Abstract
Lead (Pb) contamination in Moso bamboo forests poses a challenge in terms of sustainable development and raises concerns about the safety of bamboo shoots for consumption. However, the physiological impacts of Pb stress on Moso bamboo growth and the molecular mechanisms governing its adaptive responses remain poorly understood. This study comprehensively investigated the physiological and transcriptomic responses of Moso bamboo to Pb stress. The results showed that low concentrations (1–10 µM) of Pb stress had minimal adverse effects on biomass accumulation and the photochemical quantum yield of PSII in Moso bamboo. However, at a high Pb concentration (50 µM), the growth of roots was significantly inhibited, while Pb accumulation in the roots and shoots reached 15,611 mg·kg−1 and 759 mg·kg−1, respectively. The uptake of Pb was increased as the external Pb concentration increased, but the xylem loading of Pb reached saturation at 57.79 µM after six-hour exposure. Pb was mainly localized in the epidermis and pericycle cells in the roots, where the thickening of cell walls in these cells was found after Pb treatment. Transcriptomic profiling identified 1485 differentially expressed genes (DEGs), with significant alterations in genes associated with metal cation transporters and cell wall synthesis. These findings collectively indicate that Moso bamboo is a Pb-tolerant plant, characterized by a high accumulation capacity and efficient xylem loading. The tolerance mechanism likely involves the transcriptional regulation of genes related to heavy metal transport and cell wall biosynthesis.
1. Introduction
The rapid advancement of urbanization has exacerbated soil heavy metal contamination worldwide, mainly resulting from mining activities, industrial smelting, wastewater irrigation, and excessive agrochemical application [1,2,3]. Among these pollutants, lead (Pb) has emerged as one of the most toxic environmental contaminants, second only to arsenic [4], exhibiting persistent soil accumulation with severe ecological consequences. Pb exposure critically impairs fundamental plant physiological processes, including photosynthesis, nutrient assimilation, and metabolic homeostasis, ultimately leading to growth retardation and biomass reductions [5,6,7,8]. However, plants have evolved multiple defense mechanisms to counteract heavy metal stress, including Pb toxicity. One key strategy involves the production of various antioxidants, such as catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), and ascorbate peroxidase (APX) [9,10]. These enzymes collectively scavenge free radicals, thereby mitigating ROS-induced oxidative damage in cells. Specifically, in bamboo plants, their rapid growth rate necessitates robust ROS scavenging systems to maintain metabolic activity under metal stress. On the other hand, another crucial defense mechanism involves the regulation of heavy metal uptake and transport through membrane transporters. For instance, in rice, suppression of the expression of natural resistance-associated macrophage protein 5 (OsNRAMP5) and OsCd1 significantly reduces root Cd uptake, leading to decreased Cd accumulation throughout the plant [11,12]. Similarly, in Arabidopsis, enhancing the expression of the tonoplast-localized transporter P-type heavy metal ATPase 3 (AtHMA3) improves plant tolerance to Cd/Zn by sequestering these heavy metals in vacuoles [13]. Moreover, cell wall modification may also be involved in plant responses to heavy metal stress. When minimal quantities of Pb penetrate root cell membranes, they interact with cellular components, leading to increased cell wall thicknesses [14]. This cell wall fortification effectively restricts Pb mobility within the plant.
Moso bamboo (Phyllostachys edulis) is one of the most economically and ecologically important bamboo species in China. However, its primary distribution in Southern China coincides with regions experiencing prevalent heavy metal pollution (including Pb) [15]. Therefore, investigating the effects of heavy metal stress on Moso bamboo represents a crucial research priority. Current research on Pb in Moso bamboo has primarily focused on its impacts on the plant’s antioxidant systems and cellular ultrastructure, as well as the subcellular distribution of Pb. For example, Zhong et al. demonstrated that elevated Pb concentrations decreased thiobarbituric acid reactive substances (TBARS) and superoxide dismutase (SOD) activity while increasing peroxidase (POD) activity [16]. Their TEM-EDX analysis revealed that Pb predominantly accumulates in the cytoplasm, with lesser amounts in the cell wall and vacuoles [16]. Liu et al. [17] further observed that excessive Pb exposure caused significant chloroplast damage, including endoplasmic reticulum disappearance, nuclear and nucleolar shrinkage, and thylakoid membrane disruption. Scanning electron microscopy additionally showed that high Pb concentrations reduced the stomatal aperture. Although studies have identified Moso bamboo as exhibiting an accumulation capacity for Pb and other heavy metals, suggesting its potential for the phytoremediation of contaminated soils [16,18], critical knowledge gaps remain. In particular, the molecular mechanisms governing the Pb stress responses—including uptake, transport, accumulation, and detoxification processes—require further elucidation.
This study endeavors to elucidate the effects of Pb stress on Moso bamboo by investigating Pb uptake and accumulation through integrated physiological and transcriptomic analyses. Our findings indicate Moso bamboo’s exceptional Pb accumulation capacity, with efficient xylem loading, reaching concentrations of 15,611 mg/kg in the roots and 759 mg/kg in the shoots. The plant exhibits high Pb tolerance, which may be achieved by two key molecular mechanisms: the downregulation of metal uptake transporter genes to reduce Pb uptake and the upregulation of metal sequestration-related genes for the detoxification of Pb.
Beyond these transcription-regulatory mechanisms, our study also highlights the potential role of root cell wall modification in Moso bamboo’s Pb tolerance. These combined physiological and transcriptomic insights not only characterize Moso bamboo’s response to Pb stress but also provide valuable perspectives for future research on this important bamboo species.
2. Materials and Methods
2.1. Materials and Growth Conditions
The Moso bamboo seeds (1000 grains) used in this study were collected from Guangxi Province, China. Initially, the seeds were soaked in tap water and incubated overnight in a dark environment at 25 °C. Then, the seeds were transferred to wet filter paper and placed in a Petri dish for one week to promote germination. After germination, the seedlings were cultured in a 0.5 mM CaCl2 solution for an additional week to enhance root and shoot growth. When the seedlings developed true leaves, they were transferred to a 1 L black pot containing 1 L 1/2 Kimura B nutrient solution (pH = 5.6) and allowed to grow in a glass greenhouse, maintained at 25 °C with natural light. The nutrient solution was renewed every three days. Before Pb exposure, plants were acclimated under greenhouse conditions for 16 or 26 days to ensure physiological stabilization.
2.2. Growth Phenotype of Moso Bamboo Under Pb Stress
Moso bamboo seedlings (30 days old) were exposed to intermittent Pb treatments (0, 1, 5, 10, and 50 µM) using a two-phase cycle. Initially, seedlings were pretreated in 1/2-Kimura B nutrient solution without Pb for one day. Subsequently, they were exposed to a 0.5 mM CaCl2 solution containing varying concentrations of Pb for an additional day. This cycle was repeated for a total of 18 days, with a total of 9 days of Pb treatment. After treatment, the growth phenotypes of the seedlings were photographed, and the maximum photochemical quantum yield of PSII (Fv/Fm) of the second leaf was measured by a dual-channel modulated PAM chlorophyll fluorometer (DUAL-PAM-100, Walz, Effeltrich, Germany). Roots and shoots were then harvested, oven-dried, and weighed to determine the biomass. Experiments were performed one time with four biological replicates (one seedling per biological replicate).
2.3. Determination of Element Concentrations of Moso Bamboo Under Pb Stress
The treatment method for seedlings in this experiment was consistent with Section 2.2. After exposure to Pb, the roots of the seedling were thoroughly washed three times with a pre-cooled 5 mM CaCl2 solution. Subsequently, the roots and shoots were harvested separately and dried at 70 °C in an oven for 3 days. Then, non-ground samples were digested with 4 mL concentrated (70%) HNO3 at a temperature of up to 140 °C. Subsequently, the digested samples were diluted to a final volume of 50 mL with ultrapure water. The metal concentrations in the digested solutions were then determined by inductively coupled plasma mass spectrometry (ICP-MS) (X series 2; Thermo Scientific, Bremen, Germany). Experiments were performed two times with four biological replicates (one seedling per biological replicate).
2.4. Pb Uptake Kinetics
Moso bamboo seedlings (40 days old) were exposed to a 0.5 mM CaCl2 solution containing varying concentrations of Pb (0, 1, 5, 10, and 50 μM) for 40 min under two temperature regimes (25 °C and 4 °C). To maintain precise temperature control, the Pb treatment solutions were pre-equilibrated in temperature-controlled incubators at either 4 °C or 25 °C for 24 h. Subsequently, the roots and shoots were harvested separately and dried for the determination of the Pb concentration. The Pb net uptake was calculated by subtracting the root Pb concentration at 4 °C from that at 25 °C. Experiments were performed two times with four biological replicates (one seedling per biological replicate).
2.5. Xylem Sap Collection and Pb Concentration Determination
To investigate the Pb loading efficiency in xylem sap, Moso bamboo seedlings (40 days old) were treated with 5 μM Pb in a 0.5 mM CaCl2 solution for varying durations (0, 0.5, 1, 3, 6, 12, or 24 h) and with different Pb concentrations (0, 1, 5, 10, or 50 μM) for 6 h. Following exposure, the stems were excised approximately 2 cm above the junction of the root and the stem using a razor blade, and xylem sap was collected by natural root pressure-driven exudation without the application of external pressure for 1 h into 2 mL centrifuge tubes using a micropipette. For each biological replicate, approximately 20 µL of the xylem fluid was collected. The collected sap was then diluted to 2 mL with 5% HNO3, and the Pb concentrations were determined by using ICP-MS. Xylem sap sampling was repeated two times, with four replicate samples collected each time (two seedlings per biological replicate).
2.6. Cellular Distribution of Pb in Moso Bamboo Root
To investigate the distribution of Pb in Moso bamboo roots under different levels of Pb stress, Moso bamboo seedlings (40 days old) were exposed to a 0.5 mM CaCl2 solution containing various concentrations of Pb (0, 1, 5, 10, and 50 μM) for 2 days. After exposure, the roots were sectioned laterally into 100-μm-thick slices with a micro-slicer (VT1200S, Leica, Weztlar, Germany), focusing on the area 1–2 cm above the root tip, for staining observation. The specific methods and precautions of staining observation were adapted from Gong et al. [19]. Briefly, these sections were stained with a green dye probe specific for Pb (A10024, Molecular Probes, Invitrogen, Carlsbad, CA, USA) at room temperature for 0.5 h. After staining, the root sections were removed from the green dye and then washed twice with 1 X PBS solution, followed by one wash with a DAPI (1 μg ml−1) solution, and finally washed twice with 1 X PBS solution again. The root sections were then observed using a confocal microscope (Zeiss LSM880; Zeiss, Jena, Germany) with excitation at 488 nm and emission at 562 nm for Pb-specific signals. The Pb-specific fluorescent probe working solution was prepared by adding 50 μL of DMSO to a stock solution, followed by the addition of 1450 μL of 0.85% NaCl.
2.7. Root Cellular Morphology After Pb Stress
Moso bamboo seedlings (40 days old) were exposed to a 0.5 mM CaCl2 solution containing varying Pb concentrations (0, 5, and 50 µM) following the intermittent treatment protocol described in Section 2.2. Post-treatment, root segments (0–2 cm) were transversely sectioned into 150 µm slices using a microtome (VT1200S, Leica, Weztlar, Germany), which were then mounted on the instrument loading platform using black adhesive tape for the observation of the root cell morphology by scanning electron microscopy (TM4000Plus, Hitachi, Japan). The scanning analysis of four independent biological replicates (one seedling each) produced reproducible results.
2.8. Pb Distribution and Accumulation in Leaves
We selected seedlings of the same age (40 days old) and leaf number (five leaves) for the Pb stress treatment. After exposure, leaves from each seedling were harvested from top (newest leaf) to bottom (oldest leaf) and labeled from new to old as Leaf I through Leaf VI. Subsequently, all collected leaves were oven-dried and digested and the Pb concentration determined. Experiments were performed with four biological replicates (one leaf per biological replicate).
2.9. RNA Extraction, Transcriptome Sequencing, and qRT-PCR Analysis
To gain deeper insights into the transcriptional response mechanisms of Moso bamboo to Pb stress, total RNA was extracted from the roots of seedlings exposed to a 0 or 5 µM Pb solution in CaCl2 for a duration of 2 days. An RNA extraction kit (RNeasy Plant Mini Kit (50) (Qiagen, Hilden, Germany)) was used for total RNA extraction. The quality of the extracted RNA, including assessments of purity, concentration, and integrity, was evaluated using a spectrophotometer and the Agilent 2100/LabChip GX detection system (Agilent Technologies, Santa Clara, CA, USA). Following the quality assessment, transcriptome sequencing was performed using the Illumina NovaSeq 6000 platform (San Diego, CA, USA) with paired-end 150 bp (PE150) sequencing at BioMarker Technologies (Beijing, China). The raw sequencing data have been deposited in the NCBI Sequence Read Archive and can be accessed using the accession number PRJNA1118059. Subsequently, the raw reads were processed to generate clean data, which were then aligned with the reference genome of Moso bamboo (Phyllostachys edulis, V3.0) to produce mapped data. Differentially expressed genes (DEGs) were identified based on the criteria of fold change ≥1.5 and p-value < 0.05. Gene Ontology (GO) functional annotation was performed using the BMK Cloud online platform (www.biocloud.net (accessed on 9 February 2024)). RNA-seq was performed one time with three biological replicates, where each replicate utilized roots from one bamboo seedling for RNA isolation.
To validate the reliability of the transcriptome data, the same treatment method described above was employed for total RNA extraction from Moso bamboo roots. Then, 1 μg of total RNA was reverse-transcribed into cDNA using the reverse transcription kit from the Toyobo Company (Rever Tra Ace® qPCR RT Master Mix with gDNA Remover). The expression levels of six genes encoding metal cation transporters were quantified using quantitative fluorescence PCR. The relative expression levels of these genes were determined using the 2−ΔΔCt method, with the constitutively expressed gene PeUBQ from Moso bamboo serving as the internal control in all analyses. This experiment was performed one time with three biological replicates, where each replicate utilized roots from one bamboo seedling for RNA isolation. The primers utilized for the RT-qPCR analysis are listed in Table S1.
2.10. Statistical Analysis
Before applying parametric tests, data normality was assessed using Shapiro–Wilk tests and homoscedasticity was verified using Levene’s tests. Then, the data were analyzed using the SPSS software (version 22.0) with Duncan’s test and Student’s t-test. In the histograms, statistically significant differences (p < 0.05) between treatments are indicated by different lowercase letters above the columns (Duncan’s test). Asterisks denote significant differences in pairwise comparisons (* p< 0.05; ** p< 0.01).
3. Results
3.1. Effects of Pb Stress on the Growth of Moso Bamboo
To investigate the impact of Pb stress on Moso bamboo growth, seedlings were exposed to Pb concentrations ranging from 1 to 50 μM. Under low Pb stress (1 μM), seedling growth and biomass accumulation were not significantly different compared to the Pb-free control (Figure 1A). A slight biomass reduction in both the roots and shoots was observed for the 5 and 10 μM Pb treatments (Figure 1A–C). While 50 μM Pb toxicity significantly inhibited root growth and biomass accumulation, shoot growth and biomass were only minimally affected (Figure 1A–C). Furthermore, we examined the effects of Pb stress on the maximum photochemical quantum yield of PSII (Fv/Fm) in Moso bamboo. Compared to the Pb-free control, 50 μM Pb treatment significantly suppressed Fv/Fm, while no effect was observed under 1 to 10 μM Pb treatment (Figure 2).
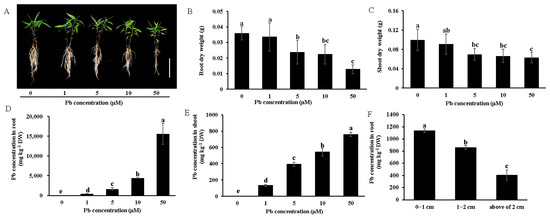
Figure 1.
Growth phenotype, biomass accumulation, and Pb accumulation of Moso bamboo seedlings under Pb stress. Growth phenotype (A), root dry weight (B), and shoot dry weight (C). Pb concentration in root (D) and shoot (E). Seedlings (30 days old) were exposed to a different concentration (0, 1, 5, 50 µM) of Pb followed by intermittent treatment for 9 days. Then, the root and shoot were harvested separately, dried, and weighed and the Pb concentration was determined. Data are presented as means ± SD from four independent replications. Scale bar = 10 cm. The Pb concentrations in different regions of the Moso bamboo root (F). Seedlings (14 days old) were exposed to 5 µM Pb in a CaCl2 solution for 2 days. After exposure, the root segments were collected separately from the root tip regions at 0–1 cm, 1–2 cm, and above 2 cm for the determination of the Pb concentration. Data are means ± SD of three independent replications. Different lowercase letters indicate significant differences at p < 0.05 by Duncan’s test.
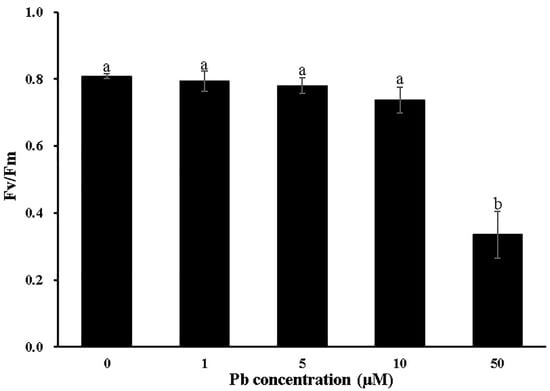
Figure 2.
The maximum photochemical quantum yield of PSII (Fv/Fm) of Moso bamboo leaves under Pb stress. Seedlings (30 days old) were exposed to a different concentration (0, 1, 5, 50 µM) of Pb followed by intermittent treatment for 9 days. Then, the Fv/Fm value of the second leaf was measured. Different lowercase letters indicate significant differences at p < 0.05 by Duncan’s test. Data are means ± SD of four independent replications.
The Pb concentration measurements revealed that both root and shoot Pb accumulation increased with rising external Pb concentrations, reaching 15,611 mg/kg in the roots and 759 mg/kg in the shoots under 50 μM Pb treatment in a 0.5 mM CaCl2 solution (Figure 1D,E). On the other hand, root tip regions (0–2 cm) demonstrated a significantly higher Pb accumulation capacity than distal regions (>2 cm), with the 0–1 cm segment exhibiting a higher Pb concentration than the 1–2 cm segment (Figure 1F).
3.2. Pb Uptake, Translocation, and Distribution
We further calculated the total Pb uptake and root-to-shoot translocation. The results showed that the total Pb uptake increased with rising external Pb concentrations under long-term Pb treatment (Figure 3A). In contrast, the root-to-shoot translocation efficiency of Pb exhibited a decreasing trend as the external Pb concentration increased (Figure 3B). Under 50 μM Pb stress, the translocation rate was approximately 14% of that observed at 1 μM Pb treatment (Figure 3B). These results indicated that a significant portion of Pb was retained in the roots. Therefore, we further investigated the short-term (40-min) Pb net uptake in bamboo, which revealed that root Pb absorption increased with higher treatment concentrations (Figure 3C). Notably, the root Pb absorption capacity showed no saturation, even at 50 μM Pb—a concentration that is considered highly toxic to plants (Figure 3C). Additionally, we assessed the impacts of Pb stress on the accumulation of other essential nutrients, including iron (Fe), manganese (Mn), copper (Cu), and zinc (Zn). Pb exposure slightly reduced the Mn, Cu, and Zn levels in both the roots and shoots while increasing Fe accumulation in these tissue types (Figure S1).
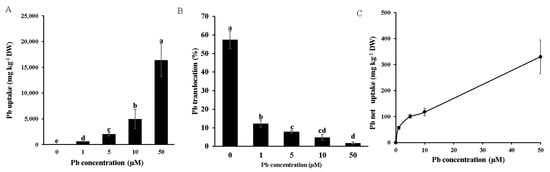
Figure 3.
Pb uptake and translocation of Moso bamboo seedlings under Pb stress. Pb uptake (A) and Pb translocation from root to shoot (B). Seedlings (30 days old) were exposed to a different concentration (0, 1, 5, 50 µM) of Pb followed by intermittent treatment for 9 days. The roots and shoots were then harvested for the determination of Pb. Subsequently, Pb uptake and Pb translocation from roots to shoots were calculated. Moso bamboo root Pb uptake kinetics (C). Seedlings (40 days old) were treated with different concentrations (0, 1, 5, 10, 50 µM) of Pb in a CaCl2 solution at 25 °C or 4 °C for 40 min. Following exposure, the roots were harvested for the determination of the Pb concentration. The net Pb uptake was calculated by subtracting the root Pb concentration at 4 °C from that at 25 °C. Data are presented as means ± SD from four independent replications. Different lowercase letters indicate significant differences at p < 0.05 according to Duncan’s test.
We further analyzed the Pb distribution across different leaves of Moso bamboo under low (1 μM) and high (50 μM) Pb treatments. From Leaf I (youngest, unexpanded blades) to Leaf VI (oldest leaves), Pb accumulation progressively increased at both concentrations (Figure S2). Strikingly, Leaf VI contained ~4.4-fold higher Pb levels than Leaf I at 1 μM Pb and ~4.8-fold higher at 50 μM Pb (Figure S2).
3.3. Xylem Loading of Pb
Pb is taken in through the plant roots and translocated into the xylem solution and subsequently accumulates in the aerial parts of the plant. Therefore, the Pb uptake capacity and xylem loading efficiency can be assessed by monitoring the xylem sap Pb concentration. The results showed a significant increase in the xylem sap Pb concentration within the first 6 h of exposure, reaching saturation thereafter, regardless of the treatment duration, which indicated the restricted capacity of the roots to transport Pb into the xylem vessels (Figure 4A). Additionally, the xylem sap Pb concentration showed a dose-dependent response, increasing proportionally with external Pb concentrations ranging from 1 to 50 μM (Figure 4B). These findings suggest that Moso bamboo exhibits a remarkable capacity for shoot Pb accumulation facilitated by effective root Pb uptake and xylem loading, even under Pb toxicity conditions.
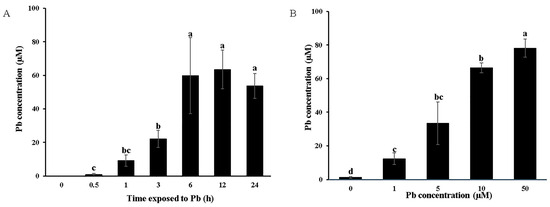
Figure 4.
Loading of Pb in xylem sap. Pb concentration in xylem sap at different treatment times (A). Seedlings (40 days old) were treated with 5 µM Pb in a 0.5 mM CaCl2 solution for different times (0, 0.5, 1, 3, 6, 12, 24 h). Pb concentration in the xylem sap at different concentrations of Pb treatment (B). Seedlings (40 days old) were treated with different concentrations (0, 1, 5, 50 µM) of Pb in a 0.5 mM CaCl2 solution for 6 h. After treatment, xylem sap was collected for the determination of the Pb concentration. Data are presented as means ± SD from four independent replications. Different lowercase letters indicate significant differences at p < 0.05 according to Duncan’s test.
3.4. Cellular Localization of Pb in Roots
We further observed the cellular localization of Pb in cross-sections of the root (1–2 cm). In the basal root zone, the green fluorescence signal of Pb was predominantly observed in the epidermis, exodermis, and pericycle under Pb stress (Figure 5F–H, Figure S3). The fluorescence intensity exhibited a clear dose-dependent response, increasing proportionally with elevated external Pb concentrations (Figure 5F–H). A colocalization analysis indicated significant overlap between Pb-specific green fluorescence and DAPI-stained cell walls (blue fluorescence) (Figure 5N–P). Notably, no green fluorescence signal was observed in the absence of Pb treatment, confirming the specificity of the Pb probe (Figure 5E). These results indicate that Pb is primarily deposited in the epidermis, exodermis, and pericycle regions of the cell wall.
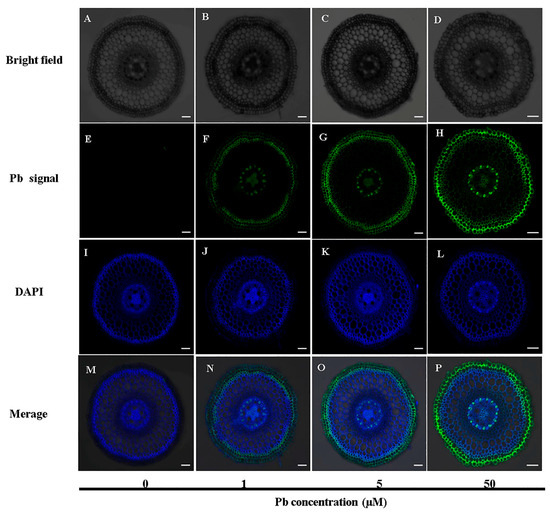
Figure 5.
Localization of Pb in the transverse section of the root (1–2 cm). Seedlings (40 days old) were treated with different concentrations (0, 1, 5, 50 µM) of Pb in a CaCl2 solution for 2 days. Subsequently, Pb localization was examined through Pb-specific probe staining under a laser scanning confocal microscope. Images (A–D) represent cellular structures observed under bright field.Green fluorescence (E–H) indicates the specific signal from Pb, whereas blue fluorescence (I–L) denotes the autofluorescence of the cell wall stained with DAPI. Images (M–P) present an overlay of the bright-field, DAPI-stained cell wall autofluorescence with the specific Pb fluorescence signal. Scale bar = 50 μm.
3.5. Effects of Pb Stress on Root Cellular Morphology
Scanning electron microscopy analysis revealed that Pb exposure did not significantly alter the overall cellular organization in root cross-sections, with both stele (Figure 6B,E,H) and exodermal cells (Figure 6C,F,I) maintaining similar structural integrity across all treatment groups. However, Pb exposure induced pronounced cell wall thickening, particularly in stele cells. This structural modification was consistently observed in Pb-treated roots but absent in control samples, suggesting a specific response to Pb stress.
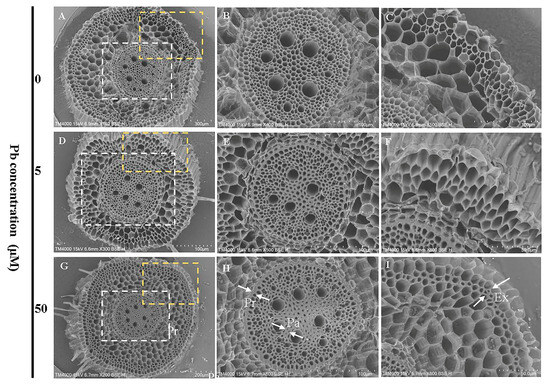
Figure 6.
Root cellular morphology of Moso bamboo seedlings under Pb stress. Seedlings (40 days old) were exposed to 0.5 mM CaCl2 solution containing different Pb concentrations (0, 5, 50 µM) followed by intermittent treatment for 9 d. Subsequently, the cellular morphology was observed using a scanning electron microscope. Images (B,C,E,F,H,I) present magnified views of the white and yellow dotted regions in (A,D,G), respectively. The thickening of the cell wall is marked by white arrows. Pericycle (Pr), Exodermal (ex), Parenchyma (Pa).
3.6. Sequencing Quality Overview and DEG Analysis
To explore the genes and metabolite pathways involved in Moso bamboo’s response to Pb stress, we conducted a eukaryotic reference transcriptome (RNA-seq) analysis using untreated (Ck) and treated (5 µM Pb) samples. The Spearman correlation coefficients among individual samples for each treatment exceeded 0.8 (Figure S4B). A total of 1485 differentially expressed genes (DEGs) were identified, with 656 genes upregulated and 829 genes downregulated based on a fold change ≥1.5 and p-value < 0.05 as screening criteria (Figure S4C). The heat map visualized the expression levels of all DEGs (Figure S4A).
To validate the functions of these DEGs, we functionally classified the gene terms using Gene Ontology (GO) analysis. The two most significantly enriched GO terms in the biological process category were metal ion transport and cell wall synthesis; in the cellular component category, they were the extracellular region and endomembrane system; and, in the molecular function category, they were heme binding and iron ion binding (Figure 7). These terms are commonly associated with plants’ adaptive responses to heavy metal stress. Previous physiological experiments have demonstrated that Moso bamboo exhibits a high capacity for Pb uptake and tolerance (Figure 1 and Figure 3A,C). Furthermore, Pb stress also promoted the thickening of the cell walls (Figure 6). Therefore, our focus was primarily on analyzing the differentially expressed genes (DEGs) involved in metal ion uptake or transport and cell wall synthesis under Pb stress conditions. A total of 16 DGs were identified that were related to metal cation uptake or transport after Pb stress, including PeIRT2, PeZIP4/8 (zinc, iron transporter), PeHIPP2/9/23/27/39/45 (heavy metal-associated isoprenylated plant protein), PeVIT2 (vacuolar iron transporter), PeHAK17/23 (high-affinity K+ transporter), PeMTP1 (metal tolerance protein), and PeMRS2-D (magnesium transporter) (Figure 8). The expression tendencies of four randomly selected genes under Pb stress were further verified by qRT-PCR. The results showed that the expression trends of these genes under Pb stress were consistent with the RNA-seq results (Figure 8B–E). We speculate that these transporters may be involved in the uptake, transport, or detoxification of Pb in Moso bamboo.
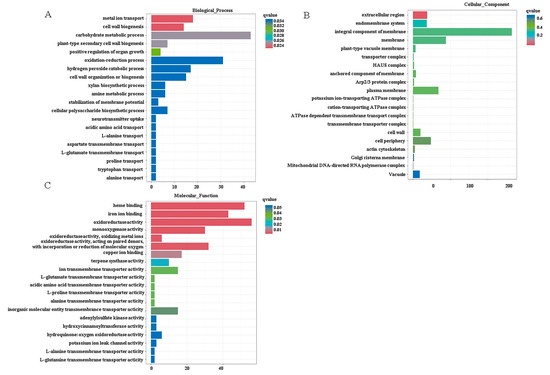
Figure 7.
Gene Ontology (GO) enrichment of DEGs in Moso bamboo roots under Pb stress. DEGs were assigned to three main categories: biological processes (A), molecular functions (B), and cellular components (C). For each category, the top 20 significantly enriched GO terms are shown.
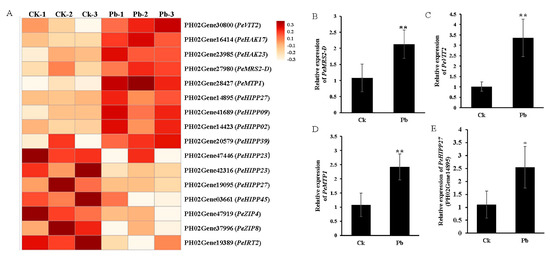
Figure 8.
DEG analysis of Moso bamboo roots under Pb stress. (A) Heat map of the expression levels of DEGs associated with heavy metal transporters. PeVIT (vacuolar iron transporter), PeHAK (high-affinity K+ transporter), PeMTP (metal tolerance protein) PeHIPP (heavy metal-associated isoprenylated plant protein), PeZIP (zinc, iron transporter). The intensity of the color in the heat map reflects the changes in gene expression levels between untreated and Pb-treated conditions. The expression levels of four randomly selected genes encoding metal cation transporters are shown in (B–E) based on qRT-PCR analysis. Asterisks indicate a significant difference (* p < 0.05 or ** p < 0.05) by Student’s t-test. Data are presented as means ± SD from three independent replications.
In addition, a total of 53 DEGs were identified that were related to cell wall synthesis after Pb stress (Table S3). These genes mainly encoded polygalacturonase, cellulose synthase, pectinesterase/pectinesterase inhibitors, galactoside 2-alpha-l-fucosyltransferase, fasciclin-like arabinogalactan protein, expansion, xyloglucan endotransglucosylase/hydrolase protein, and so on (Table S3). Changes in the expression levels of these genes may promote cell wall synthesis, resulting in increasing Pb fixation on the cell wall.
4. Discussion
4.1. Moso Bamboo Exhibits High Pb Tolerance and Accumulation Through Physiological and Anatomical Adaptations
Pb toxicity significantly disrupts plant growth and development. For instance, rice exhibits the pronounced inhibition of both aerial and root elongation after just one day of Pb exposure [20], while wheat and Sedum alfredii show considerable root growth inhibition following three days of Pb treatment [21,22]. In contrast, our study reveals that Moso bamboo displays notable tolerance to Pb toxicity. This tolerance is evidenced by the absence of visible symptoms in root and shoot growth after 9 days of exposure to 1 µM Pb, with no significant differences in dry weight between treated and control plants (Figure 1A–C). At higher concentrations (5–10 µM), the maximum photochemical quantum yield of PSII remained unaffected, and seedlings exhibited only mild growth inhibition (Figure 1A–C and Figure 2). Remarkably, while 50 µM Pb substantially inhibited root growth, shoot growth was only minimally affected (Figure 1A–C). The stronger inhibition observed in the roots compared to the shoots under 50 µM Pb treatment can be attributed to the significant accumulation of Pb in the bamboo roots, which restricts its translocation to the aboveground parts; therefore, the toxicity of Pb is much greater in the roots than in the shoots. Moreover, the root cell morphology showed no shrinkage across all Pb concentrations, even at 50 µM, indicating structural resilience to Pb-induced stress (Figure 6).
After 1 day of Pb exposure, more intense fluorescence signals were seen in the exodermal cell walls compared to cortical and stele cell walls (Figure 5), suggesting that Pb’s deposition in the exodermis restricts its movement toward the stele. This pattern mirrors observations in Moso bamboo roots exposed to Cd [23]. Following 9-day Pb exposure, Moso bamboo roots exhibited significant Pb accumulation (Figure 1D), and cell wall thickening was observed by SEM in the exodermis and endodermis, particularly in the stele region (Figure 6). As Krzesłowska has demonstrated, cell wall thickening plays a crucial role in heavy metal tolerance by sequestering metal cations [14]. We propose that Pb-induced cell wall thickening promotes Pb immobilization within cell walls, limiting its entry into root protoplasts and its subsequent translocation to shoots. On the other hand, cell wall thickening may serve as a physical barrier that significantly restricts Pb transport through the apoplastic pathway. These hypotheses are supported by three key findings: (1) transcriptome data showing the significant enrichment of GO terms related to cell wall functions (Figure 7); (2) the altered expression of 53 cell wall synthesis-related genes under Pb stress (Table S3); and (3) the significantly reduced root-to-shoot Pb translocation with increasing Pb concentrations (Figure 3B). Similar cell wall responses were reported in Chinese cabbage under Pb stress [24], further supporting Moso bamboo’s Pb tolerance mechanism.
Plant roots serve as the primary site for heavy metal uptake from soil [25], with subsequent translocation to shoots after xylem loading [26]. In the work of Zhong et al., the maximum accumulation levels of Pb in the roots and leaves were reported as 1409 mg kg−1 and 179 mg kg−1, respectively, following a 15-day hydroponic experiment with 400 μM Pb [16]. Contrastingly, in our study, we found that Moso bamboo accumulated significantly higher levels of Pb, with 15,611 mg·kg−1 in the roots and 759 mg·kg−1 in the shoots, under exposure to 50 μM Pb for 9 days (Figure 1D,E). This striking difference likely stems from our experimental design: we prepared Pb treatment solutions by adding a Pb stock solution (100 mM) to 0.5 mM CaCl2 rather than a nutrient solution, thereby minimizing Pb’s coprecipitation and enhancing its bioavailability.
The heavy metal accumulation capacity depends largely on the root uptake efficiency and xylem loading [27]. Moso bamboo displays remarkable efficiency in xylem Pb loading, reaching saturation within only six hours (Figure 4A). A similar phenomenon was also observed in Moso bamboo regarding Cd loading in xylem sap [23]. Furthermore, Moso bamboo roots demonstrate a remarkable Pb net uptake capacity even at toxic concentrations (50 µM) (Figure 3C). These findings collectively establish Moso bamboo as a Pb-accumulating and -tolerant plant species.
4.2. Transcriptomic Regulation of Metal Cation Transporters Under Pb Stress in Moso Bamboo
Pb is a toxic element for plants and lacks biological functions in their growth and development. Thus, plants do not possess specific transporters for Pb absorption. However, Pb can still be taken up by transporters for other essential elements. For example, OsNRAMP5, a Mn transporter in rice, has recently been implicated in Pb uptake [28]. Additionally, cyclic nucleotide-gated channels (CNGCs), which are permeable to calcium ions (Ca2+), may also facilitate Pb absorption [29]. Notably, our study observed significant expression changes in 16 genes encoding metal cation transporters under Pb stress.
The HIPP family, which functions as metallochaperones, has been reported to play a critical role in heavy metal homeostasis and detoxification [30]. In our study, four HIPP family members (PeHIPP02, PeHIPP09, PeHIPP27, PeHIPP39) were significantly induced by Pb (Figure 8). Similar results were also found in Chinese cabbage: two genes, HIPP05 and HIPP06, were reported to be upregulated in Chinese cabbage leaves under Pb stress [24]. Moreover, in the tea plant, CsHIPP22, CsHIPP24, and CsHIPP36 were demonstrated to be involved in heavy metal resistance [31]. On the other hand, some tonoplast transporters have also been reported to participate in heavy metal tolerance by sequestering heavy metals into vacuoles. For example, OsVIT1 and OsVIT2 mediate the vacuolar sequestration of heavy metals in yeast [32]. OsMTP1, induced by Zn/Cd, confers resistance by sequestering these metals in vacuoles [33,34]. In this study, we found that two genes, PeVIT2 and PeMTP1, which encode tonoplast transporters, were significantly upregulated by Pb exposure (Figure 8), suggesting their likely important roles in Pb tolerance. However, further studies are required to functionally characterize these two genes in Moso bamboo. Additionally, we found that three ZIP family genes (PeZIP4, PeZIP8, and PeIRT2) were significantly downregulated in the roots under Pb stress (Figure 8). As ZIP family members are known to facilitate heavy metal uptake, transport, and accumulation across the plasma membrane [35], we presume that the downregulation of PeZIP4, PeZIP8, and PeIRT2 may reduce Pb uptake in Moso bamboo. Taken together, the upregulation of PeVIT2 and PeMTP1 combined with the downregulation of uptake-related genes (PeZIP4, PeZIP8, and PeIRT2) likely play a critical role in Pb tolerance in Moso bamboo.
5. Conclusions
The remarkable Pb uptake and accumulation capacity of Moso bamboo (Phyllostachys edulis) stems from its high root absorption efficiency and effective xylem loading. Physiological analyses indicate that Moso bamboo is a Pb-tolerant plant, which is achieved through dual adaptive strategies: first, it upregulates detoxification-related genes to mitigate Pb toxicity; second, it downregulates Pb transporter genes to limit root uptake.
In addition, the thickening of cell walls in the roots may play an important role in Pb tolerance. Collectively, these findings offer novel insights into the mechanisms by which Moso bamboo tolerates and accumulates Pb, highlighting its potential for phytoremediation applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16061007/s1, Figure S1. Concentration of Fe, Mn, Cu and Zn in root and shoot of Moso bamboo seedlings under different concentration of Pb stress; Figure S2. Concentrations of Pb in different leaf of Moso bamboo; Figure S3. Diagrammatic sketch of bamboo root cellular structure; Figure S4. Statistics of differently expressed genes (DEGs) in Moso bamboo seedling roots under Pb stress; Table S1. Primer pairs for qRT-PCR analysis of four DEGs; Table S2. Metal cation related genes of DEGs; Table S3. Cell wall synthesis related genes of DEGs.
Author Contributions
F.Y.: investigation, formal analysis, writing—original draft. R.X.: investigation. C.Z.: investigation. H.J.: conceptualization. J.F.S.: conceptualization, writing—original draft, writing—review and editing, funding acquisition. K.H.: conceptualization, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant no. 31972495); the Agricultural Science and Technology Cooperation and Innovation Project of Hangzhou (202209SX13); and the College Student Innovation and Entrepreneurship Training Program (202410341035).
Data Availability Statement
All data supporting the conclusions of this study are present in the paper or Supplementary Materials.
Acknowledgments
We thank all the reviewers for their valuable time and comments.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Küpper, H. Lead toxicity in plants. Met. Ions Life Sci. 2017, 17. [Google Scholar] [CrossRef]
- Zhao, F.J.; Tang, Z.; Song, J.J.; Huang, X.Y.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant. 2022, 15, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead tolerance in plants: Strategies for phytoremediation. Environ. Sci. Pollut. Res. Int. 2013, 4, 2150–2161. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [PubMed]
- Zhang, H.; Sun, X.; Hwarari, D.; Du, X.; Wang, Y.; Xu, H.; Lv, S.; Wang, T.; Yang, L.; Hou, D. Oxidative stress response and metal transport in roots of Macleaya cordata exposed to lead and zinc. Plants 2023, 12, 516. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Adaptations to oxidative stress in Zea mays root under short-term Pb2+ exposure. Biologia 2015, 70, 190–197. [Google Scholar] [CrossRef]
- Liu, D.; Islam, E.; Li, T.Q.; Yang, X.; Jin, X.F.; Mahmood, Q. Comparison of synthetic chelators and low molecular weight organic acids in enhancing phytoextraction of heavy metals by two ecotypes of Sedum alfredii Hance. J. Hazard. Mater. 2008, 153, 114–122. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar]
- Gupta, D.K.; Nicoloso, F.T.; Schetinger, M.R.; Rossato, L.V.; Pereira, L.B.; Castro, G.Y.; Srivastava, S.; Tripathi, R.D. Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J. Hazard. Mater. 2009, 172, 479–484. [Google Scholar] [CrossRef]
- Li, X.; Bu, N.; Li, Y.; Ma, L.; Xin, S.; Zhang, L. Growth, photosynthesis and antioxidant responses of endophyte infected and non-infected rice under lead stress conditions. J. Hazard. Mater. 2012, 213, 55–61. [Google Scholar] [CrossRef]
- Shao, J.F.; Che, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. Silicon reduces cadmium accumulation by suppressing expression of transporter genes involved in cadmium uptake and translocation in rice. J. Exp. Bot 2017, 68, 5641–5651. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.Y.; Zhang, Y.; Huang, J.; Chen, Z.; Shen, R.F.; Zhu, X.F. Auxin is involved in cadmium accumulation in rice through controlling nitric oxide production and the ability of cell walls to bind cadmium. Sci. Total Environ. 2023, 904, 166644. [Google Scholar] [CrossRef] [PubMed]
- Morel, M.; Crouzet, J.; Gravot, A.; Auroy, P.; Leonhardt, N.; Vavasseur, A.; Richaud, P. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 2009, 149, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Krzesłowska, M. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, W.; Chen, Y. Spatial Variations of Soil Heavy Metal Potential Ecological Risks in Typical Moso Bamboo Forests of Southeast China. Bull. Environ. Contam. Toxicol. 2019, 102, 224–230. [Google Scholar] [CrossRef]
- Zhong, B.; Chen, J.; Shafi, M.; Guo, J.; Wang, Y.; Wu, J.; Ye, Z.; He, L.; Liu, D. Effect of lead (Pb) on antioxidation system and accumulation ability of Moso bamboo (Phyllostachys pubescens). Ecotoxicol. Environ. Saf. 2017, 138, 71–77. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Islam, E.; Chen, J.R.; Wu, J.S.; Ye, Z.Q.; Peng, D.L.; Yan, W.B.; Lu, K.P. Lead accumulation and tolerance of Moso bamboo (Phyllostachys pubescens) seedlings: Applications of phytoremediation. J. Zhejiang Univ. Sci. B 2015, 16, 123–130. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Islam, E.; Wang, Y.; Wu, J.; Ye, Z.; Yan, W.; Peng, D.; Liu, D. Cadmium-induced oxidative stress, response of antioxidants and detection of intracellular cadmium in organs of moso bamboo (Phyllostachys pubescens) seedlings. Chemosphere 2016, 153, 107–114. [Google Scholar] [CrossRef]
- Gong, X.X.; Yang, F.; Pan, X.Y.; Shao, J.F. Accumulation of silicon in shoot is required for reducing lead uptake in rice. Crop J. 2022, 11, 1261–1271. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.F.; Fan, J.H.; Li, Y.Y.; Ma, L.J.; Wang, L.L.; Li, X.M. Transcriptome modulation by endophyte drives rice seedlings response to Pb stress. Ecotoxicol. Environ. Saf. 2023, 254, 114740. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wei, X.; You, J.; Wang, W.; Lu, J.; Shi, R. Comparative antioxidative responses and proline metabolism in two wheat cultivars under short term lead stress. Ecotoxicol. Environ. Saf. 2011, 74, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Huang, H.G.; Yang, X.E.; Razafindrabe, B.H.; Inouhe, M. The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J. Hazard. Mater. 2010, 177, 437–444. [Google Scholar] [CrossRef]
- Yang, F.; Chang, Y.Z.; Zheng, Y.T.; Pan, X.Y.; Ji, H.B.; Shao, J.F. Physiological and transcriptomic characterization of cadmium toxicity in Moso bamboo (Phyllostachys edulis), a non-timber forest species. Tree Physiol. 2023, 43, 1250–1264. [Google Scholar] [CrossRef]
- Gao, P.P.; Liang, H.; Dong, Y.; Xue, P.Y.; Zhao, Q.L.; Yan, J.S.; Ma, W.; Zhao, J.J.; Liu, W.J. Transcriptomic mechanisms of reduced PM2.5-Pb retention in the leaves of the low-Pb-accumulation genotype of Chinese cabbage. J. Hazard. Mater. 2023, 444, 130385. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Qin, A.; Zain, M.; Mushtaq, Z.; Mehmood, F.; Riaz, L.; Naveed, S.; Ansari, M.J.; Saeed, M.; Ahmad, I.; et al. Pb uptake, accumulation, and translocation in plants: Plant physiological, biochemical, and molecular response: A review. Heliyon 2024, 10, e27724. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Y.; Liu, H. Editorial: Phytoremediation of heavy metal contaminated soil: Technology, mechanism, and implementation. Front. Plant Sci. 2024, 14, 1347564. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef]
- Chang, J.D.; Gao, W.; Wang, P.; Zhao, F.J. OsNRAMP5 is a major transporter for lead uptake in rice. Environ. Sci. Technol. 2022, 56, 17481–17490. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Belloeil, C.; Ianna, M.L.; Shin, R. Arabidopsis CNGC family members contribute to heavy metal ion uptake in plants. Int. J. Mol. Sci. 2019, 20, 413. [Google Scholar] [CrossRef]
- De Abreu-Neto, J.B.; Turchetto-Zolet, A.C.; De Oliveira, L.F.; Zanettini, M.H.; Margis-Pinheiro, M. Heavy metal-associated isoprenylated plant protein (HIPP): Characterization of a family of proteins exclusive to plants. FEBS J. 2013, 280, 1604–1616. [Google Scholar] [CrossRef]
- Wei, Y.; Peng, X.; Wang, X.; Wang, C. The heavy metal-associated isoprenylated plant protein (HIPP) gene family plays a crucial role in cadmium resistance and accumulation in the tea plant (Camellia sinensis L.). Ecotoxicol. Environ. Saf. 2023, 260, 115077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.H.; Yi, H.Y.; Gong, J.M. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012, 72, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yang, S.; Liu, B.; Zhang, M.; Wu, K. Molecular characterization of a rice metal tolerance protein, OsMTP1. Plant Cell Rep. 2012, 31, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Menguer, P.K.; Farthing, E.; Peaston, K.A.; Ricachenevsky, F.K.; Fett, J.P.; Williams, L.E. Functional analysis of the rice vacuolar zinc transporter OsMTP1. J. Exp. Bot. 2013, 64, 2871–2883. [Google Scholar] [CrossRef]
- Amini, S.; Arsova, B.; Hanikenne, M. The molecular basis of zinc homeostasis in cereals. Plant Cell Environ. 2022, 455, 1339–1361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).