Abstract
Habitat degradation poses a critical threat to the Malabar slender loris (Loris lydekkerianus malabaricus), yet little is known about its microhabitat requirements in intact forest. In Aralam Wildlife Sanctuary, we combined nocturnal trail surveys (337 loris sightings) with plotless sampling of 2830 trees (86 species from 35 families) to characterize both vegetation structure and loris presence. Our results show that lorises occur almost exclusively in mildly degraded wet evergreen and secondary moist deciduous subcanopies, where understory trees and climber networks provide continuous pathways. Individuals are most often encountered at heights of 5–15 m—ascending into higher strata as the night progresses—reflecting a balance between foraging access and predator avoidance. Substrate analysis revealed strong preferences for twigs ≤ 1 cm (36.98%) and small branches 2–5 cm in diameter, oriented obliquely to minimize energetic costs and maintain stability during slow, deliberate arboreal locomotion. Day-sleeping sites were overwhelmingly located within dense tangles of lianas on large-girth trees, where intertwined stems and thorny undergrowth offer concealment from both mammalian and avian predators. Vegetation surveys documented a near-equal mix of evergreen (50.6%) and deciduous (49.4%) species—including 26 endemics (18 restricted to the Western Ghats)—with Aporosa cardiosperma emerging as the most abundant riparian pioneer, suggesting both ecological resilience and potential simplification in fragmented patches. Complementing field observations, our recent habitat-suitability modeling in Aralam indicates that broad-scale climatic and anthropogenic factors—precipitation patterns, elevation, and proximity to roads—are the strongest predictors of loris occupancy, underscoring the interplay between landscape-level processes and microhabitat structure. Together, these findings highlight the imperative of multi-strata forest restoration—planting insect-hosting native trees, maintaining continuous canopy and climber networks, and integrating small “mini-forest” modules—to recreate the structural complexity vital for slender loris conservation and the broader resilience of Western Ghats biodiversity.
1. Introduction
The examination of how nonhuman primates use space is a central concern in socio-ecological research, particularly because patterns of spatial behavior are intimately linked to a species’ ability to survive, thrive, and reproduce within a given landscape [1,2]. The choices that individuals or groups make in selecting and occupying specific microhabitats within their home ranges are shaped by a variety of ecological factors—ranging from food availability to shelter, predator presence, and microclimatic conditions. Numerous studies have demonstrated that primates often exhibit a strong preference for areas of intact, minimally disturbed primary forest or mature secondary forest. These habitats are typically characterized by greater structural complexity, including well-developed canopy layers, and tend to offer more reliable and abundant food resources, both of which are crucial for the daily energetic demands and long-term reproductive success of primate populations [3,4,5].
However, across the globe, the integrity of such habitats is increasingly compromised by anthropogenic pressures, particularly habitat fragmentation. Among the various forms of disturbance that threaten primate survival, fragmentation remains one of the most pervasive and ecologically consequential [6]. Fragmentation disrupts the continuity of forest cover, leading to a patchy landscape in which the size, shape, and isolation of habitat fragments can drastically affect species richness, alter species interactions—including predator–prey dynamics—and reduce overall habitat suitability [7,8,9]. In addition to these biological consequences, fragmentation also changes the physical environment: temperature, humidity, and light penetration are all subject to alteration along forest edges and within smaller patches, which in turn affects biotic interactions in complex and often unpredictable ways [10]. Moreover, most forest fragments, especially in heavily settled regions, are not only small and isolated but also suffer from ongoing degradation due to illegal logging, livestock grazing, invasive species, and other forms of human activity [11]. For conservation efforts to be successful, particularly in fragmented and degraded landscapes, it is imperative to restore habitats in ways that are informed by a clear understanding of a species’ ecological requirements under more pristine conditions. Unfortunately, for many primate species and subspecies, particularly those that are nocturnal, cryptic, or understudied, such baseline information from intact habitats remains unavailable or insufficiently documented.
This gap in knowledge is especially critical in the context of the Western Ghats of India, a global biodiversity hotspot that harbors an exceptionally rich assemblage of flora and fauna, including several endemic primates. Despite its ecological significance, the region faces intense conservation challenges due to widespread habitat fragmentation, high human population density, and competing demands on land use [12]. While large tracts of forest in the Western Ghats have been formally brought under a protected area network, these protected zones are interspersed with, and often surrounded by, highly fragmented and human-impacted landscapes. Restoration of these degraded fragments is both ecologically urgent and logistically complex, and demands a grounded understanding of how focal species interact with forest structure in relatively undisturbed settings. Against this backdrop, the present study focuses on the habitat structure and habitat use of the slender loris, a small, nocturnal, and arboreal primate that occurs in the Western Ghats, specifically within relatively intact rainforest environments.
The genus Loris comprises two species: the Red Slender Loris (Loris tardigradus Linnaeus, 1758) and the Grey Slender Loris (Loris lydekkerianus Cabrera, 1908), both of which are distributed in southern India and Sri Lanka. Of the two, L. lydekkerianus has a broader geographic distribution [13]. Within India, L. lydekkerianus is further subdivided into two subspecies: the Mysore Slender Loris (L. l. lydekkerianus) and the Malabar Slender Loris (L. l. malabaricus), the latter being endemic to the southwestern slopes of the Western Ghats [14,15]. In India, the slender loris is accorded the highest level of protection at the national level, under Schedule I, Part I of the Wildlife (Protection) Act, 1972, while internationally, IUCN lists the species as ‘Near Threatened’ as they are significantly declining because of widespread habitat loss and hunting through much of its range [16]. Although these animals are relatively widespread in terms of their historic range, recent decades have witnessed significant declines in their populations due to forest fragmentation, habitat degradation, and direct anthropogenic threats such as hunting and trapping. As a result, many populations today are restricted to isolated patches of remnant forest, with little connectivity between them [16]. Conservation strategies and long-term management plans remain largely undeveloped, in part because of a critical lack of data on the species’ fine-scale habitat preferences within natural, undisturbed forest ecosystems [16,17].
Loris l. malabaricus is distributed throughout the western slopes of the Western Ghats, south of the Tapti River [18]. The species has been reported from a variety of habitat types, including undisturbed rainforests, rainforest scrub at higher elevations, moist deciduous forests, degraded rainforest edges, and even within human-modified landscapes such as cardamom plantations [19,20]. Despite its apparent ecological plasticity, the species is strictly arboreal and highly dependent on canopy connectivity for its locomotion, foraging, and predator avoidance. [21,22,23]. In highly fragmented habitats, slender lorises are occasionally forced to descend to the ground—a behavior that exposes them to increased risk and suggests a compromised habitat structure [17]. They are assumed to have very slow life history parameters and a low rate of reproduction due to their consumption of toxic insects [24]. Lorises employ obscure and noiseless movements, immobility, inconspicuous retreat, or rapid flight upward as modes of defence [15].
Yet, despite its ecological importance and conservation urgency, research on L. l. malabaricus remains limited, sporadic, and geographically uneven. In order to better understand the ecological needs of this species and to inform restoration efforts in fragmented landscapes, we conducted the present study in Aralam Wildlife Sanctuary—one of the few remaining areas known to support a relatively high abundance of slender lorises [25]. Our primary aim was to characterize the structure of habitats preferred by L. l. malabaricus within a largely intact rainforest setting. This is especially critical given recent predictive models suggesting that suitable habitat for the species may decline by as much as 52% by the year 2070, primarily due to ongoing land-use changes and climate-related shifts in vegetation patterns [26].
A recent study conducted in the same sanctuary identified basal area, tree species richness, and certain forms of human disturbance—such as branch lopping and tree felling—as positive determinants of loris occupancy, suggesting that the species may tolerate, or even prefer, mildly disturbed secondary forest [14]. While previous observations indicated that lorises were less likely to be active in areas with dense climber cover, we hypothesized that such tangled vegetation might serve as preferred sleeping sites due to the concealment it offers from predators and environmental exposure. Given the loris’ arboreal lifestyle and its inability to leap, we also expected the species to favor microhabitats with slender twigs and small branches that facilitate cautious, deliberate locomotion. Additionally, we predicted that lorises would prefer supports that are oblique in orientation—rather than strictly vertical or horizontal—as such structures likely offer a more energetically efficient and biomechanically feasible path through the canopy. Through this study, we aim to contribute much-needed ecological insights into the habitat preferences of L. l. malabaricus in a relatively undisturbed rainforest context, thereby informing future conservation and habitat restoration efforts across the Western Ghats.
2. Materials and Methods
2.1. Study Site
We conducted the present study in Aralam Wildlife Sanctuary in the south Indian state of Kerala (Figure 1). Spread over 55 km2, the sanctuary is situated in the western slopes of the Western Ghats range. Lying between 11°59′ N and 11°54′ N and 75°47′ E and 75°57′ E, the elevation of the site varies from 50 m to 1145 m. The vegetation consists of moist deciduous forest, semi-evergreen forest, evergreen forest, and plantations [14,27]. The temperature varies from 21 °C to 40 °C at the foothills and 8 °C to 25 °C at high altitudes, and the annual rainfall in the region is about 3000 mm [14,27]. A tribal settlement forms a fringe around the study site, with the Aralam Farm on one side and shares boundaries with three townships/human habitation on the other. The Valapattanam river creates a natural boundary line on the township area side [14,27].
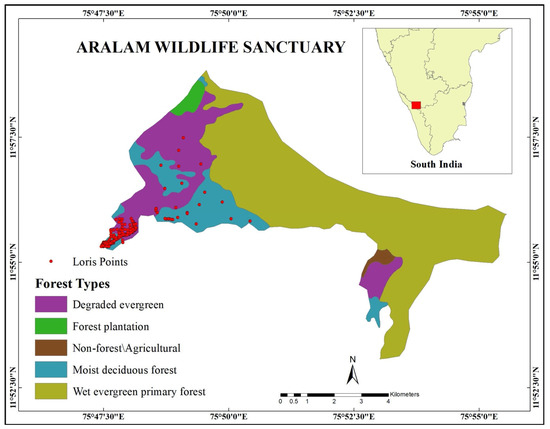
Figure 1.
Map of Aralam Wildlife Sanctuary showing the occurrence of lorises.
2.2. Observation of Slender Loris Habitat Use
SDG carried out behavioral observations of slender lorises over a period of two years from March 2014 to March 2016, for a total of 1560 h. Due to a lack of continuous visibility under the dense forest conditions, SDG recorded the behavior of the animal at the time of the first contact following the method used by Charles-Dominique and Bearder [28], Gamage [21], and Nekaris [29,30].
SDG conducted the study between 19.00 h and 6.00 h on foot with a walking speed averaging 1 km/h [25,31]. SDG carefully scanned the trees to find lorises during the walks. SDG spotted the animal by its unique orange-red tapetal reflection to light emitted by reduced intensity (~200 lumens) Petzel headlamps covered with red cellophane sheets. SDG marked the loris presence trees (trees in which lorises were spotted) using a broad satin ribbon to facilitate vegetation sampling during the day.
Lorises were detected via eyesight, and a range finder (Bushnell Medalist), was subsequently used to estimate their height on the tree. On spotting a loris, SDG recorded the data pertaining to the age class of the individual (adult male, adult female, juvenile, or infant) [32], the tree species the loris was spotted on, the height at which the loris was spotted and the tree height, the part of the tree used, clumped or single status of the tree, the size and the angular orientation (vertical, horizontal, oblique) of the substratum, and the behavioral activity of the loris. SDG also recorded the information on the presence or absence of climbers, species of climbers, and the angle of orientation of the climbers [29,32]. SDG collected the feeding ecology data only when we spotted a loris feeding or foraging for food, leading to the catching of prey and its consumption. The data included the tree species, the height at which SDG spotted the loris, and the food item it was feeding on.
2.3. Vegetation Sampling
SDG employed a plotless sampling technique [33] around 337 loris presence trees to ascertain the characteristics of the habitat used by the loris. We defined a tree as one with a girth > 10 cm. SDG divided the region around the loris presence tree into four quadrats by placing two sticks perpendicular to each other. In each quadrat, SDG measured the distance between the nearest tree (N) and the loris encountered tree (MP), and the nearest neighbor of the tree (NN) in the same direction. SDG collected all data within the area, including the four quadrats [33,34]. This area is being referred to as a plot in our study.
For classification and nomenclature of trees, we followed Sasidharan [35,36]. Data related to tree density included species and family names, distance from the previous tree (MP-N) and to the nearest neighbor (N-NN), tree height measured with a range finder, and girth (circumference) of the trunk at breast height (CBH).
As lorises cannot jump beyond 0.3 m [37], continuity of arboreal substrate is important for the slender loris’ locomotion [30,34]. We defined the arboreal connectivity as the connectivity between one tree and another through the intersection between leaves or branches (leaf connectivity) or with the help of climbers (climbers’ connectivity). We visually measured the percentage of arboreal connectivity (leaf connectivity and climbers’ connectivity) between the trees and the canopy cover using the Braun-Blanquet cover-abundance scale [34,38,39]. We examined the canopy strata of the trees based on the spread of their leaves and branches. We classified them as canopy, subcanopy, and bushes. SDG also observed lorises feeding off the ground; hence, we also measured the percentage of ground cover using the Braun-Blanquet cover-abundance scale, relative density of saplings with CBH > 10 cm, and herbaceous vegetation between MP-N and N-NN trees [34,38,39].
SDG recorded the presence or absence of climbers on each tree and the species of these climbers. Previous studies reported that Lorisiforms used dense tangles or holes as their sleeping sites [40]. So, we identified the potential sleeping sites by looking for dense climbers’ tangles, densely tangled branches forming a nest or holes on trees inside the plot or next to the plot. We recorded the presence or absence of sleeping sites, including climber tangles or branch tangles and tree holes, the tree species they were found on, and the name of the climbers they were associated with [34,41].
2.4. Data Analysis
We used the shortest distance between MP and N among the four quadrats to calculate the tree density using the T-square method [33,34]. The equation used was D = m2/(2.828 Σixi × Σizi), where D is the tree density (trees/ha), m is the number of loris presence trees (MP), xi is the distance from MP to the closest neighbor (N), and zi is the distance to the nearest neighbor (NN). We also carried out a test of random distribution using the equation t = {Σi [xi2/(xi2 + zi2)/2] − m/2}. If t is greater than +1.96, the distribution is significantly more regular than a random distribution; if it is less than −1.96, it is significantly clumped [33,34]. CBH was divided by π to give the diameter at the breast height (DBH). We calculated the basal area of the trees using the equation CBH2/4π in m2/ha.
We used chi-square tests to compare leaf and climbers’ connectivity, frequency of lorises at various heights, the size of the substrates used by the lorises, orientation of substrates and climbers, and time spent by lorises at various places. We used the Kruskal–Wallis Analysis of Variance test for temporal distribution at different time periods, and across seasons.
2.5. Ethical Note
The study was non-invasive and followed the guidelines of best practices for field Primatology from the International Primatological Society. We followed recommendations for behavioral observations on nocturnal primates, such as the use of red light and appropriate distances maintained during observations. The Principal Chief Conservator of Forests and Chief Wildlife Warden, Forest Headquarters, Vazhuthacaud, Thiruvananthapuram—695014 (Permit No. WL10-17697/2012)—approved the research protocol and adhered to the legal requirements of the Kerala Forest Department. The authors have no conflicts of interest to declare.
3. Results
3.1. Habitat Structure
3.1.1. Tree Species
The 2830 trees recorded during the vegetation study represented 86 species belonging to 35 families, with 50.57% of them being evergreen trees and 49.43% being deciduous trees. Of these, 26 species were endemic to India, among which 18 were endemic to the Western Ghats. Eight species were endemic to both India and Sri Lanka. The most abundant tree species recorded was Aporosa cardiosperma (n = 289; 10.21%). The list of floristic composition is presented in Table A1.
3.1.2. Density, Distribution, Height, Girth, and Basal Area of Trees
The tree density in the study area was 1521.04 ± 94.73 trees/ha. The tree with the highest density was Dysoxylum malabaricum with 9855.26 trees/ha. The “t” value for the test of random distribution was greater than +1.96 for three tree species (+4.04 for dead trees, +3.65 for Artocarpus hirsutus, and 2.03 for Gmelina arborea), suggesting a regular distribution, and the “t” value was less than −1.96 for five tree species, viz., Terminalia crenulata (−2.07), Dalbergia lanceolaria subsp. paniculata (−2.48), Butea monosperma (−2.34), Shorea roxburghii (−2.25), and Lagerstroemia speciosa subsp. speciosa (−2.03), suggesting significant clumping. The “t” value of the remaining 45 tree species fell between −1.96 and +1.96. While certain species showed significant deviations—either clumping or regularity—the majority of species did not deviate significantly from randomness; therefore, the overall tree distribution in the loris habitat can be interpreted as random.
The average height of the trees in the study area was 11.36 ± 0.12 m (n = 2813, range –0.30 to 67.96 m), with the largest tree being Aporosa cardiosperma with a CBH of 220 cm. Only 3 species (0.85%) out of 86 species had an average height of <5 m, viz., Terminalia catappa (3.33 ± 0.33 m), Ixora polyantha (4.99 ± 0.58 m), and Agrostistachys borneensis (5 ± 0.0 m). The average CBH was 51.71 ± 0.98 cm with a minimum of 10 cm and a maximum of 451 cm. The average DBH was 16.46 ± 0.31 cm. The average basal area was 430.41 ± 20.08 m2/ha (range 7.95 to 16,186.13). The tree with the largest basal area was Actinodaphne maderaspatana. The average basal area of the three most common families was 195.28 ± 19.47 m2/ha (n = 468; Phyllanthaceae), 856.62 ± 56.19 m2/ha (n = 260; Fabaceae), and 215.29 ± 32.80 m2/ha (n = 255; Dipterocarpaceae).
3.1.3. Canopy and Ground Cover
The study area had 40.70% of canopy tree species, 48.84% of subcanopy tree species, and 10.47% of bushy tree species. We recorded the canopy cover and the ground cover between the nearest neighbor trees 317 times, using the Braun-Blanquet scale. The canopy cover was high (50%–75%) in 43.63%, followed by moderate (25%–50%) in 24.52%, and very high (>75%) in 22.29% of the plots. Only 8.92% and 0.64% of the plots recorded low (5%–25%) and very low (<5%) canopy cover, respectively. The ground cover was low (5%–25%) in 50.79% of the plots, high (50%–75%) in 22.40% of plots, moderate (25%–50%) in 17.67%, very high (>75%) in 6.62%, and very low (<5%) in 2.52% of the plots.
3.1.4. Climbers
We identified a total of 16 species of climbers belonging to 10 families in the study area. However, we spotted the lorises on only eight species of climbers. Among them, we found the frequency of Gentum edule was found to be the highest (21.01%), followed by Acacia caesia (20.38%) (Table 1). The species richness of climbers ranged from zero to seven species in each plot. About 25.87% of the plots had two species of climbers, 23.66% had only one species, 19.56% and 19.24% of plots had three and four species of climbers, respectively. About 7.57% of the plots had five species of climbers, six species had 0.95% of the plots, only one plot had seven species of climbers, and nine plots were devoid of climbers.

Table 1.
Composition of climbers in the study area at Aralam Wildlife Sanctuary.
3.1.5. Climber and Leaf Connectivity
About 32.80% of the plots had climbers’ connectivity between < two trees, 23.25% between three and four trees, 17.83% between five and six trees, and 26.11% between seven and eight trees. About 46.50% of the plots had leaf connectivity between seven and eight trees, 35.35% between five and six trees, 12.10% between three and four trees, and 6.05% between ≤two trees. The average climbers’ connectivity between trees was 4.09 ± 0.14 (range = 0–8), and the leaf connectivity was 5.94 ± 0.10 (range = 1–8). Relatively, the leaf connectivity between trees represented by percentages of connectivity (χ2 = 58.39, df = 3, p <0.001) was more than the climbers’ connectivity (χ2 = 6.21, df = 3, p = 0.102).
3.2. Habitat Use
3.2.1. Tree Use by Lorises
We collected a total of 337 data points from the opportunistic sampling, i.e., the point at the moment of first visual contact. Out of the 86 species of trees present in the study area, the slender lorises used 51 species belonging to 28 families. They used Aporosa cardiosperma (13.38%, n = 38) and Xylia xylocarpa (11.62%, n = 33) the most, followed by Naringi crenulata (9.15%, n = 26), Holigarna arnottiana (5.28%, n = 15), Rotheca serrata (4.23%, n = 12), and Terminalia paniculata (3.87%, n = 11). We spotted lorises in the subcanopy tree species for 51.92% of our observation duration, followed by 36.54% on canopy tree species and 11.54% in bushes (χ2 = 3.00, df = 2, p = 0.223). The details on the tree species used by slender loris are summed up in Table A1. Slender lorises used trees with a mean height of 14.49 ± 0.44 m (n = 282, range 1.00–35.05 m) and were spotted at an average height of 9.57 ± 0.49 m (n = 337, range = 0.00–31.00 m). However, on height class analysis, the lorises mostly frequented trees of the 10–15 m height class. We most frequently encountered lorises in the <5 m height class (48.96%), followed by the 5–10 m height class (18.99%), and only on one occasion, above 30 m (Figure 2). The frequency of lorises at various heights varied significantly (χ2 = 131.58, df = 6, p < 0.001), showing that they were mostly at a height between 5 and 15 m. We most often encountered the slender lorises on trees with a CBH between 30 and 60 cm, having an average basal area of 220.84 ± 50.49 m2/ha (n = 91) (31.93%), followed by trees with a CBH between 60 and 90 cm having an average basal area of 418.24 ± 102.84 m2/ha (n = 57) (20%) (Figure 3).
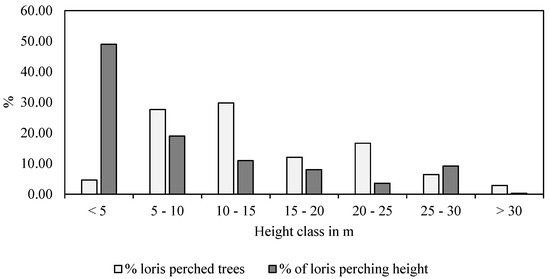
Figure 2.
Height-class analysis of loris presence in trees and perching height of slender lorises.
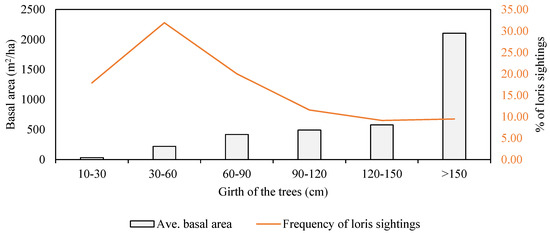
Figure 3.
Frequency of the slender loris on different trees of the basal area class.
The heights used by lorises during the three seasons did not differ (Kruskal–Wallis values H = 0.08, df = 2, p = 0.962). However, we spotted lorises at greater heights as the night progressed (Kruskal–Wallis H = 17.37, df = 3, p = 0.011). The detection also decreased as the night progressed when the lorises ascended to greater heights. We sighted lorises more during early evening and midnight than during late night and early dawn (χ2 = 88.34, df = 3, α = <0.001). Loris sightings were more during summer and monsoon than during post-monsoon (χ2 = 11.49, df = 2, p = 0.012) (Table 2).

Table 2.
Temporal distribution of slender loris sightings in different seasons.
3.2.2. Substrate Size and Orientation
Lorises (n = 338) preferred twigs of ≤1 cm (36.98%), small branches of 2–5 cm (33.43%), medium sized branches of 6–10 cm (13.02%), and the large branches > 10 cm (16.57%) (χ2 = 22.89, df = 3, p < 0.001). The orientation of these substrates (n = 330) was horizontal (27.58%), oblique (50.00%), and vertical (22.42%) (χ2 = 19.35, df = 2, p < 0.001).
3.2.3. Time Spent at Various Substrates
The lorises were sighted 42.55% of the time on climbers at a mean height of 9.83 m ± 0.77, 2.56% on the ground, and 54.89% on trees (Figure 4). There was a significant association between the proportion of sightings on different substrates (χ2 = 72.81, df = 5, p < 0.001), and nonsignificant for the height at which the loris was spotted on different substrates (χ2 = 6.00, df = 5, p = 0.306). The loris preferred to perch on the regions of the trees associated with climbers (50.89%) rather than on trees without climbers (39.94%), and on climbers not associated with trees (9.17%) (χ2 = 42.11, df = 2, p < 0.001).
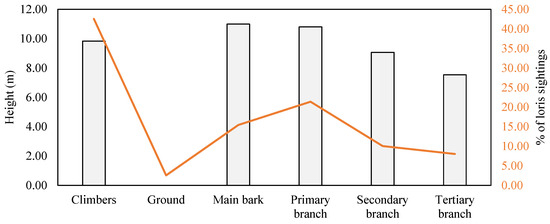
Figure 4.
Percentage of sightings of slender lorises on different substrates (orange line) and relative height where the lorises were found (grey bars).
3.2.4. Climbers Used by Lorises
Acacia caesia (33.59%), Gnetum edule (22.14%), and Aspidopterys canarensis (11.45%) were the three most important climbers used by the lorises. We most frequently found the lorises (52.94%) on climbers that were intertwined with two species, followed by single species (35.29%). The intertwining of more than two species of climbers was rare, and we found lorises on them only 11.76% of the time (Table 3). The orientation of the climbers, where the slender lorises were encountered, was oblique (47.55%), horizontal (25.17%), and vertical (27.27%).

Table 3.
Climbers used by the slender lorises at Aralam Wildlife Sanctuary.
3.2.5. Vegetation Used for Sleeping and Feeding
The sleeping sites (clumped climber tangles) were found in 87.70% of the plots sampled (n = 317) on 36 species of trees. We mostly found them on Xylia xylocarpa (15.08%), followed by Dillenia pentagyna (8.54%) (Table 4).

Table 4.
Top ten trees with sleeping sites of slender lorises.
We spotted the slender lorises feeding on 23 instances during the study period. When spotted, they were observed feeding on insects or foraging for insects. They fed on insects at an average height of 4.79 ± 0.91 m. One or more climbers (52.17%) were found most often associated with the substrate trees. However, on 30.43% of the occasions, we spotted the lorises on climbers that lacked connectivity with trees. We spotted the lorises feeding on trees without climbers on only four occasions. We observed that all the climbers, with the exception of Acacia caesia, were linked with either trees or other climbers. Slender lorises also used Acacia caesia to hold onto while feeding off the ground.
4. Discussion
4.1. Habitat Structure
The structural characteristics of the study area reflect the ecological complexity and evolutionary richness of the Western Ghats, a globally recognized biodiversity hotspot [12]. The high tree diversity and balanced composition of evergreen and deciduous species are indicative of the region’s transitional climate, altitudinal gradients, and heterogeneous microhabitats [42]. The dominance of Aporosa cardiosperma, a riparian-endemic species, underscores both its resilience to disturbance and its potential role as a keystone resource for arboreal fauna such as lorises [43]. The notable presence of endemic species further affirms the area’s biogeographic significance and aligns with floristic patterns observed in other old-growth forests across the Western Ghats [44].
The recorded tree density (1521.04 ± 94.73 trees/ha) falls within the expected range for tropical moist deciduous forests in the region (1200–1800 trees/ha) [45], suggesting that the forest retains much of its original structure. Spatial distribution patterns of dominant tree species also provide insight into forest dynamics and anthropogenic influence; the relatively uniform spacing of Artocarpus hirsutus and Gmelina arborea may reflect past selective logging or competitive exclusion mechanisms, whereas the clumped distributions of Terminalia crenulata and Shorea roxburghii could result from dispersal limitations or species-specific microhabitat preferences [46]. The overall random distribution of trees is typical of mature tropical forests, where stochastic events and ecological drift often dominate community assembly [47].
The moderate stratification in canopy height and basal area values, with emergent individuals of A. cardiosperma, suggests the presence of vertical niche availability, crucial for arboreal locomotion and predator avoidance among lorises [19]. The dominance of Fabaceae in basal area points to their role as nitrogen-fixing pioneers in recovering forests, whereas Dipterocarpaceae—associated with late-successional stages—signal forest maturity and successional complexity [48]. The limited development of the understory, with only 0.85% of species found below 5 m, implies a closed canopy that restricts light penetration, a characteristic feature of undisturbed Western Ghats rainforests [49].
4.2. Habitat Use
Our observations suggest that Malabar slender lorises preferentially occupy mildly degraded wet evergreen and secondary moist deciduous forests, particularly within subcanopy layers (5–15 m height), gradually ascending higher into the canopy as the night progresses. This vertical ascent during the night may be biologically driven by the vertical stratification of insect prey, as many nocturnal insects become more abundant in higher canopy layers later in the night, especially moths and beetles, which are key components of loris diets [50]. These findings are consistent with previous studies; Kumara et al. [19] observed lorises in the 5–20 m vertical range, with a peak at 5–15 m, and similar height preferences have been recorded in L. lydekkerianus populations in Sri Lanka, where 79% of sightings occurred below 15 m [13]. Red slender lorises (L. tardigradus tardigradus) in Sri Lanka were similarly observed in the 3.5–15 m range, with average perch heights around 8.64 ± 5.00 m [22]. Nekaris and Jayewardene also documented a predominance of activity below 12 m [51], noting that higher perch levels likely reduce detectability during nocturnal surveys. Recent phylogenetic and ecological analyses further suggest vertical niche partitioning between grey and red loris taxa, with grey subspecies often selecting higher perches—likely as a predator-avoidance tactic [52]. Altogether, these data underscore the importance of preserving multi-strata forest architecture—ensuring canopy connectivity from understory to emergent layers—to maintain the full suite of microhabitats critical for nocturnal loris behavior and conservation.
The lorises in our study were primarily found in forest patches with high tree density, high basal area, and trees in the 60–90 cm girth class. These conditions suggest low-intensity disturbance, typically limited to minor activities such as firewood collection [53]. Our findings contrast with those from Sri Lanka, where L. t. tardigradus appears to favor highly disturbed forests and even human-dominated landscapes [22,34], and L. l. nordicus, a known habitat specialist, is restricted to undisturbed montane evergreen and mist forests characterized by tall, well-connected canopies [21,54]. These differences likely reflect both ecological and anthropogenic influences, while L. t. tardigradus has adapted to fragmented, disturbed environments due to intense historical habitat loss in Sri Lanka’s wet zone, L. l. nordicus persists in climatically unique, high-elevation ecosystems with limited human access. In contrast, L. l. malabaricus appears to occupy an intermediate niche—preferring structurally complex but moderately disturbed habitats—possibly due to varying disturbance regimes, forest use patterns, and prey availability across the Western Ghats. This balance of disturbance tolerance and ecological dependency suggests both a degree of behavioral plasticity and a reliance on specific habitat features, such as vertical connectivity and microhabitat richness. These interspecific differences underscore the ecological flexibility of L. l. malabaricus and the need for site-specific conservation approaches.
4.3. Arboreal Locomotion and Substrate Use
The restricted terrestrial movement observed among slender lorises in this study aligns with their highly specialized arboreal morphology and behavioral ecology. These primates exhibit extreme adaptations for slow, deliberate quadrupedal locomotion and are anatomically incapable of leaping beyond approximately 0.3 m [37]. As such, they respond to canopy gaps by detouring rather than jumping, a strategy supported by anatomical studies of their limb and muscle structure. An important dimension of habitat use among primates is the degree of terrestriality and the use of forest strata [55,56]. From a socio-ecological perspective, forest strata use is primarily influenced by predation pressure [57], competition with sympatric species [58], and habitat structure and seasonality [59,60,61,62]. Descending to the ground exposes lorises to elevated predation risk from snakes, carnivores, and raptors, and disrupts their cryptic movement strategy—reinforcing the importance of maintaining uninterrupted canopy connectivity [18].
Instances of ground descent by lorises in fragmented landscapes have been well documented. In Sri Lanka, for example, populations inhabiting home gardens and agroforests show increased terrestriality due to broken canopy cover. Comparable patterns have been observed in Java, where installation of artificial canopy bridges for Nycticebus javanicus reduced terrestrial activity from 5.98 to 0.43 s/hour [63], and in urbanized areas where slow lorises nonetheless favored bamboo patches for movement and foraging [64]. In Kerala, similar behaviors were linked to logging and agricultural encroachment that reduced tree connectivity [17].
Our data reveal a pronounced preference for narrow substrates: twigs ≤1 cm diameter accounted for 36.98% of locomotor events, and small branches (2–5 cm) accounted for 33.43%. These diameters are consistent with the species’ need for stable yet navigable supports, enabling effective grip and reducing energetic cost [19,65]. Early natural history observations by Petter and Hladik [66] and captive studies by Subramoniam [67] support these findings, noting lorises’ avoidance of vertical supports over 2.5 cm in diameter and their inability to climb smooth trunks ≥10 cm, even in controlled environments.
Obliquely oriented substrates were used in 50% of locomotor activity, reflecting an energetically efficient strategy that balances mobility and stability. Inclined branches provide secure travel paths and serve as important escape routes from predators [51,52]. Biomechanical studies have shown that oblique substrates enable lorises to maintain a crouched posture with limbs positioned beneath the body, thereby reducing the muscular effort required to counteract gravity compared to vertical clinging or suspension. This orientation also facilitates continuous limb contact with the substrate, enhancing friction and reducing slip risk while minimizing energetic expenditure during slow, deliberate locomotion [68]. Additionally, oblique routes often present a gradient of mechanical support, allowing lorises to distribute body weight more efficiently across multiple limbs during movement [69]. Horizontal branches, in contrast, were generally used for behaviors requiring extended stasis—feeding, resting, or social interactions—while vertical clinging was limited to brief transitions due to its higher metabolic cost [15,29].
Slender lorises exhibit a highly selective pattern of sleep-site use. In our study, sleeping sites were located in 87.70% of surveyed plots—a markedly higher density than in most reported populations [29,41,51]. Lorises predominantly chose dense tangles of climbers on large-diameter trees, which offer concealment from diurnal predators and physical stability for daytime rest. These findings align with broader observations across lorisiforms, which show preferences for thorny understories, small entrance cavities, and well-connected canopies [40,65,70]. Larger trees often accumulate more extensive liana networks, creating microhabitats with both mechanical complexity and visual shielding [71,72]. These results reinforce the importance of maintaining structurally complex, vertically continuous forests to support both active and resting behaviors of slender lorises.
Our observations of loris foraging behavior further emphasize this dependence on habitat complexity. All feeding events involved insect prey, aligning with existing reports that 96% of loris diets consist of animal matter—primarily ants and termites [51]. Targeted foraging occurred on tree species known to support dense insect communities: Artocarpus hirsutus (ants, mealybugs) [73], Garcinia gummi-gutta (flea beetles) [74], Lagerstroemia microcarpa (nocturnal moths) [51], Calophyllum inophyllum (bark beetles) [75], and Antidesma bunius (hemipterans and ants) [61]. By exploiting mutualistic relationships between ants and honeydew-producing hemipterans, lorises benefit from spatially predictable prey aggregations—a strategy that requires fine-scale navigation of complex substrates [76].
Altogether, our findings underscore the central importance of preserving multi-strata forest architecture from thin, oblique twigs to dense vine tangles and large emergent trees. Such structural diversity supports the complete behavioral repertoire of the Malabar slender loris—including locomotion, foraging, and refuge use—and is essential for its conservation in both natural and human-modified landscapes.
4.4. Limitations
While this study provides valuable insights into the ecology and behavior of the Malabar slender loris (Loris lydekkerianus malabaricus), several limitations must be acknowledged. First, the study duration—although spanning two years—is relatively short for detecting long-term patterns or interannual variability, particularly in relation to reproductive cycles, habitat shifts, and population dynamics. Seasonal variation, especially the monsoon period, likely influenced insect availability and, by extension, loris foraging behavior; however, due to logistical constraints, fine-scale seasonal comparisons were limited.
Second, L. l. malabaricus is a cryptic, small-bodied nocturnal primate inhabiting dense, multilayered vegetation, making detection inherently challenging. The effectiveness of red-filtered headlamps in locating lorises relies heavily on the retro-reflective “eyeshine” produced when the animal is looking directly at the observer. If the loris is oriented away or its line of sight is obstructed, detection is virtually impossible. As such, there is a high likelihood of underestimating true encounter rates or overlooking individuals entirely.
Third, the field site is also home to large and potentially dangerous fauna—including elephants (Elephas maximus), tigers (Panthera tigris), leopards (Panthera pardus), and venomous snakes (e.g., Bungarus caeruleus, Naja naja, Daboia russelii)—which further constrained fieldwork, particularly during night surveys. Safety protocols had to be strictly followed, often reducing the duration or spatial extent of surveys.
Additional methodological limitations include the use of non-invasive visual encounter surveys without the aid of thermal imaging or radio-telemetry, which could have increased detection probabilities. Habitat data, although systematically collected, might not fully capture microhabitat use or fine-scale structural complexity due to accessibility issues in thick understory vegetation.
Taken together, these constraints suggest that while the trends reported are robust within the observed framework, future studies with longer durations, broader spatial coverage, and enhanced detection tools are essential for a more comprehensive understanding of this elusive primate’s ecology.
5. Conclusions
Our study demonstrates that Malabar slender lorises exhibit finely tuned microhabitat preferences that optimize foraging efficiency and predator avoidance. These primates forage almost exclusively on insect prey—predominantly ants and termites—with their diet spanning nine insect orders, and they select twigs ≤1 cm and small branches (2–5 cm) for locomotion, exploiting oblique substrates that minimize energy expenditure, while maintaining stability [15,40,51,53]. Diurnal refuge sites are overwhelmingly located in dense liana tangles, thorny undergrowth, and small nest holes, providing concealment from mammalian and avian predators through structural complexity [50,51,70]. These behavioral specializations underscore the critical role of multi-strata forest architecture—maintaining a mosaic of canopy, subcanopy, and understory elements—to sustain the full repertoire of loris’ ecological needs [29,66].
The Indian Institute of Science’s “mini-forest” experiment provides a compelling model for applying these insights to habitat restoration. A 1.75 ha Deccan scrub plot planted with native Western Ghats evergreen species, structurally complex woodlands developed within 25 years, now supports a thriving Mysore slender loris (L. l. lydekkerianus) population [77,78]. Beyond ex situ conservation value, this mini-forest functions as a high-performance carbon sink, lowers ambient temperatures by up to 2 °C, and has driven groundwater recharge—raising local water tables by nearly 190 ft—thereby contributing to climate resilience and hydrological stability [77]. To replicate these successes across the slender loris range, restoration initiatives should prioritize multi-strata plantings of insect-hosting trees (e.g., Artocarpus hirsutus, Garcinia gummi-gutta, Lagerstroemia microcarpa), integrate mini-forest modules in degraded and urban fringe landscapes, and embed long-term monitoring frameworks as recommended by global restoration guidelines [79]. Our recent MaxEnt-based habitat-suitability modeling in Aralam Wildlife Sanctuary identified precipitation of the warmest quarter, precipitation of the driest month, distance from roads, and elevation as the most influential predictors of Malabar slender loris (Loris lydekkerianus malabaricus) distribution, emphasizing that broad-scale climatic and anthropogenic variables—rather than local canopy connectivity measures—govern habitat suitability in this landscape [26]. Collectively, these findings validate multi-strata restoration approaches—integrating insect-hosting trees, dense climbers, and varied branch architectures—as scalable conservation strategies across the slender loris range.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16060876/s1, Table S1: Trees, loris points, and point sampling of ribboned trees.
Author Contributions
S.D.G. contributed to conceptualization, visualization, supervision, project administration, funding acquisition, investigation, resources, data curation, validation, formal analysis, and manuscript writing—original draft preparation. J.J.E. was involved in conceptualization, validation, formal analysis, and manuscript writing. M.C. contributed to visualization, resources, and writing—review and editing. M.S. contributed to conceptualization, validation, writing—review and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Women Scientist Scheme-A (WOS-A) fellowship under the Department of Science and Technology, Government of India (Grant No. SR/WOS-A/LS-89/2013) to Smitha D. Gnanaolivu.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank the Forest Department of Kerala for the permission to conduct the study. We also thank the officials of Aralam Wildlife Sanctuary, Kerala, for their timely help and support in the field work, namely Madhoosudhan, Sushant, Biju, Radhakrishna, and Laxmana, for spending long hours surveying and for keeping us safe from elephants. We thank all the volunteers who helped us collect data. Mewa Singh thanks the Science and Engineering Research Board, Government of India, for the award of SERB Distinguished Fellowship (Award number: SB/S9/YSCP/SERB-DF/2018(1).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Floristic composition of the study area of Aralam Wildlife Sanctuary.
Table A1.
Floristic composition of the study area of Aralam Wildlife Sanctuary.
| Species | All Trees | Loris Encountered | Average Height | Average DBH | Distribution | Tree Type | |
|---|---|---|---|---|---|---|---|
| Count | % | Tree (m) | Occupied (m) | (cm) | |||
| Aporosa cardiosperma | 289 | 13.38 | 9.29 ± 0.77 | 9.46 ± 1.36 | 12.77 ± 0.58 | India and Sri Lanka | Evergreen |
| Xylia xylocarpa | 226 | 11.62 | 19.02 ± 1.28 | 9.99 ± 1.41 | 29.5 ± 1.11 | Indo-Malesia | Deciduous |
| Naringi crenulata | 221 | 9.15 | 12.16 ± 1.13 | 7.74 ± 1.49 | 10.93 ± 6.46 | Indo-Malesia | Deciduous |
| Hopea parviflora | 145 | 2.46 | 11.7 ± 2.39 | 13.43 ± 4.56 | 11.93 ± 0.84 | Endemic to Western Ghats | Evergreen |
| Antides mabunius | 136 | 1.76 | 11.28 ± 2.24 | 14.93 ± 5.71 | 13.06 ± 0.91 | Indo-Malesia to Australia and South China | Evergreen |
| Gossypium herbaceum | 83 | - | 9.15 ± 5.07 | - | 10.78 ± 0.65 | India, Arab, Persia, Afghanistan, Turkey, North Africa, Spain, Ukraine, China | Evergreen |
| Actinodaphne maderaspatana | 82 | 2.46 | 8.62 ± 1.77 | 9.08 ± 2.84 | 15.5 ± 2.18 | Endemic to Western Ghats | Evergreen |
| Antiaris toxicaria | 71 | - | 17.29 ± 1.78 | - | 28.87 ± 1.79 | Paleotropics | Evergreen |
| Terminalia paniculata | 70 | 3.87 | 12.69 ± 1.87 | 8.73 ± 2.69 | 28.62 ± 2.91 | India | Deciduous |
| Schleichera oleosa | 63 | 2.82 | 22.14 ± 2.26 | 11.34 ± 2.91 | 18.3 ± 1.87 | Indo-Malesia | Deciduous |
| Chionanthus mala-elengi subsp. mala-elengi | 59 | 1.76 | 6.71 ± 0.67 | 8.41 ± 3.44 | 10.07 ± 0.48 | Endemic to Peninsular India | Evergreen |
| Xanthophyllum flavescens | 57 | 1.76 | 9.63 ± 1.23 | 11.2 ± 3.12 | 8.33 ± 0.51 | China to Indomalaysia | Deciduous |
| Strychnosnux-vomica | 55 | 0.35 | 21.95 | 9.14 | 7.47 ± 0.79 | Indo-Malesia | Deciduous |
| Chionanthus albidiflorus | 54 | - | 7.64 ± 3.95 | - | 8.83 ± 0.95 | Indo-Malesia | Evergreen |
| Dead Trees | 53 | 3.17 | 16.49 ± 1.69 | 8.41 ± 2.93 | 28.47 ± 1.98 | ||
| Polyalthia longifolia | 53 | 0.35 | 10.06 | 1 | 6.72 ± 0.71 | India and Sri Lanka | Evergreen |
| Stereospermum colais | 50 | 0.7 | 17.83 ± 7.17 | 13.5 ± 8.5 | 19.74 ± 1.66 | Indo-Malesia | Deciduous |
| Syzygium cumini | 50 | 0.7 | 19.2 ± 9.45 | 15 | 10.25 ± 0.99 | Indo-Malesia | Deciduous |
| Vateria indica | 49 | 1.06 | 16.97 ± 7.82 | 13.53 ± 8.67 | 16.98 ± 2.16 | Endemic to Western Ghats | Evergreen |
| Holigarna arnottiana | 47 | 5.28 | 17.43 ± 1.85 | 12.04 ± 2.71 | 25.85 ± 2.96 | Endemic to Western Ghats | Evergreen |
| Plumeria obtusa | 45 | 2.46 | 11.58 ± 1.91 | 5.84 ± 1.61 | 21.55 ± 3.73 | Central America, from Mexico to Panama | Deciduous |
| Sapindus trifoliatus | 44 | 1.41 | 14.86 ± 1.82 | 8.65 ± 3.87 | 13.41 ± 1.56 | South Asia | Deciduous |
| Artocarpus hirsutus | 43 | 3.52 | 21.82 ± 0.89 | 16.72 ± 3.71 | 29.53 ± 3.43 | Endemic to Western Ghats | Evergreen |
| Dillenia pentagyna | 40 | 2.11 | 27.13 ± 2.67 | 10.48 ± 4.81 | 51.41 ± 6.1 | China to Indo-Malesia | Deciduous |
| Baccaurea courtallensis | 39 | 0.35 | 8.53 | 2.74 | 7.19 ± 0.47 | Endemic to Peninsular India | Evergreen |
| Myristica beddomei | 38 | 2.82 | 12.03 ± 2.45 | 4.72 ± 2.95 | 12.81 ± 1.13 | Endemic to Peninsular India | Evergreen |
| Lagerstroemia speciosa subsp. Speciosa | 37 | 0.7 | 25.3 | 16.22 ± 13.79 | 22.07 ± 2.1 | S-China (Yunnan), India, | Evergreen |
| Shorea roxburghii | 36 | 0.7 | 7.01 | 4 ± 1 | 7.92 ± 1.16 | Indo-Malesia | Deciduous |
| Rotheca serrata | 33 | 4.23 | 10.24 ± 1.13 | 15.24 ± 3.21 | 12.26 ± 1.35 | Indo-Malesia | Deciduous |
| Drypetes venusta | 32 | 1.06 | 10.06 ± 2.14 | 13.45 ± 8.59 | 11.15 ± 1.68 | Endemic to Western Ghats | Deciduous |
| Buchanania axillaris | 30 | 2.11 | 13.56 ± 3.56 | 6.08 ± 2.45 | 13.75 ± 1.95 | India and Sri Lanka, Myanmar | Deciduous |
| Anacolosa densiflora | 26 | 0.7 | 12.04 ± 0.15 | 16.5 ± 13.5 | 17.95 ± 3.82 | India | Evergreen |
| Madhuca longifolia | 26 | 0.35 | 7.32 | 4.57 | 7.09 ± 0.69 | India and Myanmar | Deciduous |
| Lagerstroemia microcarpa | 25 | 0.35 | 22.56 | 18 | 28.01 ± 3.86 | Endemic to Western Ghats | Deciduous |
| Clausena anisata | 23 | 1.41 | 13.94 ± 0.89 | 9.93 ± 6.06 | 11.93 ± 1.53 | India, Nepal, Sri Lanka, and Africa | Evergreen |
| Butea monosperma | 20 | 0.7 | 17.53 ± 1.68 | 6.1 ± 6.1 | 19.91 ± 4.79 | India, Sri Lanka and S.E. Asia. | Deciduous |
| Gmelina arborea | 20 | 0.7 | 17.53 ± 1.37 | 6.86 ± 2.29 | 22.47 ± 4.69 | Indo-Malesia | Deciduous |
| Terminalia bellirica | 19 | 0.7 | 22.1 ± 0.15 | 6 ± 4 | 40.94 ± 5.95 | Indo-Malesia | Deciduous |
| Vatica chinensis | 18 | - | 11.08 ± 5.59 | - | 12.41 ± 1.22 | India and Sri Lanka | Evergreen |
| Ixora polyantha | 17 | - | 4.99 ± 2.37 | - | 6.13 ± 0.35 | Endemic to Western Ghats | Evergreen |
| Tarenna monosperma | 17 | - | 6.71 ± 4.18 | - | 5.82 ± 0.59 | Endemic to Western Ghats | Evergreen |
| Lannea coromandelica | 15 | 1.41 | 19.66 ± 3.55 | 8.75 ± 3.45 | 31.64 ± 5.79 | Southern Asia | Deciduous |
| Litsea coriacea | 15 | 1.06 | 10.87 ± 1.93 | 15.5 ± 8.23 | 10.12 ± 2.03 | Endemic to Western Ghats | Evergreen |
| Knema attenuata | 14 | - | 12.21 ± 7.15 | - | 8.14 ± 0.85 | Endemic to Western Ghats | Deciduous |
| Melia azedarach | 14 | - | 5.70 ± 1.73 | - | 7.55 ± 0.89 | Paleotropics | Deciduous |
| Solanum erianthum | 12 | 0.35 | 8.23 | 5.5 | 8.73 ± 0.98 | South East Asia and North Australia | Deciduous |
| Vitex altissima | 12 | 0.35 | 22.25 | 30 | 30.58 ± 4.59 | India | Deciduous |
| Terminalia crenulata | 11 | 1.06 | 25.1 ± 0.81 | 11.38 ± 8.33 | 91.56 ± 6.59 | Indo-Malesia | Deciduous |
| Olea wightiana | 11 | 0.7 | 12.19 ± 3.96 | 10 ± 5 | 10.91 ± 2.07 | Endemic to Peninsular India | Deciduous |
| Alstonia scholaris | 10 | 0.35 | 7.62 | 10 | 9.2 ± 2.41 | South and South East Asia to Australia | Evergreen |
| Artocarpus gomezianus | 10 | 0.35 | 11.89 | 9.14 | 23.78 ± 4.22 | India and Sri Lanka | Deciduous |
| Cinnamomum keralaense | 9 | 0.35 | 9.14 | 20 | 11.74 ± 2.26 | Endemic to Western Ghats | Evergreen |
| Atalantia monophylla | 8 | - | 11.05 ± 1.70 | - | 15.24 ± 2.8 | Indo-Malesia | Deciduous |
| Memecylon umbellatum | 8 | - | 9.75 ± 2.96 | - | 11.09 ± 2.03 | India and Sri Lanka | Deciduous |
| Polyalthia fragrans | 8 | - | 4.23 ± 3.11 | - | 8.12 ± 1.93 | Endemic to Western Ghats | Evergreen |
| Erythrina stricta | 8 | 1.41 | 21.11 ± 4.17 | 6.69 ± 3.77 | 26.94 ± 3.71 | India, China, Nepal, Thailand, and Vietnam | Deciduous |
| Scolopia crenata | 8 | 0.35 | 8.53 | 10 | 7.44 ± 0.76 | Indo-Malesia | Evergreen |
| Hopea ponga | 7 | - | 13.44 ± 9.11 | - | 14.23 ± 4.51 | Endemic to Western Ghats | Evergreen |
| Lepisanthes tetraphylla | 7 | - | 6.14 ± 2.14 | - | 5.5 ± 1.37 | Indo-Malesia and Africa | Evergreen |
| Sterculia villosa | 6 | - | 6.14 ± 2.14 | - | 9.97 ± 3.43 | South Asia and Myanmar | Deciduous |
| Dalbergia lanceolaria subsp. paniculata | 6 | 1.06 | 9.75 | 5.19 ± 2.62 | 12.47 ± 2.19 | India and Myanmar | Deciduous |
| Adina cordifolia | 5 | - | 15.02 ± 4.97 | - | 8.94 ± 1.23 | India, Myanmar, Sri Lanka, and Indo-China | Deciduous |
| Agrostistachys borneensis | 5 | - | 5.00 ± 1.14 | - | 9.17 ± 0.23 | Indo-Malaya | Evergreen |
| Elaeocarpus serratus | 5 | - | 10.24 ± 6.81 | - | 16.81 ± 3.34 | India, Nepal, Malaysia | Evergreen |
| Neolamarckia cadamba | 5 | - | 8.47 ± 5.48 | - | 6.18 ± 1.48 | Asia, the Pacific, and Australia | Deciduous |
| Commiphora caudata | 5 | 0.7 | 21.18 ± 1.98 | 21.5 ± 6.5 | 21.84 ± 5.25 | India and Sri Lanka | Deciduous |
| Bridelia retusa | 4 | - | 5.87 ± 1.48 | - | 8.1 ± 1.12 | Indo-Malaya | Deciduous |
| Grewia tiliifolia Vahl | 4 | - | 15.62 ± 1.2 | - | 47.35 ± 13.13 | Tropical Africa, India to Indo-China | Deciduous |
| Wrightia arborea | 4 | - | 12.10 ± 2.9 | - | 27.06 ± 7.72 | Indo-Malesia | Deciduous |
| Cinnamomum malabatrum | 4 | 0.35 | 29.57 | 10 | 18.46 ± 6.24 | Endemic to Western Ghats | Evergreen |
| Sterculia balanghas | 4 | 0.35 | 10 | 10 | 5.33 ± 1.94 | South Asia and Myanmar | Deciduous |
| Plumeria rubra | 3 | - | 7.92 ± 2.60 | - | 4.35 ± 0.46 | Native of Tropical America; widely naturalized elsewhere in the tropics | Deciduous |
| Casearia ovata | 2 | - | 5.94 ± 1.07 | - | 22.76 ± 18.62 | India and Sri Lanka | Evergreen |
| Garcinia morella | 2 | - | 6.40 ± 0 | - | 6.37 ± 0 | Indo-Malesia | Evergreen |
| Mallotus nudiflorus | 2 | - | 13.16 ± 5.84 | - | 9.55 ± 1.91 | Indo-Malaya | Evergreen |
| Terminalia alata | 2 | - | 14.62 ± 1.2 | - | 6.05 ± 0.64 | India | Deciduous |
| Terminalia catappa | 2 | - | 3.33 ± 0.46 | - | 4.3 ± 0.16 | Indo-Malesia | Deciduous |
| Azadirachta indica | 1 | - | 16 | - | 21.65 | Indo-Malesia | Evergreen |
| Calophyllum inophyllum | 1 | - | - | 35.01 | Paleotropics | Evergreen | |
| Garcinia gummi-gutta | 1 | - | 5.49 | - | 3.18 | South India and Sri Lanka | Evergreen |
| Mangifera indica | 1 | - | 8.53 | - | 10.5 | Native to India and Burma. | Evergreen |
| Manilkara roxburghiana | 1 | - | 5.23 | - | 14.01 | Endemic to Western Ghats | Evergreen |
| Manilkara zapota | 1 | - | 8.23 | - | 53.16 | India and tropical America | Evergreen |
| Scleropyrum pentandrum | 1 | - | 11.28 | - | 6.37 | India and Sri Lanka | Evergreen |
| Spondia spinnata | 1 | - | 17.37 | - | 22.92 | Indo-Malesia | Deciduous |
| Dysoxylum malabaricum | 1 | 0.35 | 11.28 | 5 | 15.92 | Endemic to Western Ghats | Evergreen |
| Syzygium mundagam | 1 | 0.35 | 12.5 | 3.04 | 18.46 | Endemic to Western Ghats | Evergreen |
References
- Overdorff, D.J. Ecological correlates to social structure in two lemur species in Madagascar. Am. J. Phys. Anthropol. 1996, 100, 487–506. [Google Scholar] [CrossRef]
- Riley, E.P. Ranging patterns and habitat use of Sulawesi Tonkean macaques (Macaca tonkeana) in a human-modified habitat. Am. J. Primatol. 2008, 70, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Heiduck, S. The use of disturbed and undisturbed forest by masked titi monkeys Callicebus personatus melanochir is proportional to food availability. Oryx 2002, 36, 133–139. [Google Scholar] [CrossRef]
- Li, Y. The effect of forest clear-cutting on habitat use in Sichuan snub-nosed monkey (Rhinopithecus roxellana) in Shennongjia Nature Reserve, China. Primates 2004, 45, 69–72. [Google Scholar] [CrossRef]
- Campera, M.; Serra, V.; Balestri, M.; Barresi, M.; Ravaolahy, M.; Randriatafika, F.; Donati, G. Effects of habitat quality and seasonality on ranging patterns of collared brown lemur (Eulemur collaris) in littoral forest fragments. Int. J. Primatol. 2014, 35, 957–975. [Google Scholar] [CrossRef]
- Marsh, L.K.; Chapman, C.A. Primates in Fragments: Complexity and Resilience; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ramsay, M.S.; Mercado Malabet, F.; Klass, K.; Ahmed, T.; Muzaffar, S. Consequences of habitat loss and fragmentation for primate behavioral ecology. In Primates in Anthropogenic Landscapes: Exploring Primate Behavioural Flexibility Across Human Contexts; Springer International Publishing: Cham, Switzerland, 2023; pp. 9–28. [Google Scholar]
- Arroyo-Rodríguez, V.; Mandujano, S. Conceptualization and measurement of habitat fragmentation from the primates’ perspective. Int. J. Primatol. 2009, 30, 497–514. [Google Scholar] [CrossRef]
- Tabarelli, M.; Gascon, C. Lessons from fragmentation research: Improving management and policy guidelines for biodiversity conservation. Conserv. Biol. 2005, 19, 734–739. [Google Scholar] [CrossRef]
- Lienert, J. Habitat fragmentation effects on fitness of plant populations—A review. J. Nat. Conserv. 2004, 12, 53–72. [Google Scholar] [CrossRef]
- Umapathy, G.; Kumar, A. Impacts of forest fragmentation on lion-tailed macaque and Nilgiri langur in Western Ghats, south India. In Primates in Fragments: Ecology and Conservation; Marsh, L.K., Ed.; Kluwer Academic: Dordrecht, The Netherlands; Plenum Press: New York, NY, USA, 2003; pp. 163–189. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Perera, M.; Sandun, J. A review of the distribution of Grey Slender Loris (Loris lydekkerianus) in Sri Lanka. Primate Conserv. 2008, 23, 89–96. [Google Scholar] [CrossRef]
- Gnanaolivu, S.D.; Kumara, H.N.; Singh, M.; Sudarsanam, D. Ecological determinants of Malabar Slender Loris (Loris lydekkerianus malabaricus, Cabrera 1908) occupancy and abundance in Aralam Wildlife Sanctuary, Western Ghats, India. Int. J. Primatol. 2020, 41, 511–524. [Google Scholar] [CrossRef]
- Schulze, H.; Meier, B. The subspecies of Loris tardigradus and their conservation status: A review. In Creatures of the Dark: The Nocturnal Prosimians; Alterman, L., Doyle, G.A., Izard, M.K., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 193–210. [Google Scholar]
- Dittus, W.; Singh, M.; Gamage, S.N.; Kumara, H.N.; Kumar, A.; Nekaris, K.A.I. Loris lydekkerianus (amended version of 2020 assessment). In The IUCN Red List of Threatened Species; IUCN Biodiversity Assessment & Knowledge Team: Red List Unit: Cambridge, UK, 2022; p. E.T44722A217741551. [Google Scholar] [CrossRef]
- Radhakrishna, S.; Singh, M. Social behaviour of the slender loris (Loris tardigradus lydekkerianus). Folia Primatol. 2002, 73, 181–196. [Google Scholar] [CrossRef]
- Nekaris, K.; Bearder, S. The lorisiform primates of Asia and mainland Africa: Diversity shrouded in darkness. In Primates in Perspective; Campbell, C.J., Fuentes, A., Mackinnon, K.C., Panger, M., Bearder, S.K., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 24–45. [Google Scholar]
- Kumara, H.N.; Singh, M.; Kumar, S. Distribution, habitat correlates, and conservation of Loris lydekkerianus in Karnataka, India. Int. J. Primatol. 2006, 27, 941–969. [Google Scholar] [CrossRef]
- Radhakrishna, S.; Kumara, H.N.; Sasi, R. Distribution patterns of slender loris subspecies (Loris lydekkerianus) in Kerala, Southern India. Int. J. Primatol. 2011, 32, 1007–1019. [Google Scholar] [CrossRef]
- Gamage, S.N.; Padmalal, U.K.G.K.; Kotagama, S.W. Montane slender loris (Loris tardigradus nycticeboides) is a critically endangered primate that needs more conservation attention. J. Dept. Wildl. Conserv. 2014, 2, 77–83. [Google Scholar]
- Gamage, S.; Liyanage, W.; Weerakoon, D.; Gunwardena, A. Habitat quality and availability of the Sri Lanka red slender Loris Loris tardigradus tardigradus (Mammalia: Primates: Lorisidae) in the Kottawa Arboretum. J. Threat. Taxa 2009, 1, 65–71. [Google Scholar] [CrossRef]
- Hettiarachchi, C.J.; Gamage, S.N.; Mahanayakage, C.A.; Padmalal, U.K.G.K.; Kotagama, S.W. Habitat suitability modelling for Montane Slender Loris in the Hakgala Strict Nature Reserve: A Geoinformatics approach. Wildlanka 2015, 3, 144–147. [Google Scholar]
- Rasmussen, D.T.; Izard, M.K. Scaling of growth and life history traits relative to body size, brain size, and metabolic rate in lorises and galagos (Lorisidae, Primates). Am. J. Phys. Anthropol. 1988, 75, 357–367. [Google Scholar] [CrossRef]
- Sasi, R.; Kumara, H.N. Distribution and relative abundance of the slender loris Loris lydekkerianus in Southern Kerala, India. Primate Conserv. 2014, 28, 165–170. [Google Scholar] [CrossRef]
- Gnanaolivu, S.D.; Erinjery, J.J.; Campera, M.; Singh, M. Distribution and habitat suitability of the Malabar Slender Loris (Lorislydekkerianus malabaricus) in the Aralam Wildlife Sanctuary, India. Land 2025, 14, 872. [Google Scholar] [CrossRef]
- Menon, A.R.R. Vegetation Mapping and Analysis of Aralam Wildlife Sanctuary Using Remote Sensing Techniques; KFRI Research Report 168; Kerala Forest Research Institute (KFRI): Kerala, India, 1999. [Google Scholar]
- Charles-Dominique, P.; Bearder, S.K. Field studies of lorisoid behaviour: Methodological aspects. In The Study of Prosimian Behaviour; Doyle, G.A., Martin, R.D., Eds.; Academic Press: New York, NY, USA, 1979; pp. 567–629. [Google Scholar]
- Nekaris, K.A. Foraging behaviour of the slender loris (Loris lydekkerianus lydekkerianus): Implications for theories of primate origins. J. Hum. Evol. 2005, 49, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Nekaris, K.A.I. Activity budget and positional behavior of the Mysore slender loris (Loris tardigradus lydekkarianus): Implications for “slow climbing” locomotion. Folia Primatol. 2001, 72, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Sterling, E.J.; Ramaroson, M.G. Rapid Assessment of the primate fauna of the eastern slopes of the Réserve Naturelle Intégrale d’Andringitra. Fieldiana Zool. 1996, 85, 293–303. [Google Scholar]
- Nekaris, K.A.I. Observations of mating, birthing and parental behaviour in three subspecies of Slender Loris (Loris tardigradus and Loris lydekkerianus) in India and Sri Lanka. Int. J. Primatol. 2003, 74, 5–6. [Google Scholar] [CrossRef]
- Sutherland, W.J. Ecological Census Techniques; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Nekaris, K.A.I.; Liyanage, W.; Gamage, S. Influence of forest structure and composition on population density of the red slender loris Loris tardigradus tardigradus in Masmullah proposed forest reserve, Sri Lanka. Mammalia 2005, 69, 201–210. [Google Scholar] [CrossRef]
- Sasidharan, N. Illustrated Manual on Tree Flora of Kerala Supplemented with Computer-Aided Identification; KFRI Research Report 282; Kerala Forest Research Institute (KFRI): Kerala, India, 2006. [Google Scholar]
- Sasidharan, N. (Ed.) Forest Trees of Kerala, a Checklist Including Exotics; KFRI Handbook No. 2; Kerala Forest Research Institute (KFRI): Kerala, India, 2010. [Google Scholar]
- Sellers, W. A biomechanical investigation into the absence of leaping in the locomotor repertoire of the slender loris (Loris tardigradus). Folia Primatol. 1996, 67, 1–14. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensociologie: Grundzuge der Vegetationskunde, 3rd ed.; Springer: Vienna, Austria, 1964. [Google Scholar]
- Kent, M.; Coker, P. Vegetation Description and Analysis: A Practical Approach; John Wiley and Sons: New York, NY, USA, 1992; pp. 167–169. [Google Scholar]
- Svensson, M.S.; Nekaris, K.A.I.; Bearder, S.K.; Bettridge, C.; Butynski, T.; Cheyne, S.M.; Das, N.; de Jong, Y.; Luhrs, A.M.; Luncz, L.; et al. Sleep patterns, daytime predation and the evolution of diurnal sleep site selection in lorisiforms. Am. J. Phys. Anthropol. 2018, 166, 563–577. [Google Scholar] [CrossRef]
- Bearder, S.K.; Nekaris, K.A.I.; Buzzell, C.A. Dangers in the night: Are some nocturnal primates afraid of the dark? In Eat or Be Eaten: Predator Sensitive Foraging Among Primates; Miller, L.E., Ed.; Cambridge University Press: Cambridge, UK, 2002; pp. 21–43. [Google Scholar]
- Pascal, J.P.; Ramesh, B.R.; Franceschi, D.D. Wet evergreen forest types of the southern Western Ghats, India. Trop. Ecol. 2004, 45, 281–292. [Google Scholar]
- Karuppusamy, S. Vegetation and Forest Types of the Western Ghats. In Biodiversity Hotspot of the Western Ghats and Sri Lanka; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 25–61. [Google Scholar]
- Shigwan, B.K.; Kulkarni, A.; Smrithy, V.; Datar, M.N. An overview of tree ecology and forest studies in the Northern Western Ghats of India. iForest 2024, 17, 213. [Google Scholar] [CrossRef]
- Ganesh, T.; Ganesan, R.; Devy, M.S.; Davidar, P.; Bawa, K.S. Assessment of plant biodiversity at a mid elevation evergreen forest of Kalakad–Mundanthurai Tiger Reserve, Western Ghats, India. Curr. Sci. 1996, 71, 379–392. [Google Scholar]
- Murali, K.S.; Shankar, U.; Shaanker, R.U.; Ganeshaiah, K.N.; Bawa, K.S. Extraction of non-timber forest products in the forests of Biligiri Rangan Hills, India. 2. Impact of NTFP extraction on regeneration, population structure, and species composition. Econ. Bot. 1996, 50, 252–269. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef]
- Sundarapandian, S.M.; Swamy, P.S. Forest structure in the Western Ghats. Proc. Indian Natl. Sci. Acad. 1999, 109, 517–529. [Google Scholar]
- Parthasarathy, N. Climber diversity in tropical forests. J. Trop. Ecol. 1999, 15, 315–332. [Google Scholar]
- Maguire, D.Y.; Robert, K.; Brochu, K.; Larrivée, M.; Buddle, C.M.; Wheeler, T.A. Vertical stratification of beetles (Coleoptera) and flies (Diptera) in temperate forest canopies. Environ. Entomol. 2014, 43, 9–17. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Jayewardene, J. Survey of the slender loris (Primates, Lorisidae) in Sri Lanka. J. Zool. 2003, 259, 327–334. [Google Scholar]
- Gamage, S.; Marikar, F.; Groves, C.; Turner, C.; Padmalal, K.; Kotagama, S. Phylogenetic relationship among slender loris species (Primates, Lorisidae: Loris) in Sri Lanka based on mtDNA CO1 barcoding. Turk. J. Zool. 2019, 43, 609–616. [Google Scholar] [CrossRef]
- Mohandass, D.; Campbell, M.J.; Hughes, A.C.; Mammides, C.; Davidar, P. Edge disturbance drives liana abundance increase and alteration of liana–host tree interactions in tropical forest fragments. Ecol. Evol. 2017, 8, 4237–4251. [Google Scholar]
- Gamage, S.N.; Hettiarachchi, C.J.; Mahanayakage, C.A.; Padmalal, U.K.G.K.; Kotagama, S.W. Factors influencing site occupancy of Montane Slender Loris (Loris tardigradus nycticeboides) in Sri Lanka. Wildlanka 2015, 3, 68–73. [Google Scholar]
- Eppley, T.M.; Hoeks, S.; Chapman, C.A.; Ganzhorn, J.U.; Hall, K.; Owen, M.A.; Adams, D.B.; Allgas, N.; Amato, K.R.; Andriamahaihavana, M.; et al. Factors influencing terrestriality in primates of the Americas and Madagascar. Proc. Natl. Acad. Sci. USA 2022, 119, e2121105119. [Google Scholar] [CrossRef]
- Estrada, G.R.; Marshall, A.J. Terrestriality across the primate order: A review and analysis of ground use in primates. Evol. Anthropol. 2024, 33, e22032. [Google Scholar] [CrossRef] [PubMed]
- McGraw, W.S.; Bshary, R. Association of terrestrial mangabeys (Cercocebus atys) with arboreal monkeys: Experimental evidence for the effects of reduced ground predator pressure on habitat use. Int. J. Primatol. 2002, 23, 311–325. [Google Scholar] [CrossRef]
- Thiel, S.; Tschapka, M.; Heymann, E.W.; Heer, K. Vertical stratification of seed-dispersing vertebrate communities and their interactions with plants in tropical forests. Biol. Rev. 2021, 96, 454–469. [Google Scholar] [CrossRef]
- Lu, S.; Lin, N.; Huang, A.; Tong, D.; Liang, Y.; Li, Y.; Lu, C. Feeding postures and substrate use of François’ langurs (Trachypithecus francoisi) in the limestone forest of Southwest China. Animals 2024, 14, 565. [Google Scholar] [CrossRef]
- Li, Y. Terrestriality and tree stratum use in a group of Sichuan snub-nosed monkeys. Primates 2007, 48, 197–207. [Google Scholar] [CrossRef]
- Porter, L.M.; Sterr, S.M.; Garber, P.A. Habitat use and ranging behavior of Callimico goeldii. Int. J. Primatol. 2007, 28, 1035–1058. [Google Scholar] [CrossRef]
- Mourthe, I.M.C.; Guedes, D.; Fidelis, J.; Boubli, J.P.; Mendes, S.L.; Strier, K.B. Ground use by northern muriquis. Am. J. Primatol. 2007, 69, 706–712. [Google Scholar] [CrossRef]
- Birot, H.; Campera, M.; Imron, M.A.; Nekaris, K.A.I. Artificial canopy bridges improve connectivity in fragmented landscapes: The case of Javan slow lorises in an agroforest environment. Am. J. Primatol. 2020, 82, e23076. [Google Scholar] [CrossRef]
- Karimloo, L.; Campera, M.; Imron, M.A.; Rakholia, S.; Mehta, A.; Hedger, K.; Nekaris, K.A.I. Habitat Use, Terrestriality and Feeding Behaviour of Javan Slow Lorises in Urban Areas of a Multi-Use Landscape in Indonesia. Land 2023, 12, 1349. [Google Scholar] [CrossRef]
- Hladik, C.M.; Charles-Dominique, P.; Petter, J. Feeding strategies of five nocturnal prosimians in the dry forest of the west coast of Madagascar. In Nocturnal Malagasy Primates; Charles-Dominique, P., Cooper, H.M., Hladik, A., Hladik, C.M., Pages, E., Pariente, G.F., Petter-Rousseaux, A., Petter, J.J., Schilling, A., Eds.; Academic Press: New York, NY, USA, 1980; pp. 41–73. [Google Scholar]
- Petter, J.J.; Hladik, C.M. Observations on the home range and population density of Loris tardigradus in the forests of Ceylon. Mammalia 1970, 34, 394–409. [Google Scholar] [CrossRef]
- Subramoniam, S. Some observations on the habits of the slender loris (Loris tardigradus). J. Bombay Nat. Hist. Soc. 1957, 54, 387–398. [Google Scholar]
- Thorpe, S.K.; Holder, R.L.; Crompton, R.H. Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science 2007, 316, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Nekaris, K.A.I. Positional behaviour and substrate preference of slow lorises, with a case study of Nycticebus bengalensis in Northeast India. In Evolution, Ecology and Conservation of Lorises and Pottos; Nekaris, K., Burrows, A., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 210–218. [Google Scholar]
- Anderson, J.R. Sleep-related behavioural adaptations in free-ranging anthropoid primates. Sleep Med. Rev. 2000, 4, 355–373. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A. Distribution and effects on tree growth of lianas and woody hemiepiphytes in a Costa Rican tropical wet forest. J. Trop. Ecol. 1990, 6, 321–336. [Google Scholar] [CrossRef]
- Malizia, A.; Grau, H.R. Liana-host tree associations in a subtropical montane forest of north-western Argentina. J. Trop. Ecol. 2006, 22, 331–339. [Google Scholar] [CrossRef]
- Deepthy, K.B.; Sunil, J.; Manoj, V.S.; Dhanya, M.K.; Maya, T.; Kuriakose, K.P.; Krishnaprasad, K.P. A new report of the myrmecophilous root mealy bug Xenococcus annandalei Silvestri (Rhizoecidae: Hemiptera)-a devastating pest. Entomon 2017, 42, 185–191. [Google Scholar]
- Prathapan, K.D.; Chaboo, C.S. Biology of Blepharida-group flea beetles with first notes on natural history of Podontia congregata Baly, 1865 an endemic flea beetle from southern India (Coleoptera, Chrysomelidae, Galerucinae, Alticini). ZooKeys 2011, 157, 95. [Google Scholar]
- Wainhouse, D.; Murphy, S.; Greig, B.; Webber, J.; Vielle, M. The role of the bark beetle Cryphalus trypanus in the transmission of the vascular wilt pathogen of takamaka (Calophyllum inophyllum) in the Seychelles. For. Ecol. Manag. 1998, 108, 193–199. [Google Scholar] [CrossRef]
- Styrsky, J.D.; Eubanks, M.D. Ecological consequences of interactions between ants and honeydew-producing insects. Proc. R. Soc. B 2007, 274, 151–164. [Google Scholar] [CrossRef]
- Sankara, R.K.; Bhat, H.R.; Kulkarni, V.A.; Ramachandra, T.V. Mini Forest—An experiment to evaluate the adaptability of Western Ghats species for afforestation. Environ. Conserv. J. 2011, 121, 79–83. [Google Scholar]
- Ramachandra, T.V.; Setturu, B.; Rajan, K.S.; Subash Chandran, M.D. Modelling the forest transition in Central Western Ghats, India. Spat. Inf. Res. 2017, 25, 117–130. [Google Scholar] [CrossRef]
- Chazdon, R.L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).