Influence of Plant Organs and Functional Traits on the Structure of Bacterial and Fungal Communities in Three Acer Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Collection
2.3. Collection of Microbial Samples
2.4. DNA Extraction and PCR Amplification
2.5. Preprocessing of Sequencing Data
2.6. Measurement of Plant Functional Traits
2.7. Statistical Analyses
3. Results
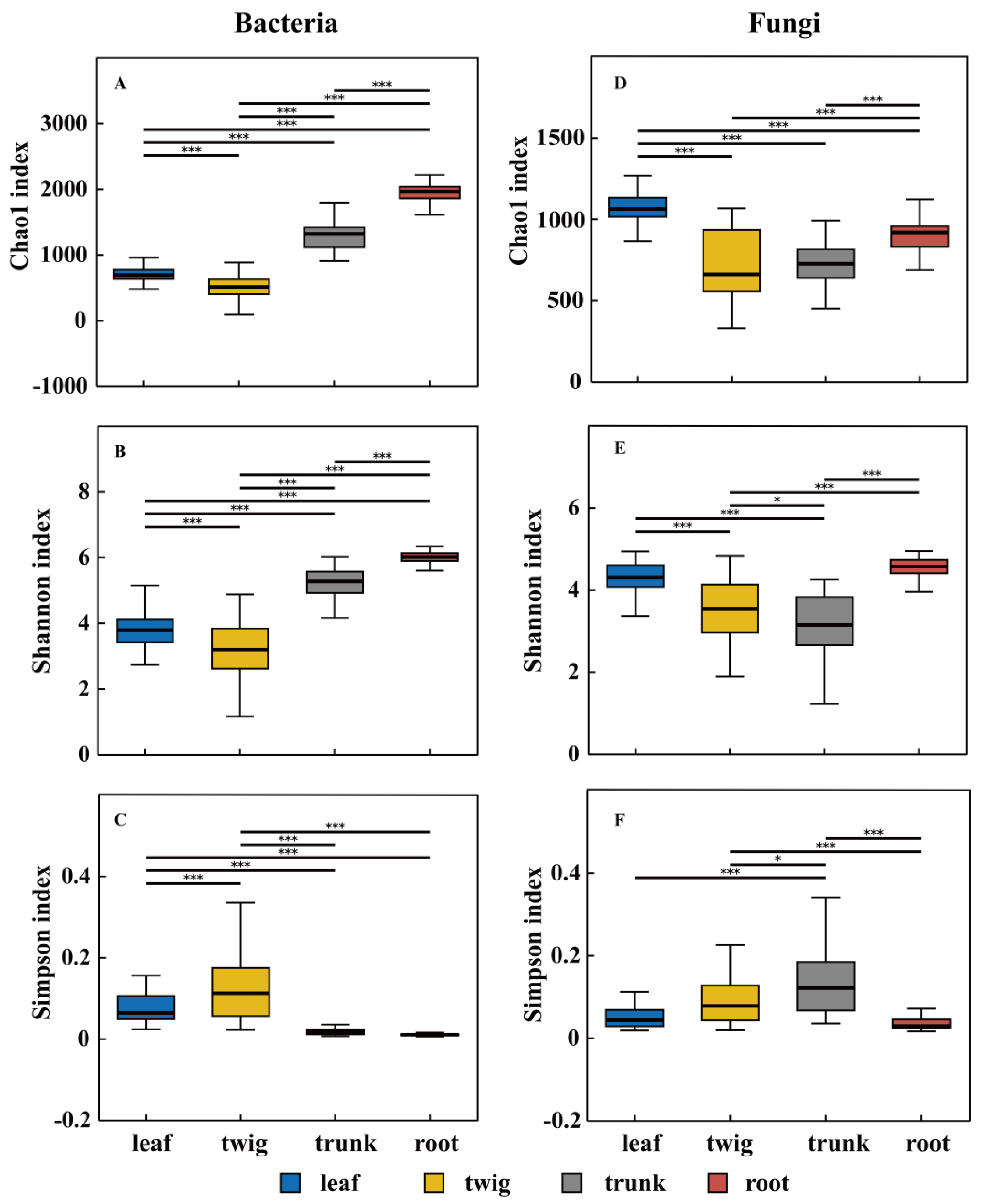
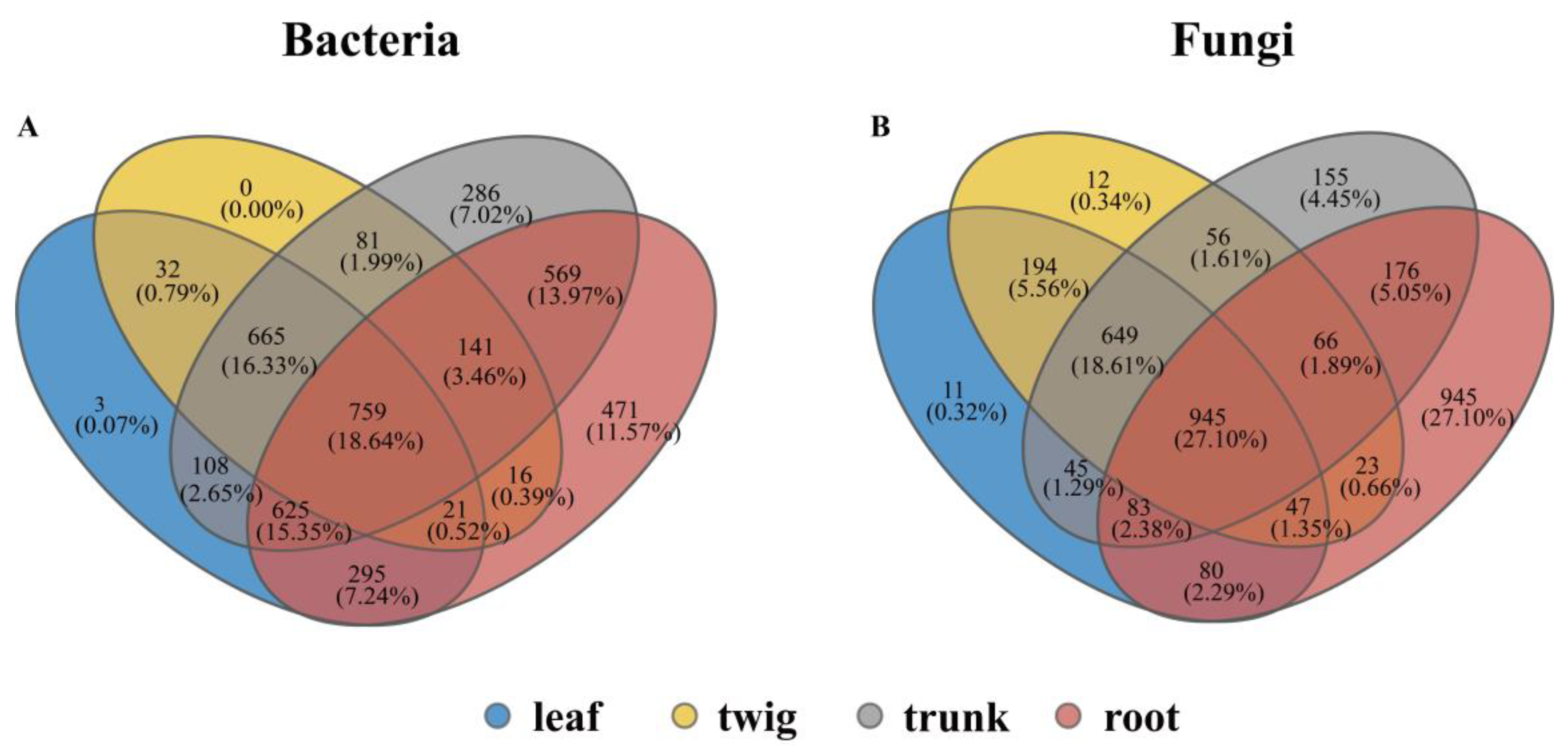
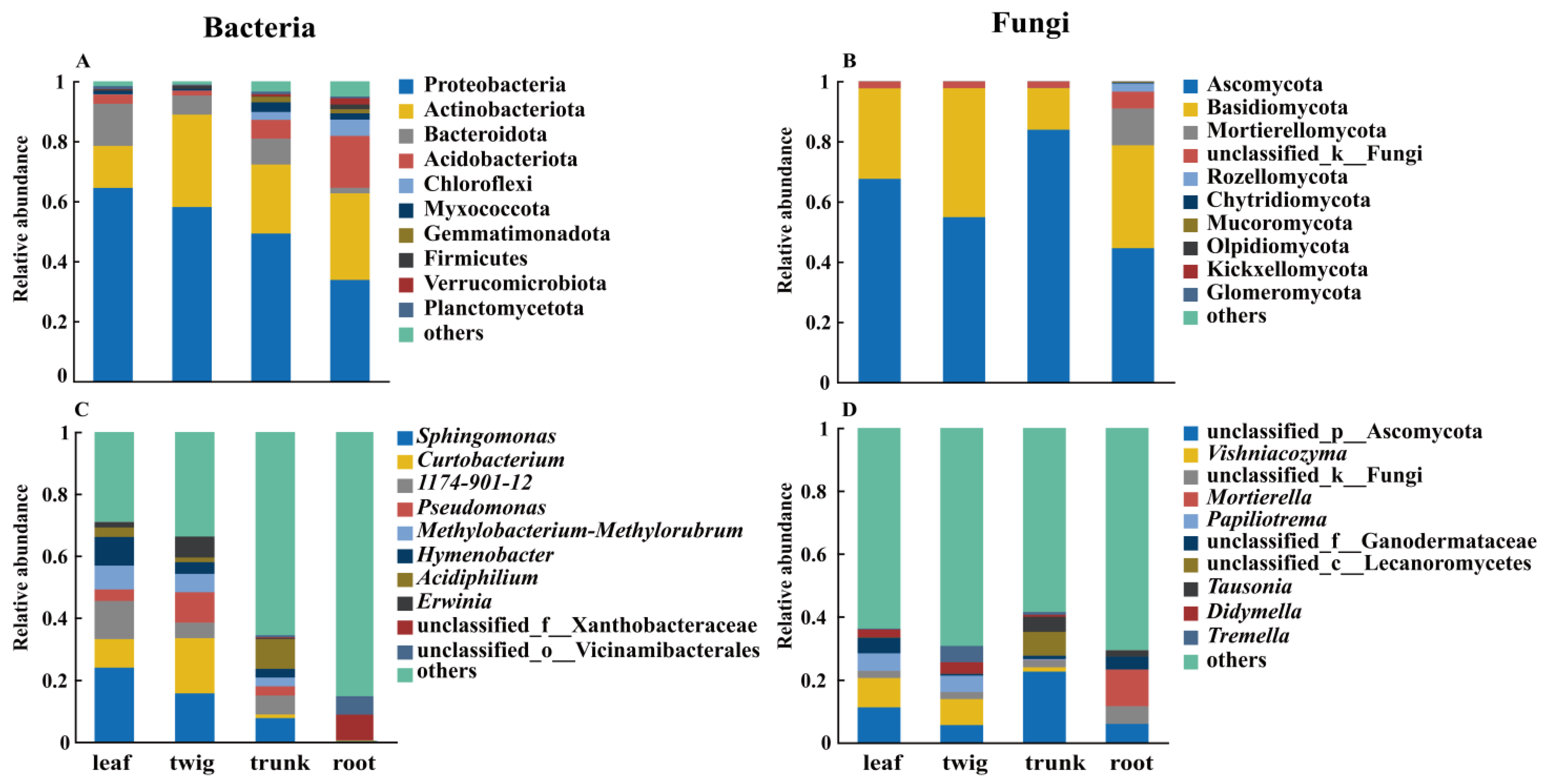
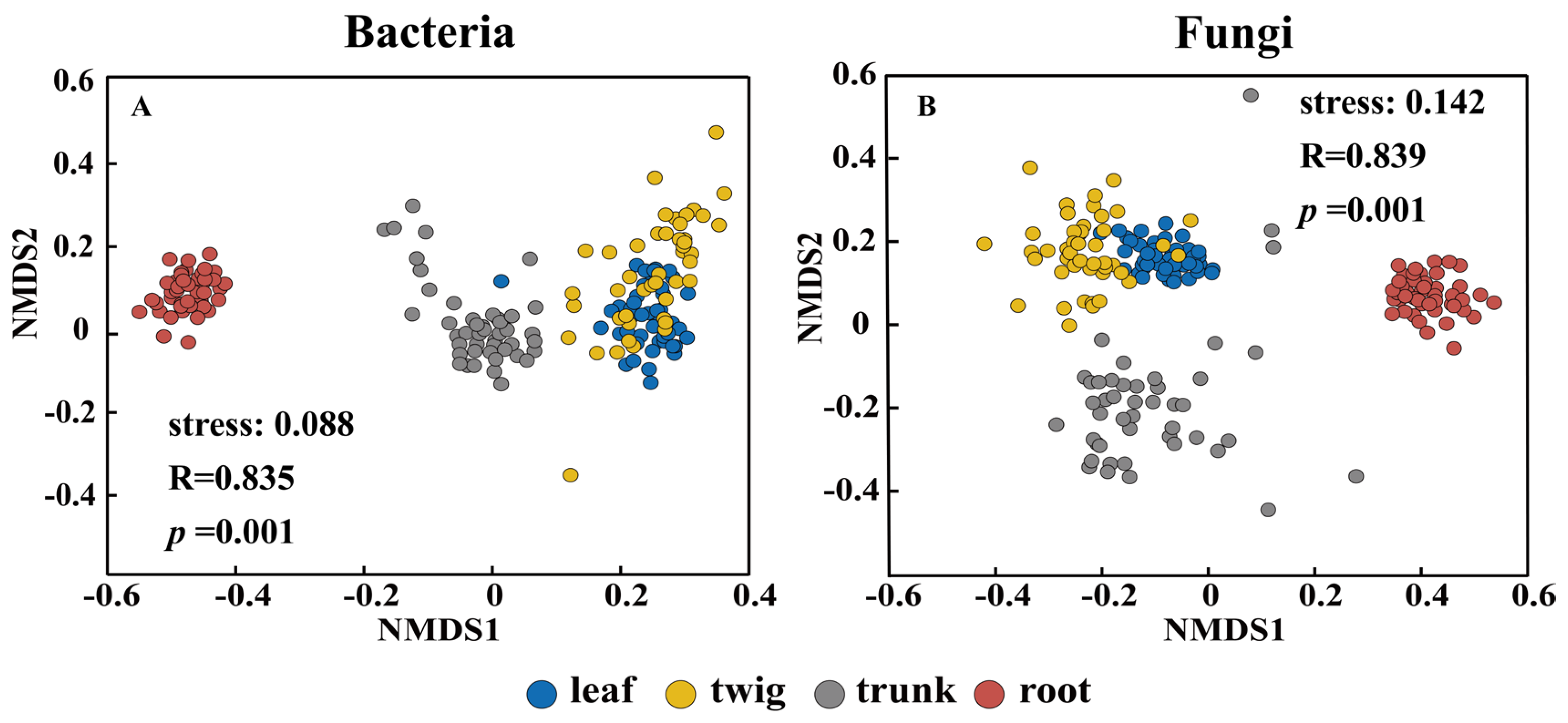
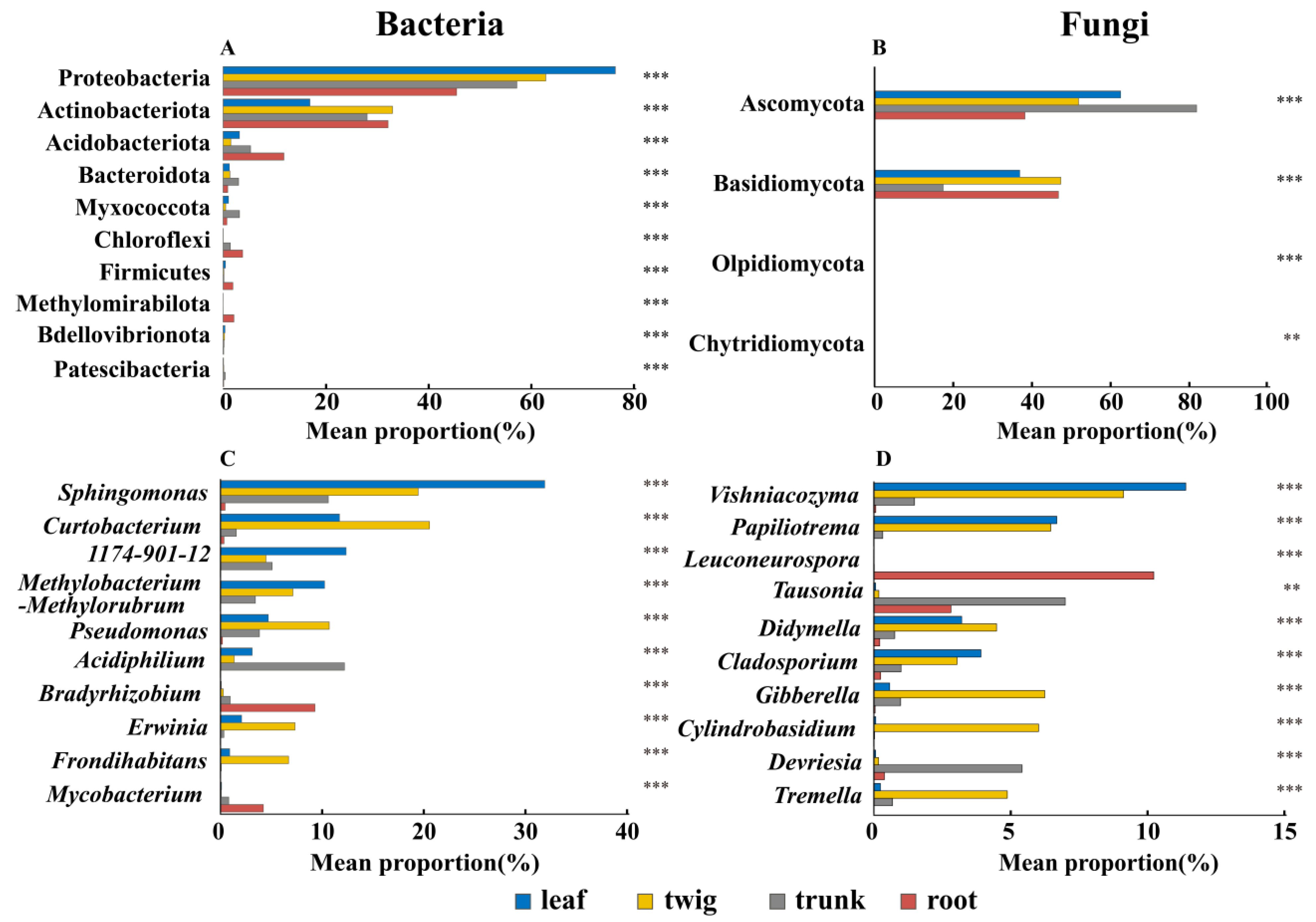
3.1. Differences in the Composition of Microbial Communities Among Different Organs
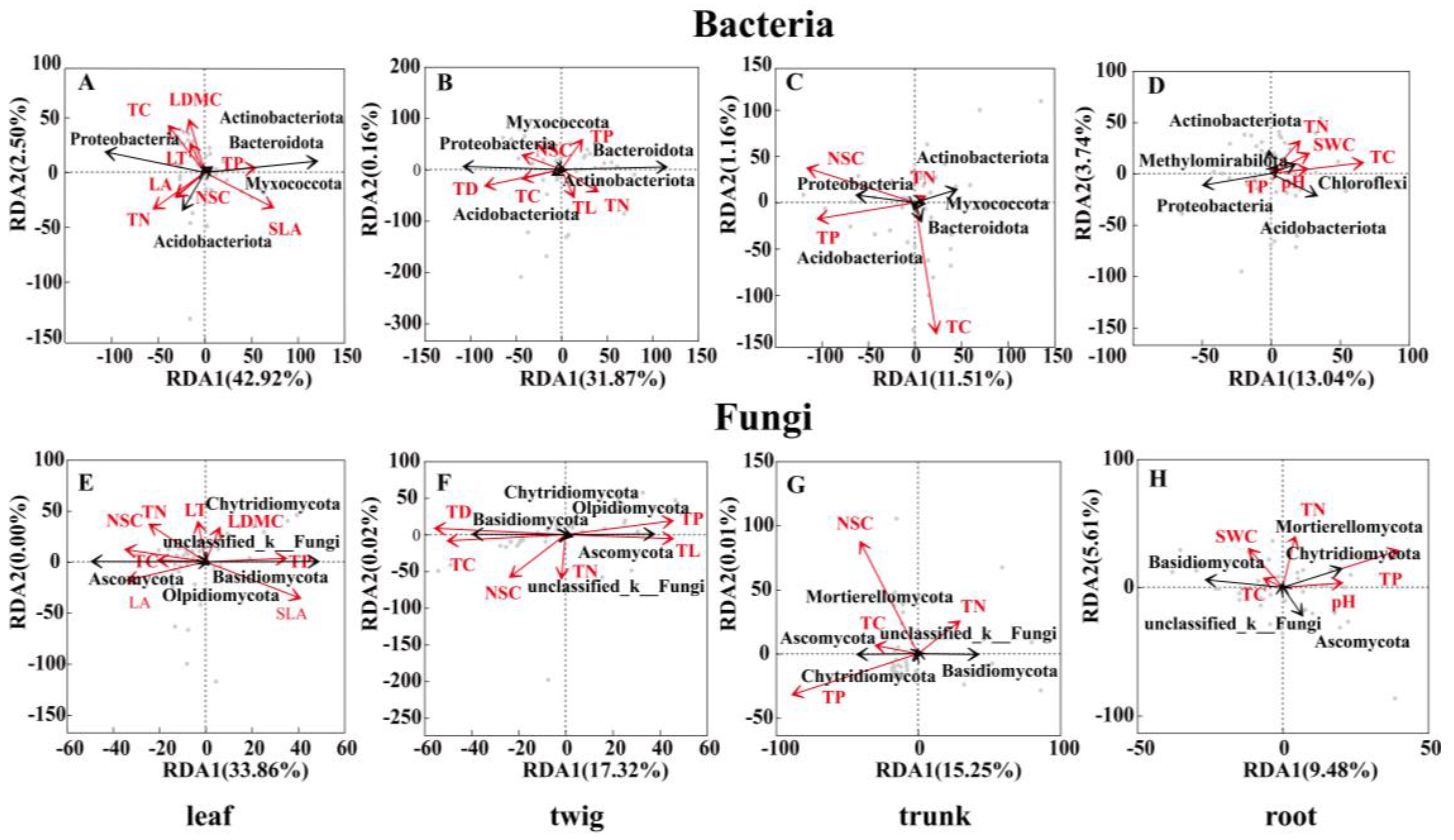
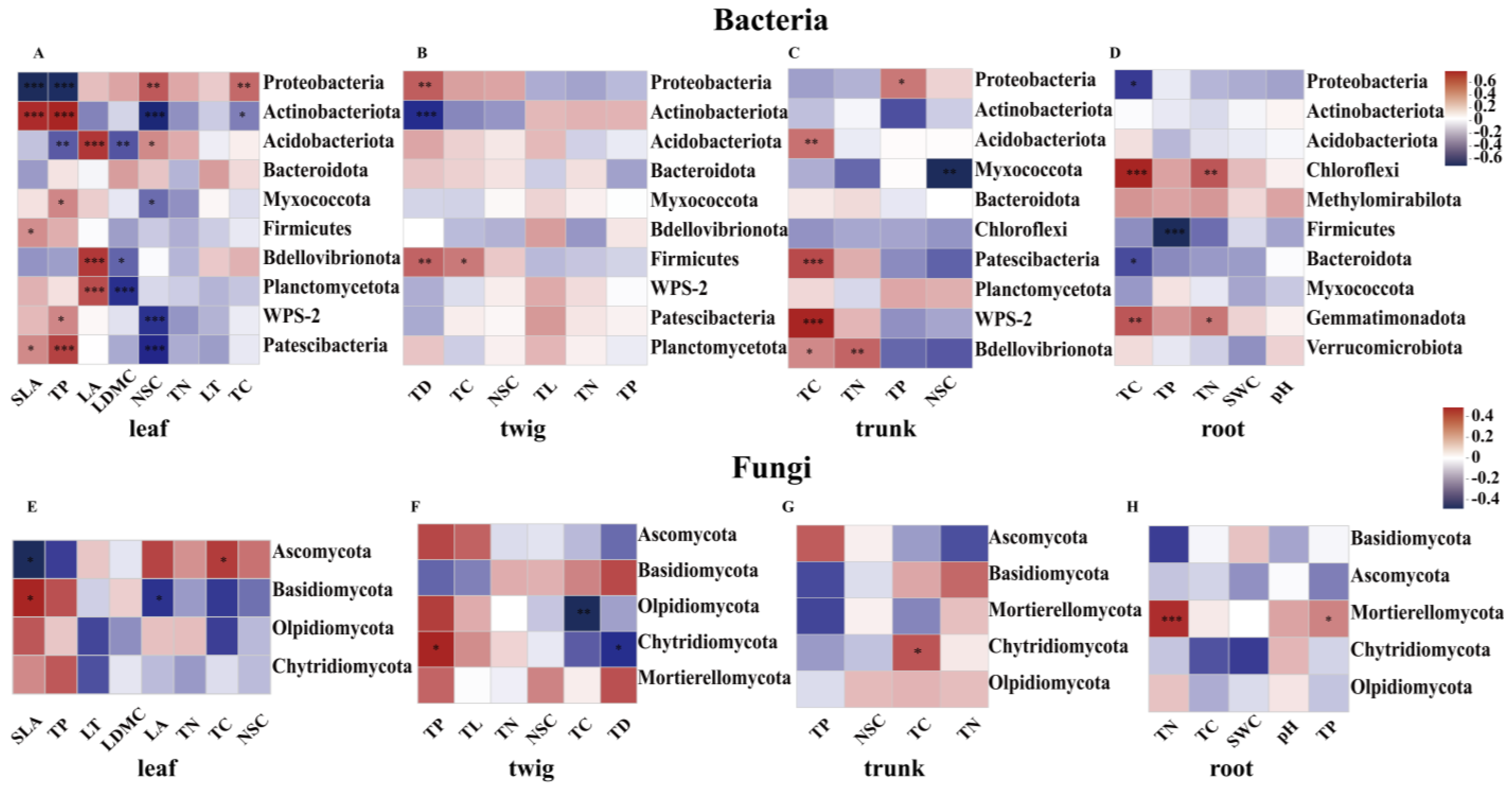
3.2. Core Microbiota and Their Relationship with the Environment and Plant Functional Traits
4. Discussion
4.1. Organ Differences Determine Microbial Diversity and Community Structure
4.2. The Structural Differences and Changes of the Core Microbial Community
4.3. The Relationship Between Plant Functional Traits and Microbial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baldrian, P.; Banin, E. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2016, 41, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.; Elsas, J.D.V.; Veen, J.A.V. Rhizosphere microbial community and its response to plant species and soil history. Plant Soil 2008, 302, 19–32. [Google Scholar] [CrossRef]
- Gao, Z.L.; Karlsson, I.; Geisen, S.; Kowalchuk, G.; Jousset, A. Protists: Puppet masters of the rhizosphere microbiome. Trends Plant Sci. 2019, 24, 165–176. [Google Scholar] [CrossRef]
- Osburn, E.D.; McBride, S.G.; Bahram, M.; Strickland, M.S. Global patterns in the growth potential of soil bacterial communities. Nat. Commun. 2024, 15, 6881. [Google Scholar] [CrossRef]
- Khan, M.F.; Chowdhary, S.; Koksch, B.; Murphy, C.D. Biodegradation of amphipathic fluorinated peptides reveals a new bacterial defluorinating activity and a new source of natural organofluorine compounds. Environ. Sci. Technol. 2023, 57, 9762–9772. [Google Scholar] [CrossRef]
- Hacquard, S.; Schadt, C.W. Towards a holistic understanding of the beneficial interactions across the Populus microbiome. New Phytol. 2014, 205, 1424–1430. [Google Scholar] [CrossRef]
- Khan, M.F.; Liao, J.; Liu, Z.; Chugh, G. Bacterial cytochrome P450 involvement in the biodegradation of fluorinated pyrethroids. J. Xenobiot. 2025, 15, 58. [Google Scholar] [CrossRef]
- Lajoie, G.; Maglione, R.; Kembel, S.W. Adaptive matching between phyllosphere bacteria and their tree hosts in a neotropical forest. Microbiome 2020, 8, 70. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liu, W.D.; Bu, J.S.; Lin, Y.B.; Bai, Y. Host genetics regulate the plant microbiome. Curr. Opin. Microbiol. 2023, 72, 102268. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, J.A.; Benhadi, J.; Lino-Neto, T.; Baptista, P. Endophytic and epiphytic phyllosphere fungal communities are shaped by different environmental factors in a Mediterranean ecosystem. Microb. Ecol. 2018, 76, 668–679. [Google Scholar] [CrossRef]
- Sweeney, C.J.; de Vries, F.T.; van Dongen, B.E.; Bardgett, R.D. Root traits explain rhizosphere fungal community composition among temperate grassland plant species. New Phytol. 2020, 229, 1492–1507. [Google Scholar] [CrossRef] [PubMed]
- Tardif, S.; Yergeau, É.; Tremblay, J.; Legendre, P.; Whyte, L.G.; Greer, C.W. The willow microbiome is influenced by soil petroleum-hydrocarbon concentration with plant compartment-specific effects. Front. Microbiol. 2016, 7, 1363. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, L.; Schmitt, I.; Dal Grande, F. Tree size drives diversity and community structure of microbial communities on the bark of beech (Fagus sylvatica). Front. For. Glob. Change 2022, 5, 858382. [Google Scholar] [CrossRef]
- Martins, F.; Pereira, J.A.; Bota, P.; Bento, A.; Baptista, P. Fungal endophyte communities in above- and belowground olive tree organs and the effect of season and geographic location on their structures. Fungal Ecol. 2016, 20, 193–201. [Google Scholar] [CrossRef]
- Li, Y.; Tian, D.S.; Pan, J.X.; Zhou, B.J.; Zhang, R.Y.; Song, L.; Wang, J.S.; Niu, S.L. Different patterns and drivers of fungal communities between phyllosphere and rhizosphere in alpine grasslands. Funct. Ecol. 2023, 37, 523–535. [Google Scholar] [CrossRef]
- Küngas, K.; Bahram, M.; Põldmaa, K. Host tree organ is the primary driver of endophytic fungal community structure in a hemiboreal forest. FEMS Microbiol. Ecol. 2019, 96, 199. [Google Scholar] [CrossRef]
- Coleman-Derr, D.; Desgarennes, D.; Fonseca-Garcia, C.; Gross, S.; Clingenpeel, S.; Woyke, T.; North, G.; Visel, A.; Partida-Martinez, L.P.; Tringe, S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol. 2015, 209, 798–811. [Google Scholar] [CrossRef]
- Fonseca-García, C.; Coleman-Derr, D.; Garrido, E.; Visel, A.; Tringe, S.G.; Partida-Martínez, L.P. The Cacti microbiome: Interplay between habitat-filtering and host-specificity. Front. Microbiol. 2016, 7, 150. [Google Scholar] [CrossRef]
- Jia, T.; Yao, Y.S.; Guo, T.Y.; Wang, R.H.; Chai, B.F. Effects of plant and soil characteristics on phyllosphere and rhizosphere fungal communities during plant development in a Copper Tailings Dam. Front. Microbiol. 2020, 11, 556002. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, Y.G.; Wang, J.T.; Singh, B.; Han, L.L.; Shen, J.P.; Li, P.P.; Wang, G.B.; Wu, C.F.; Ge, A.H.; et al. Host selection shapes crop microbiome assembly and network complexity. New Phytol. 2020, 229, 1091–1104. [Google Scholar] [CrossRef]
- Cregger, M.A.; Veach, A.M.; Yang, Z.K.; Crouch, M.J.; Vilgalys, R.; Tuskan, G.A.; Schadt, C.W. The Populus holobiont: Dissecting the effects of plant niches and genotype on the microbiome. Microbiome 2018, 6, 31. [Google Scholar] [CrossRef]
- Leff, J.W.; Del Tredici, P.; Friedman, W.E.; Fierer, N. Spatial structuring of bacterial communities within individual Ginkgo biloba trees. Environ. Microbiol. 2014, 17, 2352–2361. [Google Scholar] [CrossRef]
- Lee, N.L.Y.; Huang, D.; Quek, Z.B.R.; Lee, J.N.; Wainwright, B.J. Distinct fungal communities associated with different organs of the mangrove Sonneratia alba in the Malay Peninsula. IMA Fungus 2020, 11, 71–78. [Google Scholar] [CrossRef]
- Guo, Q.X.; Liu, L.; Liu, J.T.; Korpelainen, H.; Li, C.Y. Plant sex affects plant-microbiome assemblies of dioecious Populus cathayana trees under different soil nitrogen conditions. Microbiome 2022, 10, 191. [Google Scholar] [CrossRef]
- Grady, K.L.; Sorensen, J.W.; Stopnisek, N.; Guittar, J.; Shade, A. Assembly and seasonality of core phyllosphere microbiota on perennial biofuel crops. Nat. Commun. 2019, 10, 4135. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhong, Z.K.; Gai, X.; Ying, J.F.; Li, W.F.; Du, X.H.; Bian, F.Y.; Yang, C.B. Leaf-associated shifts in bacterial and fungal communities in response to chicken rearing under moso bamboo forests in subtropical China. Forests 2019, 10, 216. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.Y.; Liu, W.Y.; Ke, M.J.; Qu, Q.; Zhou, Z.G.; Lu, T.; Qian, H.F. Phyllosphere bacterial assemblage is affected by plant genotypes and growth stages. Microbiol. Res. 2021, 248, 126743. [Google Scholar] [CrossRef]
- Dinnage, R.; Simonsen, A.K.; Barrett, L.G.; Cardillo, M.; Raisbeck-Brown, N.; Thrall, P.H.; Prober, S.M.; Shefferson, R. Larger plants promote a greater diversity of symbiotic nitrogen-fixing soil bacteria associated with an Australian endemic legume. J. Ecol. 2018, 107, 977–991. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093–2098. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Ji, B.M.; Zhang, J.; Zhang, F.S.; Bever, J.D. Shading decreases plant carbon preferential allocation towards the most beneficial mycorrhizal mutualist. New Phytol. 2014, 205, 361–368. [Google Scholar] [CrossRef]
- Shen, Y.; Umaña, M.N.; Li, W.B.; Fang, M.; Chen, Y.X.; Lu, H.P.; Yu, S.X. Coordination of leaf, stem and root traits in determining seedling mortality in a subtropical forest. For. Ecol. Manag. 2019, 446, 285–292. [Google Scholar] [CrossRef]
- Wang, X.; Yang, T.; Mao, Z.K.; Lin, F.; Ye, J.; Fang, S.; Dai, G.H.; Hu, J.R.; Hao, Z.Q.; Wang, X.G. Community structure of phyllosphere fungi associated with dominant tree species in a broad-leaved Korean pine forest of Changbai Mountain, Northeast China. Chin. J. Appl. Ecol. 2022, 33, 2405–2412. [Google Scholar] [CrossRef]
- Kembel, S.W.; Mueller, R.C. Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities. Botany 2014, 92, 303–311. [Google Scholar] [CrossRef]
- Lamit, L.J.; Lau, M.K.; Sthultz, C.M.; Wooley, S.C.; Whitham, T.G.; Gehring, C.A. Tree genotype and genetically based growth traits structure twig endophyte communities. Am. J. Bot. 2014, 101, 467–478. [Google Scholar] [CrossRef]
- Pellitier, P.T.; Zak, D.R.; Salley, S.O. Environmental filtering structures fungal endophyte communities in tree bark. Mol. Ecol. 2019, 28, 5188–5198. [Google Scholar] [CrossRef]
- Lin, D.M.; Shen, R.; Lin, J.N.; Zhu, G.Y.; Yang, Y.C.; Fanin, N. Relationships between rhizosphere microbial communities, soil abiotic properties and root trait variation within a pine species. J. Ecol. 2024, 112, 1275–1286. [Google Scholar] [CrossRef]
- Chen, Y.; Xi, J.J.; Xiao, M.; Wang, S.L.; Chen, W.J.; Liu, F.Q.; Shao, Y.Z.; Yuan, Z.L. Soil fungal communities show more specificity than bacteria for plant species composition in a temperate forest in China. BMC Microbiol. 2022, 22, 208. [Google Scholar] [CrossRef]
- Liu, Z.L.; Jin, G.Z.; Qi, Y. Estimate of leaf area index in an old-growth mixed broadleaved-Korean pine forest in northeastern China. PLoS ONE 2012, 7, e32155. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jin, G.Z.; Liu, Z.L. Effects of needle age on leaf traits and their correlations of Pinus koraiensis across different regions. Chin. J. Plant Ecol. 2021, 45, 253–264. [Google Scholar] [CrossRef]
- Liu, Z.L.; Hikosaka, K.; Li, F.R.; Jin, G.Z. Variations in leaf economics spectrum traits for an evergreen coniferous species: Tree size dominates over environment factors. Funct. Ecol. 2020, 34, 458–467. [Google Scholar] [CrossRef]
- Liu, J.F.; Kang, F.F.; Yu, A.H.; Yang, W.J.; Chang, E.M.; Jiang, Z.P. Responses of foliar carbohydrates and nutrient status of two distinctive cypress species to shading and nitrogen addition. Glob. Ecol. Conserv. 2018, 16, e00452. [Google Scholar] [CrossRef]
- Allen, S.E. Chemical Analysis of Ecological Materials; Blackwell Science: Oxford, UK, 1989. [Google Scholar]
- Roesch, L.F.W.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.M.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.O.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Zhang, S.N.; Wang, Y.; Sun, L.T.; Qiu, C.; Ding, Y.Q.; Gu, H.L.; Wang, L.J.; Wang, Z.S.; Ding, Z.T. Organic mulching positively regulates the soil microbial communities and ecosystem functions in tea plantation. BMC Microbiol. 2020, 20, 103. [Google Scholar] [CrossRef]
- Xiong, C.; Singh, B.K.; He, J.Z.; Han, Y.L.; Li, P.P.; Wan, L.H.; Meng, G.Z.; Liu, S.Y.; Wang, J.T.; Wu, C.F.; et al. Plant developmental stage drives the differentiation in ecological role of the maize microbiome. Microbiome 2021, 9, 171. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; del Rio, T.G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef]
- Katsoula, A.; Vasileiadis, S.; Karamanoli, K.; Vokou, D.; Karpouzas, D.G. Factors structuring the epiphytic archaeal and fungal communities in a semi-arid Mediterranean ecosystem. Microb. Ecol. 2021, 82, 638–651. [Google Scholar] [CrossRef]
- Andrews, J.H.; Harris, R.F. The ecology and biogeography of microorganisms on plant surfaces. Annu. Rev. Phytopathol. 2000, 38, 145–180. [Google Scholar] [CrossRef]
- Ibrahim, M.; Sieber, T.N.; Schlegel, M. Communities of fungal endophytes in leaves of Fraxinus ornus are highly diverse. Fungal Ecol. 2017, 29, 10–19. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Mina, D.; Pereira, J.A.; Lino-Neto, T.; Baptista, P. Epiphytic and endophytic bacteria on olive tree phyllosphere: Exploring tissue and cultivar effect. Microb. Ecol. 2020, 80, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Kovalchuk, A.; Mukrimin, M.; Liu, M.X.; Zeng, Z.; Ghimire, R.P.; Kivimäenpää, M.; Holopainen, J.K.; Sun, H.; Asiegbu, F.O. Tissue microbiome of Norway spruce affected by Heterobasidion-induced wood decay. Microb. Ecol. 2018, 77, 640–650. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Yang, D.; Yue, H.T.; Wu, J.Y.; Zhao, L.Y.; Xing, X.X.; Guo, F.; Yang, J. Diversity and biological function of endophytic bacteria in Populus euphratica leaves and phloem. Acta Microbiol. Sin. 2022, 62, 213–226. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, H.; Kuang, C.T.; Gong, M.M.; Dong, L.; Cao, H. Structural and functional differentiation of soil bacterial sub-communities under different gardens. Soils 2023, 55, 1035–1043. [Google Scholar] [CrossRef]
- Du, X.F.; Deng, Y.; Li, S.Z.; Escalas, A.; Feng, K.; He, Q.; Wang, Z.J.; Wu, Y.N.; Wang, D.R.; Peng, X.; et al. Steeper spatial scaling patterns of subsoil microbiota are shaped by deterministic assembly process. Mol. Ecol. 2020, 30, 1072–1085. [Google Scholar] [CrossRef]
- Tardy, V.; Chabbi, A.; Charrier, X.; de Berranger, C.; Reignier, T.; Dequiedt, S.; Faivre-Primot, C.; Terrat, S.; Ranjard, L.; Maron, P.A. Land use history shifts In Situ fungal and bacterial successions following wheat straw input into the soil. PLoS ONE 2015, 10, e0130672. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.L.; Bres, C.; Jin, G.Z.; Fanin, N. Coniferous Tree species identity and leaf Aging alter the composition of phyllosphere communities through changes in leaf traits. Microb. Ecol. 2024, 87, 126. [Google Scholar] [CrossRef]
- Aguirre-von-Wobeser, E.; Alonso-Sánchez, A.; Méndez-Bravo, A.; Villanueva Espino, L.A.; Reverchon, F. Barks from avocado trees of different geographic locations have consistent microbial communities. Arch. Microbiol. 2021, 203, 4593–4607. [Google Scholar] [CrossRef]
- Guo, L.D.; Li, X.C.; He, C.; Sun, X.; Yao, H. Host identity is more important in structuring bacterial epiphytes than endophytes in a tropical mangrove forest. FEMS Microbiol. Ecol. 2020, 96, fiaa038. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees-from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Peng, Z.T.; Jin, G.Z.; Liu, Z.L. Leaf trait variations and relationships of three Acer species in different tree sizes and canopy conditions in Xiao Hinggan Mountains of Northeast China. Chin. J. Plant Ecol. 2024, 48, 730–743. [Google Scholar] [CrossRef]
- Balestrini, R.; Coince, A.; Cordier, T.; Lengellé, J.; Defossez, E.; Vacher, C.; Robin, C.; Buée, M.; Marçais, B. Leaf and root-associated fungal assemblages do not follow similar elevational diversity patterns. PLoS ONE 2014, 9, e100668. [Google Scholar] [CrossRef]
- Zhu, T.; Yao, J.; Liu, H.; Zhou, C.H.; Liu, Y.Z.; Wang, Z.W.; Quan, Z.X.; Li, B.; Yang, J.; Huang, W.C.; et al. Cross-phytogroup assessment of foliar epiphytic mycobiomes. Environ. Microbiol. 2021, 23, 6210–6222. [Google Scholar] [CrossRef]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.P.; Wright, S.J.; Green, J.L. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef]
- Bowsher, A.W.; Benucci, G.M.N.; Bonito, G.; Shade, A. Seasonal dynamics of core fungi in the switchgrass phyllosphere, and co-occurrence with leaf bacteria. Phytobiomes J. 2021, 5, 60–68. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.F.; Hu, Y.X.; Wang, R.; Rui, J.P.; Guo, S.L. Different selectivity in fungal communities between manure and mineral fertilizers: A study in an alkaline soil after 30 years fertilization. Front. Microbiol. 2018, 9, 2613. [Google Scholar] [CrossRef]
- Cao, H.; Chen, R.; Wang, L.; Jiang, L.; Yang, F.; Zheng, S.; Wang, G.; Lin, X. Soil pH, total phosphorus, climate and distance are the major factors influencing microbial activity at a regional spatial scale. Sci. Rep. 2016, 6, 25815. [Google Scholar] [CrossRef]
- Mou, G.F.; Bau, T. Modicella guangxiensis (Mortierellomycota, Mortierellaceae), a new species from south-western karst areas of China. Biodivers. Data J. 2024, 12, e115044. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Chen, J.J.; Zou, Y.J.; Dai, Z.X.; Xing, P.; Wu, Q.L.L. Co-occurrence pattern of bacteria and fungi on the leaves of the invasive aquatic plant Alternanthera philoxeroides. FEMS Microbiol. Ecol. 2023, 99, fiad022. [Google Scholar] [CrossRef] [PubMed]
- Adomako, M.O.; Roiloa, S.; Yu, F.H. Potential roles of soil microorganisms in regulating the effect of soil nutrient heterogeneity on plant performance. Microorganisms 2022, 10, 2399. [Google Scholar] [CrossRef]

| Taxa | Treatment | df | Sums of Sqs | F | R2 | p |
|---|---|---|---|---|---|---|
| Bacteria | Organ | 3 | 29.680 | 63.718 | 0.521 | 0.001 |
| Species | 2 | 1.593 | 2.544 | 0.028 | 0.009 | |
| Tree size | 1 | 0.468 | 1.473 | 0.008 | 0.131 | |
| Forest gap | 1 | 0.424 | 1.333 | 0.007 | 0.200 | |
| Fungi | Organ | 3 | 23.085 | 27.950 | 0.323 | 0.001 |
| Species | 2 | 2.043 | 2.601 | 0.029 | 0.001 | |
| Tree size | 1 | 0.838 | 2.109 | 0.012 | 0.007 | |
| Forest gap | 1 | 0.761 | 1.914 | 0.011 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Wang, L.; Jin, G.; Liu, Z. Influence of Plant Organs and Functional Traits on the Structure of Bacterial and Fungal Communities in Three Acer Species. Forests 2025, 16, 875. https://doi.org/10.3390/f16060875
Guo J, Wang L, Jin G, Liu Z. Influence of Plant Organs and Functional Traits on the Structure of Bacterial and Fungal Communities in Three Acer Species. Forests. 2025; 16(6):875. https://doi.org/10.3390/f16060875
Chicago/Turabian StyleGuo, Jiaxing, Lei Wang, Guangze Jin, and Zhili Liu. 2025. "Influence of Plant Organs and Functional Traits on the Structure of Bacterial and Fungal Communities in Three Acer Species" Forests 16, no. 6: 875. https://doi.org/10.3390/f16060875
APA StyleGuo, J., Wang, L., Jin, G., & Liu, Z. (2025). Influence of Plant Organs and Functional Traits on the Structure of Bacterial and Fungal Communities in Three Acer Species. Forests, 16(6), 875. https://doi.org/10.3390/f16060875





