Contribution of Different Forest Strata on Energy and Carbon Fluxes over an Araucaria Forest in Southern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area Description
2.2. Atmospheric Data Measurements
2.3. Data Processing
2.4. Flux Partitioning in Forest Strata
- -
- Ecosystem: the LE, H, and NEE fluxes were obtained by the EC system at height of 32 m;
- -
- Understory: the LE, H, and NEE fluxes were obtained by the EC system at height of 11 m;
- -
- Overstory: the LE, H, and NEE fluxes were calculated by subtracting the understory fluxes from the ecosystem fluxes, respectively.
- -
- Ecosystem: the RE and GPP from partition of NEE obtained by the EC system at a 32 m height;
- -
- Understory: the RE and GPP from partition of NEE by the EC system at an 11 m height;
- -
- Overstory: the RE and GPP were obtained by subtracting the ecosystem’s RE and GPP from the understory’s RE and GPP, respectively.
2.5. Statistical Analyses
3. Results
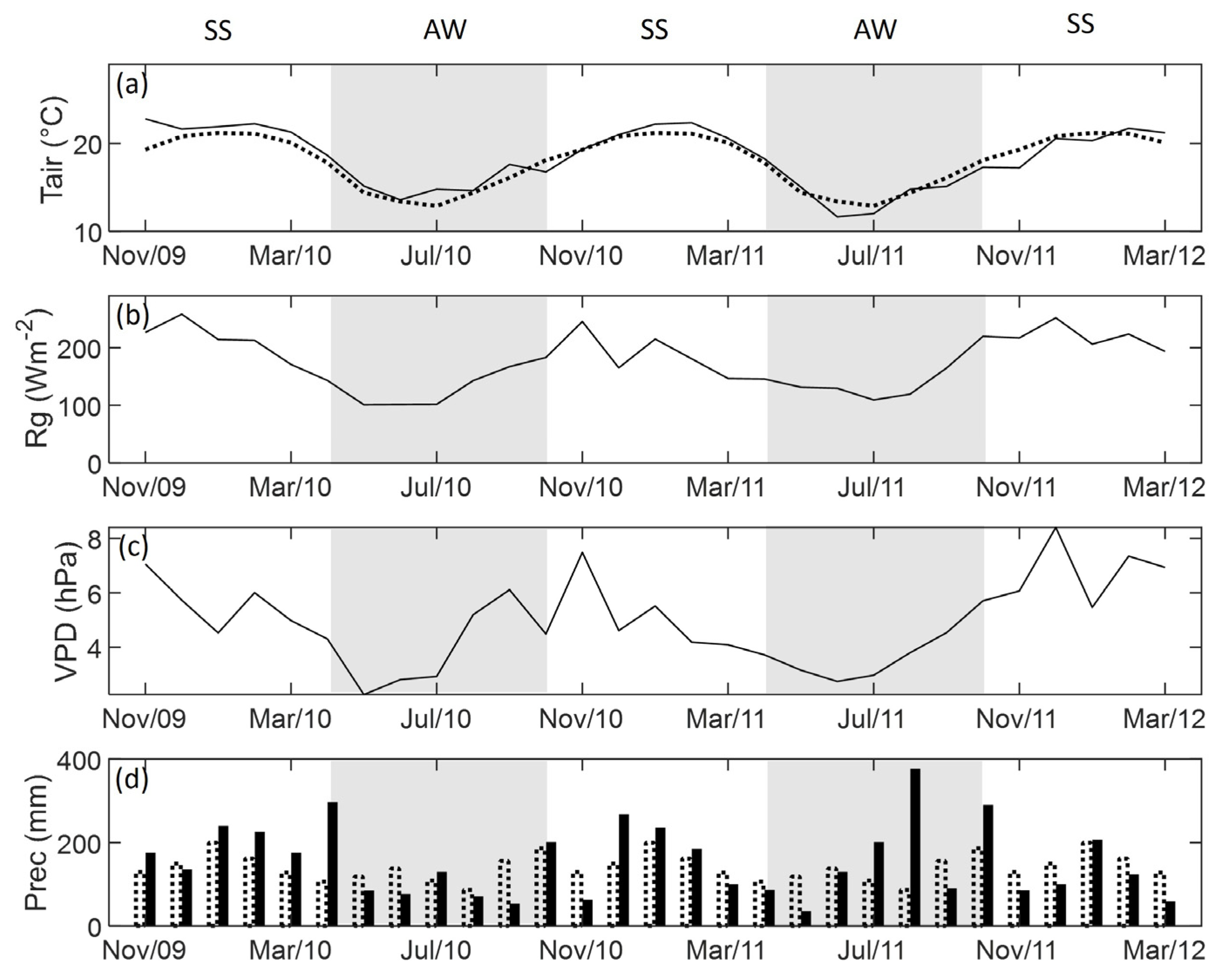
3.1. Weather Conditions
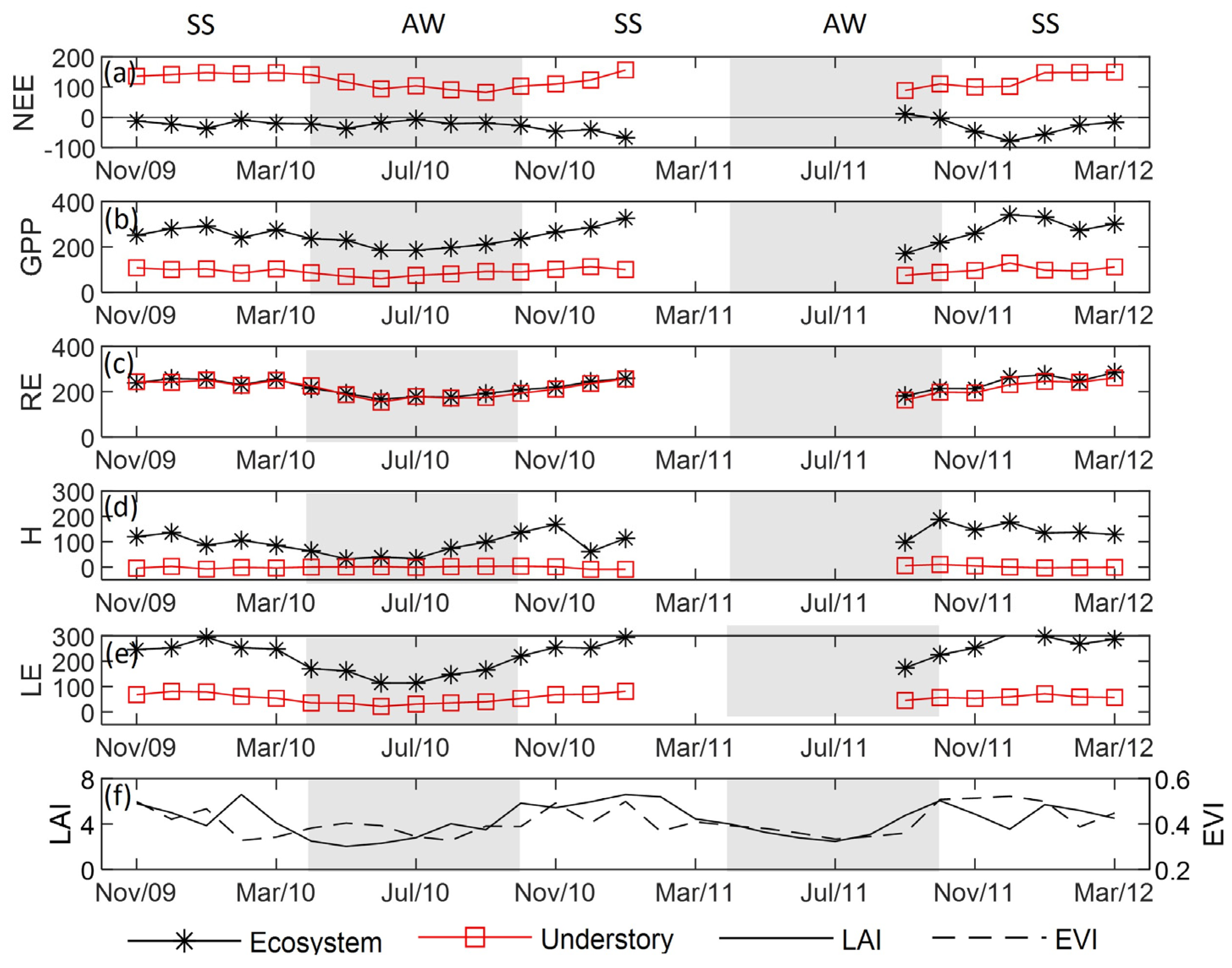
3.2. CO2 Fluxes
3.3. Energy Fluxes
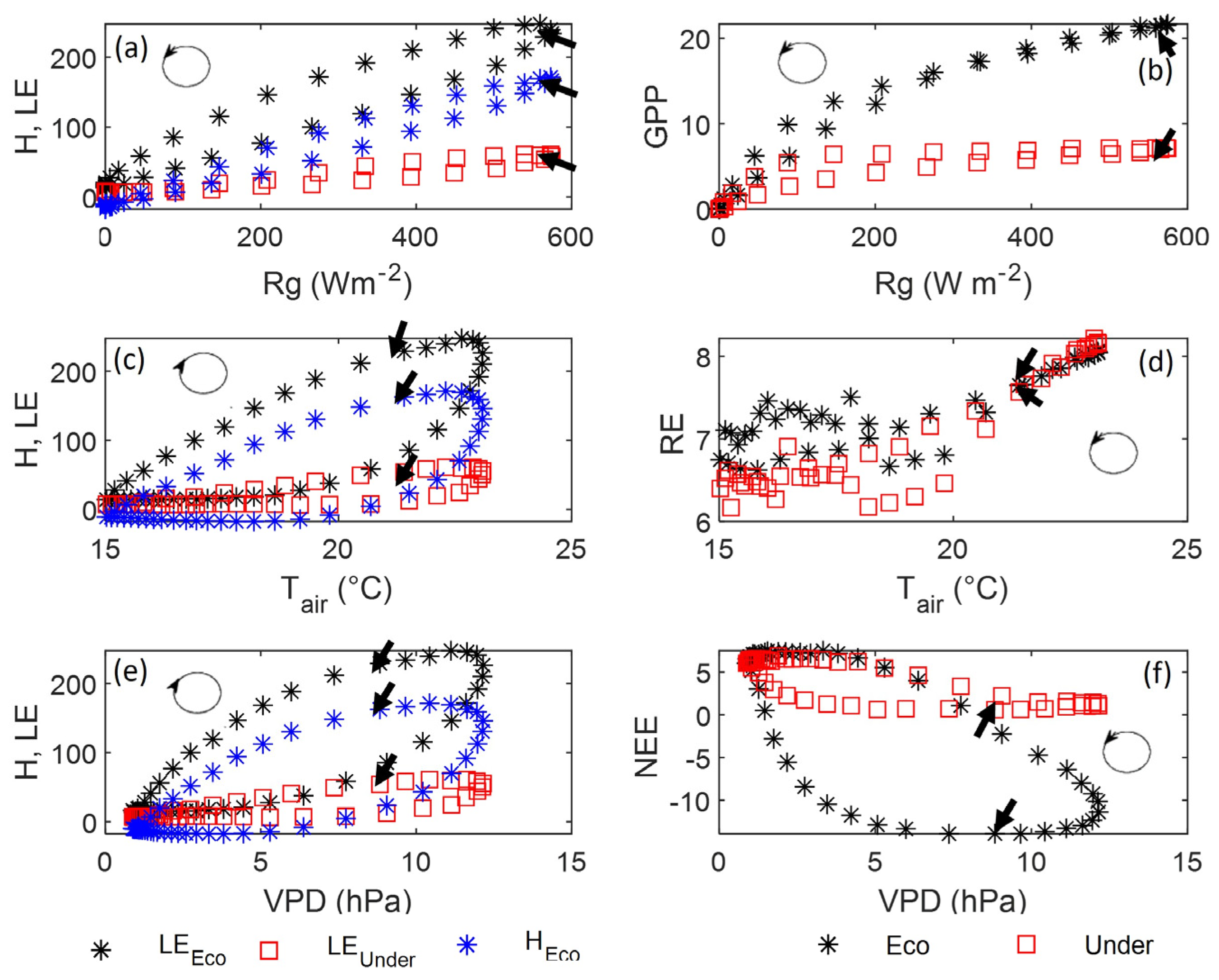
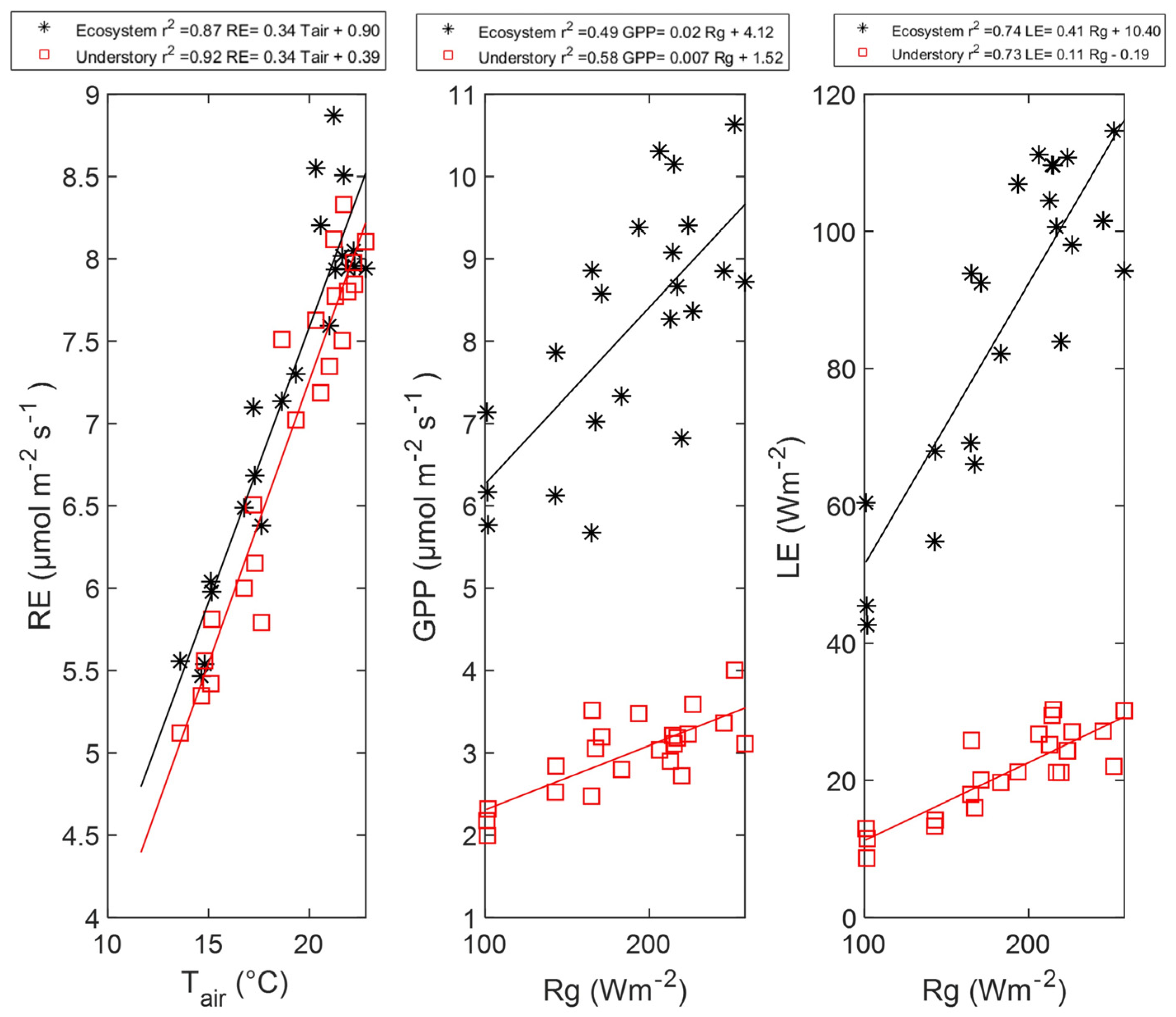
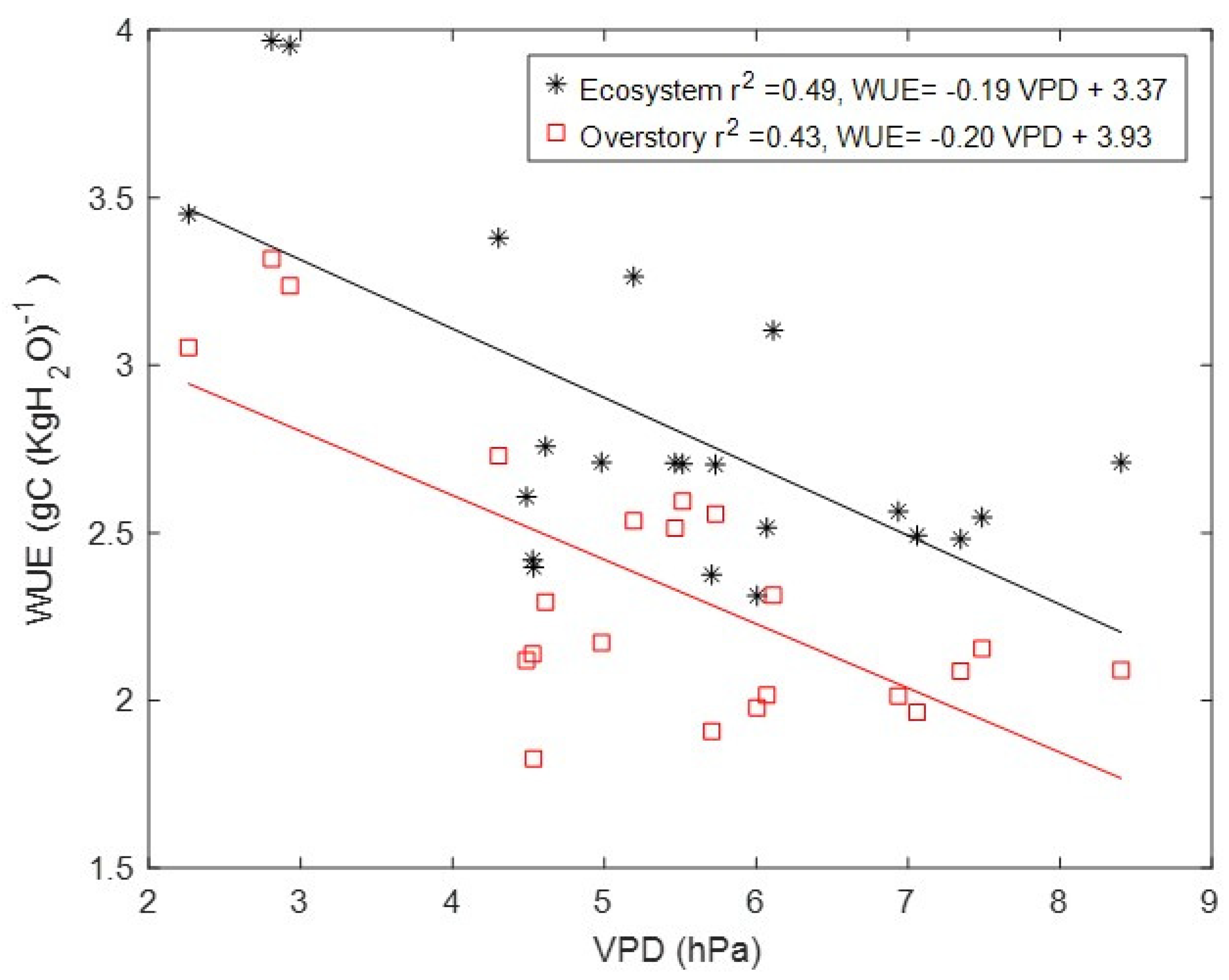
3.4. Drivers of Carbon and Energy Fluxes
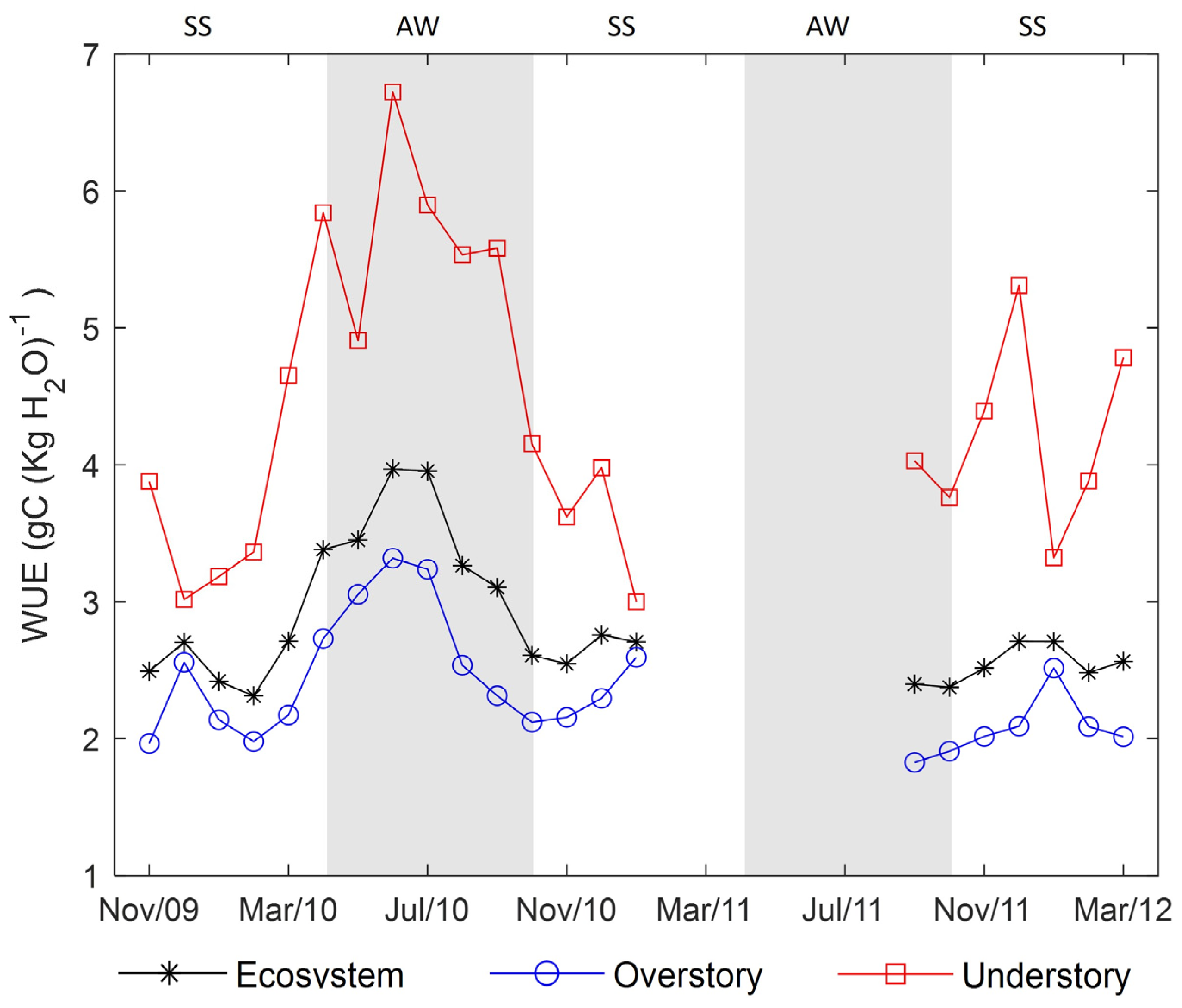
3.5. Water-Use Efficiency (WUE)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nobre, C.A.; Sampaio, G.; Borma, L.S.; Castilla-rubio, J.C.; Silva, J.S.; Cardoso, M. Land-use and climate change risks in the Amazon and the need of a novel sustainable development paradigm. Proc. Natl. Acad. Sci. USA 2016, 113, 10759–10768. [Google Scholar] [CrossRef] [PubMed]
- Haghtalab, N.; Moore, N.; Heerspink, B.P.; Hyndman, D.W. Evaluating spatial patterns in precipitation trends across the Amazon basin driven by land cover and global scale forcings. Theor. Appl. Climatol. 2020, 140, 411–427. [Google Scholar] [CrossRef]
- Fleischmann, A.S.; Laipelt, L.; Papa, F.; Paiva, R.C.D.d.; de Andrade, B.C.; Collischonn, W.; Biudes, M.S.; Kayser, R.; Prigent, C.; Cosio, E.; et al. Patterns and drivers of evapotranspiration in South American wetlands. Nat. Commun. 2023, 14, 6656. [Google Scholar] [CrossRef]
- Baker, J.C.A.; Spracklen, D.V. Climate Benefits of Intact Amazon Forests and the Biophysical Consequences of Disturbance. Front. For. Glob. Change 2019, 2, 47. [Google Scholar] [CrossRef]
- Leite-Filho, A.T.; Costa, M.H.; Fu, R. The southern Amazon rainy season: The role of deforestation and its interactions with large-scale mechanisms. Int. J. Climatol. 2020, 40, 2328–2341. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Ranta, P.; Blom, T.O.M.; Niemelä, J.; Joensuu, E.; Siitonen, M. The fragmented Atlantic rain forest of Brazil: Size, shape and distribution of forest fragments. Biodivers. Conserv. 1998, 7, 385–403. [Google Scholar] [CrossRef]
- Reis, M.S.d.; Ladio, A.; Peroni, N. Landscapes with Araucaria in South America: Evidence for a cultural dimension. Ecol. Soc. 2014, 19, art43. [Google Scholar] [CrossRef]
- Nodari, E.S. Historia de la devastación del Bosque de Araucaria en el sur del Brasil. Áreas. Rev. Int. Ciencias Soc. 2016, 35, 75–85. [Google Scholar]
- Marchioro, C.A.; Santos, K.L.; Siminski, A. Present and future of the critically endangered Araucaria angustifolia due to climate change and habitat loss. For. Int. J. For. Res. 2020, 93, 401–410. [Google Scholar] [CrossRef]
- Eisfeld, R.L.; Arce, J.E.; Sanquetta, C.R.; Braz, E.M. É economicamente viável o plantio de araucária? Uma análise entre a espécie e seu principal substituto, o pinus. Sci. For. 2020, 48, e3408. [Google Scholar] [CrossRef]
- Souza, A.F. A review of the structure and dynamics of araucaria mixed forests in southern Brazil and northern Argentina. N. Z. J. Bot. 2020, 59, 2–54. [Google Scholar] [CrossRef]
- Misson, L.; Baldocchi, D.D.; Black, T.A.; Blanken, P.D.; Brunet, Y.; Curiel Yuste, J.; Dorsey, J.R.; Falk, M.; Granier, A.; Irvine, M.R.; et al. Partitioning forest carbon fluxes with overstory and understory eddy-covariance measurements: A synthesis based on FLUXNET data. Agric. For. Meteorol. 2007, 144, 14–31. [Google Scholar] [CrossRef]
- Scott, R.; Watts, C.; Payan, J.G.; Edwards, E.; Goodrich, D.C.; Williams, D.; Shuttleworth, W.J. The understory and overstory partitioning of energy and water fluxes in an open canopy, semiarid woodland. Agric. For. Meteorol. 2003, 114, 127–139. [Google Scholar] [CrossRef]
- Xue, B.-L.; Kumagai, T.; Iida, S.; Nakai, T.; Matsumoto, K.; Komatsu, H.; Otsuki, K.; Ohta, T. Influences of canopy structure and physiological traits on flux partitioning between understory and overstory in an eastern Siberian boreal larch forest. Ecol. Modell. 2011, 222, 1479–1490. [Google Scholar] [CrossRef]
- Thiffault, N.; Fenton, N.; Munson, A.; Hébert, F.; Fournier, R.; Valeria, O.; Bradley, R.; Bergeron, Y.; Grondin, P.; Paré, D.; et al. Managing Understory Vegetation for Maintaining Productivity in Black Spruce Forests: A Synthesis within a Multi-Scale Research Model. Forests 2013, 4, 613–631. [Google Scholar] [CrossRef]
- Ikawa, H.; Nakai, T.; Busey, R.C.; Kim, Y.; Kobayashi, H.; Nagai, S.; Ueyama, M.; Saito, K.; Nagano, H.; Suzuki, R.; et al. Understory CO2, sensible heat, and latent heat fluxes in a black spruce forest in interior Alaska. Agric. For. Meteorol. 2015, 214–215, 80–90. [Google Scholar] [CrossRef]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Vangansbeke, P.; Verheyen, K.; Bernhardt-Römermann, M.; Baeten, L.; Hédl, R.; Berki, I.; Brunet, J.; et al. Forest microclimate dynamics drive plant responses to warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef]
- Cai, Y.; Tanioka, Y.; Kitawaga, T.; Ida, H.; Hirota, M. Gross primary production of dwarf bamboo, Sasa senanensis, in a mature beech forest with a substantial gap-mosaic structure. J. Plant Res. 2021, 134, 209–221. [Google Scholar] [CrossRef]
- Cai, Y.; Koido, R.; Umino, T.; Sakamoto, H.; Hasebe, Y.; Sarmah, R.; Yoneda, M.; Ida, H.; Hirota, M. Gross Primary Production of Dwarf Bamboo, Sasa senanensis, in Cool-Temperate Secondary Forests with Different Canopy Structures. Forests 2022, 13, 564. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Vogel, C.A. Energy and CO2 flux densities above and below a temperate broad-leaved forest and a boreal pine forest. Tree Physiol. 1996, 16, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.L.; Starr, G.; Clark, K.L.; Martin, T.A.; Gholz, H.L. Ecosystem and understory water and energy exchange for a mature, naturally regenerated pine flatwoods forest in north Florida. Can. J. For. Res. 2005, 35, 1568–1580. [Google Scholar] [CrossRef]
- Wang, L.; Caylor, K.K.; Villegas, J.C.; Barron-Gafford, G.A.; Breshears, D.D.; Huxman, T.E. Partitioning evapotranspiration across gradients of woody plant cover: Assessment of a stable isotope technique. Geophys. Res. Lett. 2010, 37, L09401. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Ryu, Y. A Synthesis of Forest Evaporation Fluxes—From Days to Years—As Measured with Eddy Covariance. In Forest Hydrology and Biogeochemistry; Springer: Dordrecht, The Netherlands, 2011; pp. 101–116. [Google Scholar]
- Sulman, B.N.; Roman, D.T.; Scanlon, T.M.; Wang, L.; Novick, K.A. Comparing methods for partitioning a decade of carbon dioxide and water vapor fluxes in a temperate forest. Agric. For. Meteorol. 2016, 226–227, 229–245. [Google Scholar] [CrossRef]
- Zandavalli, R.B.; Dillenburg, L.R.; de Souza, P.V.D. Growth responses of Araucaria angustifolia (Araucariaceae) to inoculation with the mycorrhizal fungus Glomus clarum. Appl. Soil Ecol. 2004, 25, 245–255. [Google Scholar] [CrossRef]
- Schaaf, L.B. Florística, Estrutura e Dinâmica no Período 1979–2000 de uma Floresta Ombrófila Mista Localizada no Sul do Paraná. Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2001. [Google Scholar]
- Bittencourt, S.; Corte, A.P.D.; Sanqueta, C.R. Estrutura da comunidade de Pteridophyta em uma floresta ombrófila mista do Paraná, Brasil. Silva Lusit. 2004, 12, 243–254. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Knyazikhin, Y.; Glassy, J.; Privette, J.L.; Tian, Y.; Lotsch, A.; Zhang, Y.; Wang, Y.; Morisette, J.T.; Votava, P.; Myneni, R.B.; et al. MODIS Leaf Area Index (LAI) and Fraction of Photosynthetically Active Radiation Absorbed by Vegetation (FPAR) Product (MOD15) Algorithm Theoretical Basis Document. 1999. Available online: https://modis.gsfc.nasa.gov/data/atbd/atbd_mod15.pdf (accessed on 28 November 2023).
- Oliveira, P.E.S.; Acevedo, O.C.; Moraes, O.L.L.; Zimermann, H.R.; Teichrieb, C. Nocturnal Intermittent Coupling Between the Interior of a Pine Forest and the Air Above It. Bound.-Layer Meteorol. 2013, 146, 45–64. [Google Scholar] [CrossRef]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Hincks, B.B.; Meyers, T.P. Measuring Biosphere-Atmosphere Exchanges of Biologically Related Gases with Micrometeorological Methods. Ecology 1988, 69, 1331–1340. [Google Scholar] [CrossRef]
- Vickers, D.; Mahrt, L. Quality control and flux sampling problems for tower and aircraft data. J. Atmos. Ocean. Technol. 1997, 14, 512–526. [Google Scholar] [CrossRef]
- Wilczak, J.M.; Oncley, S.P.; Stage, S.A. Sonic anemometer tilt correction algorithms. Bound.-Layer Meteorol. 2001, 99, 127–150. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water vapour transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Gash, J.H.C.; Culf, A.D. Applying a linear detrend to eddy correlation data in realtime. Bound.-Layer Meteorol. 1996, 79, 301–306. [Google Scholar] [CrossRef]
- Moncrieff, J.; Clement, R.; Finnigan, J.; Meyers, T. Averaging, Detrending, and Filtering of Eddy Covariance Time Series. In Handbook of Micrometeorology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 7–31. [Google Scholar]
- Moncrieff, J.B.; Massheder, J.M.; de Bruin, H.; Elbers, J.; Friborg, T.; Heusinkveld, B.; Kabat, P.; Scott, S.; Soegaard, H.; Verhoef, A. A system to measure surface fluxes of momentum, sensible heat, water vapour and carbon dioxide. J. Hydrol. 1997, 188–189, 589–611. [Google Scholar] [CrossRef]
- Foken, T.; Leuning, R.; Oncley, S.R.; Mauder, M.; Aubinet, M. Corrections and Data Quality Control. In Eddy Covariance; Springer: Dordrecht, The Netherlands, 2012; pp. 85–131. [Google Scholar]
- Béziat, P.; Ceschia, E.; Dedieu, G. Carbon balance of a three crop succession over two cropland sites in South West France. Agric. For. Meteorol. 2009, 149, 1628–1645. [Google Scholar] [CrossRef]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T.; et al. Towards a standardized processing of Net Ecosystem Exchange measured with eddy covariance technique: Algorithms and uncertainty estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Change Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Wutzler, T.; Lucas-Moffat, A.; Migliavacca, M.; Knauer, J.; Sickel, K.; Šigut, L.; Menzer, O.; Reichstein, M. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences 2018, 15, 5015–5030. [Google Scholar] [CrossRef]
- Lasslop, G.; Reichstein, M.; Papale, D.; Richardson, A.D.; Arneth, A.; Barr, A.; Stoy, P.; Wohlfahrt, G. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: Critical issues and global evaluation. Glob. Change Biol. 2010, 16, 187–208. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the Temperature Dependence of Soil Respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Zeri, M.; Sá, L.D.A.; Manzi, A.O.; Araújo, A.C.; Aguiar, R.G.; von Randow, C.; Sampaio, G.; Cardoso, F.L.; Nobre, C.A. Variability of Carbon and Water Fluxes Following Climate Extremes over a Tropical Forest in Southwestern Amazonia. PLoS ONE 2014, 9, e88130. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.R.d.; Manzi, A.O.; Cabral, O.M.; Miller, S.D.; Goulden, M.L.; Saleska, S.R.; R.-Coupe, N.; Wofsy, S.C.; Borma, L.S.; Artaxo, P.; et al. Patterns of water and heat flux across a biome gradient from tropical forest to savanna in Brazil. J. Geophys. Res. 2009, 114, G00B12. [Google Scholar]
- Giambelluca, T.W.; Scholz, F.G.; Bucci, S.J.; Meinzer, F.C.; Goldstein, G.; Hoffmann, W.A.; Franco, A.C.; Buchert, M.P. Evapotranspiration and energy balance of Brazilian savannas with contrasting tree density. Agric. For. Meteorol. 2009, 149, 1365–1376. [Google Scholar] [CrossRef]
- Rocha, H.R.d.; Goulden, M.L.; Miller, S.D.; Menton, M.C.; Pinto, L.D.V.O.; de Freitas, H.C.; Figueira, A.M.E.S. Seasonality of Water and Heat Fluxes over a Tropical Forest in Eastern Amazonia. Ecol. Appl. 2004, 14, 22–32. [Google Scholar] [CrossRef]
- Fisher, J.B.; Malhi, Y.; Bonal, D.; Da Rocha, H.R.; De Araújo, A.C.; Gamo, M.; Goulden, M.L.; Rano, T.H.; Huete, A.R.; Kondo, H.; et al. The land-atmosphere water flux in the tropics. Glob. Change Biol. 2009, 15, 2694–2714. [Google Scholar] [CrossRef]
- von Randow, C.; Manzi, A.O.; Kruijt, B.; de Oliveira, P.J.; Zanchi, F.B.; Silva, R.L.; Hodnett, M.G.; Gash, J.H.C.; Elbers, J.A.; Waterloo, M.J.; et al. Comparative measurements and seasonal variations in energy and carbon exchange over forest and pasture in South West Amazonia. Theor. Appl. Climatol. 2004, 78, 5–26. [Google Scholar] [CrossRef]
- Cabral, O.M.R.; Gash, J.H.C.; Rocha, H.R.; Marsden, C.; Ligo, M.A.V.; Freitas, H.C.; Tatsch, J.D.; Gomes, E. Fluxes of CO2 above a plantation of Eucalyptus in southeast Brazil. Agric. For. Meteorol. 2011, 151, 49–59. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H.; et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 2001, 107, 43–69. [Google Scholar] [CrossRef]
- Aubinet, M.; Feigenwinter, C.; Heinesch, B.; Laffineur, Q.; Papale, D.; Reichstein, M.; Rinne, J.; Gorsel, E. Van Nighttime Flux Correction. In Eddy Covariance; Springer: Dordrecht, The Netherlands, 2012; pp. 133–157. [Google Scholar]
- Pita, G.; Gielen, B.; Zona, D.; Rodrigues, A.; Rambal, S.; Janssens, I.A.; Ceulemans, R. Carbon and water vapor fluxes over four forests in two contrasting climatic zones. Agric. For. Meteorol. 2013, 180, 211–224. [Google Scholar] [CrossRef]
- Lasslop, G.; Migliavacca, M.; Bohrer, G.; Reichstein, M.; Bahn, M.; Ibrom, A.; Jacobs, C.; Kolari, P.; Papale, D.; Vesala, T.; et al. On the choice of the driving temperature for eddy-covariance carbon dioxide flux partitioning. Biogeosciences 2012, 9, 5243–5259. [Google Scholar] [CrossRef]
- Zhang, Q.; Manzoni, S.; Katul, G.; Porporato, A.; Yang, D. The hysteretic evapotranspiration-Vapor pressure deficit relation. J. Geophys. Res. Biogeosci. 2014, 119, 125–140. [Google Scholar] [CrossRef]
- Mallick, K.; Trebs, I.; Boegh, E.; Giustarini, L.; Schlerf, M.; Drewry, D.T.; Hoffmann, L.; von Randow, C.; Kruijt, B.; Araújo, A.; et al. Canopy-scale biophysical controls of transpiration and evaporation in the Amazon Basin. Hydrol. Earth Syst. Sci. 2016, 20, 4237–4264. [Google Scholar] [CrossRef]
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rödenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef]
- Mahrt, L.; Vickers, D. Relationship of area-averaged carbon dioxide and water vapour fluxes to atmospheric variables. Agric. For. Meteorol. 2002, 112, 195–202. [Google Scholar] [CrossRef]
- Chu, H.; Baldocchi, D.D.; John, R.; Wolf, S.; Reichstein, M. Fluxes all of the time? A primer on the temporal representativeness of FLUXNET. J. Geophys. Res. Biogeosci. 2017, 122, 289–307. [Google Scholar] [CrossRef]
- Villarreal, S.; Vargas, R. Representativeness of FLUXNET Sites Across Latin America. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006090. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Law, B.E.; Anthoni, P.M. On measuring and modeling energy fluxes above the floor of a homogeneous and heterogeneous conifer forest. Agric. For. Meteorol. 2000, 102, 187–206. [Google Scholar] [CrossRef]
- Lamaud, E.; Ogée, J.; Brunet, Y.; Berbigier, P. Validation of eddy flux measurements above the understorey of a pine forest. Agric. For. Meteorol. 2001, 106, 187–203. [Google Scholar] [CrossRef]
- Lloyd, J.; Grace, J.; Miranda, A.C.; Meir, P.; Wong, S.C.; Miranda, H.S.; Wright, I.R.; Gash, J.H.C.; McIntyre, J. A simple calibrated model of Amazon rainforest productivity based on leaf biochemical properties. Plant. Cell Environ. 1995, 18, 1129–1145. [Google Scholar] [CrossRef]
- ALTON, P.B.; NORTH, P.R.; LOS, S.O. The impact of diffuse sunlight on canopy light-use efficiency, gross photosynthetic product and net ecosystem exchange in three forest biomes. Glob. Change Biol. 2007, 13, 776–787. [Google Scholar] [CrossRef]
- Kuglitsch, F.G.; Reichstein, M.; Beer, C.; Carrara, A.; Ceulemans, R.; Granier, A.; Janssens, I.A.; Koestner, B.; Lindroth, A.; Loustau, D.; et al. Characterisation of ecosystem water-use efficiency of european forests from eddy covariance measurements. Biogeosci. Discuss. 2008, 5, 4481–4519. [Google Scholar]
- Chapin, F.S.; Woodwell, G.M.; Randerson, J.T.; Rastetter, E.B.; Lovett, G.M.; Baldocchi, D.D.; Clark, D.A.; Harmon, M.E.; Schimel, D.S.; Valentini, R.; et al. Reconciling Carbon-cycle Concepts, Terminology, and Methods. Ecosystems 2006, 9, 1041–1050. [Google Scholar] [CrossRef]
- Martínez-García, E.; Nilsson, M.B.; Laudon, H.; Lundmark, T.; Fransson, J.E.S.; Wallerman, J.; Peichl, M. Overstory dynamics regulate the spatial variability in forest-floor CO2 fluxes across a managed boreal forest landscape. Agric. For. Meteorol. 2022, 318, 108916. [Google Scholar] [CrossRef]
- Phillips, O.L.; Brienen, R.J.W. Carbon uptake by mature Amazon forests has mitigated Amazon nations’ carbon emissions. Carbon Balance Manag. 2017, 12, 1. [Google Scholar] [CrossRef]
- Hubau, W.; Lewis, S.L.; Phillips, O.L.; Affum-Baffoe, K.; Beeckman, H.; Cuní-Sanchez, A.; Daniels, A.K.; Ewango, C.E.N.; Fauset, S.; Mukinzi, J.M.; et al. Asynchronous carbon sink saturation in African and Amazonian tropical forests. Nature 2020, 579, 80–87. [Google Scholar] [CrossRef]
- Tang, X.; Li, H.; Desai, A.R.; Nagy, Z.; Luo, J.; Kolb, T.E.; Olioso, A.; Xu, X.; Yao, L.; Kutsch, W.; et al. How is water-use efficiency of terrestrial ecosystems distributed and changing on Earth? Sci. Rep. 2014, 4, 7483. [Google Scholar] [CrossRef]
- de Oliveira, G.; Brunsell, N.A.; Moraes, E.C.; Yosio, E.; Bertani, G.; Santos, T.V.; Aragao, L.E.O.C.; Oliveira, G.D.; Brunsell, N.A.; Moraes, E.C.; et al. Evaluation of MODIS-based estimates of water-use efficiency in Amazonia. Int. J. Remote Sens. 2017, 38, 5291–5309. [Google Scholar] [CrossRef]
- Katerji, N.; Mastrorilli, M.; Rana, G. Water use efficiency of crops cultivated in the Mediterranean region: Review and analysis. Eur. J. Agron. 2008, 28, 493–507. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz, M.B.; de Oliveira, P.E.S.; Souza, V.d.A.; Teichrieb, C.A.; Zimermann, H.R.; Veeck, G.P.; Mergen, A.; Pinheiro, M.E.O.; Stefanello, M.B.; de Moraes, O.L.L.; et al. Contribution of Different Forest Strata on Energy and Carbon Fluxes over an Araucaria Forest in Southern Brazil. Forests 2025, 16, 1008. https://doi.org/10.3390/f16061008
Diaz MB, de Oliveira PES, Souza VdA, Teichrieb CA, Zimermann HR, Veeck GP, Mergen A, Pinheiro MEO, Stefanello MB, de Moraes OLL, et al. Contribution of Different Forest Strata on Energy and Carbon Fluxes over an Araucaria Forest in Southern Brazil. Forests. 2025; 16(6):1008. https://doi.org/10.3390/f16061008
Chicago/Turabian StyleDiaz, Marcelo Bortoluzzi, Pablo Eli Soares de Oliveira, Vanessa de Arruda Souza, Claudio Alberto Teichrieb, Hans Rogério Zimermann, Gustavo Pujol Veeck, Alecsander Mergen, Maria Eduarda Oliveira Pinheiro, Michel Baptistella Stefanello, Osvaldo L. L. de Moraes, and et al. 2025. "Contribution of Different Forest Strata on Energy and Carbon Fluxes over an Araucaria Forest in Southern Brazil" Forests 16, no. 6: 1008. https://doi.org/10.3390/f16061008
APA StyleDiaz, M. B., de Oliveira, P. E. S., Souza, V. d. A., Teichrieb, C. A., Zimermann, H. R., Veeck, G. P., Mergen, A., Pinheiro, M. E. O., Stefanello, M. B., de Moraes, O. L. L., de Oliveira, G., Santos, C. A. G., & Roberti, D. R. (2025). Contribution of Different Forest Strata on Energy and Carbon Fluxes over an Araucaria Forest in Southern Brazil. Forests, 16(6), 1008. https://doi.org/10.3390/f16061008







