Vegetation Restoration Significantly Increased Soil Organic Nitrogen Mineralization and Nitrification Rates in Karst Regions of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Samples Collection
2.3. Soil Nitrogen Transformation Rates
2.4. Soil Physicochemical Properties
2.5. Soil Microbial Community
2.6. Data Statistics and Analysis
3. Results
3.1. Soil Physical and Chemical Properties
3.2. Soil Microbial Community Composition
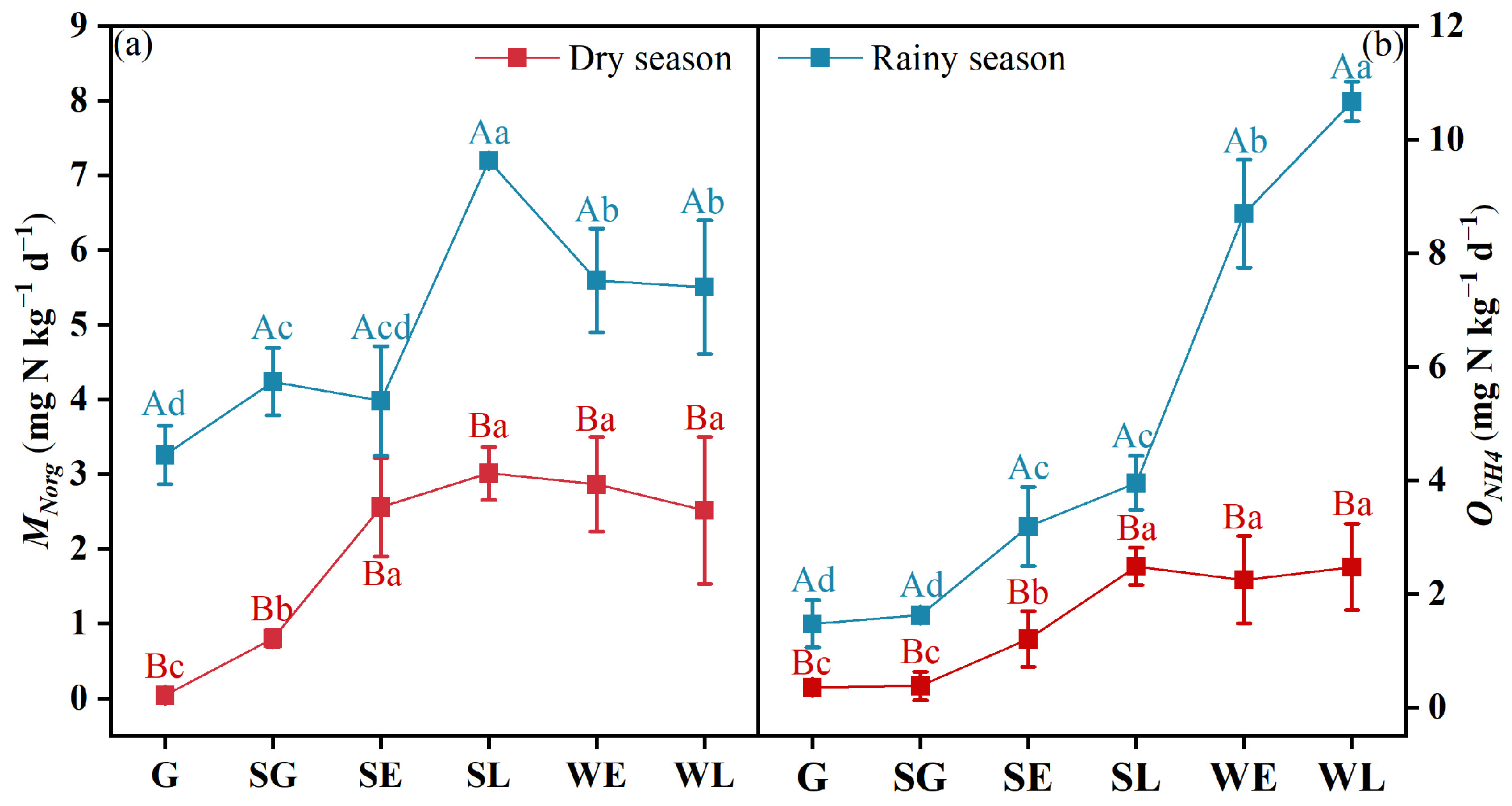
3.3. Soil Organic Nitrogen Mineralization and Nitrification Rates
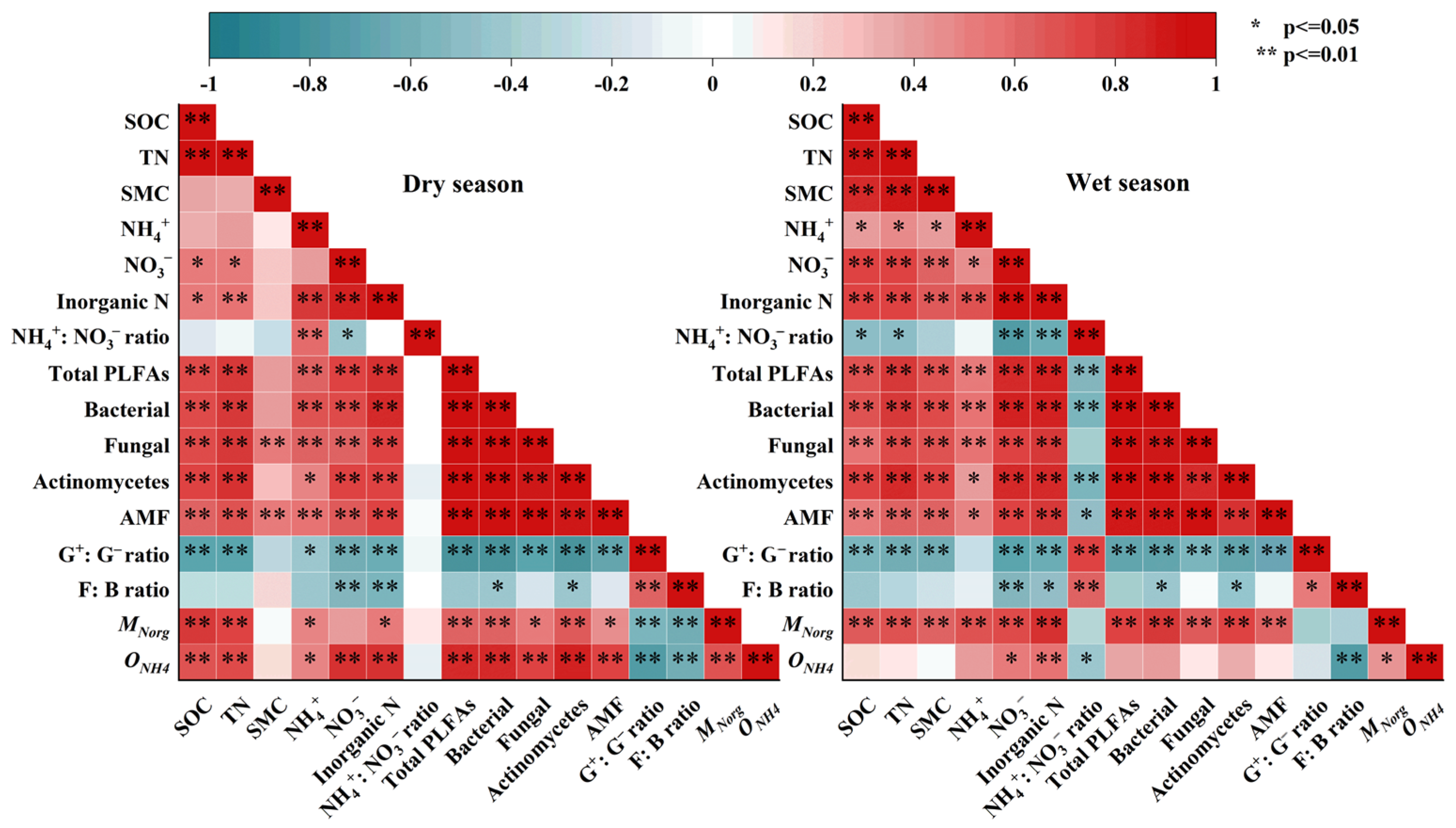
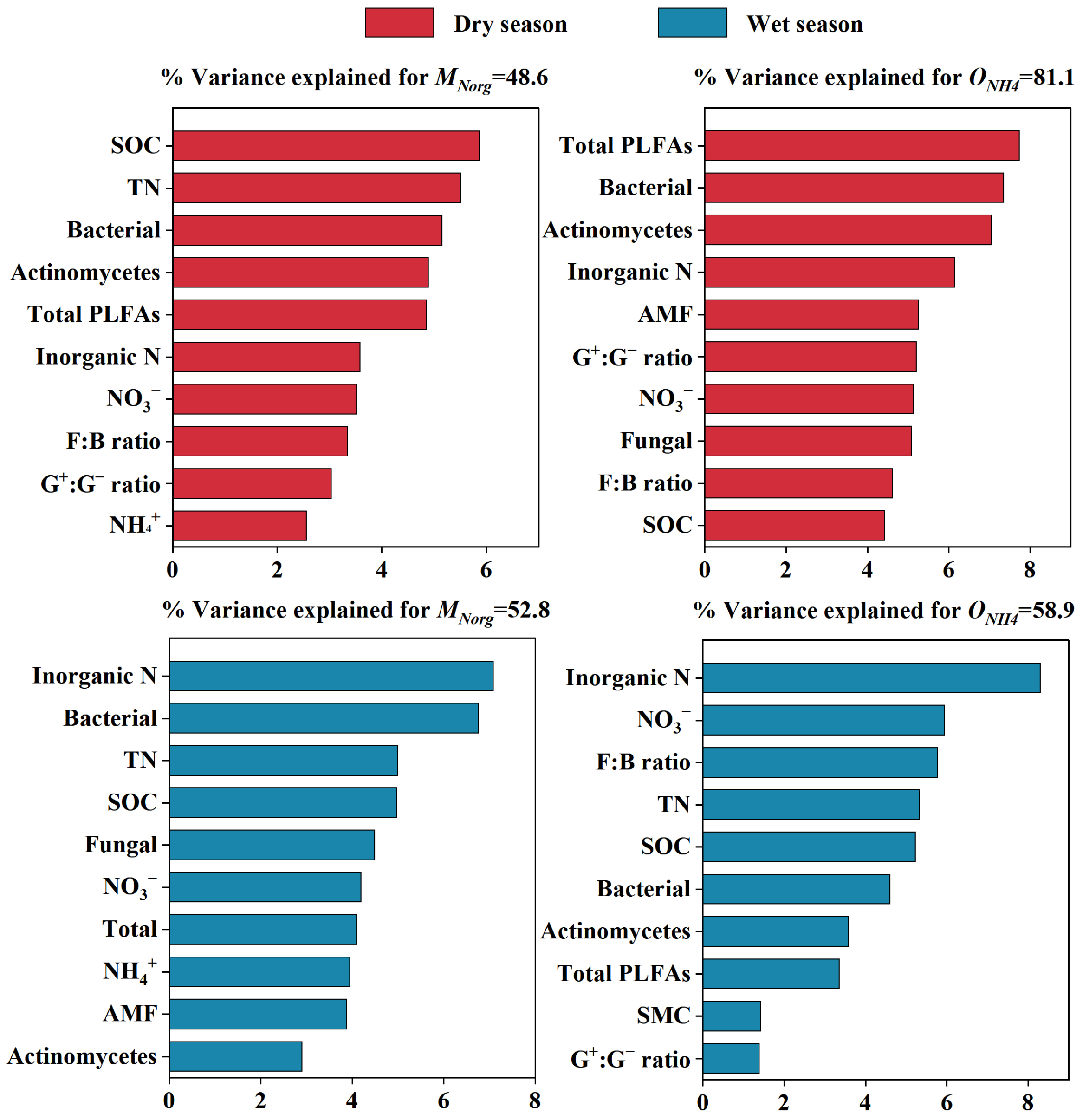
3.4. Factors Affecting Soil Organic Nitrogen Mineralization and Nitrification Rates
4. Discussion
4.1. Changes in Soil Microbial Community During Vegetation Restoration in Karst Region
4.2. Soil Organic Nitrogen Mineralization and Nitrification Rates During Vegetation Restoration in Karst Region
4.3. Factors Influencing Soil Organic Nitrogen Mineralization and Nitrification Rates During Vegetation Restoration in Karst Region
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.J.; Zhu, Q.L.; Yang, L.; Elrys, A.S.; Sun, J.F.; Ni, K.; Meng, L.; Zhu, T.B. Afforestation increases soil inorganic N supply capacity and lowers plant N limitation in subtropical karst areas. Geoderma 2024, 443, 116848. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Lian, Y.Q.; Qin, X.Q. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- D’Ettorre, U.S.; Liso, I.S.; Parise, M. Desertification in karst areas: A review. Earth-Sci. Rev. 2024, 253, 104786. [Google Scholar] [CrossRef]
- Guo, B.; Yang, F.; Fan, Y.W.; Zang, W.Q. The dominant driving factors of rocky desertification and their variations in typical mountainous karst areas of Southwest China in the context of global change. Catena 2023, 220, 106674. [Google Scholar] [CrossRef]
- Wang, L.J.; Luo, N.N.; Shi, Q.L.; Sheng, M.Y. Responses of soil labile organic carbon fractions and enzyme activities to long-term vegetation restorations in the karst ecosystems, Southwest China. Ecol. Eng. 2023, 194, 107034. [Google Scholar] [CrossRef]
- Cheng, H.T.; Zhou, X.H.; Dong, R.H.; Wang, X.M.; Liu, G.D.; Li, Q.F. Natural vegetation regeneration facilitated soil organic carbon sequestration and microbial community stability in the degraded karst ecosystem. Catena 2023, 222, 106856. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, C.H.; Chen, H.S.; Yue, Y.M.; Zhang, W.; Zhang, M.Y.; Qi, X.K.; Fu, Z.Y. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef]
- Lu, Z.X.; Wang, P.; Ou, H.B.; Wei, S.X.; Wu, L.C.; Jiang, Y.; Liu, Z.M. Effects of different vegetation restoration on soil nutrients, enzyme activities, and microbial communities in degraded karst landscapes in southwest China. For. Ecol. Manag. 2022, 508, 120002. [Google Scholar] [CrossRef]
- Mushia, N.M.; Ramoelo, A.; Ayisi, K.K. The impact of the quality of coal mine stockpile soils on sustainable vegetation growth and productivity. Sustainability 2016, 8, 546. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Perring, M.P.; Hedin, L.O.; Levin, S.A.; McGroddy, M.; Mazancourt, C.D. Increased plant growth from nitrogen addition should conserve phosphorus in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2008, 105, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.N.; Cheng, Y.; Yang, L.; Qin, X.H.; Meng, L.; He, Q.X.; Zhu, T.B.; Müller, C. Effects of agricultural cultivation on soil organic nitrogen mineralization in karst regions. Carsol. Sin. 2020, 29, 189195. [Google Scholar]
- Elrys, A.S.; Wang, J.; Metwally, M.A.; Cheng, Y.; Zhang, J.B.; Cai, Z.C.; Chang, S.X.; Müller, C. Global gross nitrification rates are dominantly driven by soil carbon-to-nitrogen stoichiometry and total nitrogen. Glob. Change Biol. 2021, 27, 6512–6524. [Google Scholar] [CrossRef]
- Tao, Y.L.; Xie, W.J.; Xu, L.; Zhang, L.C.; Wang, G.M.; Wang, X.N.; Shi, C.L. The characteristics of soil salinization effects on nitrogen mineralization and nitrification in upland fields. Front. Environ. Sci. 2024, 12, 1369554. [Google Scholar] [CrossRef]
- Martens, R. Current methods for measuring microbial biomass C in soil: Potentials and limitations. Biol. Fertil. Soils 1995, 19, 87–99. [Google Scholar] [CrossRef]
- Daly, A.B.; Jilling, A.; Bowles, T.M.; Buchkowski, R.W.; Frey, S.D.; Kallenbach, C.M.; Keiluweit, M.; Mooshammer, M.; Schimel, J.P.; Grandy, A.S. A holistic framework integrating plant-microbemineral regulation of soil bioavailable nitrogen. Biogeochemistry 2021, 154, 211–229. [Google Scholar] [CrossRef]
- Skubała, K.; Chowaniec, K.; Stanek, M.; Błaszkowski, J.; Móll, M.; Zubek, S. Soil and vegetation drivers of microbial attributes in a microhabitat mosaic at different successional stages after restoration of inland sand dunes. Appl. Soil Ecol. 2025, 206, 105832. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.B.; Zhang, J.Y.; Xue, S. Long-term effects of vegetational restoration on soil microbial communities on the Loess Plateau of China. Restor. Ecol. 2016, 24, 794–804. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Du, H.; Gao, K.; Fang, Y.T.; Wang, K.L.; Zhu, T.B.; Zhu, J.; Cheng, Y.; Li, D.J. Plant species diversity enhances soil gross nitrogen transformations in a subtropical forest, southwest China. J. Appl. Ecol. 2023, 60, 1364–1375. [Google Scholar] [CrossRef]
- Chen, Y.C.; Ma, S.Q.; Jiang, H.M.; Hu, Y.; Lu, X.Y. Influences of litter diversity and soil moisture on soil microbial communities in decomposing mixed litter of alpine steppe species. Geoderma 2020, 377, 114577. [Google Scholar] [CrossRef]
- Roa-Fuentes, L.L.; Martínez-Garza, C.; Etchevers, J.; Campo, J. Recovery of soil C and N in a tropical pasture: Passive and active restoration. Land Degrad. Dev. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Singha, D.; Brearley, F.Q.; Tripathi, S.K. Fine root and soil nitrogen dynamics during stand development following shifting agriculture in Northeast India. Forests 2020, 11, 1236. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Zhao, Y.Q.; Zhang, S.H.; Xu, Y.L.; Han, X.H.; Yang, G.h.; Ren, C.J. N-cycle gene abundance determination of N mineralization rate following re-afforestation in the Loess Plateau of China. Soil Ecol. Lett. 2024, 6, 230188. [Google Scholar] [CrossRef]
- Liang, J.H.; Cui, X.D.; Wen, L.Y.; Liu, D.; Yi, C.X.; Huang, K.Z.; Wang, J. Comparison of soil calcium and magnesium fractions transport in classic karst and non-karst region, Guilin. Carsol. Sin. 2022, 41, 220–227. (In Chinese) [Google Scholar]
- Rowley, M.C.; Grand, S.; Verrecchia, E.P. Calcium-mediated stabilization of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Rütting, T.; Schleusner, P.; Hink, L.; Prosser, J.I. The contribution of ammonia–oxidizing archaea and bacteria to gross nitrification under different substrate availability. Soil Boil. Biochem. 2021, 160, 108353. [Google Scholar] [CrossRef]
- Banning, N.C.; Grant, C.D.; Jones, D.L.; Murphy, D.V. Recovery of soil organic matter, organic matter turnover and nitrogen cycling in a post–mining forest rehabilitation chronosequence. Soil Boil. Biochem. 2008, 40, 2021–2031. [Google Scholar] [CrossRef]
- Zhu, X.A.; Jiang, X.J.; Singh, A.K.; Zeng, H.H.; Chen, C.F.; Lu, E.F.; Liu, W.J. Reduced litterfall and decomposition alters nutrient cycling following conversion of tropical natural forests to rubber plantations. Ecol. Indic. 2022, 138, 108819. [Google Scholar] [CrossRef]
- Lv, P.; Zuo, X.; Sun, S.; Zhang, J.; Zhao, S.; Hu, Y. Seasonal changes of soil nitrogen mineralization along restoration gradient of sandy grassland, northern China. Pol. J. Ecol. 2021, 68, 283–295. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.; Ghemoh, C.J.; Subramanian, S.; Tanga, C.M. In situ nitrogen mineralization and nutrient release by soil amended with black soldier fly frass fertilizer. Sci. Rep. 2021, 11, 14799. [Google Scholar] [CrossRef]
- Pandey, R.; Bargali, S.S.; Bargali, K. Seasonal dynamics of soil Inorganic N and N-mineralization in sub-tropical Sal forest in Central Himalaya, India. J. Sci. Res. 2022, 66, 161–175. [Google Scholar]
- Wang, W.X.; Cheng, R.M.; Shi, Z.M.; Ingwersen, J.; Luo, D.; Liu, S.R. Seasonal dynamics of soil respiration and nitrification in three subtropical plantations in southern China. IForest 2016, 9, 813. [Google Scholar] [CrossRef]
- Zhu, X.; Zou, X.; Lu, E.; Deng, Y.; Luo, Y.; Chen, H.; Liu, W. Litterfall biomass and nutrient cycling in karst and nearby non-karst forests in tropical China: A 10-year comparison. Sci. Total Environ. 2021, 758, 143619. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Li, S.; Yue, F.; Cheng, Q.; Peng, T.; Soulsby, C. Linking nitrate dynamics to water age in underground conduit flows in a karst catchment. J. Hydrol. 2021, 596, 125699. [Google Scholar] [CrossRef]
- Rolo, V.; Olivier, P.I.; Guldemond, R.A.R.; van Aarde, R.J. Validating space-for-time substitution in a new-growth coastal dune forest. Appl. Veg. Sci. 2016, 19, 235–243. [Google Scholar] [CrossRef]
- Dor, M.; Fan, L.C.; Zamanian, K.; Kravchenko, A.N. Long-term land use conversion influence on soil pore structure and organic carbon. Agric. Ecosyst. Environ. 2025, 387, 109633. [Google Scholar] [CrossRef]
- Guo, X.J.; Gong, X.P.; Shi, J.S.; Guo, J.; Domínguez-Villar, D.; Lin, Y.S.; Wang, H.W.; Yuan, D.X. Temporal variations and evaporation control effect of the stable isotope composition of precipitation in the subtropical monsoon climate region, Southwest China. J. Hydrol. 2021, 599, 126278. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zang, R.G.; Wang, G.Y.; Huang, X.R. Classification of landscape types based on land cover, successional stages and plant functional groups in a species-rich forest in Hainan Island, China. Trop. Conserv. Sci. 2016, 9, 135–152. [Google Scholar] [CrossRef]
- Li, D.J.; Wen, L.; Xiao, K.C.; Song, T.Q.; Wang, K.L. Responses of soil gross nitrogen transformations to three vegetation restoration strategies in a subtropical karst region. Land Degrad. Dev. 2021, 32, 2520–2527. [Google Scholar] [CrossRef]
- Kirkham, D.; Bartholomew, W.V. Equations for following nutrient transformations in soil utilizing tracer data. Soil Sci. Soc. Am. Proc. 1954, 18, 33–34. [Google Scholar] [CrossRef]
- Bremner, J.M.; Keeney, D.R. Determination and isotope-ratio analysis of different forms of nitrogen in soils: 3. Exchangeable ammonium, nitrate, and nitrite by extraction-distillation methods. Soil Sci. Soc. Am. J. 1966, 30, 577–582. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Li, C.; Cano, A.; Acosta-Martinez, V.; Veum, K.S.; Moore-Kucera, J. A comparison between fatty acid methyl ester profiling methods (PLFA and EL-FAME) as soil health indicators. Soil Sci. Soc. Am. J. 2020, 84, 1153–1169. [Google Scholar] [CrossRef]
- Lewe, N.; Hermans, S.; Lear, G.; Kelly, L.T.; Thomson-Laing, G.; Weisbrod, B.; Deslippe, J.R. Phospholipid fatty acid (PLFA) analysis as a tool to estimate absolute abundances from compositional 16S rRNA bacterial metabarcoding data. J. Microbiol. Methods 2021, 188, 106271. [Google Scholar] [CrossRef]
- Cai, X.W.; Zhang, D.; Wang, Y.Q.; Diao, L.F.; Cheng, X.L.; Luo, Y.Q.; An, S.Q.; Yang, W. Shift in soil microbial communities along ~160 years of natural vegetation restoration on the Loess Plateau of China. Appl. Soil Ecol. 2022, 173, 104394. [Google Scholar] [CrossRef]
- Hu, P.L.; Xiao, J.; Zhang, W.; Xiao, L.M.; Yang, R.; Xiao, D.; Zhao, J.; Wang, K.L. Response of soil microbial communities to natural and managed vegetation restoration in a subtropical karst region. Catena 2020, 195, 104849. [Google Scholar] [CrossRef]
- Brown, R.W.; Chadwick, D.R.; Bending, G.D.; Collins, C.D.; Whelton, H.L.; Daulton, E.; Jones, D.L. Nutrient (C, N and P) enrichment induces significant changes in the soil metabolite profile and microbial carbon partitioning. Soil Boil. Biochem. 2022, 172, 108779. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Boil. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Smith, A.P.; Marín-Spiotta, E.; Balser, T. Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: A multiyear study. Glob. Change Biol. 2015, 21, 3532–3547. [Google Scholar] [CrossRef]
- Yokobe, T.; Hyodo, F.; Tokuchi, N. Seasonal effects on microbial community structure and nitrogen dynamics in temperate forest soil. Forests 2018, 9, 153. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L.; Zhu, T.B.; Yang, H.; Zhang, J.B.; Yang, J.L.; Cao, J.H.; Bai, B.; Jiang, Z.C.; Liang, Y.M.; et al. Rapid recovery of nitrogen retention capacity in a subtropical acidic soil following afforestation. Soil Boil. Biochem. 2018, 120, 171–180. [Google Scholar] [CrossRef]
- Li, Z.L.; Zeng, Z.Q.; Tian, D.S.; Wang, J.S.; Fu, Z.; Zhang, F.Y.; Zhang, W.N.; Luo, Y.Q.; Niu, S.L. Global patterns and controlling factors of soil nitrification rate. Glob. Change Biol. 2020, 26, 4147–4157. [Google Scholar] [CrossRef]
- Su, W.Q.; Yu, M.J.; Lin, J.H.; Tang, C.X.; Xu, J.M. Fire decreases gross mineralization rate but does not alter gross nitrification rate in boreal forest soils. Soil Biol. Biochem. 2022, 175, 108838. [Google Scholar] [CrossRef]
- Xu, Y.B.; Xu, Z.H. Effects of land use change on soil gross nitrogen transformation rates in subtropical acid soils of Southwest China. Environ. Sci. Pollut. Res. 2015, 22, 10850–10860. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Mou, Z.J.; Kuang, L.H.; Wu, W.J.; Zhang, J.; Wang, F.M.; Hui, D.F.; Peñuelas, J.; Sardans, J.; et al. Phosphorus addition decreases microbial residual contribution to soil organic carbon pool in a tropical coastal forest. Glob. Change Biol. 2021, 27, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes Benefaction Role in Soil and Plant Health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef]
- Zhang, K.R.; Li, X.S.; Cheng, X.L.; Zhang, Z.H.; Zhang, Q.F. Changes in soil properties rather than functional gene abundance control carbon and nitrogen mineralization rates during long-term natural revegetation. Plant Soil 2019, 443, 293–306. [Google Scholar] [CrossRef]
- Li, D.J.; Yang, Y.; Chen, H.; Xiao, K.C.; Song, T.Q.; Wang, K.L. Soil gross nitrogen transformations in typical karst and nonkarst forests, southwest China. J. Geophys. Res.-Biogeosci. 2017, 122, 2831–2840. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, F.; Cheng, X.L. Effects of land use change type on soil microbial attributes and their controls: Data synthesis. Ecol. Indic. 2022, 138, 108852. [Google Scholar] [CrossRef]
- Mo, Q.; Li, Z.A.; Zhu, W.; Zou, B.; Li, Y.; Yu, S.; Wang, F. Reforestation in southern China: Revisiting soil N mineralization and nitrification after 8 years restoration. Sci. Rep. 2016, 6, 19770. [Google Scholar] [CrossRef]
- Nie, Y.; Han, X.; Chen, J.; Wang, M.; Shen, W. The simulated N deposition accelerates net N mineralization and nitrification in a tropical forest soil. Biogeosciences 2019, 16, 4277–4291. [Google Scholar] [CrossRef]
- Singh, J.S.; Kashyap, A.K. Dynamics of viable nitrifier community, N-mineralization and nitrification in seasonally dry tropical forests and savanna. Microbiol. Res. 2006, 161, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Santiago, S.; Geisseler, D. Effects of moisture contents in incorporated residues and soil on net nitrogen mineralization in a laboratory study. Agrosys. Geosci. Environ. 2022, 5, e20268. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, M.; Chen, H.; Zhao, H.; Huang, Y.; Su, W.; Xu, J. Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob. Change Biol. 2020, 26, 5267–5527. [Google Scholar] [CrossRef]
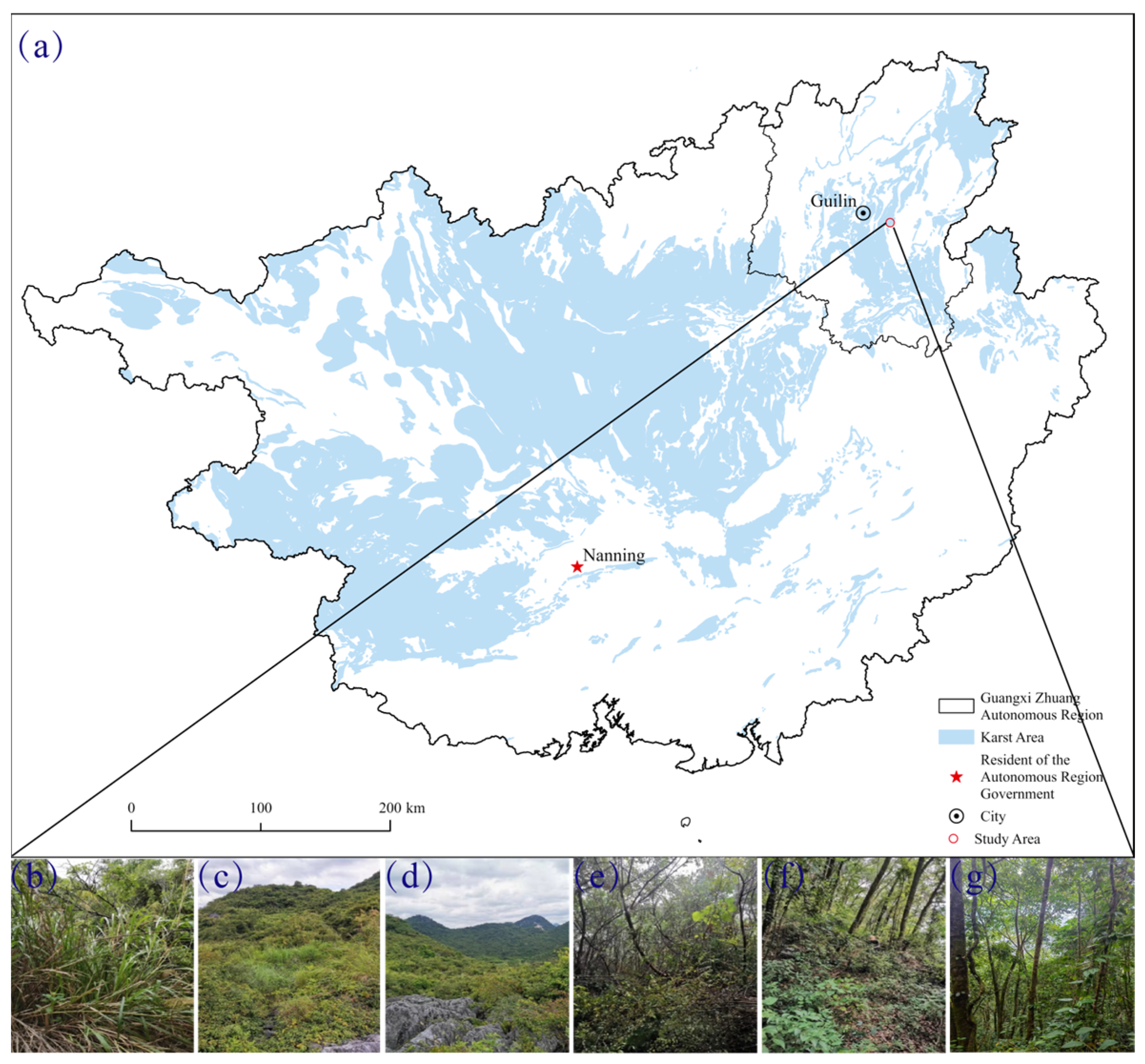
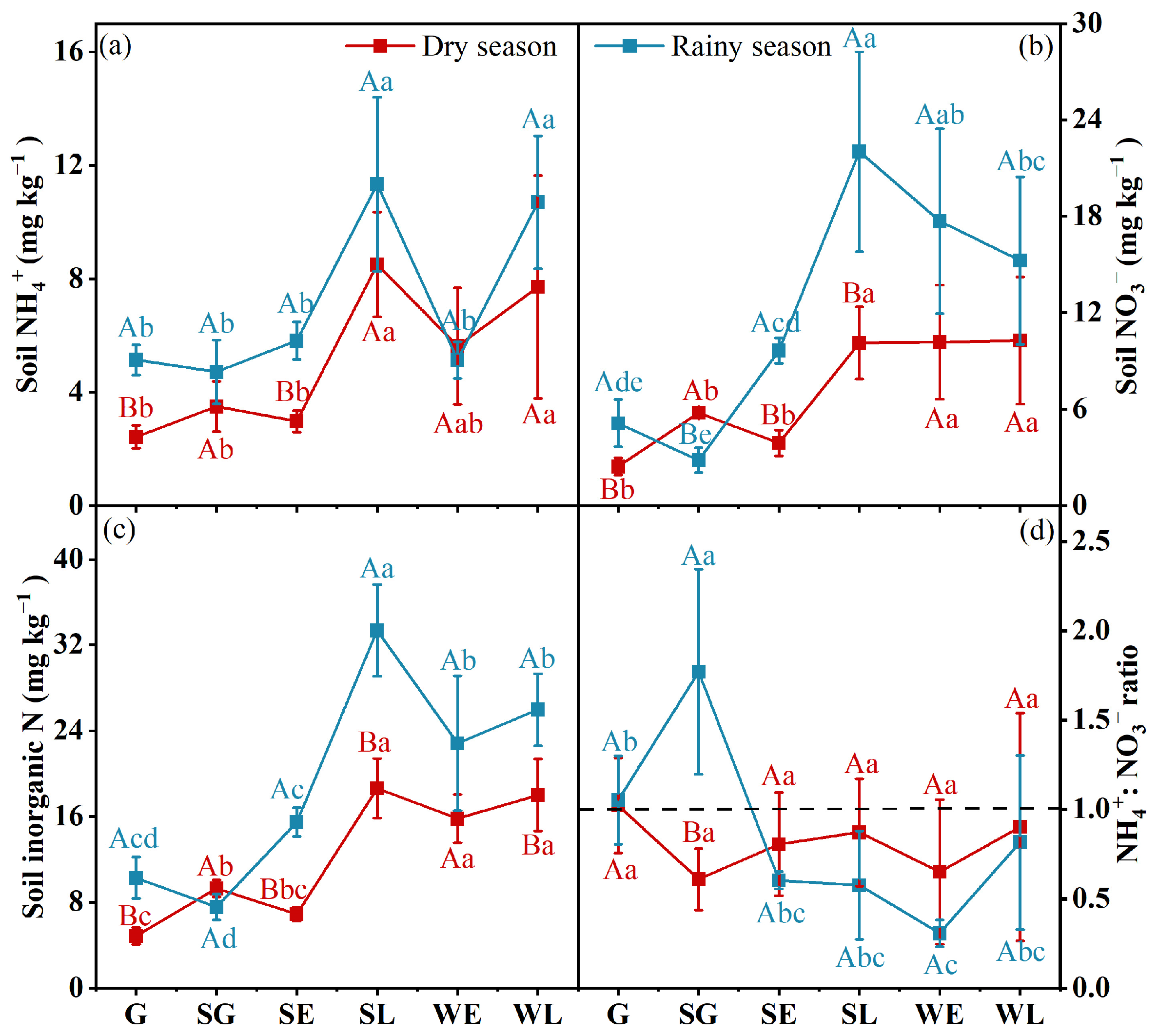
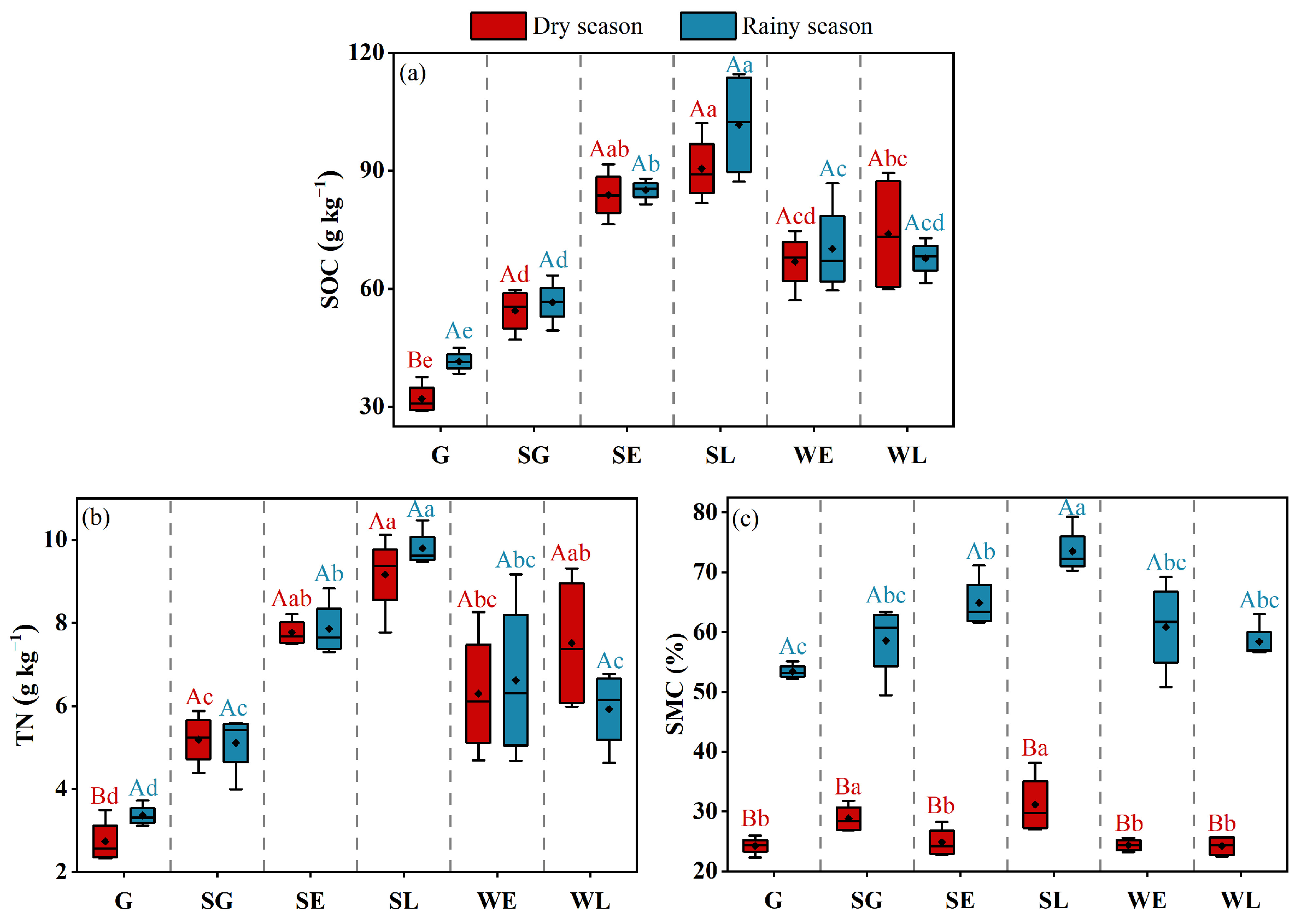
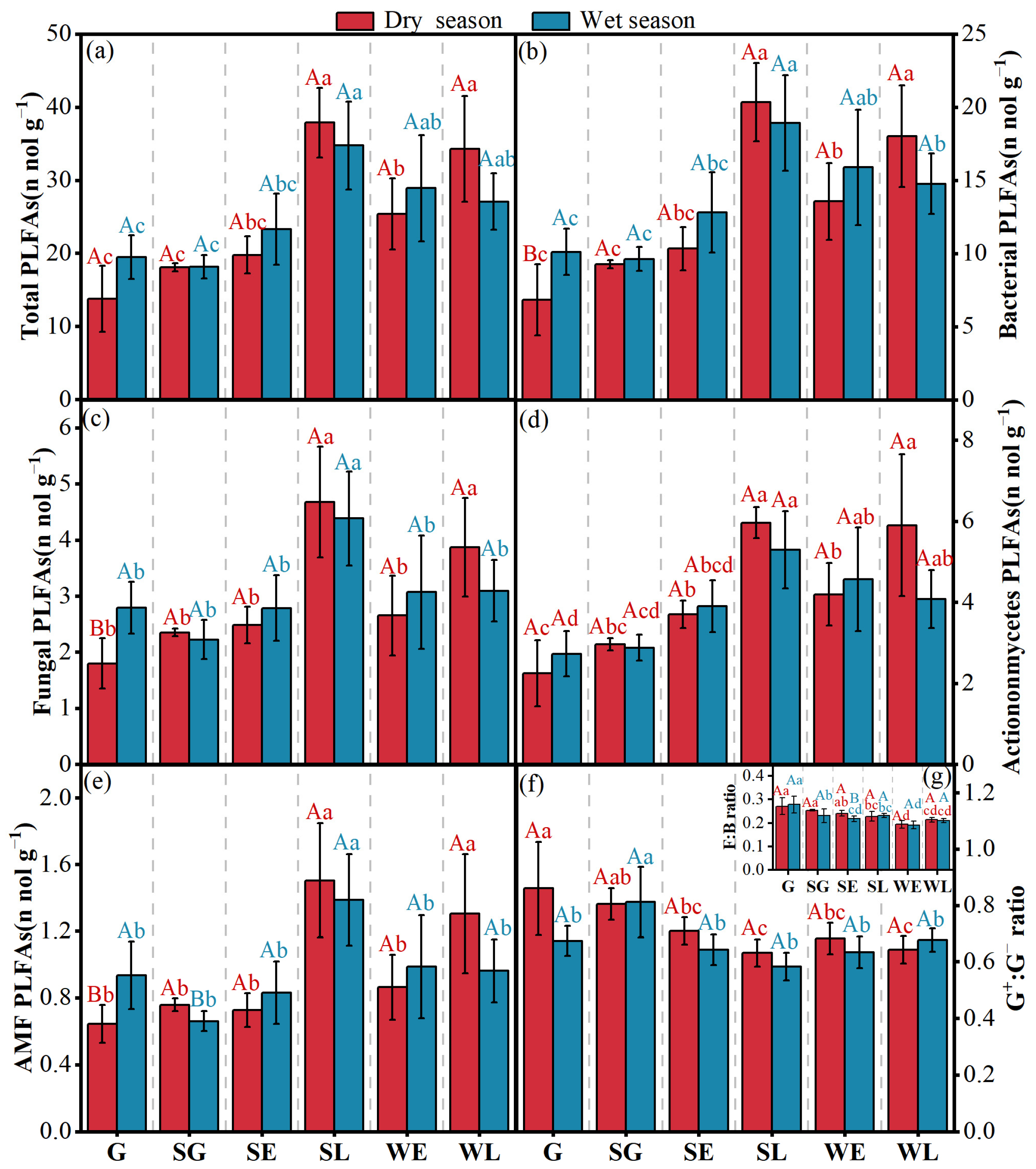
| Restoration Stages | Longitude and Latitude | Altitude (m) | Slope (°) | Soil Depth (cm) | Interference Conditions |
|---|---|---|---|---|---|
| Grassland | 110°32′ E 25°12′ N | 324–341 | 2–5 | 0–30 | Abandoned, no interference for 3–5 years |
| Shrub-grassland | 110°32′ E 25°12′ N | 322–348 | 4–8 | 0–30 | Abandoned, no interference for 7–10 years |
| Early-stage shrubland | 110°31′ E 25°12′ N | 337–345 | 6–13 | 0–25 | Abandoned, no interference for 15–20 years |
| Late-stage shrubland | 110°53′ E 25°21′ N | 321–343 | 12–15 | 0–25 | Abandoned, no interference for 30–35 years |
| Early-stage woodland | 110°53′ E 25°12′ N | 335–376 | 24–30 | 0–25 | Abandoned, no interference for 45–50 years |
| Late-stage woodland | 110°52′ E 25°20′ N | 246–300 | 20–25 | 0–20 | Abandoned, no interference for 70–80 years |
| Factors | Soil Organic N Mineralization Rate | Soil Nitrification Rates | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean Square | df | F | p Values | Mean Square | df | F | p Values | |
| Vegetation restoration stage | 61.5 | 5 | 12.3 | <0.01 ** | 221 | 5 | 152 | <0.01 ** |
| season | 117 | 1 | 117 | <0.01 ** | 140 | 1 | 480 | <0.01 ** |
| Vegetation restoration stage × season | 9.70 | 5 | 1.94 | 0.03 * | 95.8 | 5 | 65.8 | <0.01 ** |
| Error | 25.4 | 36 | 0.71 | 10.5 | 36 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Yang, H.; Liu, L.; Yang, S.; Wen, D.; Li, X.; Meng, L.; Deng, Z.; Liang, J.; Lu, D.; et al. Vegetation Restoration Significantly Increased Soil Organic Nitrogen Mineralization and Nitrification Rates in Karst Regions of China. Forests 2025, 16, 1006. https://doi.org/10.3390/f16061006
Yang L, Yang H, Liu L, Yang S, Wen D, Li X, Meng L, Deng Z, Liang J, Lu D, et al. Vegetation Restoration Significantly Increased Soil Organic Nitrogen Mineralization and Nitrification Rates in Karst Regions of China. Forests. 2025; 16(6):1006. https://doi.org/10.3390/f16061006
Chicago/Turabian StyleYang, Lin, Hui Yang, Lijun Liu, Shuting Yang, Dongni Wen, Xuelan Li, Lei Meng, Zhong Deng, Jian Liang, Danmei Lu, and et al. 2025. "Vegetation Restoration Significantly Increased Soil Organic Nitrogen Mineralization and Nitrification Rates in Karst Regions of China" Forests 16, no. 6: 1006. https://doi.org/10.3390/f16061006
APA StyleYang, L., Yang, H., Liu, L., Yang, S., Wen, D., Li, X., Meng, L., Deng, Z., Liang, J., Lu, D., & Zhu, T. (2025). Vegetation Restoration Significantly Increased Soil Organic Nitrogen Mineralization and Nitrification Rates in Karst Regions of China. Forests, 16(6), 1006. https://doi.org/10.3390/f16061006






