Wood Anatomy Properties and Global Climate Change Constraints of Forest Species from the Natural Forest of Mozambique
Abstract
1. Introduction
1.1. Main Forest Formations
1.1.1. Miombo Woodland
1.1.2. Mopane Forest
1.1.3. Mecrusse Forest
1.2. Higly Valued Wood Forest Species
1.2.1. Spirostachys africana
1.2.2. Afzelia quanzensis
1.2.3. Millettia stuhlmannii
1.2.4. Pterocarpus angolensis
1.2.5. Colophospermum mopane
1.3. Environmental and Human Pressure Affecting Forest Trees
2. Materials and Methods
3. Results
3.1. Spirostachys africana Sond.
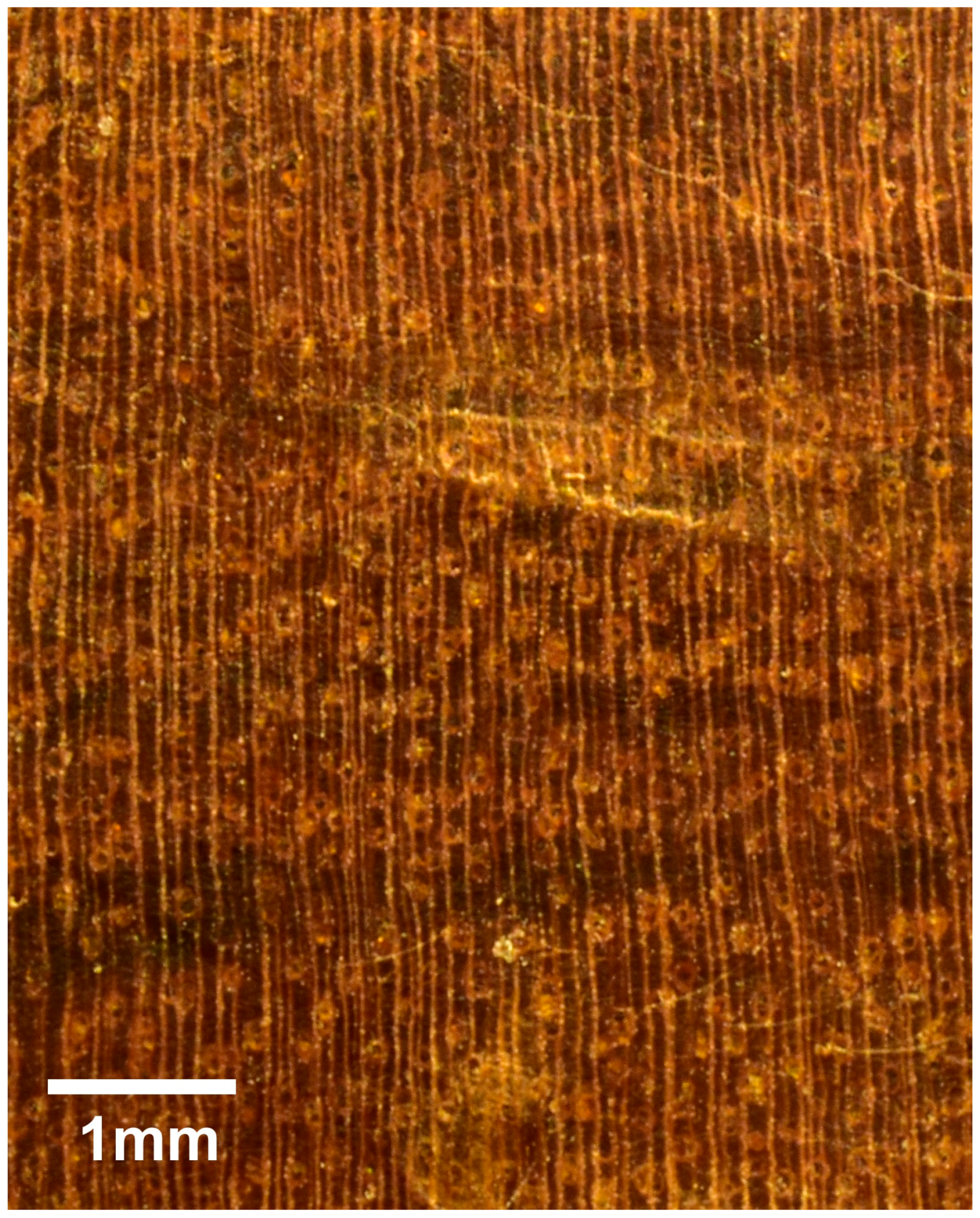
3.1.1. Macroscopic Features
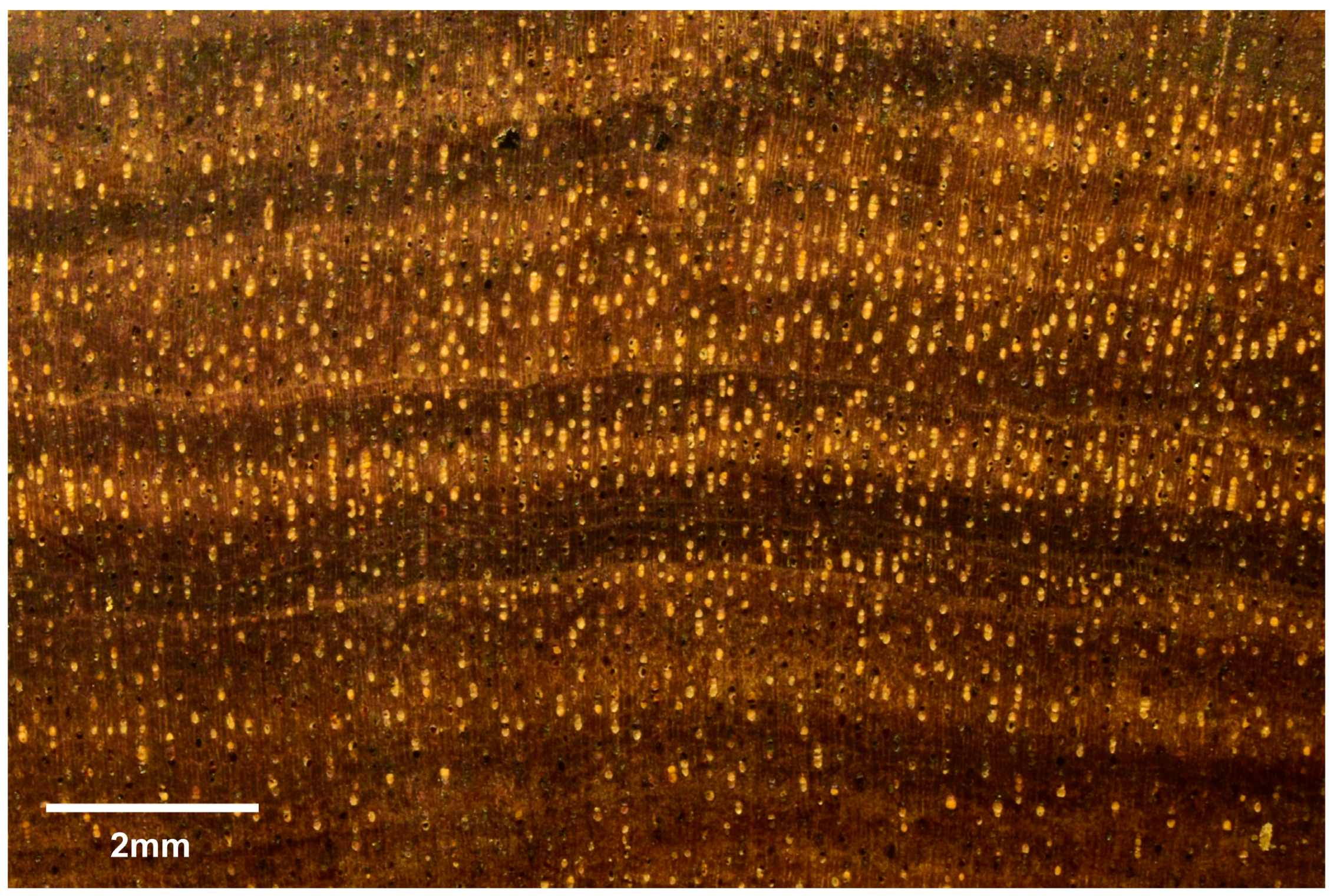
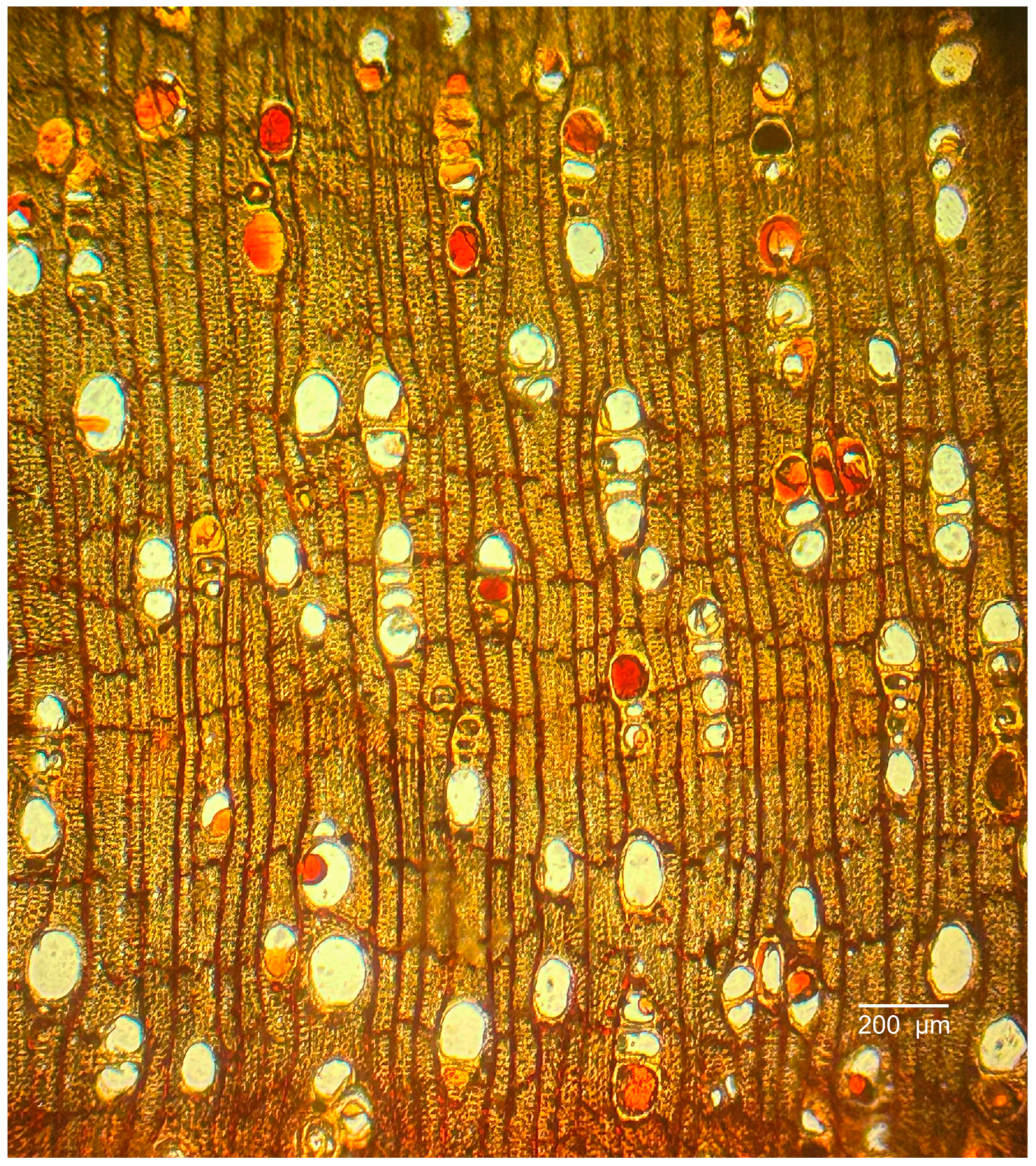
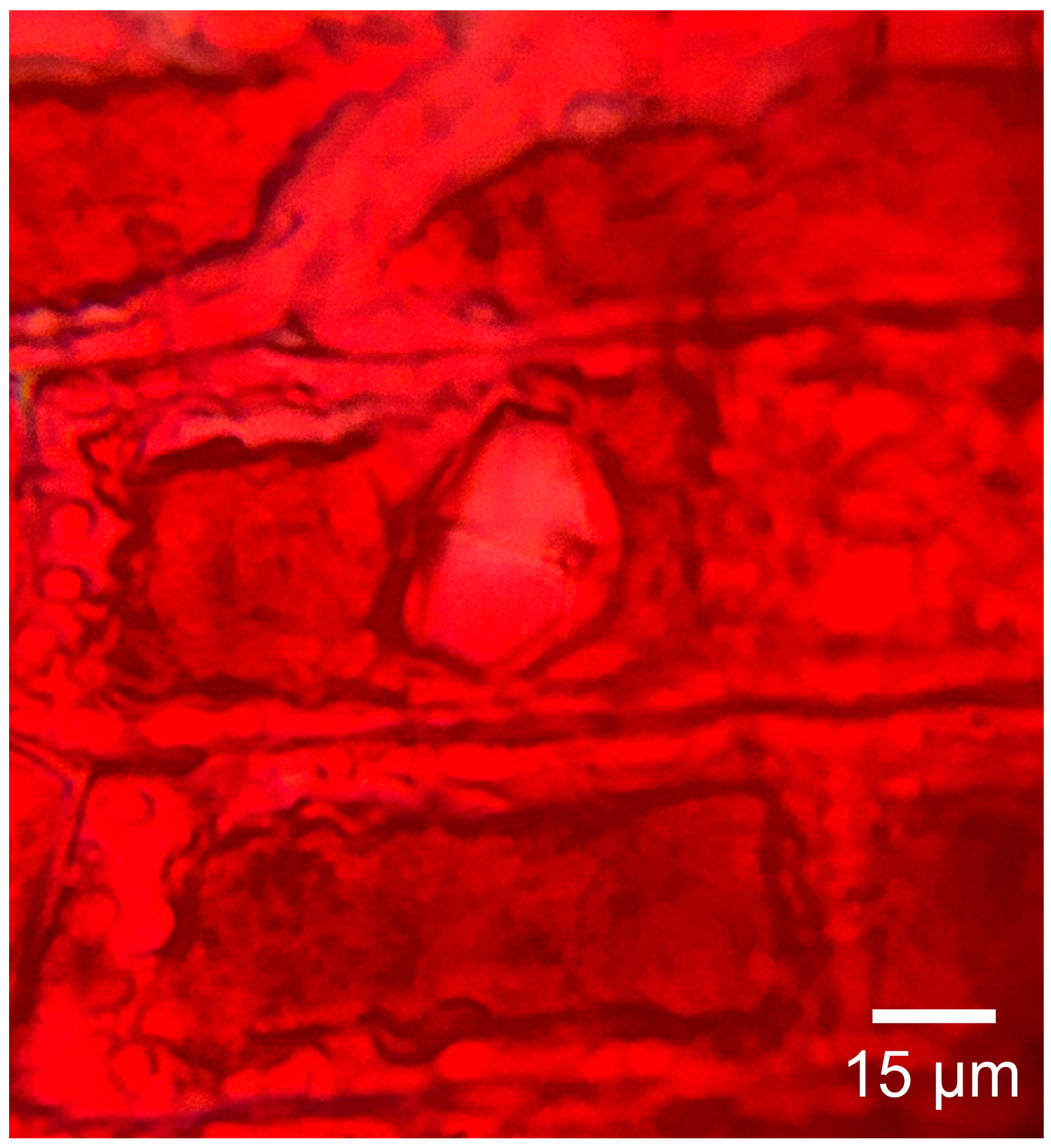
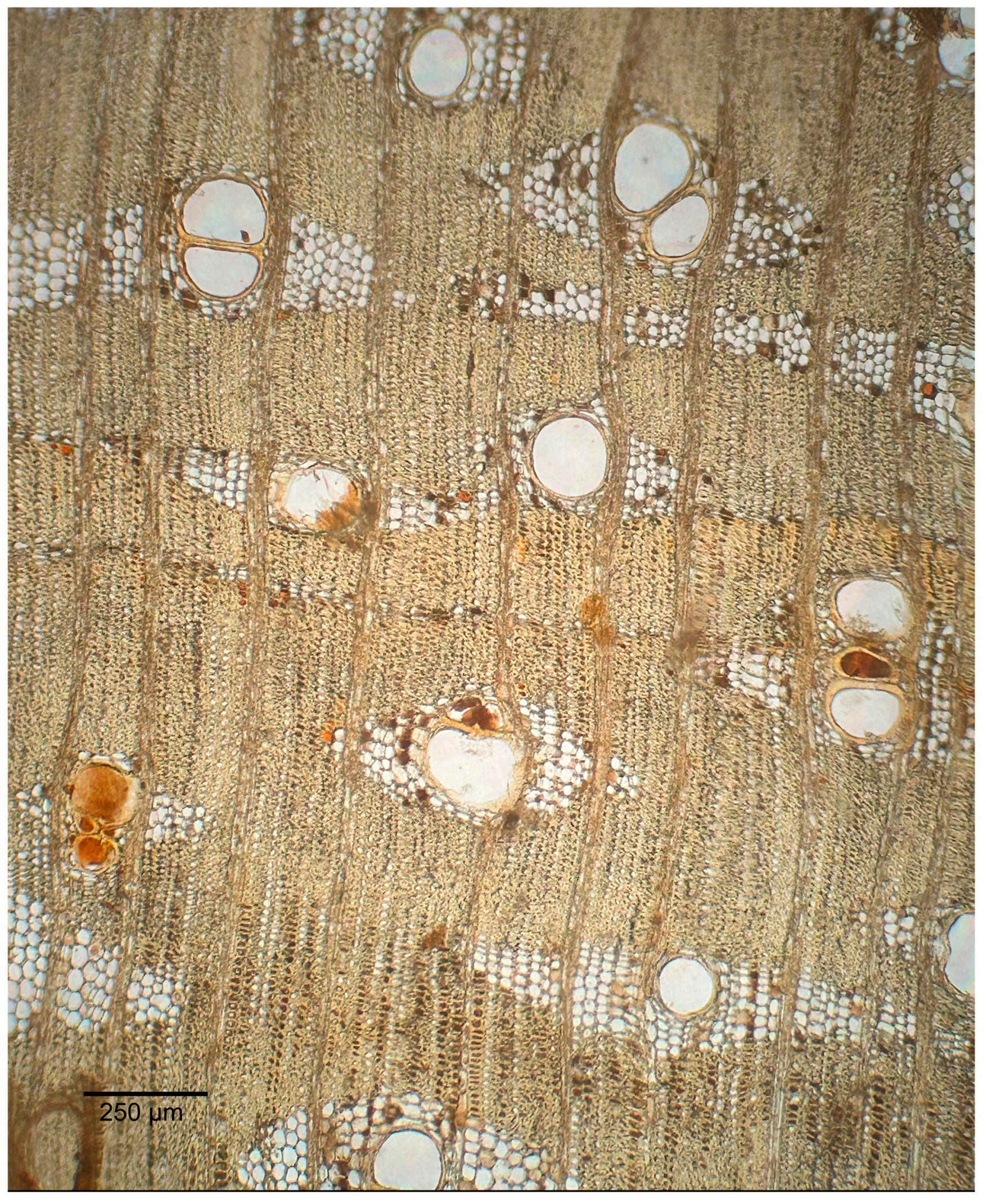
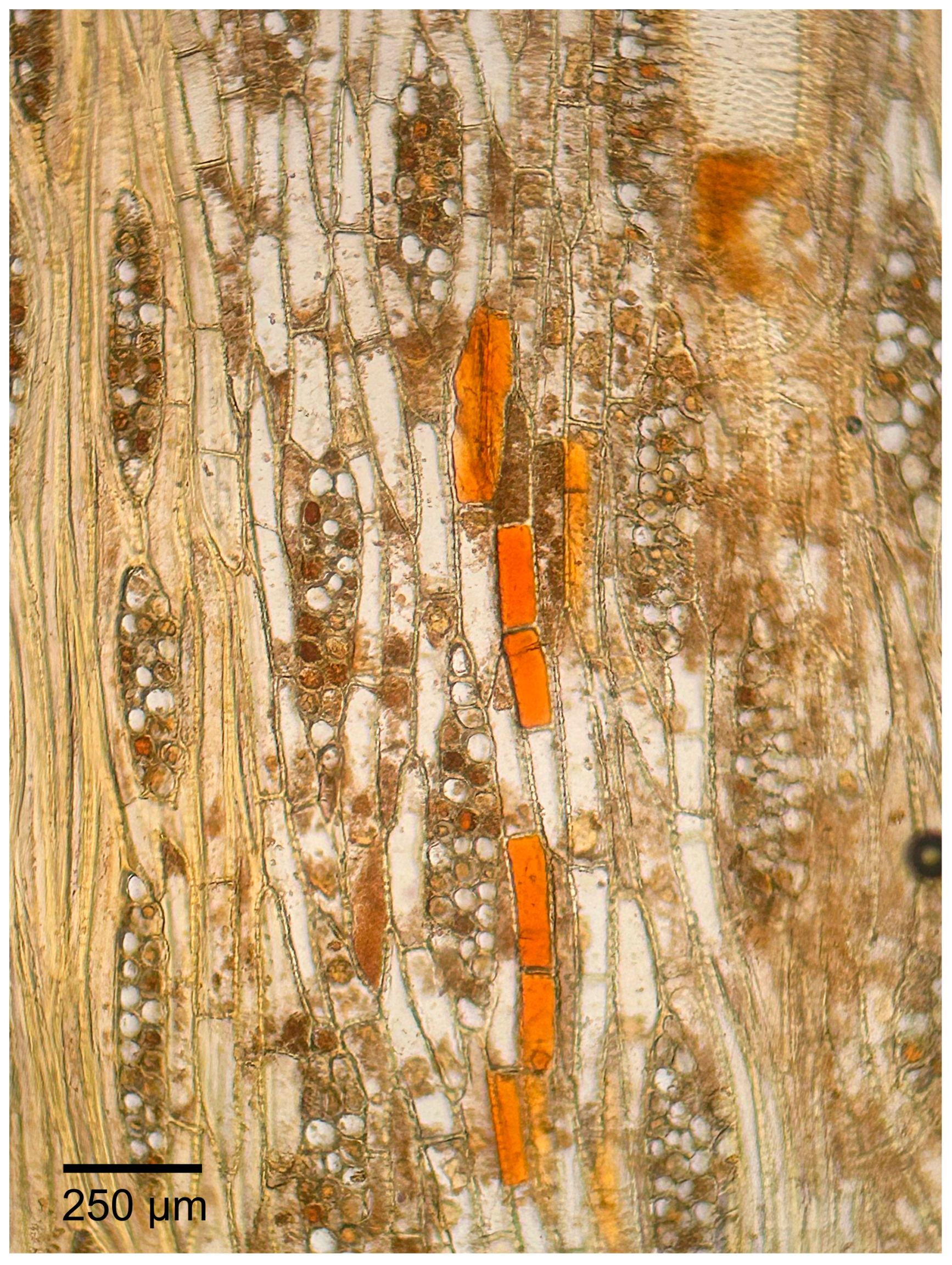
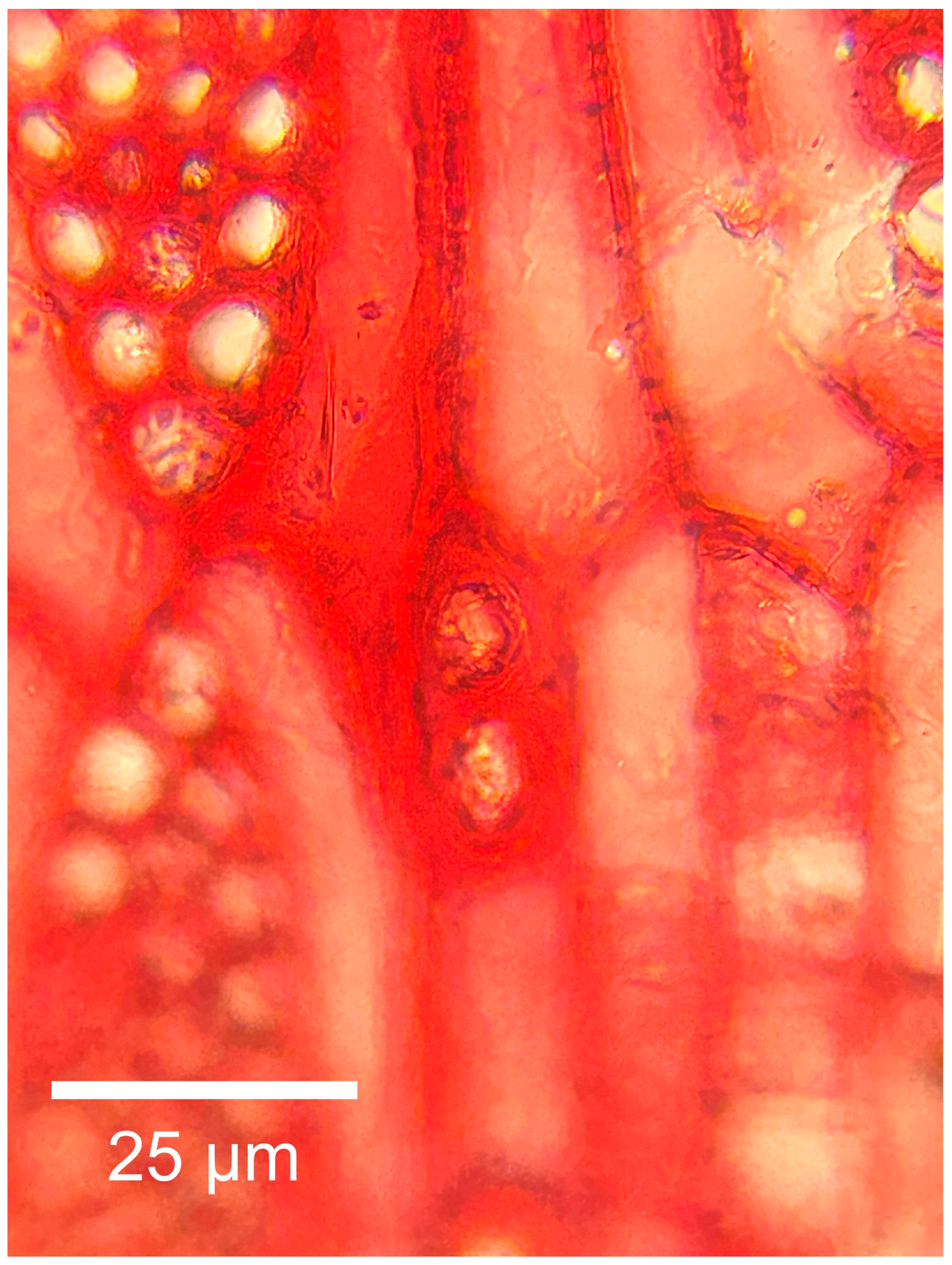
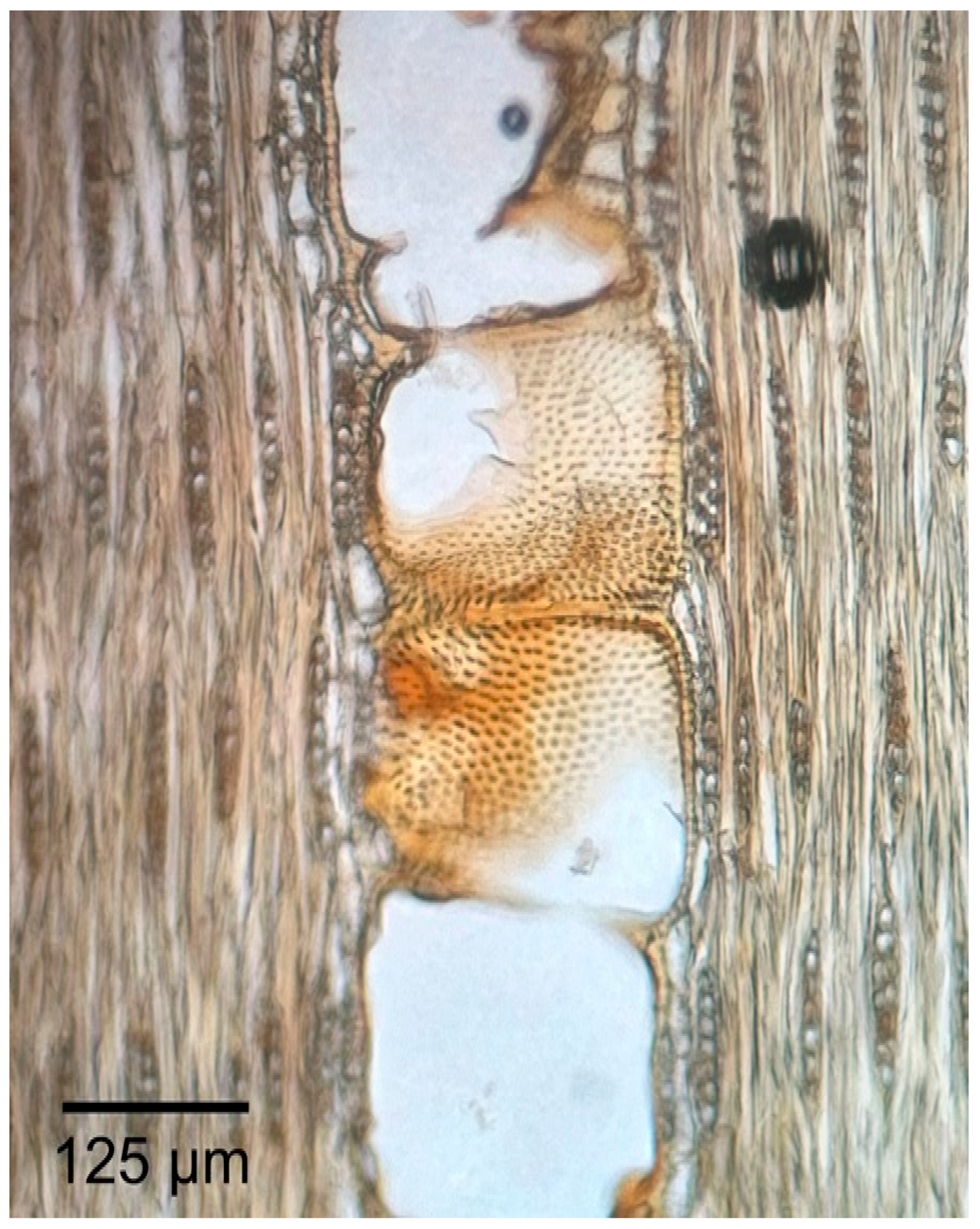
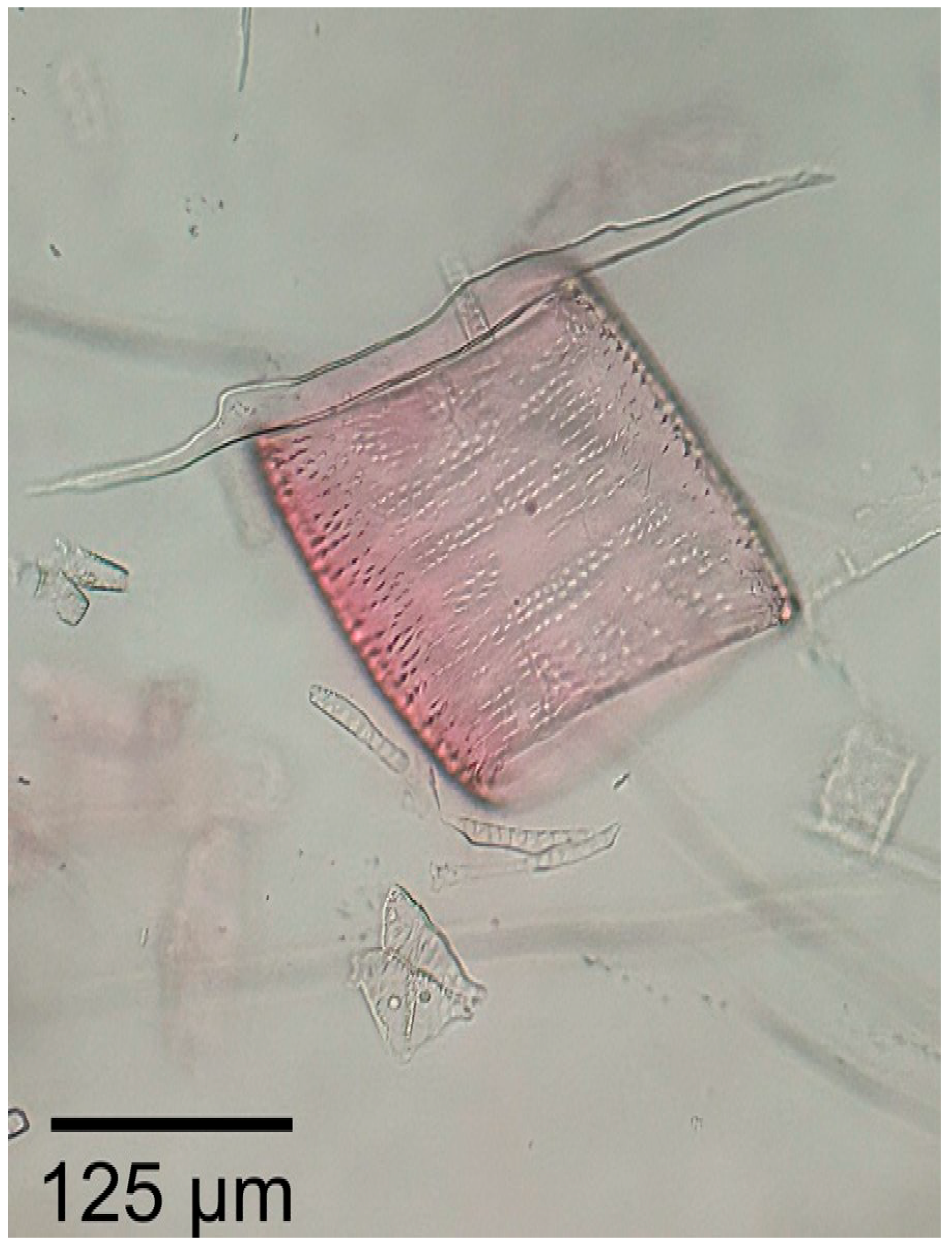
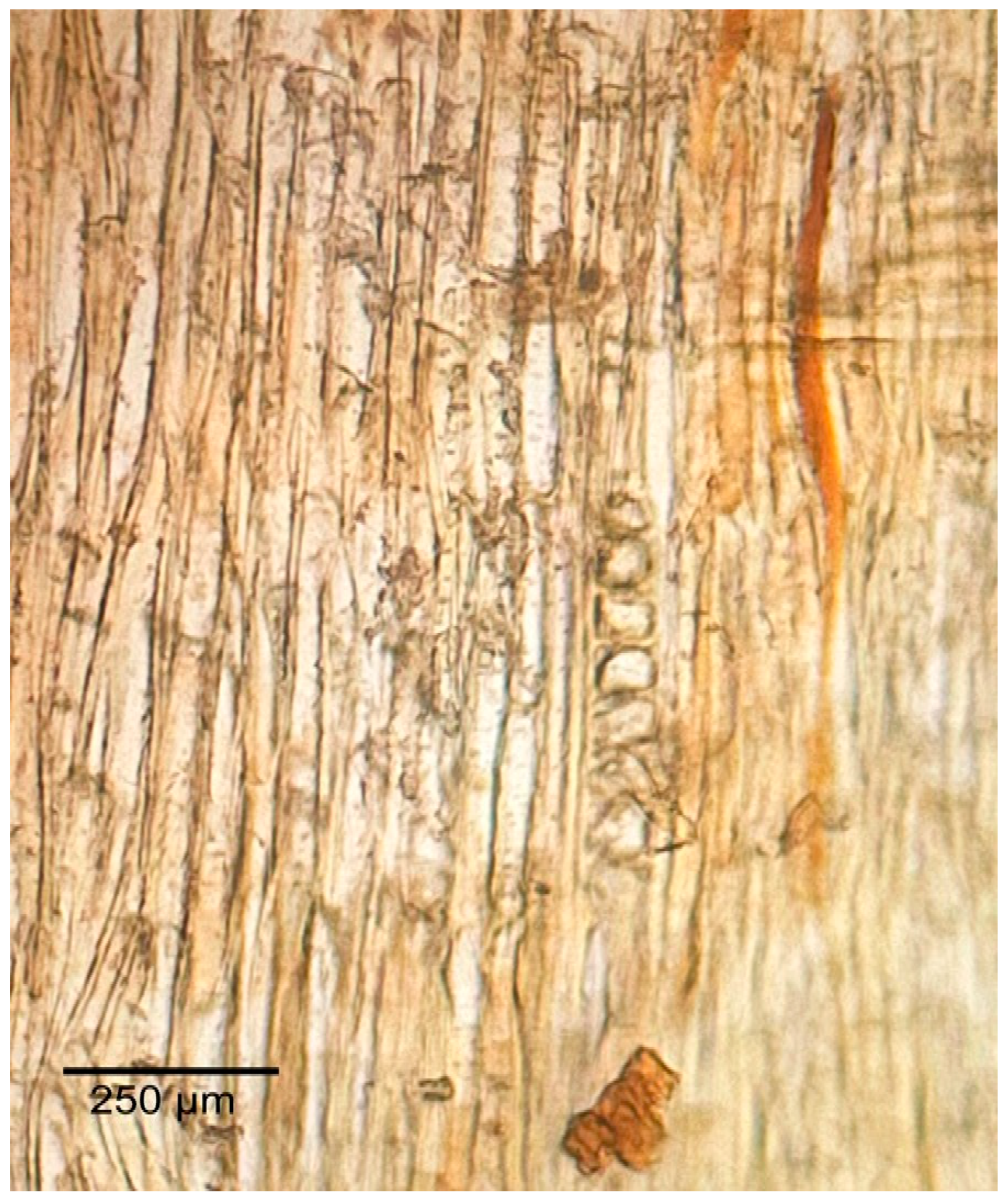
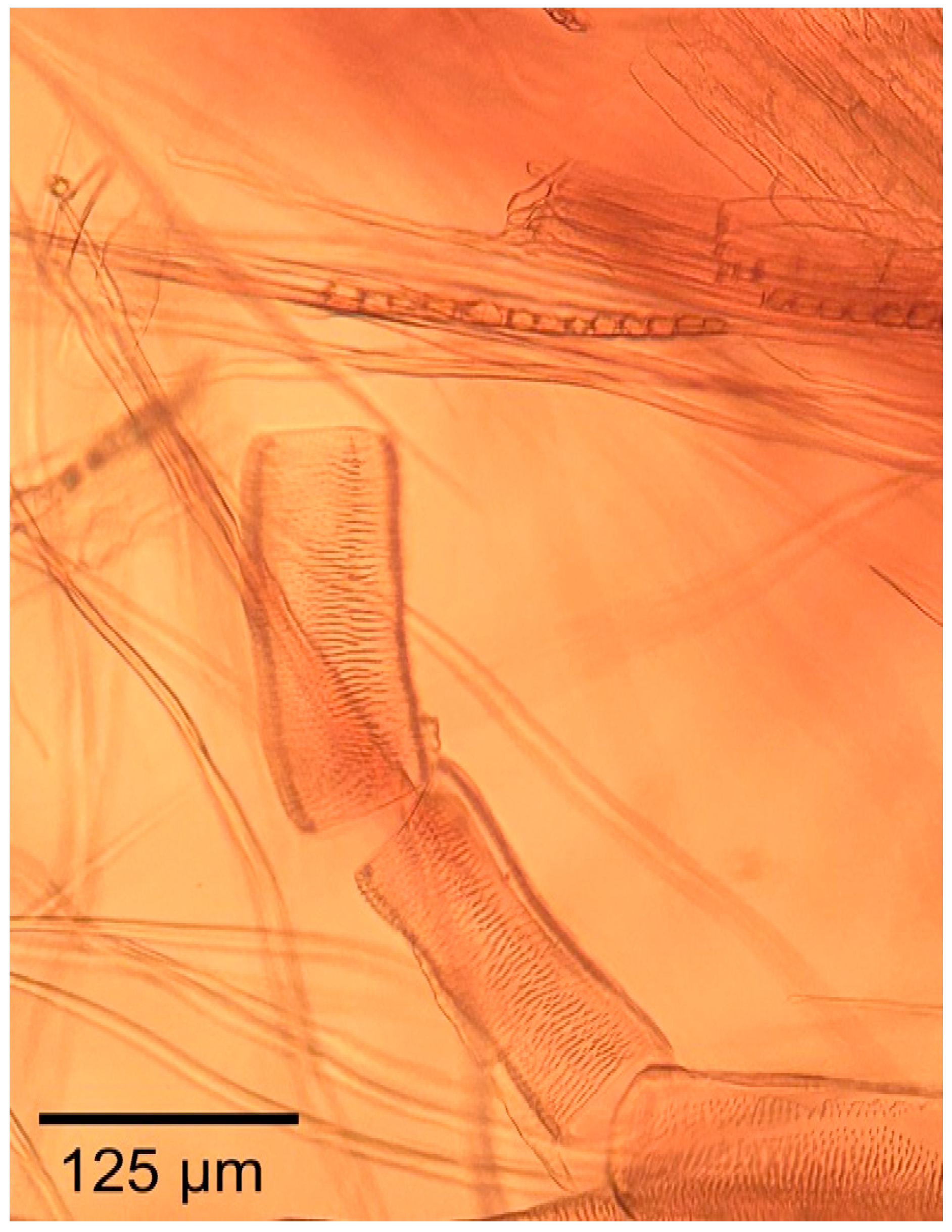
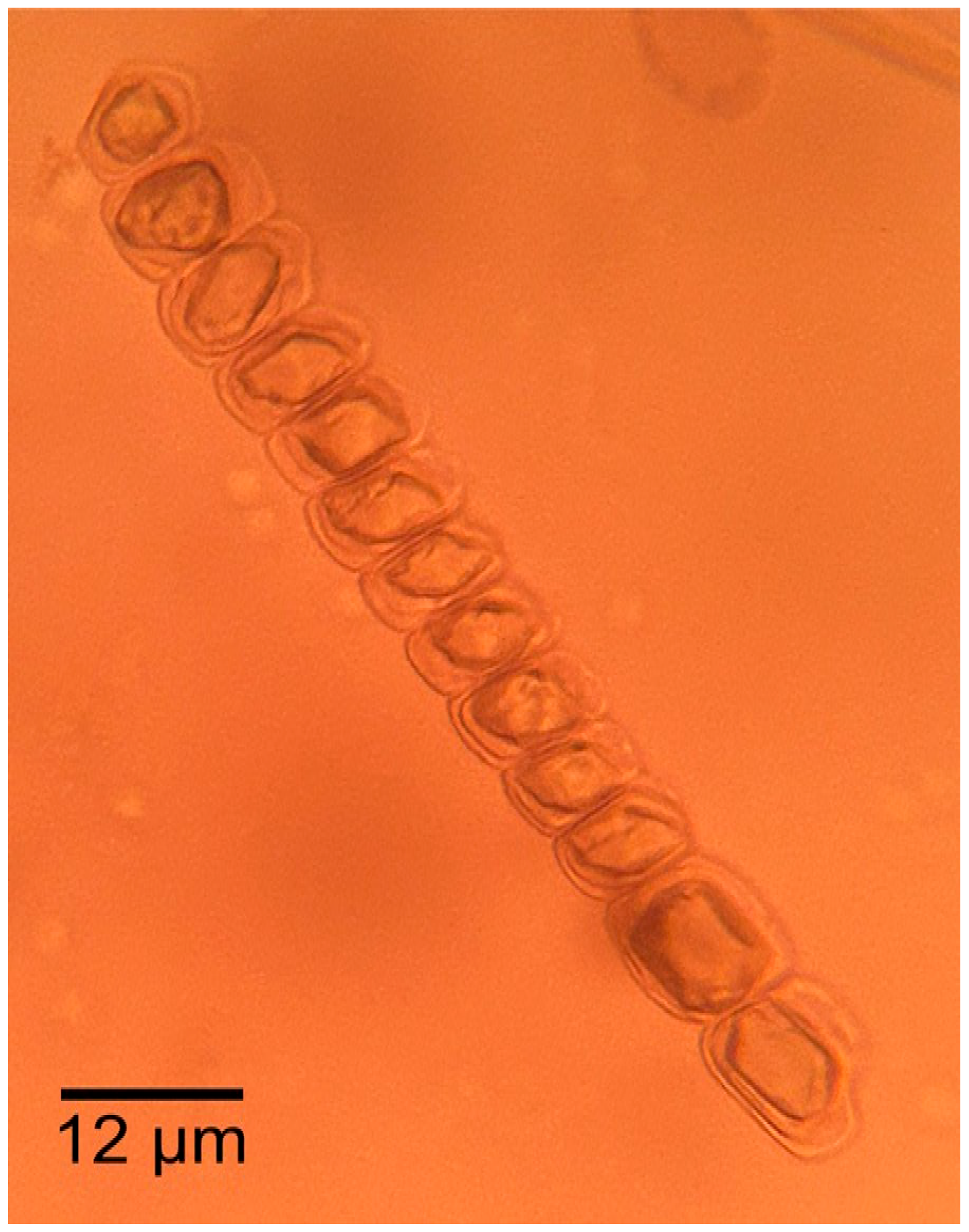
3.1.2. Microscopic Features
3.2. Afzelia quanzensis Welw.
3.2.1. Macroscopic Features
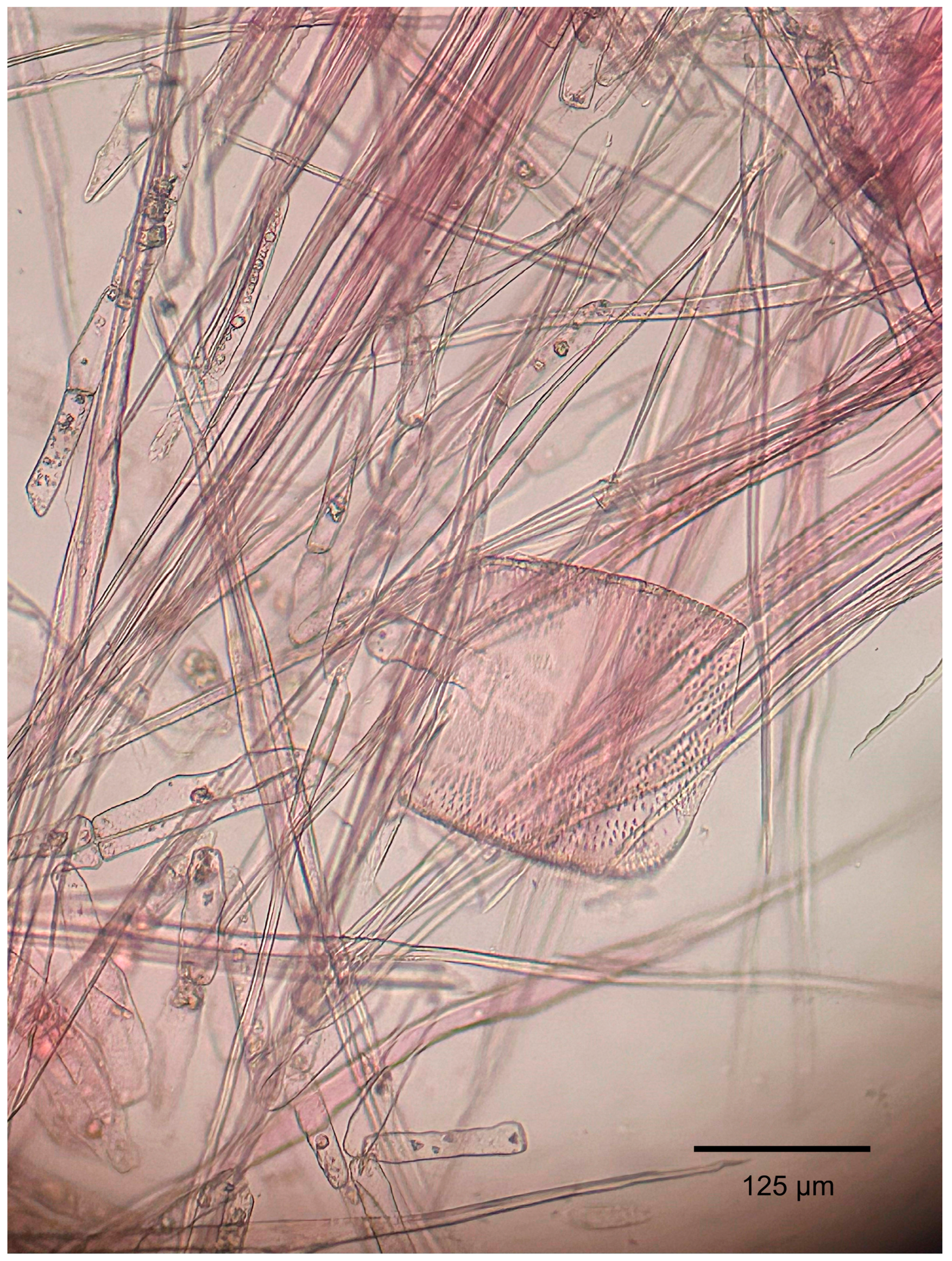
3.2.2. Microscopic Features
3.3. Millettia stuhlmannii Taub.
3.3.1. Macroscopic Features
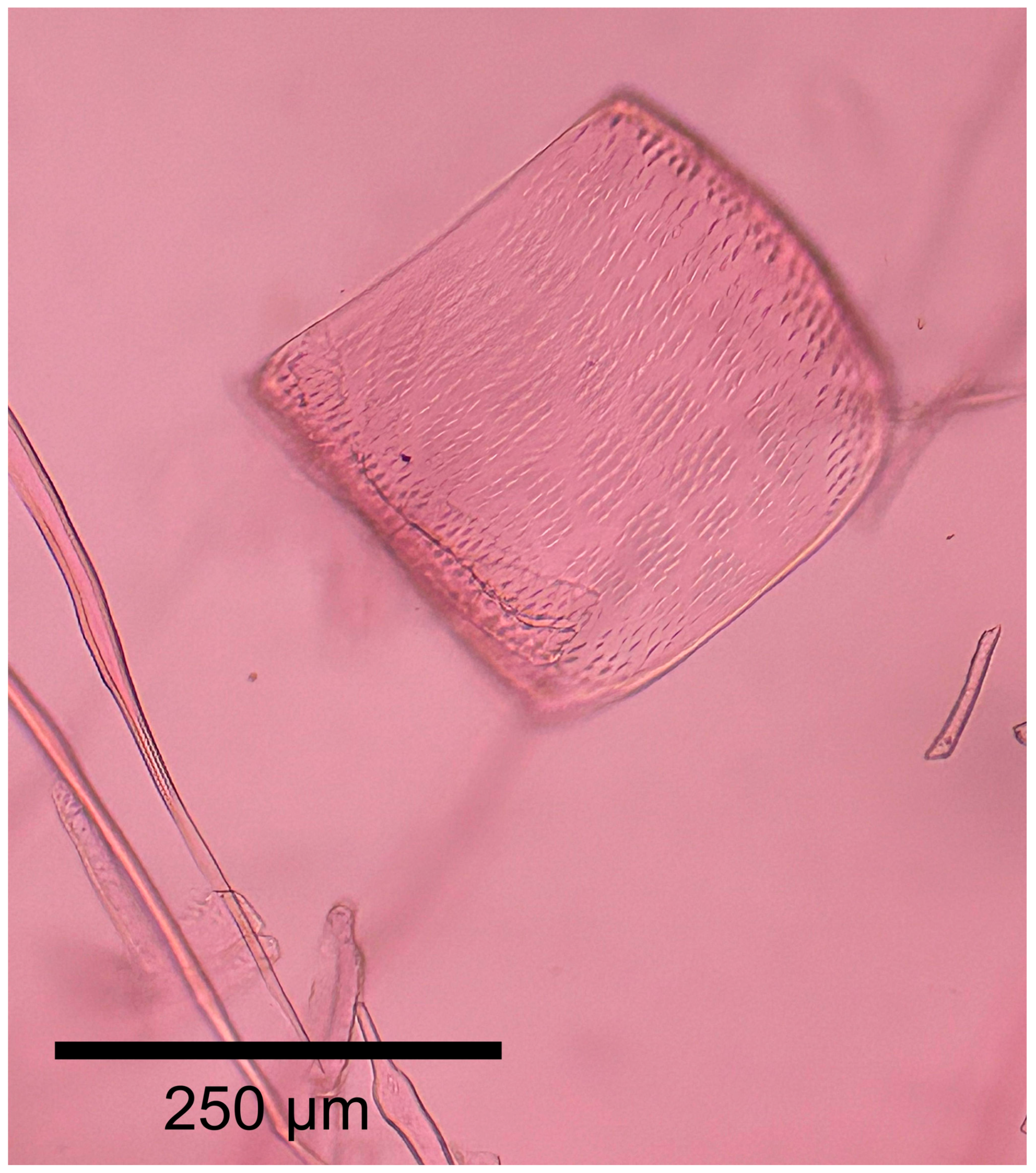
3.3.2. Microscopic Features
3.4. Pterocarpus angolensis DC.
3.4.1. Macroscopic Features
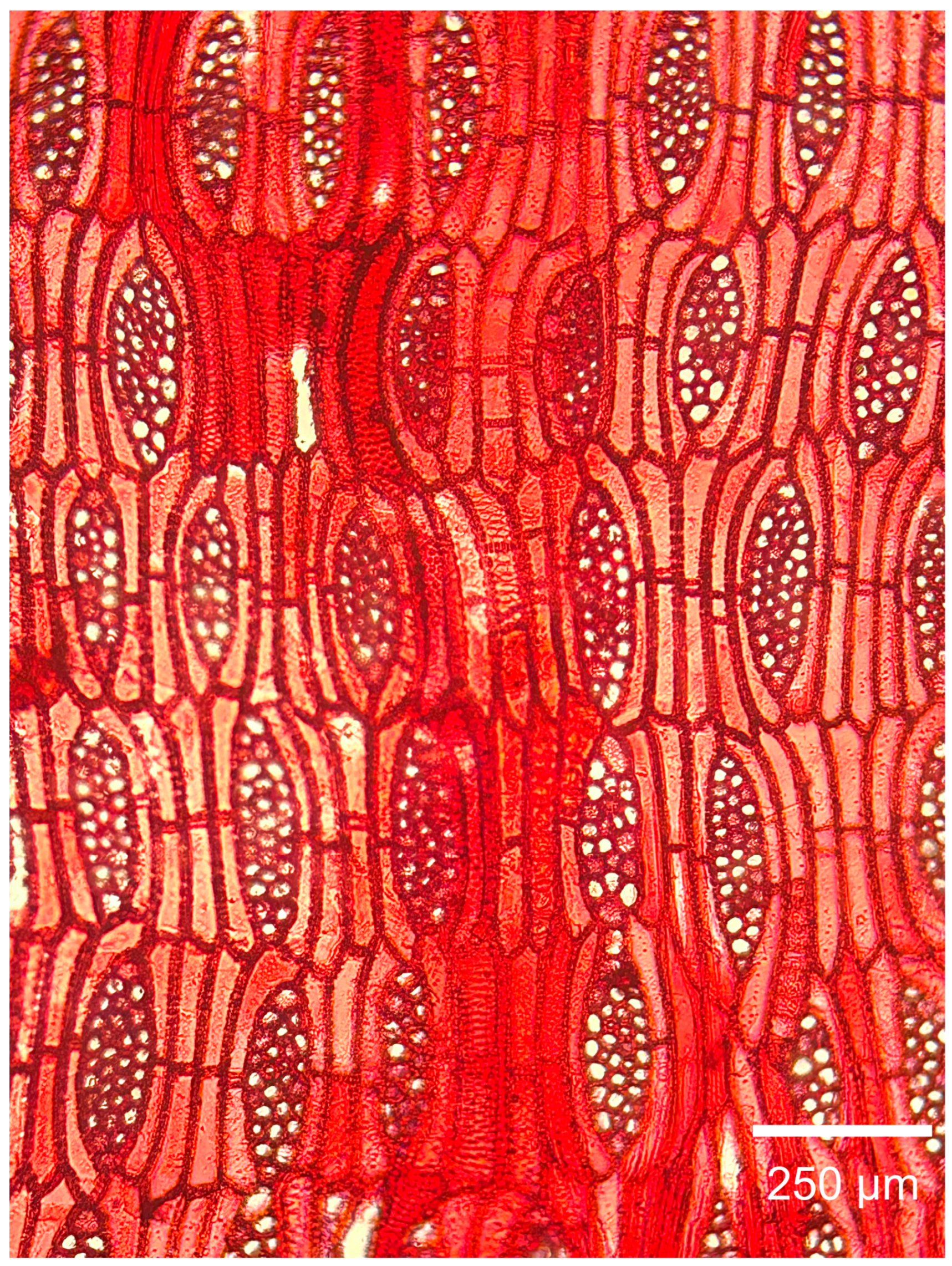
3.4.2. Microscopic Features
3.5. Colophospermum mopane (J. Kirk ex Benth.) J. Léonard
3.5.1. Macroscopic Features
3.5.2. Microscopic Features
4. Discussion
4.1. Summary of Wood Anatomical Features
4.2. Relationships Between Wood Anatomy and Species Vulnerability to Extreme Weather Events
4.2.1. Drought Resilience
- A low frequency of relatively large, solitary vessels that allow high water flow;
- Thick-walled fibers surrounding vessels, mostly solitary, reinforcing them against embolism;
- Simple perforation plates and vestured pits, optimizing hydraulic efficiency under drought stress.
4.2.2. Flooding Resilience
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Conselho de Ministros. Plano Director Para Prevenção e Mitigação Das Calamidades Naturais; FAOLEX: Rome, Italy, 2017. [Google Scholar]
- Aquino, A.; Lim, J.; Kaechele, K.; Taquidir, M. Mozambique Country Forestry Note; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- MITADER. Strategic Forestry Agenda of Mozambique 2019-2035 and National Forestry Programme; FAOLEX: Rome, Italy, 2019. [Google Scholar]
- Mansur, E.; Zacarias, A. From Policies to Practices: Lessons from Community Forestry in Mozambique. In Proceedings of the XII World Forestry Congress, Québec City, QC, Canada, 21–28 September 2003. [Google Scholar]
- Ryan, C.M.; Pritchard, R.; McNicol, I.; Owen, M.; Fisher, J.A.; Lehmann, C. Ecosystem Services from Southern African Woodlands and Their Future under Global Change. Phil. Trans. R. Soc. B 2016, 371, 20150312. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Zorrilla-Miras, P.; Baumert, S.; Luz, A.C.; Woollen, E.; Grundy, I.; Artur, L.; Ribeiro, N.; Mahamane, M.; Patenaude, G. Charcoal Income as a Means to a Valuable End: Scope and Limitations of Income from Rural Charcoal Production to Alleviate Acute Multidimensional Poverty in Mabalane District, Southern Mozambique. World Dev. Perspect. 2017, 7–8, 43–60. [Google Scholar] [CrossRef]
- Joaquim-Meque, E.; Lousada, J.; Liberato, M.L.R.; Fonseca, T.F. Forest in Mozambique: Actual Distribution of Tree Species and Potential Threats. Land 2023, 12, 1519. [Google Scholar] [CrossRef]
- Ribeiro, N.S.; Syampungani, S.; Matakala, N.M.; Nangoma, D.; Ribeiro-Barros, A.I. Miombo Woodlands Research Towards the Sustainable Use of Ecosystem Services in Southern Africa. In Biodiversity in Ecosystems—Linking Structure and Function; Lo, Y.-H., Blanco, J.A., Roy, S., Eds.; InTech: London, UK, 2015; ISBN 978-953-51-2028-5. [Google Scholar]
- Ribeiro, N.S.; Matos, C.N.; Moura, I.R.; Washington-Allen, R.A.; Ribeiro, A.I. Monitoring Vegetation Dynamics and Carbon Stock Density in Miombo Woodlands. Carbon Balance Manag. 2013, 8, 11. [Google Scholar] [CrossRef]
- Noé Dos Santos Ananias Hofiço. Frederico Dimas Fleig Diversity and Structure of Miombo Woodlands in Mozambique Using a Range of Sampling Sizes. J. Agric. Sci. Technol. 2015, 5, 679–690. [Google Scholar] [CrossRef]
- Makhado, R.; Potgieter, M.; Luus-Powell, W. Nutritional Value of Colophospermum Mopane as Source of Browse and Its Chemical Defences against Browsers: A Review. J. Anim. Plant Sci. 2016, 26, 569–576. [Google Scholar]
- Bila, J.M.; Mabjaia, N. Crescimento e Fitossociologia de Uma Floresta Com Colophospermum Mopane, Em Mabalane, Província de Gaza, Moçambique. Pesq. Flor. Bras. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Bila, J.M.; Sanquetta, C.R.; Corte, A.P.D.; De Freitas, L.J.M. Distribuição Diamétrica e Principais Espécies Arbóreas Presentes Nos Ecossistemas de Miombo, Mopane e Mecrusse Em Moçambique. Pesq. Flor. Bras. 2018, 38. [Google Scholar] [CrossRef]
- Magalhães, T.M.; Seifert, T. Estimation of Tree Biomass, Carbon Stocks, and Error Propagation in Mecrusse Woodlands. Open J. For. 2015, 05, 471–488. [Google Scholar] [CrossRef]
- Ali, A.C.; Uetimane, E.; Lhate, I.A.; Terziev, N. Anatomical Characteristics, Properties and Use of Traditionally Used and Lesser-Known Wood Species from Mozambique: A Literature Review. Wood Sci. Technol. 2008, 42, 453–472. [Google Scholar] [CrossRef]
- Malimbwi, R.; Chidumayo, E.N.; Zahabu, E.; Kingazi, S.; Misana, S.; Luoga, E.; Nduwamungu, J. Woodfuel. In The dry forests and woodlands of Africa: Managing for Products and Services; The Earthscan Forest Library; Earthscan: London, UK, 2010; pp. 155–177. ISBN 978-1-84971-131-9. [Google Scholar]
- Sitoe, A.; Chidumayo, E.N.; Alberto, M. Timber and Wood Products. In The Dry Forests and Woodlands of Africa: Managing for Products and Services; The earthscan forest library; Earthscan: London, UK, 2010; pp. 131–153. ISBN 978-1-84971-131-9. [Google Scholar]
- Meier, E. Wood! Identifying and Using Hundreds of Woods Worldwide. 2015. Available online: http://www.wood-database.com (accessed on 10 March 2025).
- PlantZAfrica. Spirostachys Africana. Available online: https://pza.sanbi.org/spirostachys-africana (accessed on 10 March 2025).
- Singh, K.; Baijnath, H. Spirostachys Africana: A Review of Phytochemistry, Traditional and Biological Uses and Toxicity. Indilinga Afr. J. Indig. Knowl. Syst. 2020, 19, 176–188. [Google Scholar]
- Lennox, S.J.; Bamford, M. Use of Wood Anatomy to Identify Poisonous Plants: Charcoal of Spirostachys Africana. S. Afr. J. Sci. 2015, 111, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Victor, P.M.; Tshisikhawe, M.P.; Magwede, K. Population Dynamics of Spirostachys Africana Sond. a Species Highly Exploited amongst Some Communities of Limpopo Province, South Africa. Biodiversitas 2023, 24, 492–497. [Google Scholar] [CrossRef]
- PlantZAfrica. Afzelia Quanzensis. Available online: https://pza.sanbi.org/afzelia-quanzensis (accessed on 11 March 2025).
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Afzelia Quanzensis. In Agroforestree Database: A Tree Reference and Selection Guide, Version 4.0; CIFOR-ICRAF: Nairobi, Kenya, 2009. [Google Scholar]
- Louppe, D.; Gérard, J. Afzelia Quanzensis. In Prota Database; PROTA4U: Nairobi, Kenya, 2011. [Google Scholar]
- Tropical Plants Database. Millettia Stuhlmannii. Available online: https://tropical.theferns.info/viewtropical.php?id=Millettia%20stuhlmannii (accessed on 15 March 2025).
- Lemmens, R. Millettia Stuhlmannii Taub. In Prota Database; PROTA4U: Nairobi, Kenya, 2008; Volume 7. [Google Scholar]
- Uetimane, E.; Jebrane, M.; Terziev, N.; Daniel, G. Comparative Wood Anatomy and Chemical Composition of Millettia Mossambicensis and Millettia Stuhlmannii from Mozambique. BioRes 2018, 13, 3335–3345. [Google Scholar] [CrossRef]
- Ekman, S.-M.S.; Wenbin, H.; Langa, E. Chinese Trade and Investment in the Mozambican Timber Industry: A Case Study from Cabo Delgado Province; Center for International Forestry Research (CIFOR): Nairobi, Kenya, 2013. [Google Scholar]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Pterocarpus Angolensis. In Agroforestree Database: A Tree Reference and Selection Guide; Version 4.0; CIFOR-ICRAF: Nairobi, Kenya, 2009. [Google Scholar]
- Tropical Plants Database. Pterocarpus Angolensis. Available online: https://tropical.theferns.info/viewtropical.php?id=Pterocarpus+angolensis (accessed on 15 March 2025).
- Graz, F.P. Description and Ecology of Pterocarpus Angolensis in Namibia. Dinteria 2004, 29, 27–39. [Google Scholar]
- Caro, T.M.; Sungula, M.; Schwartz, M.W.; Bella, E.M. Recruitment of Pterocarpus Angolensis in the Wild. For. Ecol. Manag. 2005, 219, 169–175. [Google Scholar] [CrossRef]
- Mojeremane, W.; Lumbile, A.U. A Review of Pterocarpus Angolensis DC. (Mukwa) an Important and Threatened Timber Species of the Miombo Woodlands. Res. J. For. 2016, 10, 8–14. [Google Scholar] [CrossRef]
- Santos, E.S.; Luís, Â.; Gonçalves, J.; Rosado, T.; Pereira, L.; Gallardo, E.; Duarte, A.P. Julbernardia Paniculata and Pterocarpus Angolensis: From Ethnobotanical Surveys to Phytochemical Characterization and Bioactivities Evaluation. Molecules 2020, 25, 1828. [Google Scholar] [CrossRef]
- Timberlake, J.R. Colophospermum Mopane: Annotated Bibliography and Review; The Zimbabwe Bulletin of Forestry Research; Forestry Commission: Harare, Zimbabwe, 1995; ISBN 978-0-7974-1420-4.
- Jinga, P.; Mureva, A.; Manyangadze, T. Mopane (Colophospermum mopane): A Potential Winner under Climate Change in Southern Africa. Austral Ecol. 2023, 48, 1848–1864. [Google Scholar] [CrossRef]
- Mashabane, L.G.; Wessels, D.C.J.; Potgieter, M.J. The Utilisation of Colophospermum Mopane by the Vatsonga in the Gazankulu Region (Eastern Northern Province, South Africa). S. Afr. J. Bot. 2001, 67, 199–205. [Google Scholar] [CrossRef]
- Stevens, N. What Shapes the Range Edge of a Dominant African Savanna Tree, Colophospermum Mopane ? A Demographic Approach. Ecol. Evol. 2021, 11, 3726–3736. [Google Scholar] [CrossRef] [PubMed]
- Madzibane, J.; Potgieter, M.J. Uses of Colophospermum Mopane (Leguminosae: Caesalpinioideae) by the Vhavenda. S. Afr. J. Bot. 1999, 65, 440–444. [Google Scholar] [CrossRef]
- Makhado, R.A.; Potgieter, M.J.; Wessels, D.C.J. Colophospermum Mopane Wood Utilisation in the Northeast of the Limpopo Province, South Africa. Ethnobot. Leafl. 2009, 13, 921–945. [Google Scholar]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Colophospermum Mopane. In Agroforestree Database: A Tree Reference and Selection Guide; Version 4.0; CIFOR-ICRAF: Nairobi, Kenya, 2009. [Google Scholar]
- Steyn, J. The Mopane Tree–Colophospermum Mopane, Often Despised but Having a Lot to Offer. Kruger2Canyon. 2023. Available online: https://kruger2canyon.co.za/the-mopane-tree-colophospermum-mopane-often-despised-but-having-a-lot-to-offer/ (accessed on 18 March 2025).
- Brigham, T.; Chihong, A.; Chidumayo, E. Trade in Woodland Products from Miombo Region. In Trade in Woodland Products from Miombo Region; Center for International Forestry Research: Kepong, Malaysia, 1997; p. 273. ISBN 979-8764-07-2. [Google Scholar]
- Mbanze, A.A.; Shuangao, W.; Mudekwe, J.; Dias, C.R.; Sitoe, A. The Rise and Fall of Plantation Forestry in Northern Mozambique. Trees For. People 2022, 10, 100343. [Google Scholar] [CrossRef]
- Di Matteo, F.; Schoneveld, G. Agricultural Investments in Mozambique: An Analysis of Investment Trends, Business Models and Social and Environmental Conduct; Center for International Forestry Research (CIFOR): Nairobi, Kenya, 2016. [Google Scholar]
- Noe dos Santos Ananias, H. Potencial de regeneração natural e crescimento de Millettia stuhlmannii Taub. em floresta de miombo como subsídio para o manejo sustentável. Master’s Thesis, Universidade Federal de Santa Maria, Santa Maira, Brazil, 2021. [Google Scholar]
- Thompson, H.E.; Berrang-Ford, L.; Ford, J.D. Climate Change and Food Security in Sub-Saharan Africa: A Systematic Literature Review. Sustainability 2010, 2, 2719–2733. [Google Scholar] [CrossRef]
- Nikodemus, A.; Abdollahnejad, A.; Kapuka, A.; Panagiotidis, D.; Hájek, M. Socio-Economic Benefits of Colophospermum Mopane in a Changing Climate in Northern Namibia. Forests 2023, 14, 290. [Google Scholar] [CrossRef]
- Tucker, J.; Daoud, M.; Oates, N.; Few, R.; Conway, D.; Mtisi, S.; Matheson, S. Social Vulnerability in Three High-Poverty Climate Change Hot Spots: What Does the Climate Change Literature Tell Us? Reg. Environ. Change 2015, 15, 783–800. [Google Scholar] [CrossRef]
- Wlokas, H.L. The Impacts of Climate Change on Food Security and Health in Southern Africa. J. Energy South. Afr. 2008, 19, 12–20. [Google Scholar] [CrossRef]
- Osbahr, H.; Twyman, C.; Neil Adger, W.; Thomas, D.S.G. Effective Livelihood Adaptation to Climate Change Disturbance: Scale Dimensions of Practice in Mozambique. Geoforum 2008, 39, 1951–1964. [Google Scholar] [CrossRef]
- Brown, M.E.; Hintermann, B.; Higgins, N. Markets, Climate Change, and Food Security in West Africa. Environ. Sci. Technol. 2009, 43, 8016–8020. [Google Scholar] [CrossRef]
- Baarsch, F.; Granadillos, J.R.; Hare, W.; Knaus, M.; Krapp, M.; Schaeffer, M.; Lotze-Campen, H. The Impact of Climate Change on Incomes and Convergence in Africa. World Dev. 2020, 126, 104699. [Google Scholar] [CrossRef]
- World Bank Group World Bank Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/ (accessed on 18 March 2025).
- USAID Climate Risk Profile: Mozambique—Fact Sheet—Mozambique|ReliefWeb. Available online: https://reliefweb.int/report/mozambique/climate-risk-profile-mozambique-fact-sheet (accessed on 16 April 2025).
- Ministry of Foreign Affairs of the Netherlands. Climate Change Profile Mozambique; Ministry of Foreign Affairs of the Netherlands: The Hague, The Netherlands, 2018; p. 20.
- Midgley, G.F.; Bond, W.J. Future of African Terrestrial Biodiversity and Ecosystems under Anthropogenic Climate Change. Nature Clim. Change 2015, 5, 823–829. [Google Scholar] [CrossRef]
- Sintayehu, D.W. Impact of Climate Change on Biodiversity and Associated Key Ecosystem Services in Africa: A Systematic Review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- Janssen, T.A.J.; Hölttä, T.; Fleischer, K.; Naudts, K.; Dolman, H. Wood Allocation Trade-offs between Fiber Wall, Fiber Lumen, and Axial Parenchyma Drive Drought Resistance in Neotropical Trees. Plant Cell Environ. 2020, 43, 965–980. [Google Scholar] [CrossRef]
- Baas, P.; Ewers, F.W.; Davis, S.D.; Wheeler, E.A. Evolution of Xylem Physiology. In The Evolution of Plant Physiology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 273–295. ISBN 978-0-12-339552-8. [Google Scholar]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in Wood Density and Structure Are Linked to Prevention of Xylem Implosion by Negative Pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Tyree, M.T.; Sperry, J.S. Vulnerability of Xylem to Cavitation and Embolism. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1989, 40, 19–36. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H. Hydraulic Architecture of Whole Plants and Plant Performance. In Xylem Structure and the Ascent of Sap; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 2002; pp. 175–214. ISBN 978-3-642-07768-5. [Google Scholar]
- Ellerby, D.J.; Ennos, A.R. Resistances to Fluid Flow of Model Xylem Vessels with Simple and Scalariform Perforation Plates. J. Exp. Bot. 1998, 49, 979–985. [Google Scholar] [CrossRef]
- Feild, T.S.; Wilson, J.P. Evolutionary Voyage of Angiosperm Vessel Structure-Function and Its Significance for Early Angiosperm Success. Int. J. Plant Sci. 2012, 173, 596–609. [Google Scholar] [CrossRef]
- Jansen, S. Vestured pits: A diagnostic character in the secondary xylem of myrtales. J. Trop. For. Sci. 2008, 20, 328–339. [Google Scholar]
- Franklin, G.L. Preparation of Thin Sections of Synthetic Resins and Wood-Resin Composites, and a New Macerating Method for Wood. Nature 1945, 155, 51. [Google Scholar] [CrossRef]
- IAWA. Committee Standard List of Characters Suitable For Computerized Hardwood Identification. IAWA Bull. 1989, 2, 99–110. [Google Scholar]
- Wheeler, E.; Baas, P.; Gasson, P. IAWA List of Microcopie Features for Hardwood Identification. IAWA J. Int. Assoc. Wood Anat. 1989, 10, 219–332. [Google Scholar]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The Determinants of Leaf Turgor Loss Point and Prediction of Drought Tolerance of Species and Biomes: A Global Meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Dias, A.T.C.; Ackerly, D.D.; Aerts, R.; Van Bodegom, P.M.; Cornwell, W.K.; Dong, M.; Kurokawa, H.; Liu, G.; Onipchenko, V.G.; et al. Global to Community Scale Differences in the Prevalence of Convergent over Divergent Leaf Trait Distributions in Plant Assemblages: Global Patterns in Plant Species Assembly. Glob. Ecol. Biogeogr. 2011, 20, 755–765. [Google Scholar] [CrossRef]
- Gartner, B.L.; Bullock, S.H.; Mooney, H.A.; Brown, V.B.; Whitbeck, J.L. Water transport properties of vine and tree stems in a tropical deciduous forest. Am. J Bot. 1990, 77, 742–749. [Google Scholar] [CrossRef]
- Mcculloh, K.A.; Johnson, D.M.; Meinzer, F.C.; Voelker, S.L.; Lachenbruch, B.; Domec, J. Hydraulic Architecture of Two Species Differing in Wood Density: Opposing Strategies in Co-occurring Tropical Pioneer Trees. Plant Cell Environ. 2012, 35, 116–125. [Google Scholar] [CrossRef]
- Méndez-Alonzo, R.; Paz, H.; Zuluaga, R.C.; Rosell, J.A.; Olson, M.E. Coordinated Evolution of Leaf and Stem Economics in Tropical Dry Forest Trees. Ecology 2012, 93, 2397–2406. [Google Scholar] [CrossRef]
- Jansen, S.; Baas, P.; Gasson, P.; Lens, F.; Smets, E. Variation in Xylem Structure from Tropics to Tundra: Evidence from Vestured Pits. Proc. Natl. Acad. Sci. USA 2004, 101, 8833–8837. [Google Scholar] [CrossRef]
- Jansen, S.; Baas, P.; Gasson, P.; Smets, E. Vestured Pits: Do They Promote Safer Water Transport? Int. J. Plant Sci. 2003, 164, 405–413. [Google Scholar] [CrossRef]
- Wardrop, A.B.; Ingle, H.D.; Davies, G.W. Nature of Vestured Pits in Angiosperms. Nature 1963, 197, 202–203. [Google Scholar] [CrossRef]
- Rabaey, D.; Lens, F.; Smets, E.; Jansen, S. The Phylogenetic Significance of Vestured Pits in Boraginaceae. Taxon 2010, 59, 510–516. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of Tree Mortality under Drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Alder, N.N.; Eastlack, S.E. The Effect of Reduced Hydraulic Conductance on Stomatal Conductance and Xylem Cavitation. J. Exp. Bot. 1993, 44, 1075–1082. [Google Scholar] [CrossRef]
- Tyree, M.T.; Engelbrecht, B.M.J.; Vargas, G.; Kursar, T.A. Desiccation Tolerance of Five Tropical Seedlings in Panama. Relationship to a Field Assessment of Drought Performance. Plant Physiol. 2003, 132, 1439–1447. [Google Scholar] [CrossRef]
- Case, M.F.; Wigley, B.J.; Wigley-Coetsee, C.; Carla Staver, A. Could Drought Constrain Woody Encroachers in Savannas? Afr. J. Range Forage Sci. 2020, 37, 19–29. [Google Scholar] [CrossRef]
- Muianga, M.; Norfolk, S. Investimento Chinês no Sector Florestal Moçambicano; International Institute for Environment and Development: London, UK, 2017; p. 60. [Google Scholar]
- Swenson, N.G.; Enquist, B.J. Ecological and Evolutionary Determinants of a Key Plant Functional Trait: Wood Density and Its Community-wide Variation across Latitude and Elevation. Am. J Bot. 2007, 94, 451–459. [Google Scholar] [CrossRef]
- Putz, F.E.; Coley, P.D.; Lu, K.; Montalvo, A.; Aiello, A. Uprooting and Snapping of Trees: Structural Determinants and Ecological Consequences. Can. J. For. Res. 1983, 13, 1011–1020. [Google Scholar] [CrossRef]
- ter Steege, H.; Hammond, D.S. Character Convergence, Diversity, and Disturbance in Tropical Rain Forest in Guyana. Ecology 2001, 82, 3197–3212. [Google Scholar] [CrossRef]
- Physiological Plant Ecology II: Water Relations and Carbon Assimilation; Lange, O.L., Ed.; Encyclopedia of Plant Physiology; Springer: Berlin, Germany, 1982; ISBN 978-0-387-10906-0. [Google Scholar]
- Kozlowski, T.T.; Kramer, P.J.; Pallardy, S.G. The Physiological Ecology of Woody Plants; Physiological Ecology; Academic Press Inc.: San Diego, CA, USA, 1991; ISBN 978-0-323-13800-0. [Google Scholar]
- Tsoumis, G. Science and Technology of Wood: Structure, Properties, Utilization; Van Nostrand Reinhold New York: New York, NY, USA, 1991; Volume 115. [Google Scholar]
- Geiger, D.R.; Servaites, J.C. 5—Carbon Allocation and Response to Stress. In Response of Plants to Multiple Stresses; Mooney, H.A., Winner, W.E., Pell, E.J., Eds.; Pergamon Programmed Texts; Academic Press: San Diego, CA, USA, 1991; pp. 103–127. ISBN 978-0-08-092483-0. [Google Scholar]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How Do Trees Die? A Test of the Hydraulic Failure and Carbon Starvation Hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural Leaf Carbohydrate Dynamics of P Inus Edulis during Drought-induced Tree Mortality Reveal Role for Carbon Metabolism in Mortality Mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef]
- Pallardy, S.G. Enzymes, Energetics, and Respiration. In Physiology of Woody Plants; Elsevier: Amsterdam, The Netherlands, 2008; pp. 169–197. ISBN 978-0-12-088765-1. [Google Scholar]
- Evans, D.E. Aerenchyma Formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]












| Scientific Name | Family | Common Name | Forest Type | Commercial Class |
|---|---|---|---|---|
| Spirostachys africana | Euphorbiaceae | Tambotie | Near the water | Precious |
| Afzelia quanzensis | Fabaceae | Pod Mahogany | Miombo | First |
| Millettia stuhlmannii | Fabaceae | Panga Panga | Miombo | First |
| Pterocarpus angolensis | Fabaceae | Umbila | Miombo | First |
| Colophospermum mopane | Fabaceae | Mopane | Mopane | First |
| Feature | Spirostachys africana | Afzelia quanzensis | Millettia stuhlmannii | Pterocarpus angolensis | Colophospermum mopane |
|---|---|---|---|---|---|
| Perforation plates | Simple | Simple | Simple | Simple | Simple |
| Inter-vessel pits | Alternate | Alternate | Alternate | Alternate | Alternate |
| Vestured pits | No | Yes | Yes | Yes | Yes |
| Vessels arrangement | Multiple (2–4) | 1–3 | 1–3 | 1 | 1–3 |
| Vessels frequency/mm2 | 49 | <9 | <6 | <6 | 11 |
| Vessels diameter (µm) | 78 | 161 | 223 | 148 | 91 |
| Axial parenchyma | Difuse | Lozenge-aliform, confluent, marginal | Lozenge-aliform, confluent, band, marginal | Aliform, confluent, band | Vasicentric, lozenge-aliform, confluent |
| Rays width | 1 | 1–3 | 3–4 | 1–2 | 1–3 |
| Rays cell composition | All procumbent | All procumbent | All procumbent | All procumbent | All procumbent |
| Storied structure | Absent | Absent | Rays, axial parenchyma, vessels, fibers | Rays, axial parenchyma, vessels, fibers | Absent |
| Mineral inclusions | Yes | Yes | Yes | Yes | Yes |
| Wood density (kg.m−3) * | 810 | 840 | 870 | 600 | 1080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joaquim-Meque, E.; Louzada, J.; Mady, F.T.M.; Souza, V.C.F.d.; Liberato, M.L.R.; Fonseca, T.F. Wood Anatomy Properties and Global Climate Change Constraints of Forest Species from the Natural Forest of Mozambique. Forests 2025, 16, 1018. https://doi.org/10.3390/f16061018
Joaquim-Meque E, Louzada J, Mady FTM, Souza VCFd, Liberato MLR, Fonseca TF. Wood Anatomy Properties and Global Climate Change Constraints of Forest Species from the Natural Forest of Mozambique. Forests. 2025; 16(6):1018. https://doi.org/10.3390/f16061018
Chicago/Turabian StyleJoaquim-Meque, Eugénia, José Louzada, Francisco Tarcísio Moraes Mady, Valquíria Clara Freire de Souza, Margarida L. R. Liberato, and Teresa Fidalgo Fonseca. 2025. "Wood Anatomy Properties and Global Climate Change Constraints of Forest Species from the Natural Forest of Mozambique" Forests 16, no. 6: 1018. https://doi.org/10.3390/f16061018
APA StyleJoaquim-Meque, E., Louzada, J., Mady, F. T. M., Souza, V. C. F. d., Liberato, M. L. R., & Fonseca, T. F. (2025). Wood Anatomy Properties and Global Climate Change Constraints of Forest Species from the Natural Forest of Mozambique. Forests, 16(6), 1018. https://doi.org/10.3390/f16061018







