Abstract
A possible tactic to survey and control Pine Wilt Disease is the use of semiochemical-baited traps to capture the insect-vector, the pine sawyer Monochamus galloprovincialis (Olivier) (Coleoptera: Cerambycidae). The most common chemical lure used is the Galloprotect Pack, which includes the aggregation pheromone ([2-undecyloxy] ethanol), a host monoterpene (α-pinene), and bark-beetle pheromones (ipsenol and 2-methyl-3-buten-1-ol). This lure also attracts non-target species, including bark beetles (Coleoptera: Curculionidae: Scolytinae) that use ipsenol (Ips sexdentatus (Boerner)) and 2-methyl-3-buten-1-ol (Orthotomicus erosus (Wollaston)) as pheromones, but also large numbers of their natural enemies, Temnoscheila caerulea (Olivier) (Coleoptera: Trogossitidae), Aulonium ruficorne (Olivier) (Coleoptera: Colydiidae), and Thanasimus formicarius (L.) (Coleoptera: Cleridae), and other saproxylic insects (Coleoptera: Cerambycidae). These catches cause a decrease in biodiversity of the forest insect communities, and the removal of predatory insects may favour bark beetle outbreaks. Thus, our project objective was to test trap modifications to try to reduce catches of non-target insects. Modifying the multifunnel trap’s collection cup by placing a 0.5 cm mesh in the drainage hole allowed the escape of all predator beetles (Cleridae, Trogossitidae, Colydiidae, and Histeridae) in 2020, and retained only two Trogossitidae in 2021, against 249 specimens caught in the non-modified collection cup. This simple modification thus allowed the escape of almost all predators, while maintaining the traps’ efficiency at catching the target species, M. galloprovincialis.
1. Introduction
Pine wilt disease (PWD) is a complex system that involves the infection of susceptible host trees by the pinewood nematode (PWN) Bursaphelenchus xylophilus (Steiner & Buhrer 1934; Nickle, 1970), through the activity of its insect vectors, the pine sawyer from the Monochamus genus. PWN is native to the North American east coast, but has now invaded many parts of the world, including Japan (1905), China (1982), Korea (1988) [1,2,3,4], and more recently, Europe. Since its detection in Portugal in 1999 [5], it has dispersed to Spain [6,7] and Madeira [8].
As a quarantine organism in Europe, strict measures have been implemented to prevent the progression of PWN to new areas. Globally, in almost all countries with PWN infestations, detection efforts are carried out annually, both for symptomatic trees [1,2,9,10] and for its insect-vectors during their flight periods [1,2,9,10,11,12,13,14,15,16,17], and all the material collected (wood samples and captured insects) is sent to a specialized laboratories for PWN screening.
Aerial or ground application of chemical insecticides has been used to control Monochamus adults in PWD-affected Asian countries [15]. However, in addition to the cost of insecticide applications over large areas, such applications can cause serious side effects, such as environmental contamination and toxicity to non-target organisms [14,18].
The strategy to control PWD caused by PWN worldwide focuses on infested host trees, with winter surveys of conifer forests for the detection and removal of infested trees, initially implemented in Japan [1] and followed in China [2,9], Korea [10], and Portugal [11]. A complementary measure targets the insect-vector populations by trapping during their flight periods, to decrease vector populations and slow the disease spread [14,17].
For the capture of Monochamus spp. in Europe, standard protocols use Teflon-coated black Lindgren multifunnel or black cross-vane traps [19], spaced 250–300 m apart [20] and lured with Galloprotect Pack (SEDQ, Barcelona, Spain), which includes the host volatile α-pinene, two bark beetle pheromones which act as synergists (ipsenol and 2-methyl-3-buten-1-ol) and the insects’ aggregation pheromone ([2-undeciloxyl] ethanol), known as monochamol [21]. Although developed for M. galloprovincialis (Olivier), this combination proved to be an effective lure for other Monochamus species in Europe [22] and in the PWN native range of eastern North America [23,24,25].
The Monochamus species evolved to be attracted by bark beetle pheromones [26,27,28,29,30,31,32,33], because they need weakened or recently dead hosts for egg-laying. If Monochamus spp. arrive while a tree is still under bark beetle attack, not only are the tree tissues more nutritious, but the larvae can enhance their nourishment through intraguild predation on bark beetle larvae [27,34].
However, when a trap is placed to capture Monochamus spp., using lures that include host tree volatiles and bark beetles’ pheromones, many other non-target Coleoptera are caught. Non-target species usually found in these traps include other large wood borers (Cerambycidae and Buprestidae) and smaller Cleridae, Colydiidae, Histeridae, and Trogossitidae, which include beneficial predators of xylophagous and bark beetles [23,35,36]. Catches of non-target species have been reported while attempting to control a number of forest pest insects [37,38,39]. These unintended catches of non-target species can have considerable negative impacts, reducing biodiversity and populations of beneficial predatory insects. These difficult issues have led some to question the wisdom of using baited traps to assess the spread of PWD and other diseases in several European countries [40,41,42,43]. Attempts have been made to reduce bycatch by modifying conventional traps, but with very limited results [39,44].
In this context, our objectives were to carry out a quantitative assessment of the negative effects of the M. galloprovincialis trapping methodology on non-target insects and to test modifications to the insect collection containers to allow smaller insects to escape. Here, we report that a simple modification to the collection cup can eliminate by-catch of most nontarget insects.
2. Materials and Methods
2.1. Assay Location and Time of Experiment
Field experiments were carried out over two consecutive years (24 July–21 October 2020; 5 July–27 September 2021) in a pure 40 years old maritime pine (Pinus pinaster) stand in Central Portugal (Seia municipality) (40°16′22″ N 7°42′12″ W), infested by PWN and with confirmed presence of insect vectors.
2.2. Experimental Design
The traps used in the assays were variations of black 12-element multi-funnel traps (WITASEK PflanzenSchutz GmbH, Vienna, Austria). All traps were baited with Galloprotect 2D Plus (SEDQ, Barcelona, Spain), and placed 50 m apart, hung from live branches in the lower canopy of dominated pines, at 6 an average height, in a Latin-square experimental design (3 different types × 3 repetitions). The distance between traps overlapped the effective areas of neighbouring traps (100–150 m radius) [20] in order to maximize the probability of catching beetles. Traps were serviced and rotated weekly (indicated by arrows in Figure 1) to minimize the location effect on captures. Lures were renewed after 6 wk, according to the manufacturer’s recommendation, and the experiment was terminated after 12 wk, after each trap type was exposed in every position, on four different occasions (Figure 1).
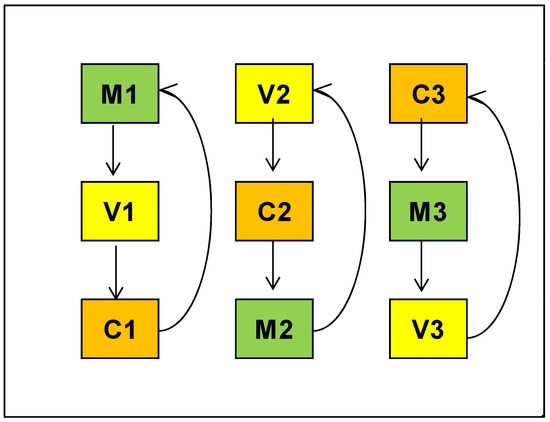
Figure 1.
Latin-square experimental design for comparing three models of multi-funnel traps in their ability to retain Monochamus galloprovincialis (target insect) and allow the escape of non-target insects. 2020 assays trap placement: C—Commercial 2019 model; M—Commercial 2019 model with modified collection cup; V—Commercial 2012 model. 2021 assays trap placement: C—Commercial 2019 model; M—Commercial 2019 model with modified collection cup; V—Commercial 2019 model with prototype collection cup.
Traps used in 2020 trials were the model commercially available in 2012 (V), the 2019 commercial model (C) (Figure 2), and the same 2019 model with a modified collection cup (M), where the bottom of the cup was removed and replaced by a 0.5 cm plastic mesh (Figure 3). The number of funnels and consequently the size of the 2012 and 2019 trap versions were similar, although there were some minor differences in the funnel connections and the collection cup design, which was larger and shorter in the 2012 model.
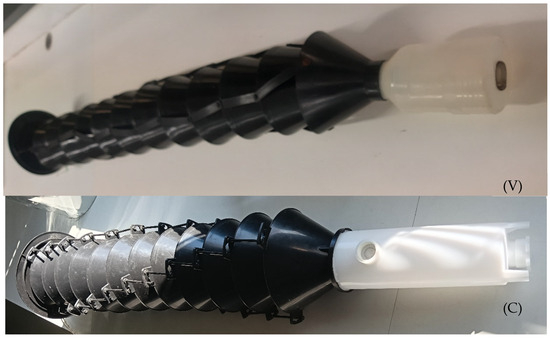
Figure 2.
Black 12-element multi-funnel traps used in the 2020 assay. (V) Commercial 2012 model; (C) Commercial 2019 model.

Figure 3.
Collection cups of black 12-element multi-funnel commercial 2019 model traps, used in 2021 assay. (Left): Commercial 2019 model collection cup; (center): Modified collection cup; (right): Prototype collection cup.
In 2021 assay, three versions of the 2019 model were tested with the same Latin-square experimental design (Figure 1): the 2019 commercial model (C), the modified collection cup tested in 2020 (M) and a prototype collection cup made with 0.5 cm metallic net in the drainage hole (V), prepared by the manufacturer, WITASEK PflanzenSchutz GmbH, (Vienna, Austria) (Figure 3).
In both years, insects captured weekly in each trap were identified to species level, using morphological taxonomic keys [45,46,47], and counted.
2.3. Data Analysis
The results of each experiment were statistically analysed to determine significant differences between the evaluated parameters (number of specimens caught in the traps of Cerambycidae, Scolytinae, Trogossitidae, Colydiidae, Cleridae, and Histeridae). Since the number of different insect species caught per trap and per date of collection was frequently zero and total values were quite small, a square root transformation was applied to the raw data [48]. One-way analysis of variance (ANOVA) was used to compare mean values of captured beetles per trap and collection, followed by post-hoc least significant difference (LSD) tests to establish homogeneous groups with a confidence level of 95% (α = 0.05), using the Statistica software package, version 12 [49].
3. Results
In 2020, a total of 67 specimens of the target M. galloprovincialis were caught, with a female-biased sex ratio (3.8:1). No statistically significant differences between the number of specimens caught among the tested trap types were found, F(2, 105) = 0.7932; p = 0.4551. The multifunnel 2012 model and the 2019 model with a modified cup caught 27 and 25 specimens, respectively, compared to the 2019 model (12 beetles). The results obtained by the modified cup revealed that replacement of the cup bottom with plastic mesh did not allow the target species to escape, thus not affecting the main purpose of this trapping procedure.
In this assay, several other cerambycid beetles were caught, in lower numbers, and were equally distributed among the trap types. These included 21 Arhopalus syriacus (Reitter), 9 A. ferus (Mulsant), 9 Spondylis buprestoides (Linnaeus), and 2 Acanthocinus griseus (Fabricius), which were found in the 2012 trap (Figure 4).
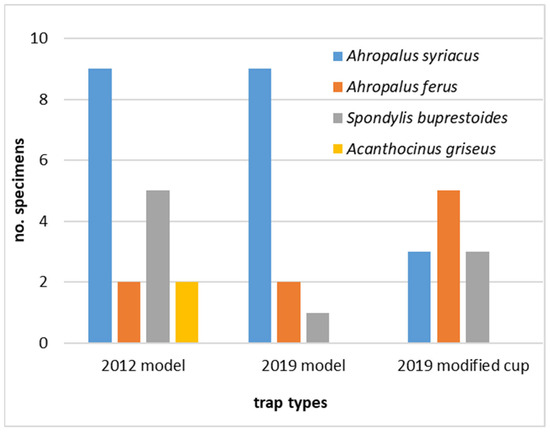
Figure 4.
Number of cerambycids, other than Monochamus galloprovincialis, caught per trap during the assay of 2020, in the different trap models tested (commercial 2012 model; commercial 2019 model with standard cup and with modified cup).
Of the 2385 scolytids caught, most were Orthotomicus erosus (Wollaston) (92.1%) and Ips sexdentatus (Boerner) (5.5%), with few remaining specimens being Hylurgus ligniperda (Fabricius) (32 beetles) and Tomicus sp. (10 beetles) (Figure 5A,B). As expected, no scolytids were found inside the modified collection cup, because all of the above bark beetle adults are less than 3 mm in length, and so could easily escape through the plastic mesh, resulting in highly significant statistical differences when compared with the other trap models, F(2, 105) = 16.0165; p < 0.0001 (Figure 5C). The 2012 model was more efficient at catching these nontarget species, capturing more than twice as many (1596 specimens) as the 2019 trap (789 specimens) (raw data is available in the supplemental material file).
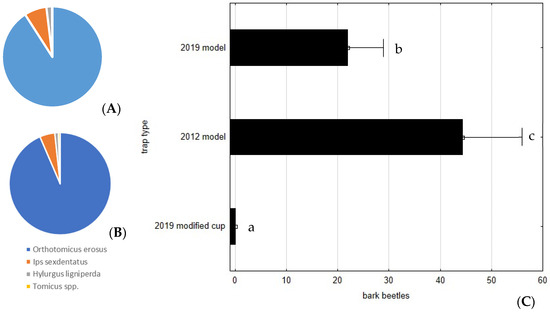
Figure 5.
Average number of bark beetles (Scolytinae) caught per trap and per week, during the assay of 2020, in the Commercial 2019 model (A) and Commercial 2012 model (B). The 2019 model trap with a modified cup did not capture any specimens. (C) Average number of bark beetles caught per trap. Bars represent the mean value and whiskers the standard error. Letters near each whisker represent LSD post-hoc homogeneous groups after ANOVA.
During the 2020 assay, 133 predatory beetles were caught in the traps, including 99 Temnoscheila caerulea (Olivier) (Trogossitidae), 28 Aulonium ruficorne (Olivier) (Colydiidae), 5 Thanasimus formicarius (L.) (Cleridae), and 1 Histeridae specimen (Figure 6).
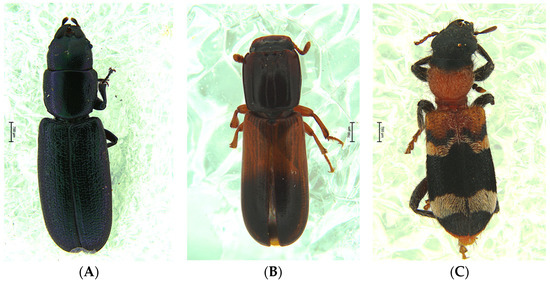
Figure 6.
Nontarget coleopteran predatory species, (A) Temnoscheila caerulea (Trogossitidae); (B) Aulonium ruficorne (Colydiidae); (C) Thanasimus formicarius (Cleridae), caught in the traps assays during 2020 and 2021.
The results obtained for the predatory beetles were analogous to those described for scolytids, with no specimens recovered from the traps with the modified cup, although T. caerulea is a considerably bigger beetle than the scolytids and other predatory species (Figure 6). Statistically significant differences were observed between trap types, F(2, 105)= 7.4906; p = 0.0009, with the modified collection cup being different than the two other trap types (Figure 7).
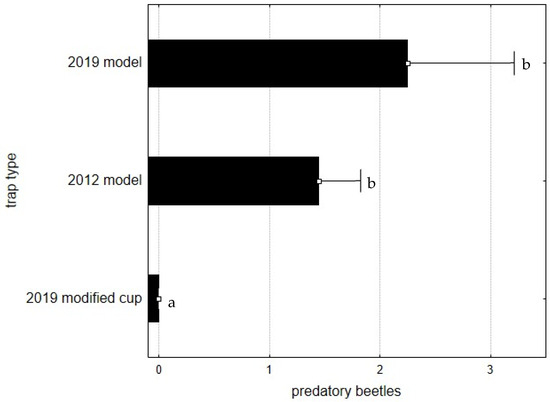
Figure 7.
Average number of predatory beetles caught per trap and per week, during 2020. Bars represent the mean value and whiskers the standard error. Letters near each whisker represent LSD post-hoc homogeneous groups after ANOVA.
In the 2021 assays, fewer M. galloprovincialis were caught (43 adult beetles), with no statistical differences in numbers collected in the different trap types, F(2, 105) = 1.5591; p = 0.2152. Eight specimens were found in the traps with the modified cup (the same used in 2020), while in the 2019 trap model and the traps with the prototype cup (with a net in the drainage hole), 18 and 17 beetles were caught, respectively.
Regarding the other cerambycid species trapped in 2021, higher numbers of A. syriacus, A. ferus, and S. buprestoides were captured in the 2019 trap model, thus revealing these saproxylic beetles were also able to escape from modified cups (Figure 8). No specimens of A. griseus were found.
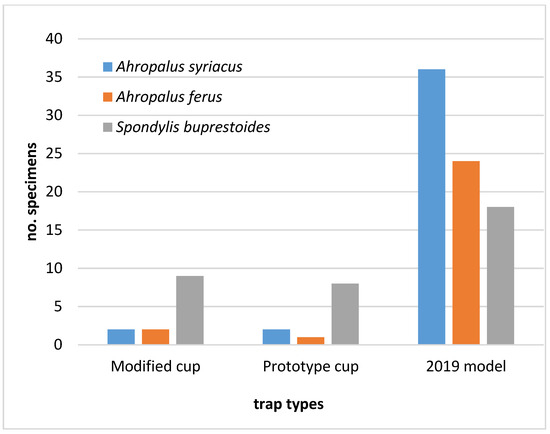
Figure 8.
Number of cerambycids, other than Monochamus galloprovincialis, caught per trap during the assay of 2021, in the different collection cups used in the multifunnel commercial 2019 model.
In the 2021 assay, the collection cup with mesh on the bottom or in the drain hole (Modified and Prototype) allowed the escape of all scolytids, as in 2020, resulting in highly significant differences when compared to the commercial 2019 model, F(2, 105) = 15.0404; p < 0.0001. The number of scolytids caught in 2021 was considerably less (44 specimens) than in the 2020 assay (7 O. erosus, 29 I. sexdentatus, 7 H. ligniperda, and 1 Tomicus spp.) (Figure 9).
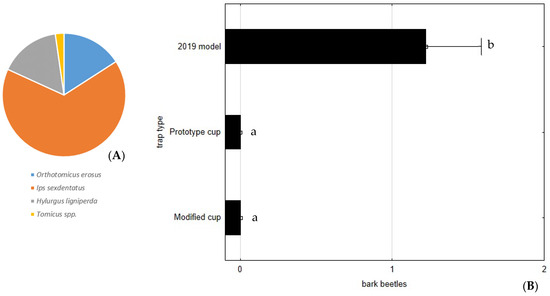
Figure 9.
Average number of bark beetles (Scolytinae) caught per trap and per week, during the assay of 2021, in the Commercial 2019 model (A). No bark beetles were found in traps with modified and prototype cups; (B) Average number of bark beetles caught per trap. Bars represent the mean value and whiskers the standard error. Letters near each whisker represent LSD post-hoc homogeneous groups after ANOVA.
Finally, during the 2021 assay, 249 predators were caught, with the Trogossitidae family (T. caerulea) contributing 88% (219 specimens), followed by Colydiidae (23 specimens), 6 Cleridae (T. formicarius), and 1 Histeridae specimen.
Almost all specimens were caught in the commercial 2019 model cup, with the exception of two T. caerulea found in the prototype cup. Consequently, there were highly significant differences, F(2, 105) = 30.0779; p < 0.0001, between the number of predator specimens caught in the different trap types (Figure 10).
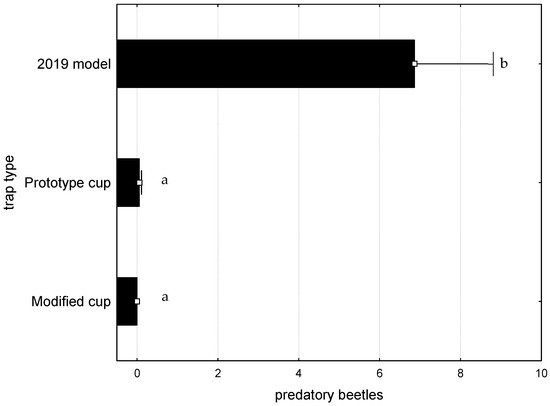
Figure 10.
Average number of predatory beetles caught per trap and per week, during the assay of 2021. Bars represent the mean value and whiskers the standard error. Letters near each whisker represent LSD post-hoc homogeneous groups after ANOVA.
4. Discussion and Conclusions
One strategy for the management of PWD-affected pines involves trapping its insect vectors, cerambycids in the genus Monochamus. Typically, pine sawyers are considered secondary species, needing other abiotic agents (e.g., severe drought or forest fires) or biotic agents (e.g., bark beetles) to weaken host trees so that they can be successfully colonized. The co-evolution of these species to respond to host volatiles and bark beetles’ pheromones allows them to efficiently locate suitable host trees still under attack, and this selectivity is likely chemically mediated [50,51,52].
Traps used for pine sawyers have included water pan traps, cross-vane traps with a black silhouette [53,54], black multi-funnel traps [19,55], or transparent cross-vane traps [17,19]. Trials helped demonstrate that either a 12-element black multi-funnel or a black cross-vane trap performed similarly in capturing M. galloprovincialis [19].
North American researchers determined that M. titillator (F.) was attracted to a blend of bark beetle pheromones and turpentine tree host volatiles [56,57]. Analogous attraction was later confirmed with other Monochamus species, such as M. clamator (leConte), M. scutellatus (Say) [27,51], and the European M. galloprovincialis [17,29].
However, lures consisting of a combination of host tree volatiles and bark beetle pheromones, not surprisingly, also attract significant numbers of non-target insects, including useful saproxylic species, and beneficial natural enemies of pest species [44,58,59,60,61,62].
In forests, a plethora of organisms contribute to nutrient cycling, canopy thinning, gap dynamics, soil structure, hydrology, and successional pathways [63]. Useful predatory species can exploit the pheromones of their prey, or other indirect chemical cues such as the volatiles emitted by trees under attack, to locate their prey [40,41,42]. Bakke and Kvamme [64] suggested that predators may also be attracted to volatiles emitted by captured beetles inside a trap.
The main insect predator species caught in our study, including T. caerulea (Trogossitidae), A. ruficorne (Colydiidae), and T. formicarius (Cleridae), had previously been collected in traps baited with pheromone blends of I. sexdentatus [41,60,65,66,67,68] and are considered important bark beetle natural enemies, contributing to their population control by preying on the last instar larvae and callow adults under the bark [62,68,69].
Catching these predators constitutes a problem for bark beetle management and forest insect diversity and equilibrium. Trapping control techniques must strive to be sustainable, impacting primarily or preferably exclusively the target species. Significant catches of nark beetle predators in commercial trap designs can contribute to undesirable effects on pest populations [70]. For example, it was estimated that a reduction of predator densities could lead, within 4–20 bark-beetle generations, to a doubling of the pest population, prolonging bark beetle outbreaks [71]. The use of commercial multifunnel traps to control the PWN insect-vector has this unwanted effect on the bark beetles’ populations that already benefit from PWN-infested trees as breeding substrate, since all surveyed pines were colonized by bark beetles, O. erosus, at least [72].
The results of our study demonstrate the low specificity of the Galloprotect Pack lure, as well as its possible negative effects against beneficial insects when used with the commercially available traps. However, simple modifications to the collection cups essentially eliminated catches of all non-target species, including other saproxylic cerambycids (Arhopalus spp. and S. buprestoides), bark beetles, and predatory species.
However, the fact that the modified cup does not retain bark beetles is apparently not desirable for general pine forest management, because I. sexdentatus and O. erosus are pests in the Mediterranean area that cause considerable mortality and must be controlled. The inclusion of Monochamus pheromone in the Galloprotect Pack lure has been demonstrated to have a strong repellent effect on bark beetles, drastically reducing their catches [23]. Therefore, for the integrated protection of pine forests, it will probably be necessary to simultaneously deploy traps with specific lures for bark beetles.
Finally, considering that the average size of most Monochamus species is similar, the modified collection cup can be effective worldwide in retaining mainly the target Monochamus and allowing the escape of smaller non-target species. However, testing of the modified collection cup in new areas with new species would probably be prudent before deploying the modified trap in operational programs.
Author Contributions
Conceptualization, L.B. and E.S.; methodology, L.B. and E.S.; software, L.B.; validation, L.B. and E.S.; formal analysis, L.B. and E.S.; investigation, L.B. and E.S.; resources, L.B. and E.S.; data curation, L.B. and E.S.; writing—original draft preparation, L.B.; writing—review and editing, L.B. and E.S.; visualization, L.B. and E.S.; supervision, L.B. and E.S.; project administration, L.B. and E.S.; funding acquisition, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by project PDR2020-101-032086—GO Gi(Pin), GREEN-IT “BioResources 4 Sustainability” https://doi.org/10.54499/UIDB/04551/2020, and Witasek PflanzenSchutz GmbH provided prototype collection cups.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors extend their sincere gratitude to Barbara Machado from FLORGENESE, for the link with WITASEK and field work logistic support. Telma Briote (FNAPF), Adérito Matos and Miguel Pimpão (INIAV), for helping with trap placement and insect collection. We acknowledge Jocelyn Millar (University of California, Riverside) for reviewing and editing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Futai, K. Pine Wilt in Japan: From First Incidence to the Present. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 5–12. [Google Scholar]
- Zhao, B.G. Pine wilt disease in China. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 18–25. [Google Scholar]
- Back, M.A.; Bonifácio, L.; Inácio, M.L.; Mota, M.; Boa, E. Pine wilt disease: A global threat to forestry. Plant Pathol. 2024, 73, 1026–1041. [Google Scholar] [CrossRef]
- EPPO. Bursaphelenchus xylophilus. EPPO Datasheets on Pests Recommended for Regulation. 2025. Available online: https://gd.eppo.int (accessed on 6 May 2025).
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, Causal Agent of Pine Wilt Disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Alonso, M.; Menéndez, M.; Diaz, R. Constitutive chemical compounds in different tissues of seven pine species and their relationship with susceptibility to pinewood nematode (Bursaphelenchus xylophilus). Environ. Sci. Proc. 2021, 3, 68. [Google Scholar] [CrossRef]
- Fonseca, L.; Cardoso, J.M.S.; Lopes, A.; Pestana, M.; Abreu, F.; Nunes, N.; Mota, M.; Abrantes, I. The pinewood nematode, Bursaphelenchus xylophilus, in Madeira Island. Helminthologia 2012, 49, 96–103. [Google Scholar] [CrossRef]
- Kwon, T.S.; Shin, J.H.; Lim, J.H.; Kim, Y.K.; Lee, E.J. Management of pine wilt disease in Korea through preventative silvicultural control. For. Ecol. Manag. 2011, 261, 562–569. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Sousa, E.; Abrantes, I. Pine wilt disease historical review. In Pine Wilt Disease in Europe: Biological Interactions and Integrated Management; FNAPF-Federação Nacional das Associações de Proprietários Florestais: Lisbon, Portugal, 2015; pp. 13–32. [Google Scholar]
- Li, M.; Dai, Y.; Wang, Y.; Wang, L.; Sun, S.; Chen, F. New insights into the life history of Monochamus saltuarius (Cerambycidae: Coleoptera) can enhance surveillance strategies for pine wilt disease. J. For. Res. 2021, 32, 2699–2707. [Google Scholar] [CrossRef]
- Ikeda, T.; Oda, K.; Yamane, A.; Enda, N. Volatiles from pine logs as the attractants for the Japanese Pine Sawyer Monochamus alternatus Hope (Coleoptera: Cerambycidae). J. Jap. For. Soc. 1980, 62, 150–152. [Google Scholar]
- Ikeda, T.; Yamane, A.; Enda, N.; Oda, K.; Makihara, H.; Ito, K.; Ôkochi, I. Attractiveness of each volatile components of felled pine trees for Monochamus alternatus (Coleoptera: Cerambycidae). J. Jap. For. Soc. 1986, 68, 15–19. [Google Scholar]
- Sousa, E.; Bonifácio, L.; Naves, P.; Vieira, M. Pine wilt disease management. In Pine Wilt Disease in Europe: Biological Interactions and Integrated Management; FNAPF-Federação Nacional das Associações de Proprietários Florestais: Lisbon, Portugal, 2015; pp. 221–295. [Google Scholar]
- Shin, S.C. Pine wilt disease in Korea. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 26–32. [Google Scholar]
- Lee, H.R.; Lee, S.C.; Lee, D.H.; Jung, M.; Kwon, M.J.; Huh, M.J.; Kim, D.S.; Lee, J.E.; Park, I.K. Identification of aggregation-sex pheromone of the Korean Monochamus alternatus (Coleoptera: Cerambycidae) population, the main vector of Pine Wood Nematode. J. Econ. Entomol. 2018, 111, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, L.; Praias, F.; Sousa, E. Trapping Monochamus galloprovincialis (Coleoptera: Cerambycidae), vector of the pine wood nematode, with pine allelochemicals in Portugal. Silva Lusit. 2012, 20, 39–53. [Google Scholar]
- Holmes, S.B.; MacQuarrie, C.J.K. Chemical control in forest pest management. Can. Entomol. 2016, 148, S270–S295. [Google Scholar] [CrossRef]
- Alvarez, G.; Etxebeste, I.; Gallego, D.; David, G.; Bonifacio, L.; Jactel, H.; Sousa, E.; Pajares, J.A. Optimization of traps for live trapping of Pine Wood Nematode vector Monochamus galloprovincialis. J. Appl. Entomol. 2014, 139, 618–626. [Google Scholar] [CrossRef]
- Jactel, H.; Bonifácio, L.; van Halder, I.; Vétillard, F.; Robinet, C.; David, G. A novel, easy method for estimating pheromone trap attraction range: Application to the pine sawyer beetle Monochamus galloprovincialis. Agric. For. Entomol. 2019, 21, 8–14. [Google Scholar] [CrossRef]
- Pajares, J.A.; Alvarez, G.; Ibeas, F.; Gallego, D.; Hall, D.; Farman, D. Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. J. Chem. Ecol. 2010, 36, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Pajares, J.A.; Álvarez, G.; Hall, D.R.; Ibarra, N.; Hoch, G.; Halbig, P.; Schroeder, M. Attractants for management of the pine sawyer beetle Monochamus sutor, a potential vector of Bursaphelenchus xylophilus. J. Appl. Entomol. 2016, 141, 97–111. [Google Scholar] [CrossRef]
- Miller, D.R.; Allison, J.D.; Crowe, C.M.; Dickinson, D.M.; Eglitis, A.; Hofstetter, R.W.; Munson, A.S.; Poland, T.M.; Reid, L.S.; Steed, B.E.; et al. Pine sawyers (Coleoptera: Cerambycidae) attracted to α-pinene, monochamol, and ipsenol in North America. J. Econ. Entomol. 2016, 109, 1205–1214. [Google Scholar] [CrossRef]
- Allison, J.D.; McKenney, J.L.; Millar, J.G.; McElfresh, J.S.; Mitchell, R.F.; Hanks, L.M. Response of the woodborers Monochamus carolinensis and Monochamus titillator to known cerambycid pheromones in the presence and absence of the host plant volatile alpha-pinene. Environ. Entomol. 2012, 41, 1587–1596. [Google Scholar] [CrossRef]
- Allison, J.D.; Guignard, Q.; Ochoa, I.; Sousa, E.; Bonifácio, L. Asymmetric semiochemical-mediated interactions of Monochamus spp. (Coleoptera: Cerambycidae) and associated bark beetles in Portugal and Canada. Environ. Entomol. 2024, 54, 46–53. [Google Scholar] [CrossRef]
- Allison, J.D.; Borden, J.H.; McIntosh, R.L.; de Groot, P.; Gries, R. Kairomonal response by four Monochamus species (Coleoptera: Cerambycidae) to bark beetle pheromones. J. Chem. Ecol. 2001, 27, 633–646. [Google Scholar] [CrossRef]
- Allison, J.D.; Morewood, W.D.; Borden, J.H.; Hein, K.E.; Wilson, I.M. Differential bio-activity of Ips and Dendroctonus (Coleoptera: Scolytidae) pheromone components for Monochamus clamator and M. scutellatus (Coleoptera: Cerambycidae). Environ. Entomol. 2003, 32, 23–30. [Google Scholar] [CrossRef]
- De Groot, P.; Nott, W. Response of the whitespotted sawyer beetle, Monochamus scutellatus, and associated woodborers to pheromones of some Ips and Dendroctonus bark beetles. J. Appl. Entomol. 2004, 128, 483–487. [Google Scholar] [CrossRef]
- Pajares, J.A.; Ibeas, F.; Diez, J.J.; Gallego, D. Attractive responses by Monochamus galloprovincialis (Col., Cerambycidae) to host and bark beetle semiochemicals. J. Appl. Entomol. 2004, 128, 633–638. [Google Scholar]
- Pajares, J.A.; Alvarez, G.; Hall, D.R.; Douglas, P.; Centeno, F.; Ibarra, N.; Schroeder, M.; Teale, S.A.; Wang, Z.; Yan, S.; et al. 2-(Undecyloxy)-ethanol is a major component of the male-produced aggregation pheromone of Monochamus sutor. Entomol. Exp. Appl. 2013, 149, 118–127. [Google Scholar] [CrossRef]
- Miller, D.R.; Asaro, C. Ipsenol and Ipsdienol attract Monochamus titillator (Coleoptera: Cerambycidae) and associated large pine woodborers in Southeastern United States. J. Econ. Entomol. 2005, 98, 2033–2040. [Google Scholar] [CrossRef]
- Miller, D.R.; Asaro, C.; Crowe, C.; Duerr, D. Bark beetle pheromones and pine volatiles: Attractant kairomone lure blend for longhorn beetles (Cerambycidae) in pine stands of the Southeastern United States. J. Econ. Entomol. 2011, 104, 1245–1257. [Google Scholar] [CrossRef]
- Miller, D.R.; Crowe, C.M.; Barnes, B.F.; Gandhi, K.J.K.; Duerr, D.A. Attaching lures to multiple-funnel traps targeting saproxylic beetles (Coleoptera) in pine stands: Inside or outside funnels? J. Econ. Entomol. 2013, 106, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Dodds, K.J.; Graber, C.; Stephen, F.M. Facultative intraguild predation by larval Cerambycidae (Coleoptera) on bark beetle larvae (Coleoptera: Scolytidae). Environ. Entomol. 2001, 30, 17–22. [Google Scholar] [CrossRef]
- Jaworski, T.; Plewa, R.; Dziuk, A.; Sukovata, L. Different responses of Monochamus galloprovincialis and three non-target species to trap type, colour, and lubricant treatment. Ann. For. Res. 2022, 65, 31–46. [Google Scholar] [CrossRef]
- Mellin, M.; Vihervuori, L.; Koivula, M.; Velmala, S. Pheromone-based monitoring of invasive alien insects along the border of Finland and Russia—Methods and unintentionally caught species. Balt. For. 2023, 28, 639. [Google Scholar] [CrossRef]
- Tamutis, V.; Zalubas, P. Non-target beetles trapped in Ips typographus L. pheromone traps. Balt. J. Coleopterol. 2001, 1, 65–70. [Google Scholar]
- Galko, J.; Nikolov, C.; Kunca, A.; Vakula, J.; Gubka, A.; Zubrik, M.; Konopka, B. Effectiveness of pheromone traps for the European spruce bark beetle: A comparative study of four commercial products and two new models. Lesn. Cas. For. J. 2016, 62, 207–215. [Google Scholar] [CrossRef]
- Bracalini, M.; Croci, F.; Ciardi, E.; Mannucci, G.; Papucci, E.; Gestri, G.; Tiberi, R.; Panzavolta, T. Ips sexdentatus Mass-Trapping: Mitigation of Its Negative Effects on Saproxylic Beetles Larger Than the Target. Forests 2021, 12, 175. [Google Scholar] [CrossRef]
- Chiverton, P.A.; Sotherton, N.W. The effects on beneficial arthropods of the exclusion of herbicides from cereal crop edges. J. Appl. Ecol. 1991, 28, 1027–1039. [Google Scholar] [CrossRef]
- Aukema, B.H.; Dahlsten, D.L.; Raffa, K.F. Exploiting behavioral disparities among predators and prey to selectively remove pests: Maximizing the ratio of bark beetles to predators removed during semiochemically based trap-out. Environ. Entomol. 2000, 29, 651–660. [Google Scholar] [CrossRef]
- Paradise, C.J.; Penev, Y.; Yu, P.; Grant, D.; Stanback, M. Accumulation of non-target arthropods on sticky tree bands. J. N. C. Acad. Sci. 2016, 132, 1–5. [Google Scholar] [CrossRef]
- Fite, T.; Damte, T.; Tefera, T.; Negeri, M. Evaluation of commercial trap types and lures on the population dynamics of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) and its effects on non-targets insects. Cogent Food Agric. 2020, 6, 1771116. [Google Scholar] [CrossRef]
- Martín, A.; Etxebeste, I.; Pérez, G.; Álvarez, G.; Sánchez, E.; Pajares, J. Modified pheromone traps help reduce bycatch of bark-beetle natural enemies. Agric. For. Entomol. 2013, 15, 86–97. [Google Scholar] [CrossRef]
- Chinery, M. Guide to the Insects of Britain and Western Europe; Collins Education: Glasgow, UK, 1986; 320p. [Google Scholar]
- Ferreira, M.C.; Cabral, M.T. Pragas do Pinhal; Estação Florestal Nacional: Lisbon, Portugal, 1999; 159p. (In Portuguese) [Google Scholar]
- Ferreira, M.C.; Ferreira, G.W.S. Pragas das Resinosas. Guia de Campo, 3rd ed.; D.G.P.A./Ministério Agricultura, Pescas e Alimentação: Lisbon, Portugal, 1990; 108p. (In Portuguese) [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prantice-Hall International Inc.: New Jersey, NJ, USA, 1984; 718p. [Google Scholar]
- Stat Soft Inc. Statistica 64, version 12; Stat Soft Inc.: Tulsa, OK, USA, 2014.
- Linsley, E.G. Ecology of cerambycidae. Ann. Rev. Entomol. 1959, 4, 99–138. [Google Scholar] [CrossRef]
- Hanks, L.M. Influence of the larval host plant on reproductive strategies of cerambycid beetles. Annu. Rev. Entomol. 1999, 44, 483–505. [Google Scholar] [CrossRef]
- Allison, J.D.; Borden, J.H.; Seybold, S.J. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 2004, 14, 123–150. [Google Scholar] [CrossRef]
- De Groot, P.; Nott, R. Response of Monochamus (Col., Cerambycidae) and some Buprestidae to flight intercept traps. J. Appl. Entomol. 2003, 127, 548–552. [Google Scholar] [CrossRef]
- Morewood, W.D.; Heln, K.E.; Katanic, P.J.; Borden, J.H. An improved trap for large wood-boring insects with special reference to Monochamus scutellatus (Coleoptera: Cerambycidae). Can. J. For. Res. 2002, 32, 519–525. [Google Scholar] [CrossRef]
- Ibeas, F.; Gallego, D.; Diez, J.J.; Pajares, J.A. An operative kairomonal lure for managing pine sawyer beetle Monochamus galloprovincialis (Coleoptera: Cerymbycidae). J. Appl. Entomol. 2007, 131, 13–20. [Google Scholar] [CrossRef]
- Billings, R.F. Southern pine bark beetles and associated insects. Effects of rapidly-released host volatiles on response to aggregation pheromones. J. Appl. Ent. 1985, 99, 483–491. [Google Scholar]
- Billings, R.F.; Cameron, R.S. Kairomonal responses of coleoptera, Monochamus titillator (Cerambycidae), Thanasimus dubius (Cleridae), and Temnochila virescens (Trogossitidae), to behavioral chemicals of southern pine bark beetles (Coleoptera: Scolytidae). Environ. Entomol. 1984, 13, 1542–1548. [Google Scholar] [CrossRef]
- Jurc, M.; Hauptman, T.; Pavlin, R.; Borkovic, D. Target and non-target beetles in semiochemical-baited cross vane funnel traps used in monitoring Bursaphelenchus xylophilus (PWN) vectors in pine stands. Phytoparasitica 2016, 44, 151–164. [Google Scholar] [CrossRef]
- Etxebeste, I.; Álvarez, G.; Pérez, G.; Pajares, J.A. Field response of the six-toothed pine bark beetle, Ips sexdentatus (Col.: Curculionidae, Scolytinae), to pheromonal blend candidates. J. Appl. Entomol. 2012, 136, 431–444. [Google Scholar] [CrossRef]
- Panzavolta, T.; Bracalini, M.; Bonuomo, L.; Croci, F.; Tiberi, R. Field response of non-target beetles to Ips sexdentatus aggregation pheromone and pine volatiles. J. Appl. Entomol. 2014, 138, 586–599. [Google Scholar] [CrossRef]
- Seybold, S.J.; Huber, D.P.W.; Lee, J.C.; Graves, A.D.; Bohlmann, J. Pine monoterpenes and pine bark beetles: A marriage of convenience for defense and chemical communication. Phytochem. Rev. 2006, 5, 143–178. [Google Scholar] [CrossRef]
- Serez, M. Verwendung des Aggregationspheromon-Präparats “Ipslure” gegen den mediterranen Kiefernborkenkäfer, Ips (Orthotomicus) erosus (Woll.) (Col., Scolytidae). Schadlingskd. Pfl. 1987, 60, 94–95. [Google Scholar] [CrossRef]
- Raffa, R.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Kolb, T.E. Responses of tree-killing bark beetles to a changing climate. In Climate Change and Insect Pests; Björkman, C., Niemelä, P., Eds.; CABI: Wallingford, UK, 2015; pp. 173–201. [Google Scholar]
- Bakke, A.; Kvamme, T. Kairomone response in Thanasimus predators to pheromone components of Ips typographus. J. Chem. Ecol. 1981, 7, 305–312. [Google Scholar] [CrossRef]
- Mendel, Z.; Podoler, H.; Livne, H. Interactions between Aulonium ruficorne (Coleoptera: Colydiidae) and other natural enemies of bark beetles (Coleoptera: Scolytidae). Entomophaga 1990, 35, 99–105. [Google Scholar] [CrossRef]
- Larrañaga, I.E.; Hernández, A.B.M.; Escolar, G.P.; Fernández, M.F.; Casero, J.J.D.; Alonso, J.A.P. Evaluación de compuestos semioquímicos para su empleo en estrategias de aumento de enemigos naturales de Ips sexdentatus (Col.: Scolytidae). Cuad. Soc. Esp. Cienc. Forestales. 2008, 32, 27–32. [Google Scholar]
- Etxebeste, I.; Lencina, J.L.; Pajares, J. Saproxylic community, guild and species responses to varying pheromone components of a pine bark beetle. Bull. Entomol. Res. 2013, 103, 497–510. [Google Scholar] [CrossRef]
- Schroeder, L.M. Prolonged development time of the bark beetle predator Thanasimus formicarius (Col.; Cleridae) in relation to its prey species Tomicus piniperda (L.) and Ips typographus (L.) (Col.; Scolytidae). Agr. For. Entomol. 1999, 1, 127–135. [Google Scholar] [CrossRef]
- Warzée, N.; Grégoire, J.C. Biodiversité forestiére et annemies naturels des scolytes: Le cas examplaire de Thanasimus formicarius. Forêt Wallonne. 2003, 66, 2–6. [Google Scholar]
- Piper, R. UK Saproxylic Beetles. Available online: https://www.rosspiper.net/2020/01/10/saproxylic-beetles/ (accessed on 18 October 2022).
- Krista, L.; Ryall, L.F. Habitat loss decreases predator-prey ratios in a pine-bark beetle system. Oikos 2005, 110, 265–270. [Google Scholar]
- Bonifácio, L. Evolution and Impact of the Pine Wilt Disease in the Affected Zone South of Tagus River. Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 2009; 208p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).