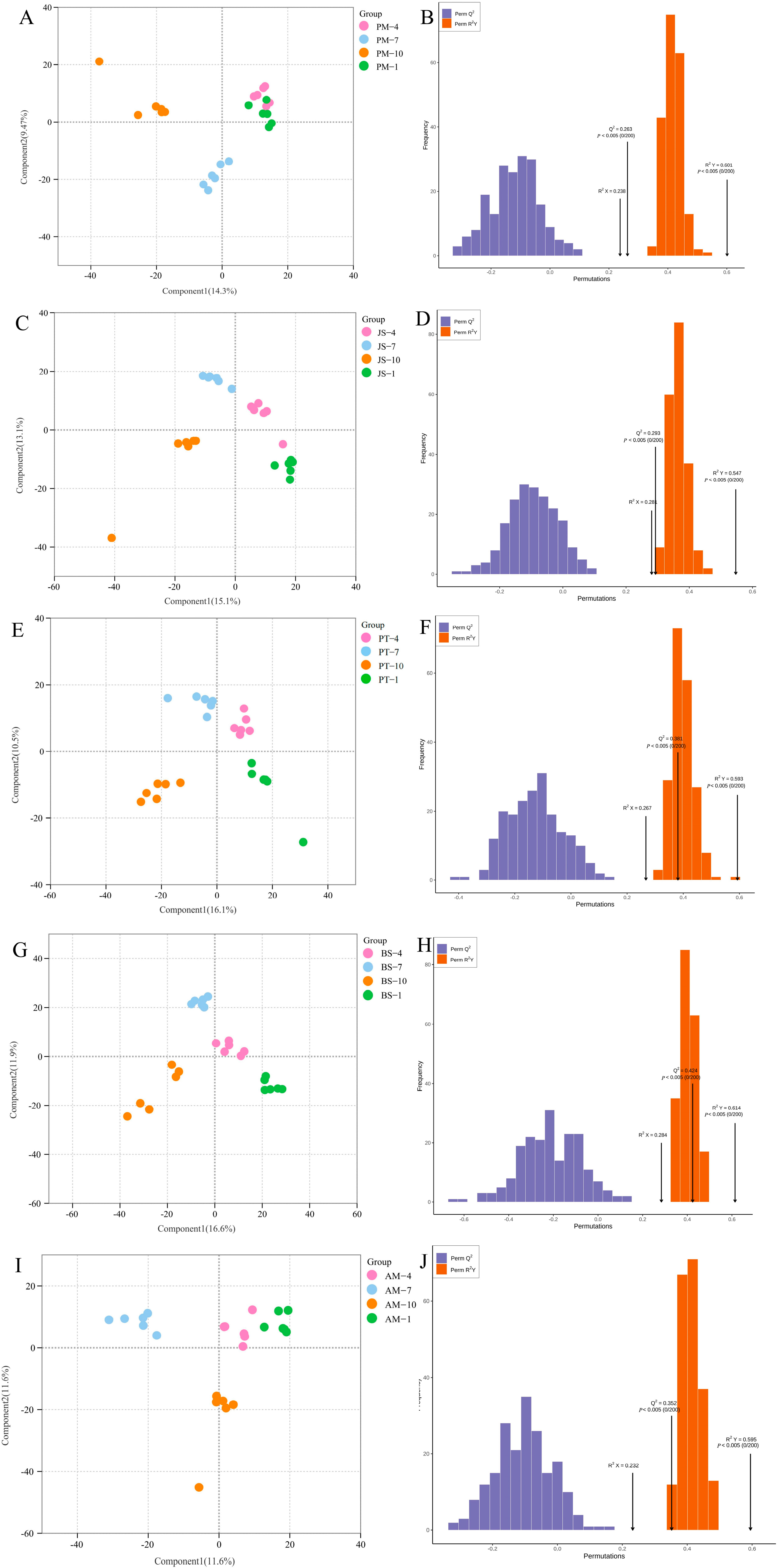
2.2. GC-TOF-MS Analysis and Identification of Differentially Expressed Metabolites
2.2.1. GC-TOF-MS Analysis Results
2.2.2. Identification of Differentially Expressed Metabolites
2.3. Analysis of Differentially Expressed Metabolites
2.3.1. Seasonal Response Patterns of Differentially Expressed Metabolites in Coniferous Species
2.3.2. Seasonal Response Patterns of Differentially Expressed Metabolites in Broad-Leaved Species
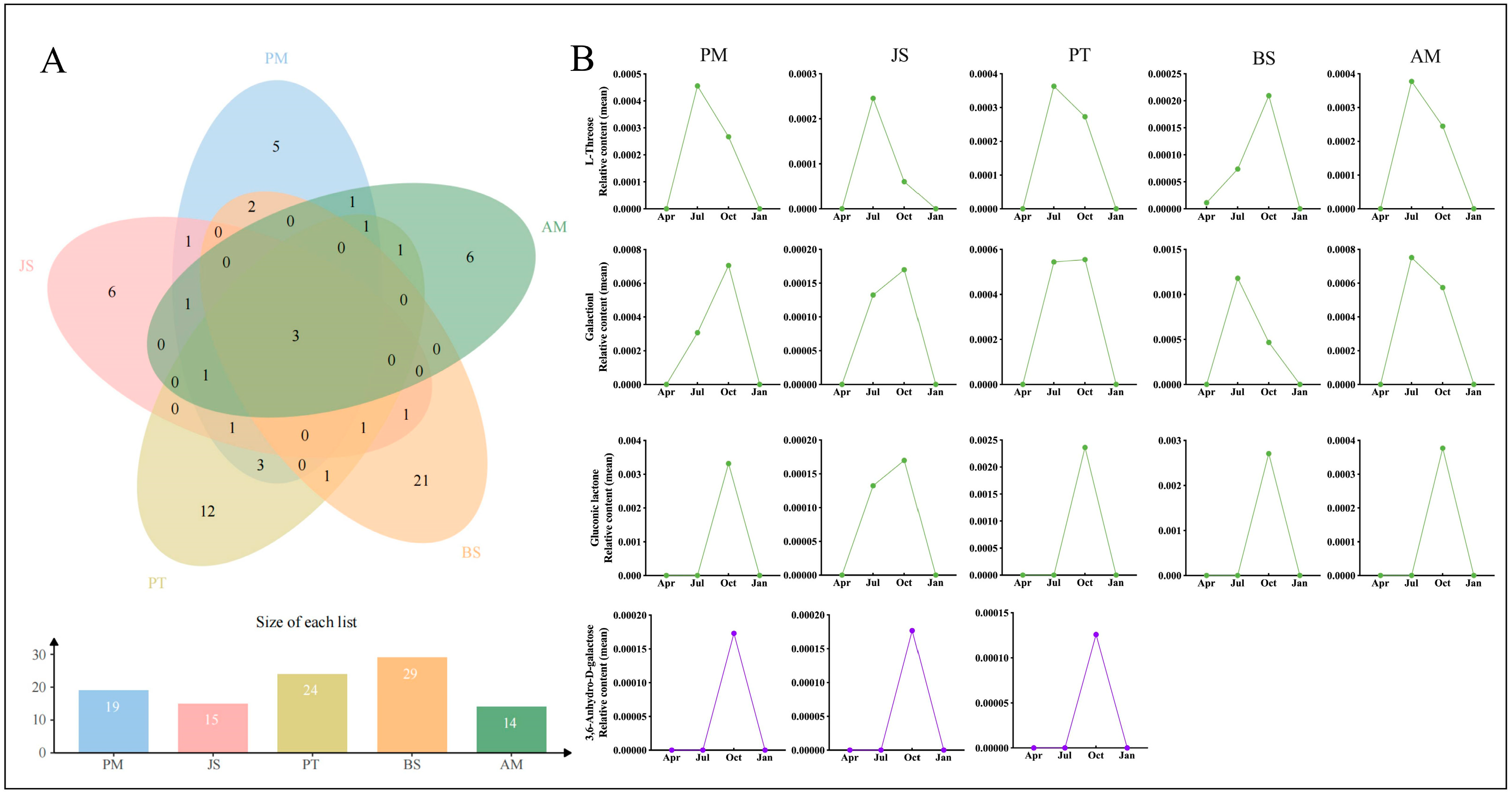
2.3.3. Venn Analysis of Differentially Expressed Metabolites
2.3.4. Identification of Core Differentially Expressed Metabolites in Five Evergreens
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chlorophyll Content Determination
4.3. GC-TOF-MS Metabolomic Analysis
4.3.1. Metabolite Extraction
4.3.2. GC-TOF-MS Analysis
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banks, H.P. Time of Appearance of Some Plant Biocharacters during Siluro-Devonian Time. Can. J. Bot. 1981, 59, 1292–1296. [Google Scholar] [CrossRef]
- Stewart, W.N.; Rothwell, G.W. Paleobotany and the Evolution of Plants; Cambridge University Press: Cambridge, UK; New York, NY, USA; Port Chester, NY, USA; Melbourne, Australia; Sydney, Australia, 1993. [Google Scholar]
- Ensminger, I.; Busch, F.; Huner, N.P.A. Photostasis and Cold Acclimation: Sensing Low Temperature through Photosynthesis. Physiol. Plant. 2010, 126, 28–44. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.H.; Zhu, J.K. Methods and Concepts in Quantifying Resistance to Drought, Salt and Freezing, Abiotic Stresses That Affect Plant Water Status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The Link between Genotypes and Phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Allen, J.; Davey, H.M.; Broadhurst, D.; Heald, J.K.; Rowland, J.J.; Oliver, S.G.; Kell, D.B. High-Throughput Classification of Yeast Mutants for Functional Genomics Using Metabolic Footprinting. Nat. Biotechnol. 2003, 21, 692–696. [Google Scholar] [CrossRef]
- Duan, L.X.; Qi, X.Q. Study on Plant Metabolomics Based on GC-MS. Life Sci. 2015, 27, 971–977. [Google Scholar]
- Taylor, J.; King, R.D.; Altmann, T.; Fiehn, O. Application of Metabolomics to Plant Genotype Discrimination Using Statistics and Machine Learning. Bioinformatics 2002, 18 (Suppl. S2), 241–248. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Fernie, A.R. The Use of Metabolomics to Dissect Plant Responses to Abiotic Stresses. Cell Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Nischal, P.; Sharma, A.D. Chemical Fingerprint Based Involvement of Plant Metabolites and Osmoregulatory Solutes in Providing Abiotic Stress Tolerance to Invasive Plant Lantana Camara. J. Stress. Physiol. Biochem. Zhurnal Stress-Fiziol. I Biokhimii 2019, 15, 93–102. [Google Scholar]
- Fürtauer, L.; Weiszmann, J.; Weckwerth, W.; Nagele, T. Dynamics of Plant Metabolism during Cold Acclimation. Int. J. Mol. Sci. 2019, 20, 5411–5426. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Mumtaz, M.A.; Zhou, Y.; Yang, Z.; Shu, H.; Zhu, J.; Bao, W.; Cheng, S.; Yin, L.; Huang, J.; et al. Integrated Transcriptomic and Metabolomic Analyses of Cold-Tolerant and Cold-Sensitive Pepper Species Reveal Key Genes and Essential Metabolic Pathways Involved in Response to Cold Stress. Int. J. Mol. Sci. 2022, 23, 6683–6701. [Google Scholar] [CrossRef]
- Chai, F.; Liu, W.; Xiang, Y.; Meng, X.; Sun, X.; Cheng, C.; Liu, G.; Duan, L.; Xin, H.; Li, S. Comparative Metabolic Profiling of Vitis Amurensis and Vitis Vinifera during Cold Acclimation. Hortic. Res. 2019, 6, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, C.; Ren, J.; Dong, J.; Shi, X.; Zhao, X.; Wang, X.; Wang, J.; Zhong, C.; Zhang, S.; et al. An Advanced Lipid Metabolism System Revealed by Transcriptomic and Lipidomic Analyses Plays a Central Role in Peanut Cold Tolerance. Front. Plant Sci. 2020, 11, 1110–1128. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid Signalling in Plant Responses to Abiotic Stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Ivanov, L.A.; Migalina, S.V.; Ronzhina, D.A.; Tumurjav, S.; Gundsambuu, T.; Bazha, S.N.; Ivanova, L.A. Altitude-Dependent Variation in Leaf Structure and Pigment Content Provides the Performance of a Relict Shrub in Mountains of Mongolia. Ann. Appl. Biol. 2022, 181, 321–331. [Google Scholar] [CrossRef]
- Li, Y.Y.; Pang, L.; Chen, Q.W.; Zhou, Y.Q.; Jiang, C.J. Effects of Low Temperature Stress on the Physiological Characteristics of Tea Leaves. J. Northwest AF Univ. Nat. Sci. Ed. 2012, 40, 134–138. [Google Scholar]
- Hincha, D.K.; Zuther, E. Plant Cold Acclimation: Methods and Protocols; Springer: New York, NY, USA, 2014. [Google Scholar]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the Temperature-Stress Metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of Flavonoids and Effects of Stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Zuther, E.; Lee, Y.P.; Erban, A.; Kopka, J.; Hincha, D.K. Natural Variation in Freezing Tolerance and Cold Acclimation Response in Arabidopsis thaliana and Related Species. Adv. Exp. Med. Biol. 2018, 1081, 81–98. [Google Scholar]
- Cook, D.; Fowler, S.; Fiehn, O.; Thomashow, M.F. A Prominent Role for the Cbf Cold Response Pathway in Configuring the Low-Temperature Metabolome of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 15243–15248. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, V.J.; Gakière, B.; Kempa, S.; Adamik, M.; Willmitzer, L.; Hesse, H.; Hoefgen, R. Towards Dissecting Nutrient Metabolism in Plants: A Systems Biology Case Study on Sulphur Metabolism. J. Exp. Bot. 2004, 55, 1861–1870. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, Salt, and Temperature Stress-Induced Metabolic Rearrangements and Regulatory Networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Valerio, C.; Costa, A.; Marri, L.; Issakidis-bourguet, E.; Pupillo, P.; Trost, P.; Sparla, F. Thioredoxin-Regulated Beta-Amylase (Bam1) Triggers Diurnal Starch Degradation in Guard Cells, and in Mesophyll Cells under Osmotic Stress. J. Exp. Bot. 2011, 62, 545–555. [Google Scholar] [CrossRef]
- Zhao, C.X.; Zhang, R.; Niu, K.J.; Zhu, R.T.; Wang, Y.; Ma, X.; Ma, H.L. Metabolomic Study of Qinghai Wild Poa pratensis in Response to Low Temperature Stress. Acta Agrestia Sin. 2020, 28, 904–914. [Google Scholar]
- Bao, Y.Z.; Yang, N.; Cang, J.; Feng, M.F.; Lv, Y.; Peng, K.K.; Tian, Y.; Zhang, D.; Wang, J.H.; Meng, Q. Metabolomic Analysis of Winter Wheat Dongnongdongmai No. 1 under Different Temperatures. J. Triticeae Crops 2017, 37, 647–655. [Google Scholar]
- Duan, E.L.; Ma, L.Y.; Yang, Y.; Deng, S.J.; Ma, J.; Duan, X.Q.; Zhou, M.M. Metabolomic Analysis of Cold Stress in Magnolia wufengensis and Magnolia denudata. Mol. Plant Breed. 2019, 17, 1771–1779. [Google Scholar]
- Jin, J.; Zhang, H.; Zhang, J.; Liu, P.; Cheng, X.; Li, Z.; Xu, Y.; Lu, P.; Cao, P. Integrated Transcriptomics and Metabolomics Analysis to Characterize Cold Stress Responses in Nicotiana tabacum. BMC Genom. 2017, 18, 496. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Manzi, M.; Ollas, C.; Gomez-cadenas, A. Metabolomics as a Tool to Investigate Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential Biochemical Indicators of Salinity Tolerance in Plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Klotke, J.; Kopka, J.; Gatzke, N.; Heyer, G.A. Impact of Soluble Sugar Concentrations on the Acquisition of Freezing Tolerance in Accessions of Arabidopsis thaliana with Contrasting Cold Adaptation—Evidence for a Role of Raffinose in Cold Acclimation. Plant Cell Environ. 2004, 27, 1395–1404. [Google Scholar] [CrossRef]
- Liu, Y.F.; Li, T.L.; Jiao, X.C. Effects of Short-Term Nighttime Sub-Low Temperature and Recovery on Photosynthesis and Sucrose Metabolism in Tomato. Acta Hortic. Sin. 2011, 38, 683–691. [Google Scholar]
- Joshi, V.; Joung, J.G.; Fei, Z.; Jander, G. Interdependence of Threonine, Methionine and Isoleucine Metabolism in Plants: Accumulation and Transcriptional Regulation under Abiotic Stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef]
- Zhang, W.F.; Gong, Z.H.; Wu, M.B.; Chan, H.; Yuan, Y.J.; Tang, N.; Zhang, Q.; Miao, M.J.; Chang, W.; Li, Z.; et al. Integrative Comparative Analyses of Metabolite and Transcript Profiles Uncovers Complex Regulatory Network in Tomato (Solanum lycopersicum L.) Fruit Undergoing Chilling Injury. Sci. Rep. 2019, 9, 4470. [Google Scholar] [CrossRef]
- Angelcheva, L.; Mishra, Y.; Antti, H.; Kjellsen, T.D.; Funk, C.; Strimbeck, R.G.; Schroder, W.P. Metabolomic Analysis of Extreme Freezing Tolerance in Siberian Spruce (Picea obovata). New Phytol. 2014, 204, 545–555. [Google Scholar] [CrossRef]
- Yang, X.; Liu, C.; Li, M.; Li, Y.; Yan, Z.; Feng, G.; Liu, D. Integrated Transcriptomics and Metabolomics Analysis Reveals Key Regulatory Network That Response to Cold Stress in Common Bean (Phaseolus vulgaris L.). BMC Plant Biol. 2023, 23, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, L.; Zhou, J.; Lyu, D.; Zhao, D.; Qin, S. Comparison of Transcriptome and Metabolome Analysis Revealed Differences in Cold Resistant Metabolic Pathways in Different Apple Cultivars under Low Temperature Stress. Hortic. Plant J. 2023, 9, 183–198. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Yu, G. The Dominant Glutamic Acid Metabolic Flux to Produce γ-Amino Butyric Acid over Proline in Nicotiana tabacum Leaves under Water Stress Relates to Its Significant Role in Antioxidant Activity. J. Integr. Plant Biol. 2011, 53, 608–618. [Google Scholar] [CrossRef]
- Yang, C.W.; Li, C.Y.; Zhang, M.L.; Liu, J.; Shi, D.C. Ph and Ion Balance in Wheat-Wheatgrass under Salt- or Alkali Stress. Chin. J. Appl. Ecol. 2008, 19, 1000–1005. [Google Scholar]
- Zhao, X.Q.; Wang, W.S.; Fan, Z.; Zhang, T.; Li, Z.K. Temporal Profiling of Primary Metabolites under Chilling Stress and Its Association with Seedling Chilling Tolerance of Rice (Oryza sativa L.). Rice 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach Towards Plant Defense. Pathogens 2020, 9, 312–337. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. Contribution to Flower Colors of Flavonoids Including Anthocyanins: A Review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef]
- Green, B.R.; Durnford, D.G. The Chlorophyll-Carotenoid Proteins of Oxygenic Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 685–714. [Google Scholar] [CrossRef]
- Zhang, S.X.; Nima, P.C.; Xu, Y.M.; Miao, Y.J.; Bao, S.H.N.; Zhang, W.H. Physiological Responses of Three Elymus Grasses to Low Temperature Stress and Evaluation of Seedling Cold Resistance. Pratacult. Sci. 2016, 33, 1154–1163. [Google Scholar]
- Zhang, A.O.; Cui, Z.H.; Yu, J.L.; Hu, Z.J.; Zhang, L.J. Dissipation of Excess Excitation Energy of the Needle Leaves in Pinus Trees during Cold Winters. Int. J. Biometeorol. 2016, 60, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Lu, T.Y.; Shen, H.L.; Wang, Y.X.; Zhang, P. Differences in Photosynthetic and Physiological Parameters of Needles between Long-Term Cone-Bearing and Non-Cone-Bearing Korean Pine Trees. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2023, 47, 137–146. [Google Scholar]
- Galvagno, M.; Rossini, M.; Migliavacca, M.; Cremonese, E.; Colombo, R.; Marra di Cella, U. Seasonal Course of Photosynthetic Efficiency in Larix decidua Mill. In Response to Temperature and Change in Pigment Composition during Senescence. Int. J. Biometeorol. 2013, 57, 871–880. [Google Scholar] [CrossRef]
- Dai, H.Y.; Hua, J.S.; Zhang, R.P.; Cai, G.Z.; Chen, L.Q. Effects of Low Temperature Stress on Seedling Growth of Plateau Japonica Rice. Plant Dis. Pests Res. Engl. Ed. 2015, 6, 39–43. [Google Scholar]
- Tan, J.H. Physiological and biochemical responses of superior provenances of Masson pine seedlings to artificial low temperature stress. Sci. Silvae Sin. 2013, 49, 51–55. [Google Scholar]
- Yamazaki, J.Y.; Tsuchiya, S.; Nagano, S.; Maruta, E. Photoprotective Mechanisms against Winter Stresses in the Needles of Abies mariesii Grown at the Tree Line on Mt. Norikura in Central Japan. Photosynthetica 2007, 45, 547–554. [Google Scholar] [CrossRef]
- Cao, P.P.; Jia, G.X. Changes in Winter Leaf Color of Two Variants of Sabina vulgaris and Their Relationship with the Antioxidant System. Plant Physiol. J. 2015, 51, 763–770. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in Flavonoid Research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Kerio, L.C.; Wachira, F.N.; Wanyoko, J.K.; Rotich, M.K. Total Polyphenols, Catechin Profiles and Antioxidant Activity of Tea Products from Purple Leaf Coloured Tea Cultivars. Food Chem. 2013, 136, 1405–1413. [Google Scholar] [CrossRef]
- Hughes, N.M.; Burkey, K.O.; Cavender-Bares, J.; Smith, W.K. Xanthophyll Cycle Pigment and Antioxidant Profiles of Winter Red (Anthocyanic) and Winter Green (Acyanic) Angiosperm Evergreen Species. J. Exp. Bot. 2012, 63, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Hughes, N.M. Winter Leaf Reddening in ‘Evergreen’ Species. New Phytol. 2011, 190, 573–581. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148C, 350–382. [Google Scholar]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. Fiehnlib: Mass Spectral and Retention Index Libraries for Metabolomics Based on Quadrupole and Time-of-Flight Gas Chromatography/Mass Spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-Mclntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for Large-Scale Metabolic Profiling of Serum and Plasma Using Gas Chromatography and Liquid Chromatography Coupled to Mass Spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]

| Classification | Picea meyeri | Juniperus sabina | Pinus tabuliformis | Buxus sinica var. parvifolia | Ammopiptanthus mongolicus |
|---|---|---|---|---|---|
| Vitamins | 0 | 0 | 2 | 0 | 0 |
| Phenylpropanoids and polyketides | 0 | 0 | 1 | 2 | 0 |
| Others | 1 | 0 | 1 | 1 | 1 |
| Organic acids and derivatives | 2 | 4 | 7 | 5 | 2 |
| Lipids | 3 | 2 | 0 | 1 | 1 |
| Carbohydrates | 11 | 8 | 10 | 9 | 5 |
| Benzene and substituted derivatives | 1 | 1 | 2 | 1 | 2 |
| Amino acids and derivatives | 0 | 0 | 1 | 7 | 1 |
| Alcohols and polyols | 1 | 0 | 0 | 3 | 2 |
| Total | 19 | 15 | 24 | 29 | 14 |
| Fold Change (January vs. July) | |||||
|---|---|---|---|---|---|
| DEMs | Picea meyeri | Juniperus sabina | Pinus tabuliformis | Buxus sinica var. parvifolia | Ammopiptanthus mongolicus |
| Threitol | 8.251 | - | - | - | - |
| D-Glyceric acid | 8.565 | - | - | - | - |
| 1,5-Anhydroglucitol | 4.033 | - | - | - | - |
| D-Glucose | 0.523 | - | - | - | - |
| Butyraldehyde | 0.896 | - | - | - | - |
| (2R,3S)-2-hydroxy-3-isopropylbutanedioic acid | 1.031 | - | - | - | - |
| Indanone | - | 8.836 | - | - | - |
| Citraconic acid | - | 3.018 | - | - | - |
| Fumaric acid | - | 0.196 | - | 1.957 | - |
| Glutaric Acid | - | 4.044 | - | - | - |
| Sarcosine | - | - | 1.491 | - | - |
| Glucoheptonic acid | - | - | 245,813.318 | - | - |
| Hexenedioic acid | - | - | 10.077 | - | - |
| Oxamic acid | - | - | 1.207 | - | - |
| 4-Hydroxy-3-methoxycinnamaldehyde | - | - | 3.943 | - | - |
| Adenine | - | - | 4.393 | - | - |
| (R)-(-)-carvone | - | - | 6.506 | - | - |
| Bis2-hydroxypropyl amine | - | - | - | 117,241.384 | - |
| Scopoletin | - | - | - | 4.337 | - |
| Phenyllactic | - | - | - | 0.372 | - |
| Phenylphosphoric acid | - | - | - | 19.296 | - |
| Lactic acid | - | - | - | 10.505 | - |
| Acetol | - | - | - | 0.326 | - |
| Cis-1,2-Dihydronaphthalene-1,2-diol | - | - | - | 0.459 | - |
| Isocitric acid | - | - | - | 1.221 | - |
| 1-Hydroxy-2-naphthoic acid | - | - | - | 0.811 | - |
| Digitoxose | - | - | - | 7.587 | - |
| Mucic acid | - | - | - | 3.207 | - |
| Valine | - | - | - | 283,766.217 | - |
| 4-Aminobutyric acid | - | - | - | 6.087 | - |
| 2,4-Diaminobutyric acid | - | - | - | 0.404 | - |
| Asparagine | - | - | - | 0.868 | - |
| Cycloleucine | - | - | - | 0.060 | - |
| 4-hydroxybenzaldehyde | - | - | - | - | 471,021.059 |
| 2-Amino-2-methylpropane-1,3-diol | - | - | - | - | 4.341 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Li, T.; Zhang, X.; Zhang, Y.; He, Z.; Shang, X.; Li, G.; Wang, R. Metabolomic Investigations Reveal Properties of Natural Low-Temperature Adaptation Strategies in Five Evergreen Trees. Forests 2025, 16, 886. https://doi.org/10.3390/f16060886
Liu B, Li T, Zhang X, Zhang Y, He Z, Shang X, Li G, Wang R. Metabolomic Investigations Reveal Properties of Natural Low-Temperature Adaptation Strategies in Five Evergreen Trees. Forests. 2025; 16(6):886. https://doi.org/10.3390/f16060886
Chicago/Turabian StyleLiu, Bin, Tao Li, Xuting Zhang, Yanxia Zhang, Zhenping He, Xiaorui Shang, Guojing Li, and Ruigang Wang. 2025. "Metabolomic Investigations Reveal Properties of Natural Low-Temperature Adaptation Strategies in Five Evergreen Trees" Forests 16, no. 6: 886. https://doi.org/10.3390/f16060886
APA StyleLiu, B., Li, T., Zhang, X., Zhang, Y., He, Z., Shang, X., Li, G., & Wang, R. (2025). Metabolomic Investigations Reveal Properties of Natural Low-Temperature Adaptation Strategies in Five Evergreen Trees. Forests, 16(6), 886. https://doi.org/10.3390/f16060886





