Climate-Induced Shift in the Population Dynamics of Tortrix viridana L. in Ukraine
Abstract
1. Introduction
- -
- to analyze the dynamics of T. viridana outbreaks in the forests of Ukraine for 1947–2025 and individual 20-year intervals of this period;
- -
- to compare the main parameters of T. viridana outbreaks in forests of individual regions for 1978–2001 and 2002–2025 (outbreak incidence, severity, duration, and mean interval between outbreaks);
- -
- to search for an explanation for the observed changes in the main parameters of T. viridana outbreaks
- -
- by the shift of T. viridana as part of the flush feeders’ complex;
- -
- by the shift in the suitability of forest subcompartments for T. viridana;
- -
- by phenological mismatch in oak bud-flushing and T. viridana hatching.
2. Materials and Methods
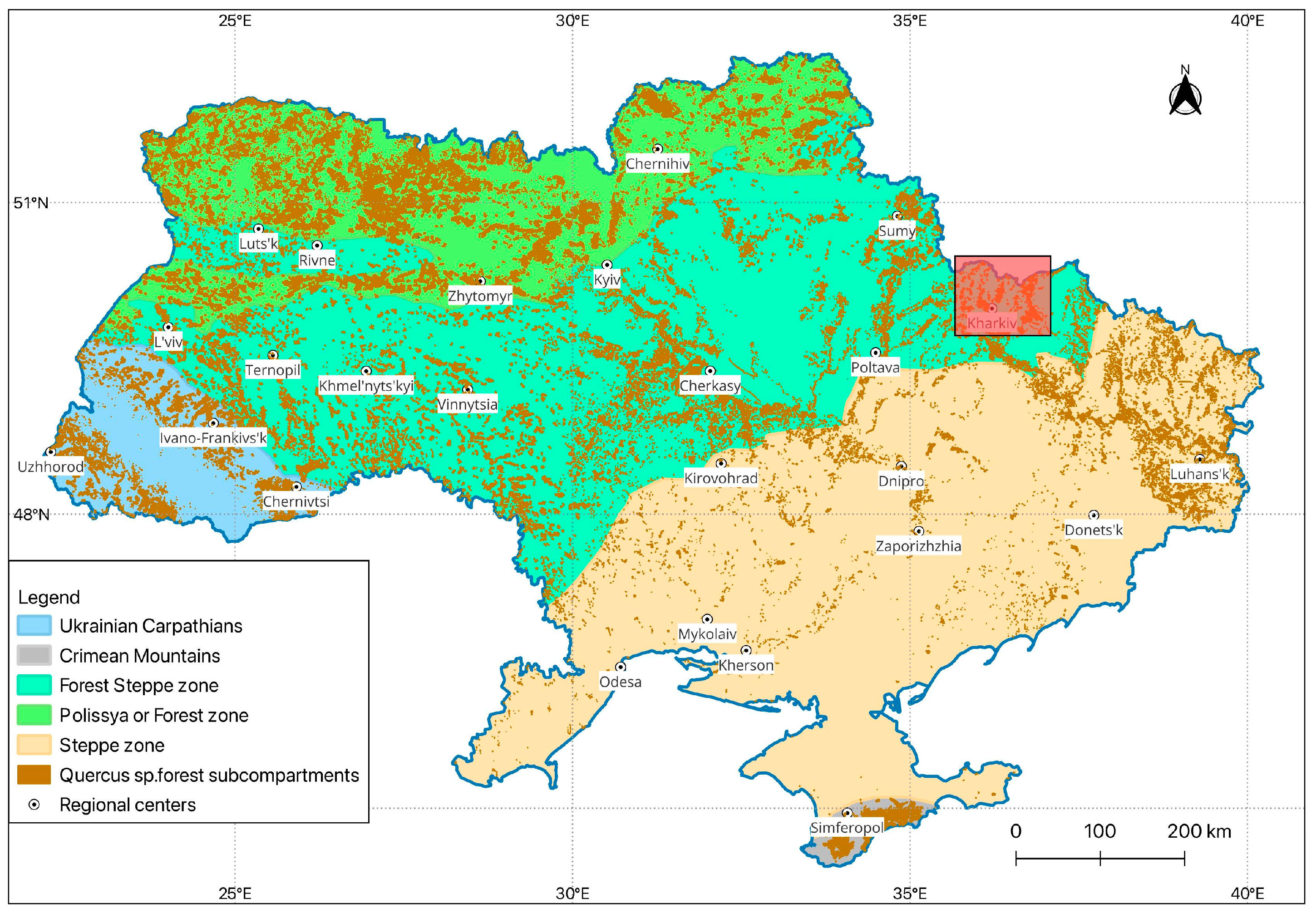
2.1. Study Region and Data Sources
2.2. Spatio-Temporal Dynamics of Tortrix viridana Outbreaks
2.3. The Suitability of the Forest Sites for Tortrix viridana
2.4. Data Processing
3. Results
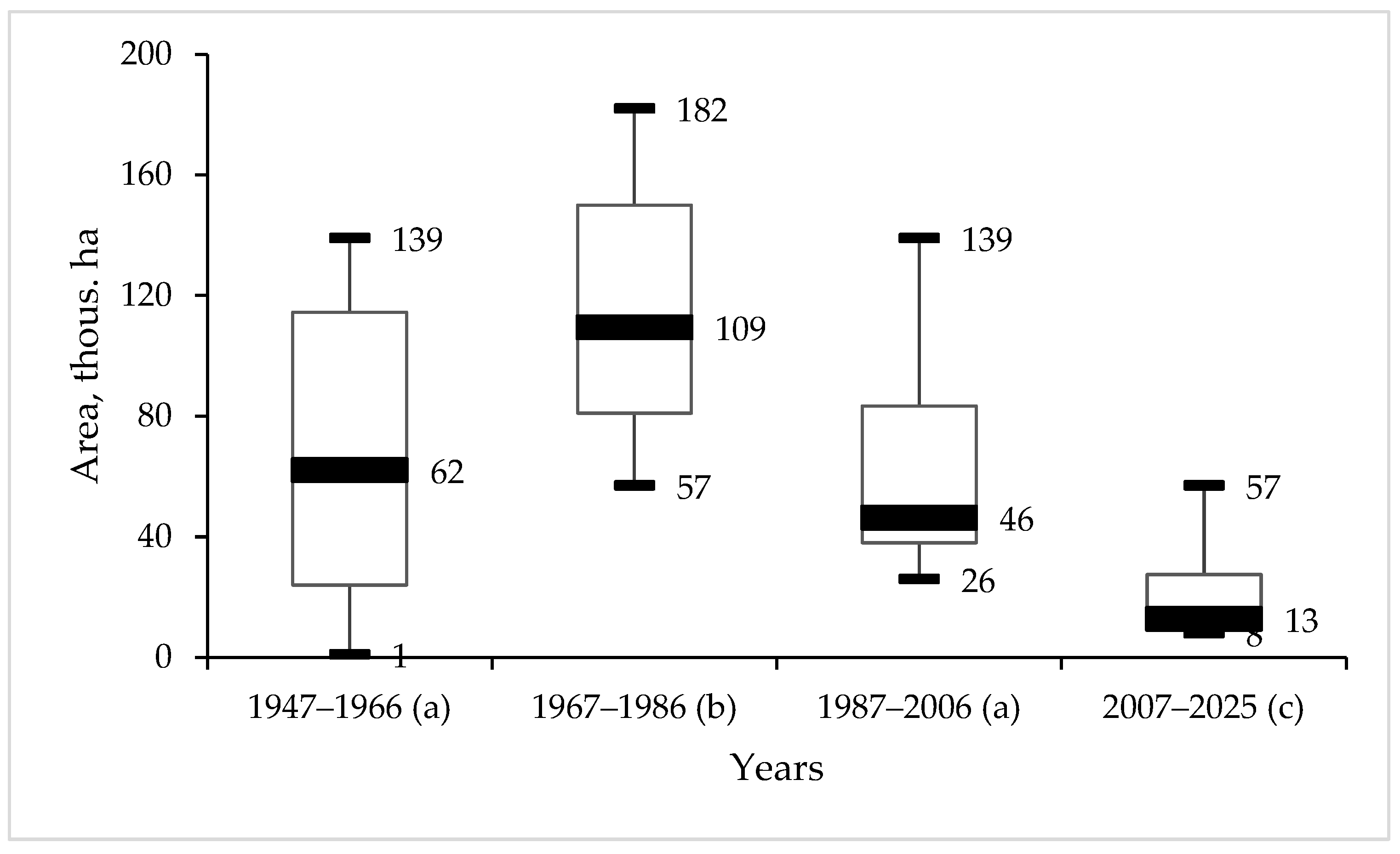
3.1. Dynamics of T. viridana Outbreaks in the Forests of Ukraine for 1947–2025
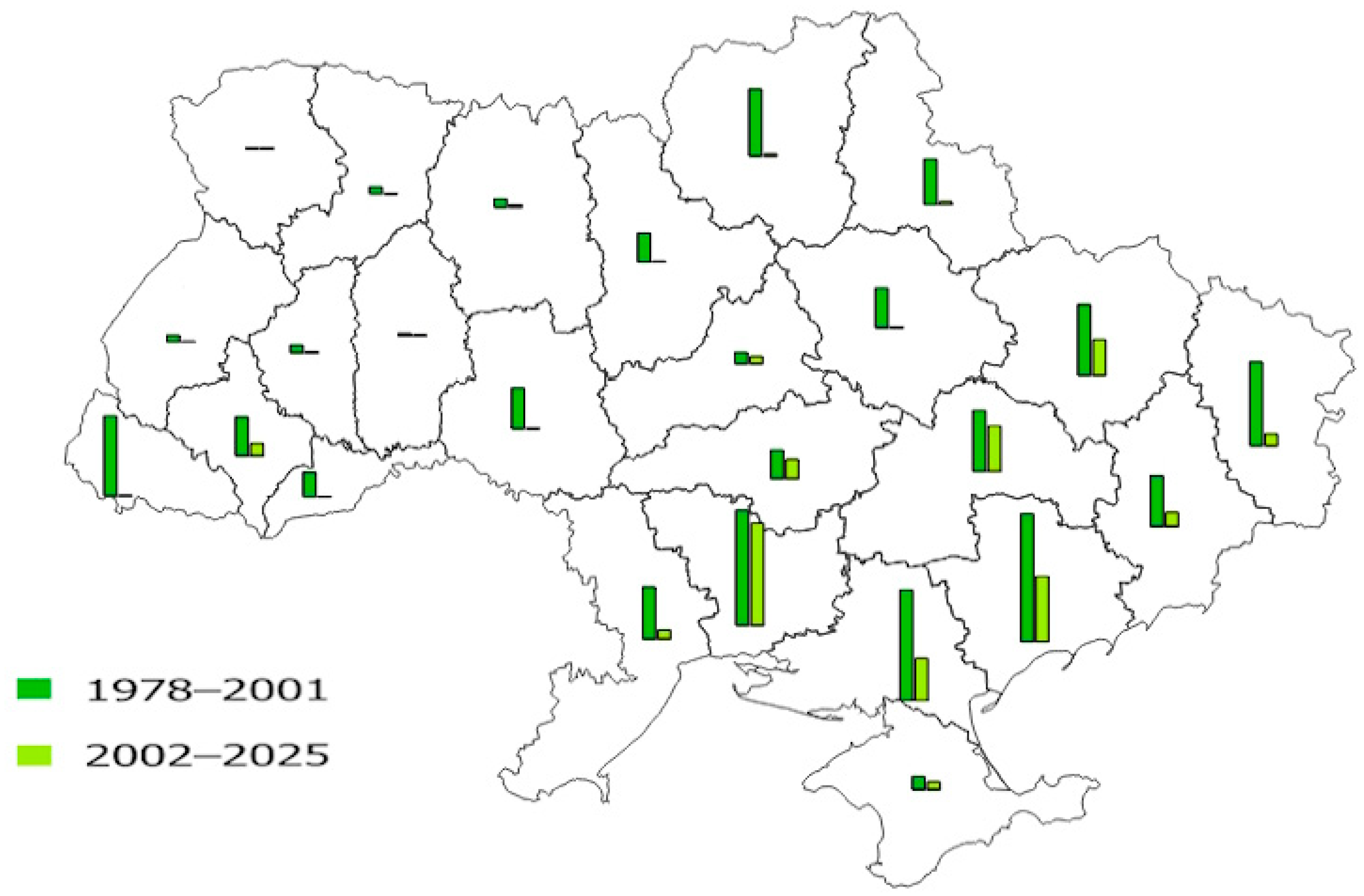
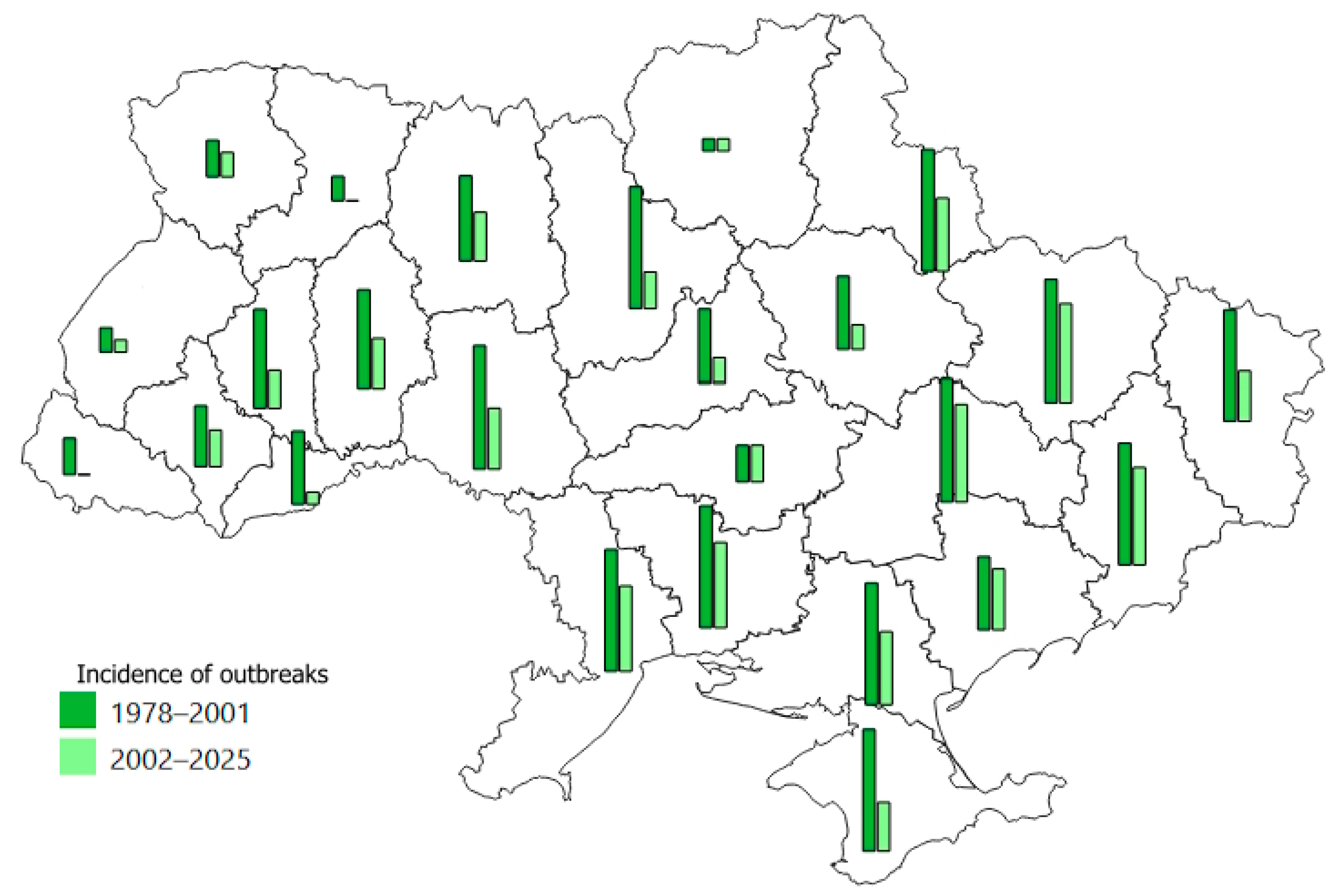
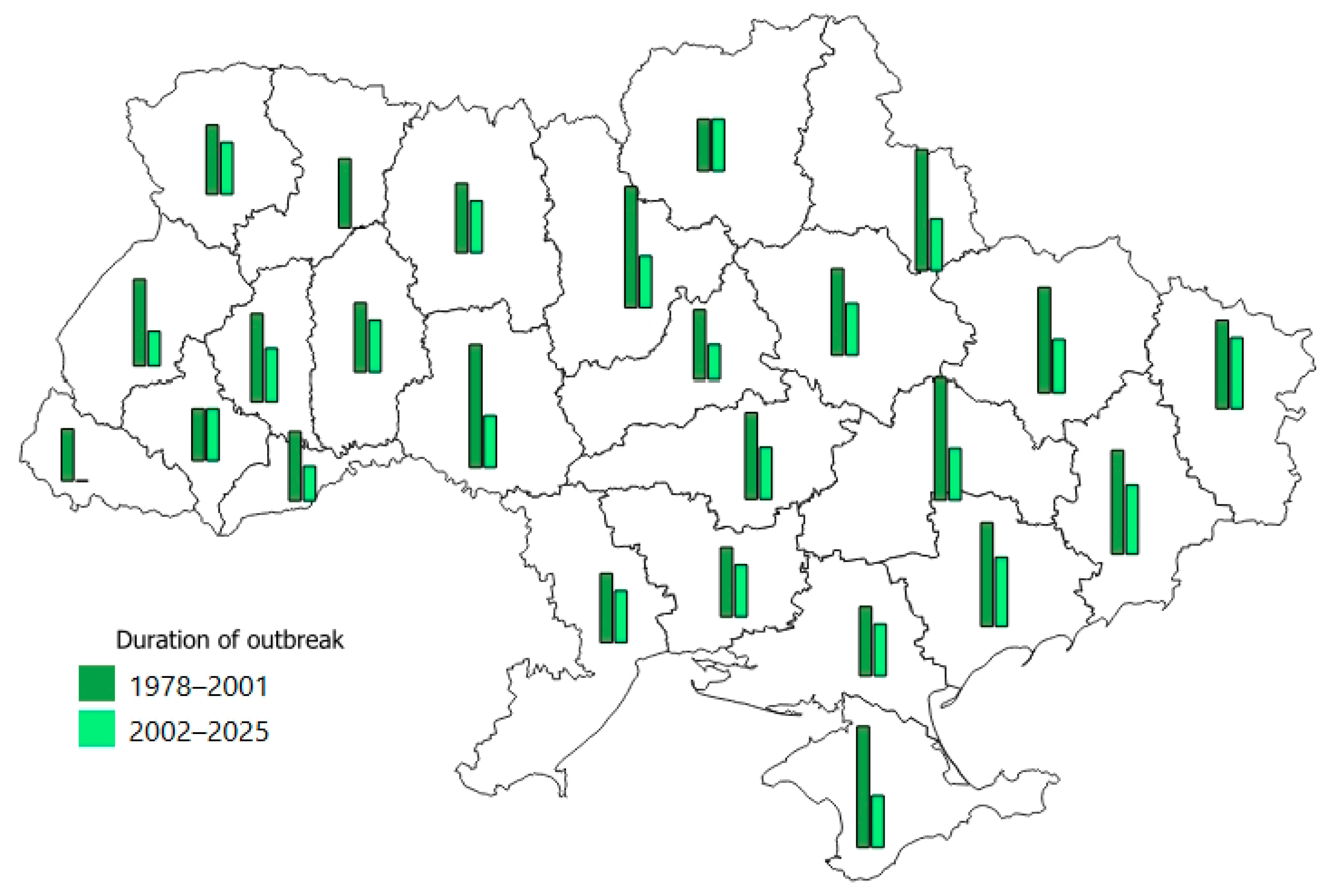
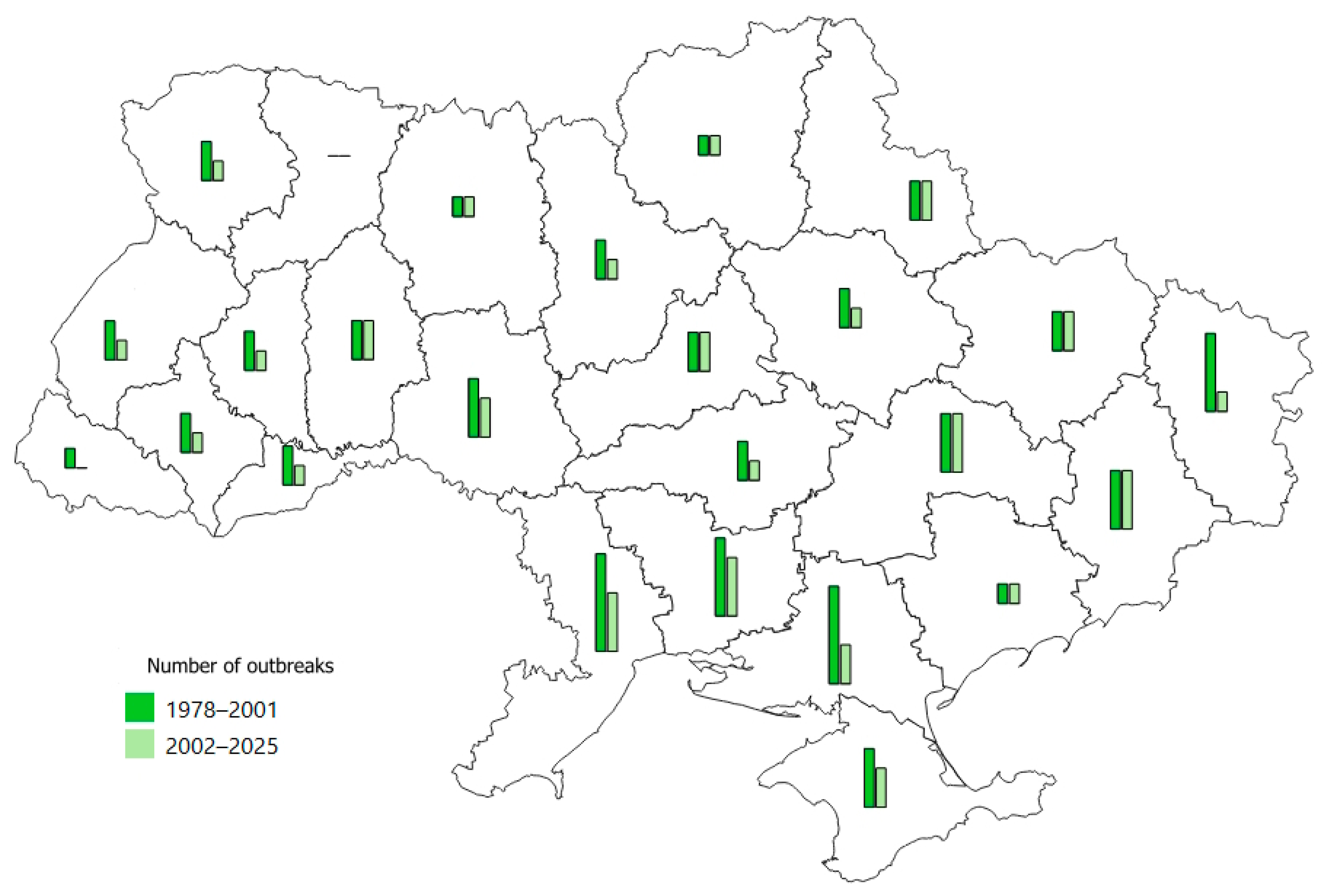
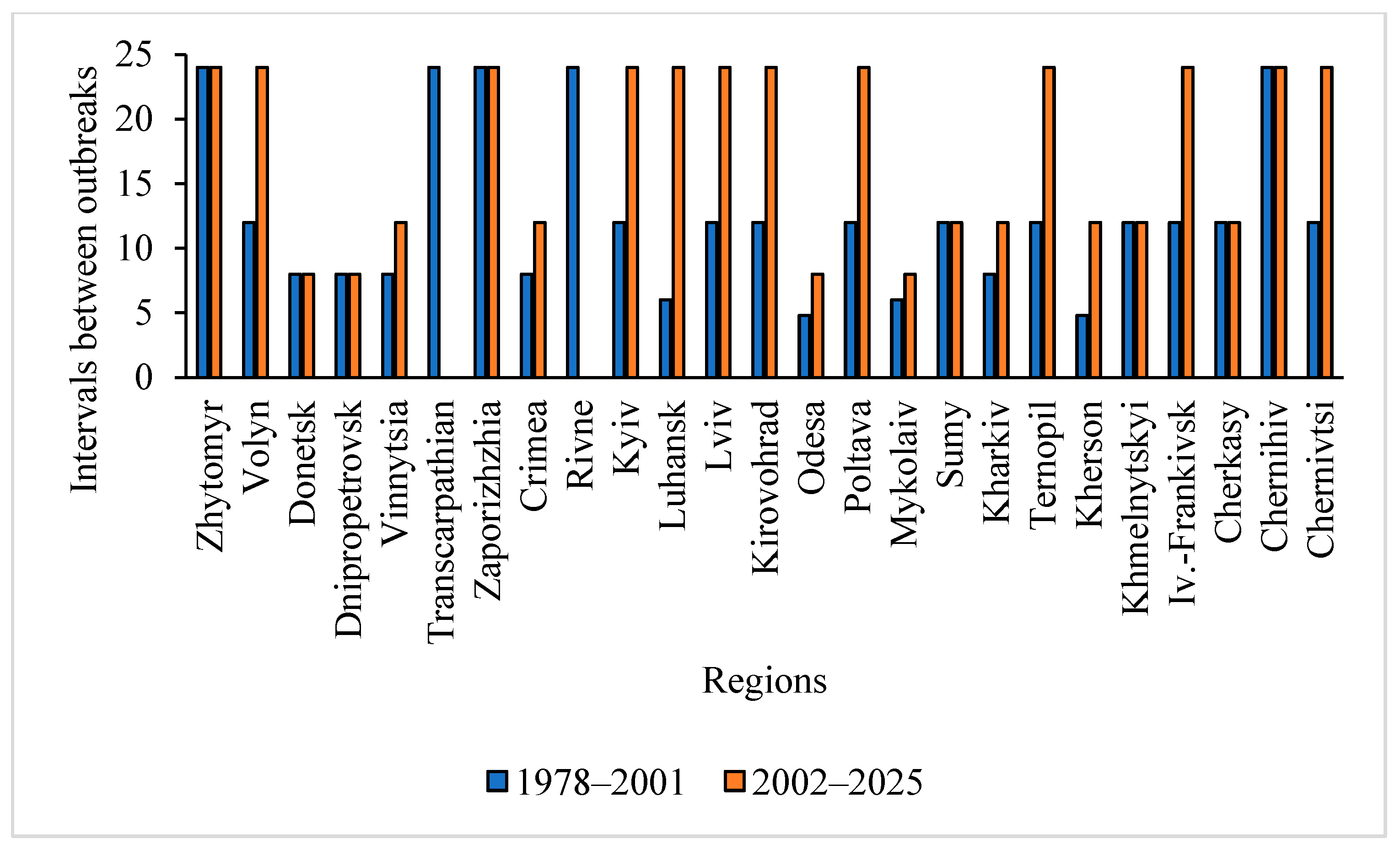
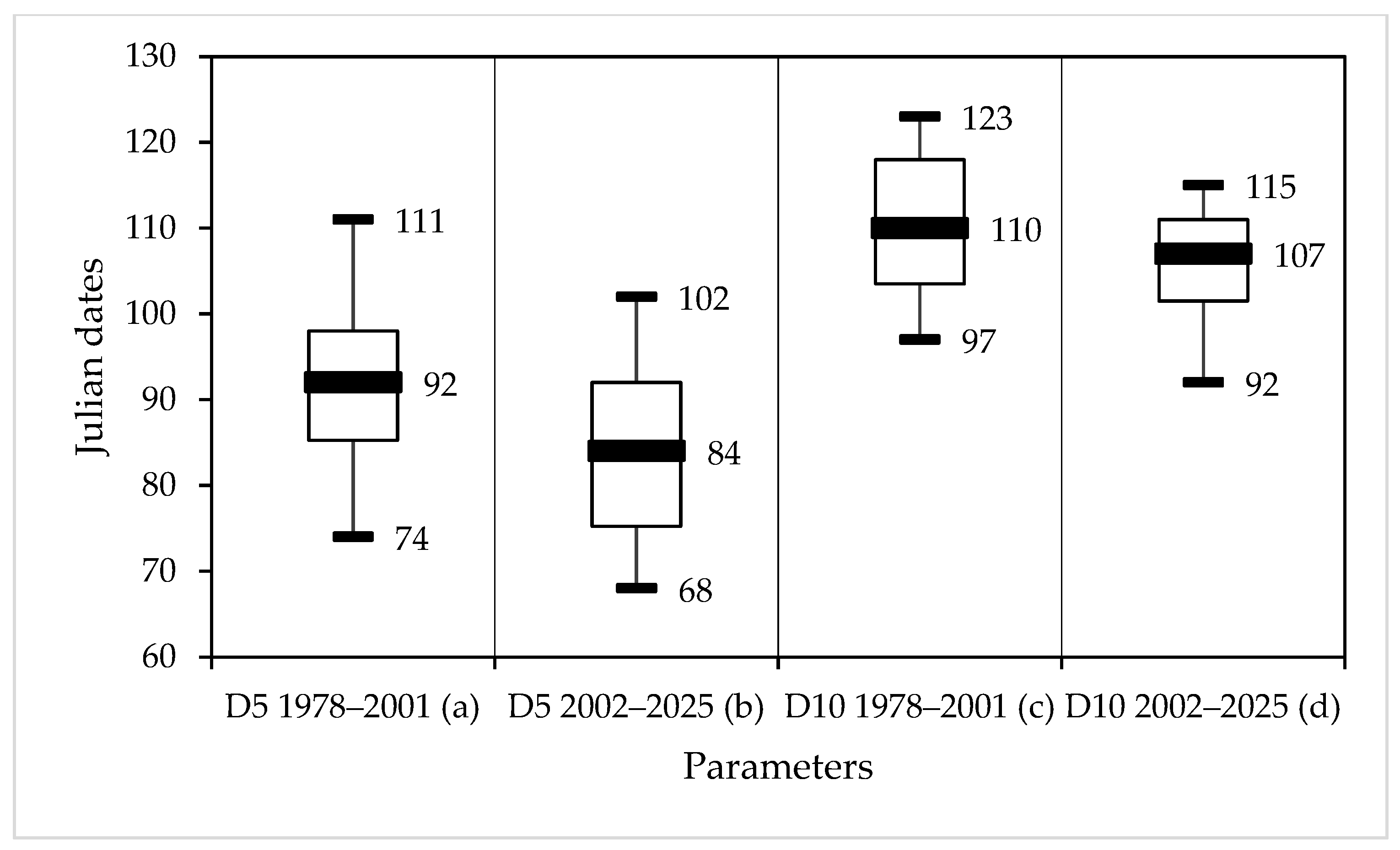
3.2. The Main Parameters of T. viridana Outbreaks in Forests of Individual Regions
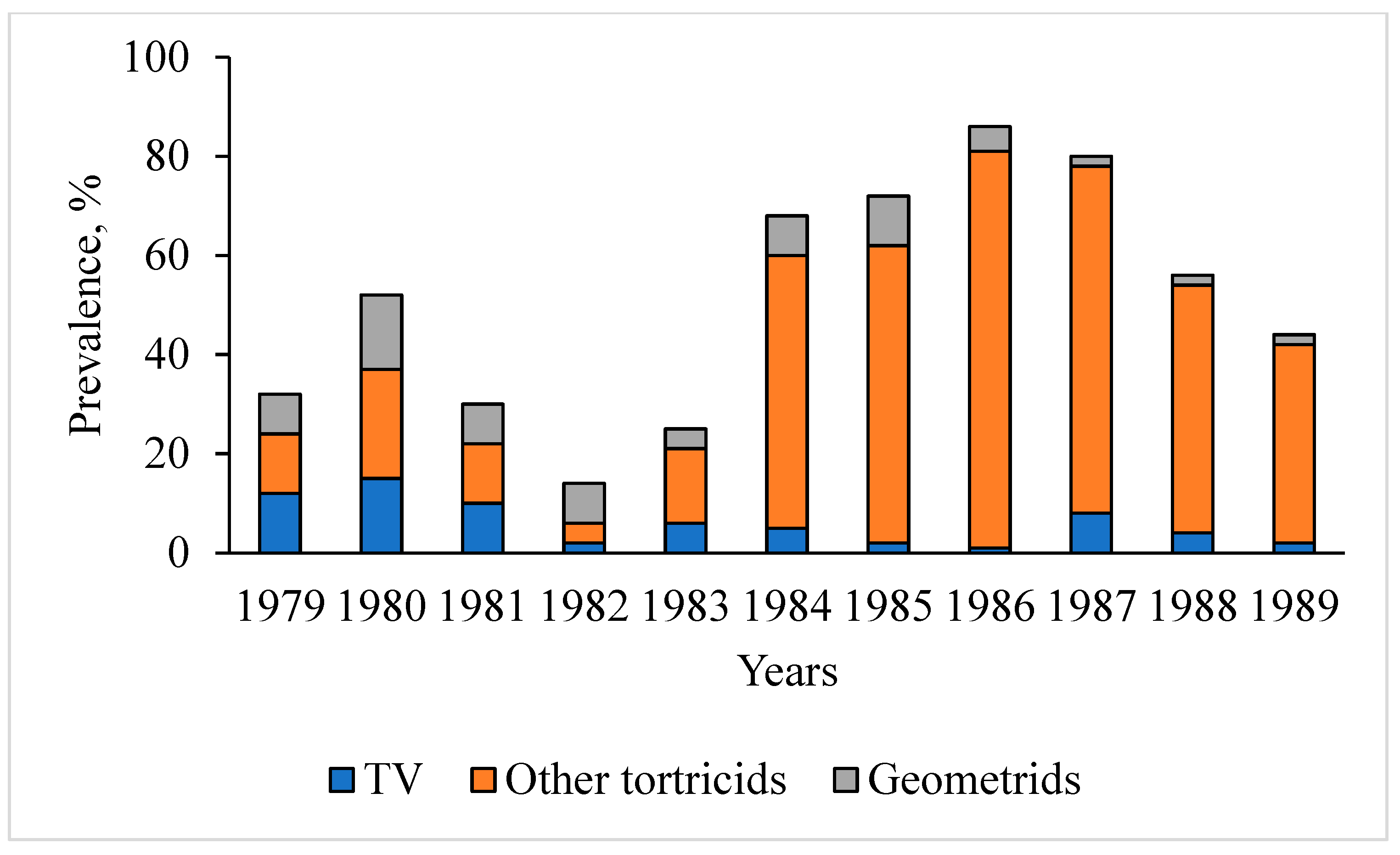
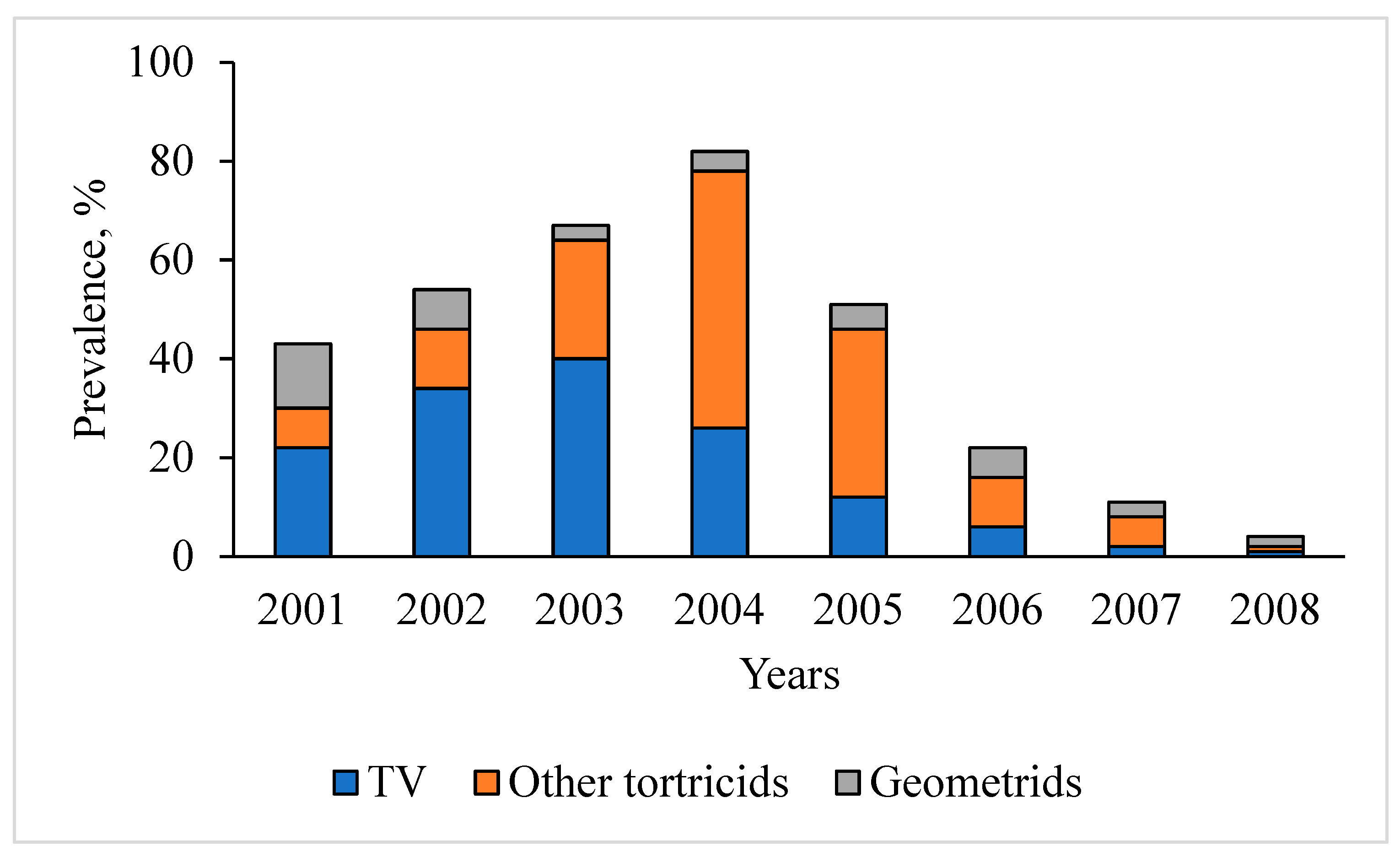
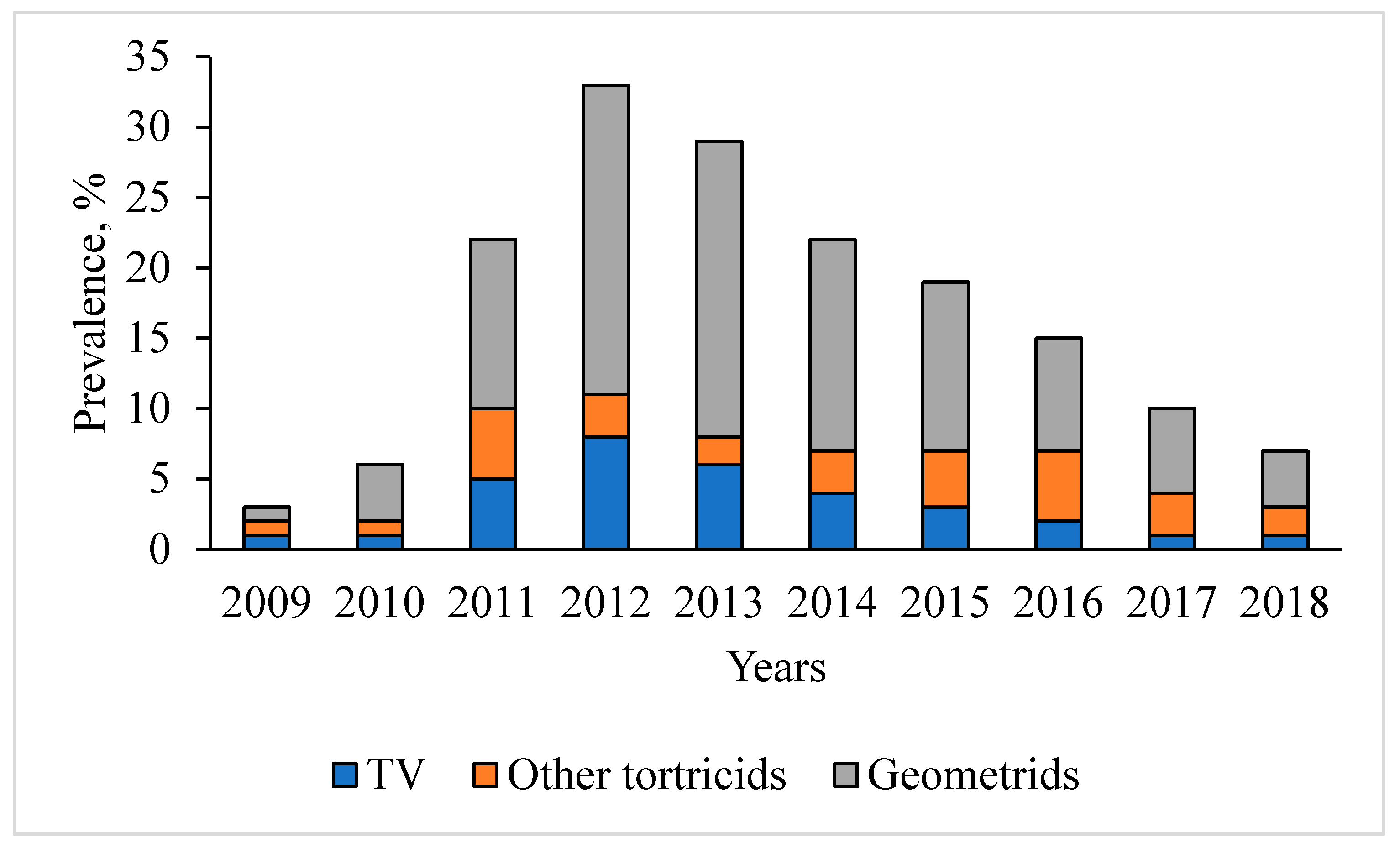
3.3. Tortrix Viridana as Part of the Flush Feeder Complex in Long-Term Dynamics
3.4. Change in the Suitability of Forest Subcompartments for Tortrix viridana
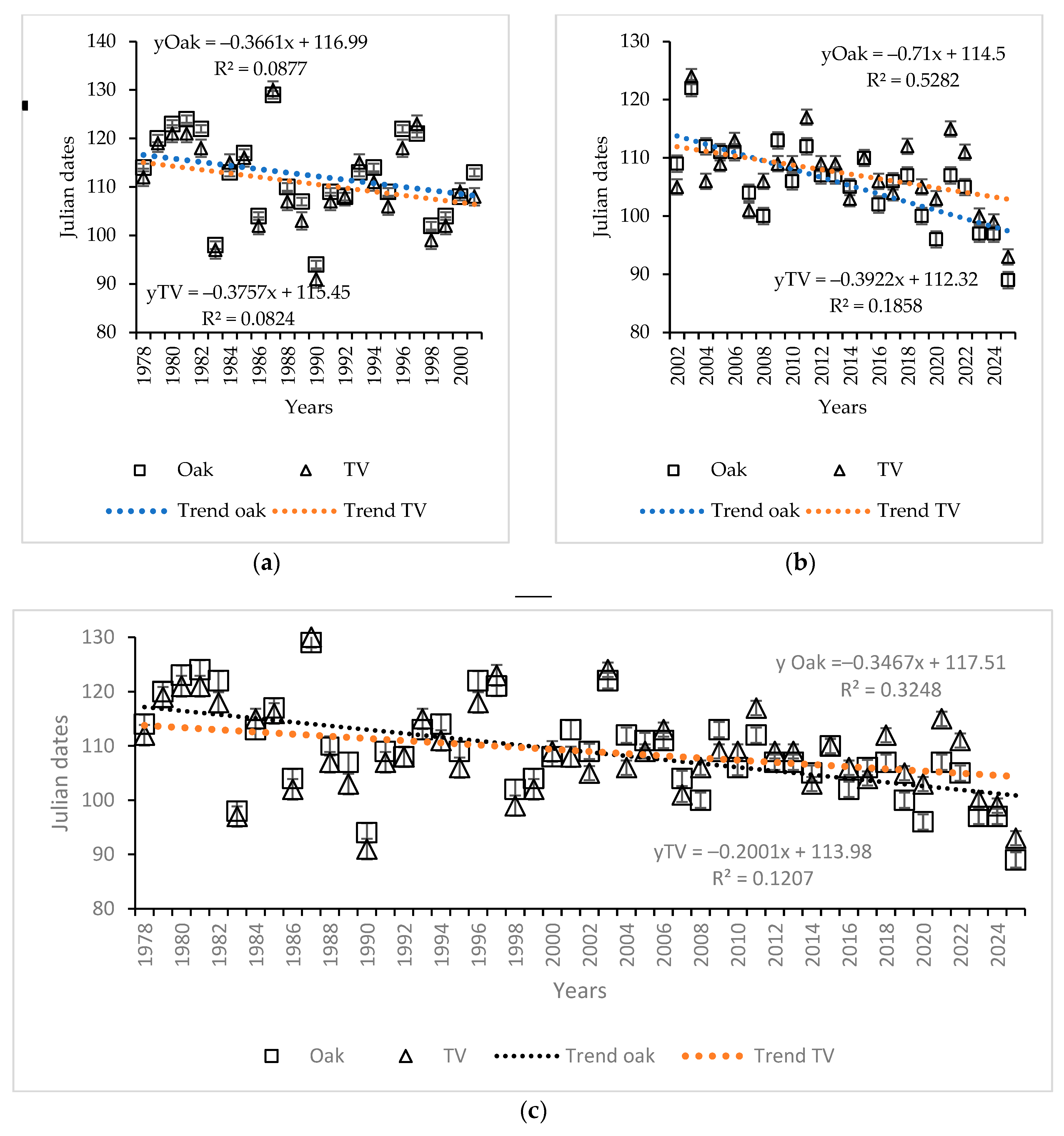
3.5. Phenological Mismatch in Oak Bud-Flushing and T. viridana Hatch Dates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gasov, H. Der grűne Eichenwickler (Tortrix viridana) als Forstschädling. Arb. Biol. Reichsamsalt 1925, 12, 355–508. [Google Scholar]
- Fankhänel, H. Über die Massenvermehrung des Grünen Eichenwicklers (Tortrix viridana L.) in der DDR in den Jahren 1957–1959 und über Maßnahmen zur Überwachung des Schädlings. Anz. Schädlingskunde 1962, 35, 158. [Google Scholar] [CrossRef]
- Schwenke, W. Die Forstschädlinge Europe; Bd.3; Paul Parey: Hamburg, Germany; Berlin, Germany, 1978; pp. 76–85. [Google Scholar]
- Du Merle, P.; Mazet, R. Dynamique intracyclique d’une population méditerranéenne de tordeuse verte du chêne, Tortrix viridana (Lepidoptera: Tortricidae). Ecol. Mediterr. 1990, 16, 73–91. [Google Scholar] [CrossRef]
- Bogenschütz, H. Eurasian species in forestry. In World Crop Pests. 5: Tortricid Pests; van der Geest, L.P.S., Evenhuis, H.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 673–709. [Google Scholar]
- Berryman, A.A. Forest Insects: Principles and Practice of Population Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Beletsky, E.N. Mass Propagation of Insects. History, Theory, Forecasting: Monograph; Maidan: Kharkov, Ukraine, 2011; 172p. (In Russian) [Google Scholar]
- Meshkova, V. History and Geography of Foliage Browsing Insect Pests Outbreaks; Maidan: Majdan, Serbia, 2002; 244p. (In Ukrainian) [Google Scholar]
- Meshkova, V. Seasonal Development of Foliage Browsing Insects; Planeta-Print: Kharkov, Ukraine, 2009; 382p. (In Russian) [Google Scholar]
- Rubtsov, V.V.; Rubtsova, N.N. Analysis of the Interaction of Leaf-Eating Insects with Oak; Nauka: Moscow, Russia, 1984; 183p. (In Russian) [Google Scholar]
- Turčáni, M.; Patočka, J.; Kulfan, M. How do lepidopteran seasonal guilds differ on some oaks (Quercus spp.)–A case study. J. For. Sci. 2009, 55, 578–590. [Google Scholar] [CrossRef]
- Turčáni, M.; Patočka, J.; Kulfan, M. Which factors explain lepidopteran larvae variance in seasonal guilds on some oaks? J. For. Sci. 2010, 56, 68–76. [Google Scholar] [CrossRef]
- Seifi, S.; Madadi, H.; Ghobari, H.; Bavaghar, M.P. Studying the effective factors of spatial distribution of Tortrix viridana L. in Mariwan oak forests. Iran. J. For. Range Prot. Res. 2023, 21, 337–349. [Google Scholar]
- Gholami, H.; Ghobari, H.; Badakhshan, H.; Rafie, J.N.; Salehi, H.; Namayandeh, A. Patterns of genetic variation among host-plant associated populations of the green oak leaf roller moth, Tortrix viridana (Lepidoptera: Tortricidae) in oak forests of northwestern Iran. J. Entomol. Soc. Iran 2022, 42, 67–80. [Google Scholar]
- Bertić, M.; Orgel, F.; Gschwendtner, S.; Schloter, M.; Moritz, F.; Schmitt-Kopplin, P.; Zimmer, I.; Fladung, M.; Schnitzler, J.P.; Schroeder, H.; et al. European oak metabolites shape digestion and fitness of the herbivore Tortrix viridana. Funct. Ecol. 2023, 37, 1476–1491. [Google Scholar] [CrossRef]
- Bertić, M.; Schroeder, H.; Kersten, B.; Fladung, M.; Orgel, F.; Buegger, F.; Schnitzler, J.P.; Ghirardo, A. European oak chemical diversity–from ecotypes to herbivore resistance. New Phytol. 2021, 232, 818–834. [Google Scholar] [CrossRef]
- Yousefi, S.; Haghighian, F.; Jahromi, M.N.; Pourghasemi, H.R. Pest-infected oak trees identify using remote sensing-based classification algorithms. In Computers in Earth and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 363–376. [Google Scholar]
- Du Merle, P. Phenological resistance of oaks to the green oak leafroller Tortrix viridana (Lepidoptera: Tortricidae). In Mechanism of Woody Plant Defenses Against Insects; Mattson, W.J., Levieux, J., Bernard-Dagan, C., Eds.; Springer: New York, NY, USA, 1988; pp. 215–226. [Google Scholar]
- Du Merle, P.; Mazet, R. Phenological stages and infestation by Tortrix viridana L. (Lep., Tortricidae) of the buds of two oaks (Quercus pubescens and Q. ilex). Acta Oecol. (Oecol. Appl.) 1983, 4, 47–53. [Google Scholar]
- Hunter, M.D. Differential susceptibility to variable plant phenology and its role in competition between two insect herbivores on oak. Ecol. Entomol. 1990, 15, 401–408. [Google Scholar] [CrossRef]
- Feeny, P.P. Plant apparency and chemical defence. In Recent Advances in Phytochemistry. Biochemical Interactions Between Plants and Insects; Wallace, J.W., Mansell, R.L., Eds.; Plenum Press: New York, NY, USA, 1978; Volume 10, pp. 1–40. [Google Scholar]
- Feeny, P.P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- Visser, M.E.; Holleman, L.J.M. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. Biol. Sci. 2001, 268, 89–94. [Google Scholar] [CrossRef] [PubMed]
- van Asch, M.; Van Tienderen, P.H.; Holleman, L.J.M.; Visser, M. Predicting adaptation of phenology in response to climate change, an insect herbivore example. Glob. Change Biol. 2007, 13, 1596–1604. [Google Scholar] [CrossRef]
- van Asch, M.; Visser, M.E. Phenology of forest caterpillars and their host trees: The importance of synchrony. Annu. Rev. Entomol. 2007, 52, 37–55. [Google Scholar] [CrossRef]
- van Dis, N.E.; Sieperda, G.-J.; Bansal, V.; van Lith, B.; Wertheim, B.; Visser, M.E. Phenological mismatch affects individual fitness and population growth in the winter moth. Proc. R. Soc. B 2023, 290, 414. [Google Scholar] [CrossRef]
- Weir, J.C. Trophic generalism in the winter moth: A model species for phenological mismatch. Oecologia 2024, 206, 225–239. [Google Scholar] [CrossRef]
- Weir, J.C.; Phillimore, A.B. Buffering and phenological mismatch: A change of perspective. Glob. Change Biol. 2024, 30, e17294. [Google Scholar] [CrossRef]
- Patocka, J.; Kristin, A.; Kulfan, J.; Zach, P. Die Eichenschadlinge und ihre Feinde. Zvolen, Institute fur Waldokologie der Slowakishen Akademie der Wissenschaften. 1999. 396p. ISBN 8096723839. Available online: https://agris.fao.org/search/en/providers/122436/records/675ac75a0ce2cede71d146f2 (accessed on 9 June 2025).
- Ivashov, A.V.; Boyko, G.E.; Simchuk, A.P. The role of host plant phenology in the development of the oak leafroller moth, Tortrix viridana L. (Lepidoptera: Tortricidae). For. Ecol. Manag. 2002, 157, 7–14. [Google Scholar] [CrossRef]
- Tkach, V.P.; Tarnopilska, O.M.; Orlov, O.O. Types of Forest Formations of Ukraine in the System of European Classifications; Madryd: Kharkiv, Ukraine, 2024; 415p, ISBN 978-617-8254-23-0. [Google Scholar] [CrossRef]
- Wesołowski, T.; Rowiński, P. Late leaf development in pedunculate oak (Quercus robur): An antiherbivore defence? Scand. J. For. Res. 2008, 23, 386–394. [Google Scholar] [CrossRef]
- Wesołowski, T.; Rowiński, P. Timing of bud burst and tree-leaf development in a multispecies temperate forest. For. Ecol. Manag. 2006, 237, 387–393. [Google Scholar] [CrossRef]
- Boulanger, Y.; Desaint, A.; Martel, V.; Marchand, M.; Massoda Tonye, S.; Saint-Amant, R.; Régnière, J. Recent climate change strongly impacted the population dynamic of a North American insect pest species. PLoS Clim. 2025, 4, e0000488. [Google Scholar] [CrossRef]
- Belitz, M.W.; Larsen, E.A.; Hurlbert, A.H.; Di Cecco, G.J.; Neupane, N.; Ries, L.; Tingley, M.W.; Guralnick, R.P.; Youngflesh, C. Potential for bird–insect phenological mismatch in a tri-trophic system. J. Anim. Ecol. 2025, 94, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Damien, M.; Tougeron, K. Prey-predator phenological mismatch under climate change. Curr. Opin. Insect Sci. 2019, 35, 60–68. [Google Scholar] [CrossRef]
- Renner, S.S.; Zohner, C.M. Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 165–182. [Google Scholar] [CrossRef]
- Moldavan, L.; Pimenowa, O.; Wasilewski, M.; Wasilewska, N. Sustainable Development of Agriculture of Ukraine in the Context of Climate Change. Sustainability 2023, 15, 10517. [Google Scholar] [CrossRef]
- Buras, A.; Menzel, A. Projecting Tree Species Composition Changes of European Forests for 2061–2090 Under RCP 4.5 and RCP 8.5 Scenarios. Front. Plant Sci. 2019, 9, 1986. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Levkovska, L. Modern Trends in the Development of the Risky Farming Zone in the Context of Climate Change. Effective Economy. Dnipro State Agrarian and Economic University, LLC “DCS Centre”. 2021, Volume 9. Available online: http://www.economy.nayka.com.ua/pdf/9_2021/14.pdf (accessed on 10 May 2025).
- Buksha, I.F.; Pyvovar, T.S.; Buksha, M.I.; Pasternak, V.P.; Buksha, T.I. Modelling and forecasting the impact of climate change on forests of Ukraine for 21st century time horizon. For. Ideas 2021, 27, 470–482. [Google Scholar]
- Didukh, Y.P. World of Plants of Ukraine in Aspect of the Climate Change; Naukova Dumka: Kyiv, Ukraine, 2023; 176p, ISBN 978-966-00-1868-6. (In Ukrainian) [Google Scholar] [CrossRef]
- General Characteristics of Ukrainian Forests. 2016. Available online: https://forest.gov.ua/napryamki-diyalnosti/lisi-ukrayini/zagalna-harakteristika-lisiv-ukrayini (accessed on 2 March 2025). (In Ukrainian)
- Didukh, Y.P.; Vynokurov, D.S. Spatial-temporal changes of bioclimate factors in Europe. Hydrol. Hydrochem. Hydroecology 2021, 1, 64–75. [Google Scholar] [CrossRef]
- Zepner, L.; Karrasch, P.; Wiemann, F.; Bernard, L. ClimateCharts. Net—An interactive climate analysis web platform. Int. J. Digit. Earth 2021, 14, 338–356. [Google Scholar] [CrossRef]
- Rumiantsev, M.; Luk’yanets, V.; Musienko, S.; Mostepanyuk, A.; Obolonyk, I. Main problems in natural seed regeneration of pedunculate oak (Quercus robur L.) stands in Ukraine. For. Stud.|Metsanduslikud Uurim. 2018, 69, 7–23. [Google Scholar] [CrossRef]
- Hayda, Y.; Mohytych, V.; Bidolakh, D.; Kuzovych, V.; Sulkowska, M. The introduction of red oak (Quercus rubra L.) in Ukrainian forests: Advantages of productivity versus disadvantages of invasiveness. Folia For. Polonica. Ser. A For. 2022, 64, 245–252. [Google Scholar] [CrossRef]
- Ukrainian State Forest Management Planning Association. Available online: https://lisproekt.gov.ua/ (accessed on 2 March 2025).
- QGIS 3.40.5. Available online: https://qgis.org/en/site/ (accessed on 2 March 2025).
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Peck, R.; Short, T.; Olsen, C. Introduction to Statistics and Data Analysis; Cengage Learning: Boston, MA, USA, 2020; Available online: http://www.statisticslectures.com/topics/ztestproportions/ (accessed on 2 March 2025).
- Boychenko, S.; Kuchma, T.; Karamushka, V.; Maidanovych, N.; Kozak, O. Wildfires and Climate Change in the Ukrainian Polissia During 2001–2023. Sustainability 2025, 17, 2223. [Google Scholar] [CrossRef]
- Gosling, R.H.; Jackson, R.W.; Elliot, M.; Nichols, C.P. Oak declines: Reviewing the evidence for causes, management implications and research gaps. Ecol. Solut. Evid. 2024, 5, e12395. [Google Scholar] [CrossRef]
- Langer, G.J.; Bußkamp, J.; Burkardt, K.; Hurling, R.; Plašil, P.; Rohde, M. Review on temperate oak decline and oak diseases with a focus on Germany. J. Kult. 2025, 77, 36–49. [Google Scholar]
- Liu, M.; Jiang, P.; Chase, J.M.; Liu, X. Global insect herbivory and its response to climate change. Curr. Biol. 2024, 34, 2558–2569. [Google Scholar] [CrossRef]
- Logan, J.; Régnière, J.; Powell, J. Assessing the impacts of global warming on forest pest dynamics. Front. Ecol. Environ. 2003, 1, 130–137. [Google Scholar] [CrossRef]
- Jepsen, J.; Hagen, S.; Ims, R.; Yoccoz, N. Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: Evidence of a recent outbreak range expansion. J. Anim. Ecol. 2008, 77, 257–264. [Google Scholar] [CrossRef]
- Robinet, C.; Roques, A. Direct impacts of recent climate warming on insect populations. Int. Zool. 2010, 5, 132–142. [Google Scholar] [CrossRef]
- Kardash, Y.S. Changes in the complex of phyllophagous insects in deciduous trees of Kharkiv city for 50 years. Balt. Coast. Zone 2021, 24, 27–39. [Google Scholar]
- Meshkova, V. Foliage-browsing Lepidoptera (Insecta) in deciduous forests of Ukraine for the last 70 years. Proc. For. Acad. Sci. Ukr. 2021, 22, 173–179. [Google Scholar] [CrossRef]
- Pureswaran, D.; Roques, A.; Battisti, A. Forest Insects and Climate Change. Curr. For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef]
- Ekholm, A.; Tack, A.; Pulkkinen, P.; Roslin, T. Host plant phenology, insect outbreaks and herbivore communities—The importance of timing. J. Anim. Ecol. 2020, 89, 829–841. [Google Scholar] [CrossRef]
- Stireman, J.O., III; Dyer, L.A.; Janzen, D.H.; Singer, M.S.; Lill, J.T.; Marquis, R.J.; Ricklefs, R.E.; Gentry, G.L.; Hallwachs, W.; Coley, P.D.; et al. Climatic unpredictability and parasitism of caterpillars: Implications of global warming. Proc. Natl. Acad. Sci. USA 2005, 102, 17384–17387. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, H. Climate change-associated tree mortality increases without decreasing water availability. Ecol. Lett. 2015, 18, 1207–1215. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, C.; Schneider, R.; Cyr, D.; McDowell, N.G.; Kneeshaw, D. Drought-induced increase in tree mortality and corresponding decrease in the carbon sink capacity of Canada’s boreal forests from 1970 to 2020. Glob. Change Biol. 2023, 29, 2274–2285. [Google Scholar] [CrossRef] [PubMed]
- Liebhold, A.M. Forest pest management in a changing world. Int. J. Pest Manag. 2012, 58, 289–295. [Google Scholar] [CrossRef]
- Gamayunova, S.G. Bioecological features of parasitoids of green oak leaf roller. For. For. Melior. 1988, 76, 51–53. [Google Scholar]
- Betz, E.; Schwerdtfeger, F. Vergleichende Untersuchungen an der Kronenfauna der Eichen in Latenz-und Gradationsgebieten des Eichenwicklers (Tortrix viridana L.) 2. Die Parasiten der Lepidopteren. Z. Angew. Entomol. 1971, 67, 149–170. [Google Scholar] [CrossRef]
- Du Merle, P. Les facteurs de mortalité des oeufs de Tortrix viridana L. (Lep., Tortricidae). II. Parasitisme par un Trichogramma (Hym., Trichogrammatidae) et “maladies”. Agronomie 1983, 3, 359–367. [Google Scholar] [CrossRef]
- Andreieva, O.; Martynchuk, I.; Zhytova, O.; Vyshnevskyi, A.; Zymaroieva, A. Features of forecasting of foliage-browsing insects distribution in the forests of Zhytomyr Polissia. Sci. Horiz. 2021, 24, 68–76. [Google Scholar]
- Borysenko, O.I.; Meshkova, V.L. Prediction of Fires and Insect Pests Foci Spread in the Pine Stands by Means of GIS; Planeta-Print: Kharkiv, Ukraine, 2021; 148p, ISBN 978-617-7897-67-4. [Google Scholar]
- Crimmins, T.M.; Gerst, K.L.; Huerta, D.G.; Marsh, R.L.; Posthumus, E.E.; Rosemartin, A.H.; Switzer, J.; Weltzin, J.F.; Coop, L.; Dietschler, N.; et al. Short-term forecasts of insect phenology inform pest management. Ann. Entomol. Soc. Am. 2020, 113, 139–148. [Google Scholar] [CrossRef]
- Jones, B.C.; Despland, E. Effects of synchronization with host plant phenology occur early in the larval development of a spring folivore. Can. J. Zool. 2006, 84, 628–633. [Google Scholar] [CrossRef]
- Sarvašová, L.; Zach, P.; Parák, M.; Saniga, M.; Kulfan, J. Infestation of Early- and Late-Flushing Trees by Spring Caterpillars: An Associational Effect of Neighbouring Trees. Forests 2021, 12, 1281. [Google Scholar] [CrossRef]
- Malyshev, A.V.; Blume-Werry, G.; Spiller, O.; Smiljanić, M.; Weigel, R.; Kolb, A.; Nze, B.Y.; Märker, F.; Sommer, F.C.F.J.; Kinley, K.; et al. Warming nondormant tree roots advances aboveground spring phenology in temperate trees. New Phytol. 2023, 240, 2276–2287. [Google Scholar] [CrossRef]
- Silvestro, R.; Deslauriers, A.; Prislan, P.; Rademacher, T.; Rezaie, N.; Richardson, A.D.; Vitasse, Y.; Rossi, S. From Roots to Leaves: Tree Growth Phenology in Forest Ecosystems. Curr. For. Rep. 2025, 11, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Fu, Z.; Zhang, D.; Liu, L. Soil temperature dominates forest spring phenology in China. Agric. For. Meteorol. 2024, 355, 110141. [Google Scholar] [CrossRef]
- Raimondo, S.; Turcáni, M.; Patoèka, J.; Liebhold, A.M. Interspecific synchrony among foliage-feeding forest Lepidoptera species and the potential role of generalist predators as synchronizing agents. Oikos 2004, 107, 462–470. [Google Scholar] [CrossRef]
- Chaplygina, A.B. Dendrophilic Passerines (Passeriformes) as a Structural-Functional Element of Anthropogenically Transformed Forest Biogeocoenoses of North-Eastern Ukraine. Doctoral Thesis, Oles Honchar Dnipro National University, Dnipro, Ukraine, 2018. [Google Scholar]
- Biquet, J.; Bonamour, S.; De Villemereuil, P.; De Franceschi, C.; Teplitsky, C. Phenotypic plasticity drives phenological changes in a Mediterranean blue tit population. J. Evol. Biol. 2022, 35, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Hinks, A.E.; Cole, E.F.; Daniels, K.J.; Wilkin, T.A.; Nakagawa, S.; Sheldon, B.C. Scale-dependent phenological synchrony between songbirds and their caterpillar food source. Am. Nat. 2015, 186, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.E.; Holleman, L.J.M.; Gienapp, P. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 2006, 147, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.E.; Lindner, M.; Gienapp, P.; Long, M.C.; Jenouvrier, S. Recent natural variability in global warming weakened phenological mismatch and selection on seasonal timing in great tits (Parus major). Proc. R. Soc. B 2021, 288, 20211337. [Google Scholar] [CrossRef] [PubMed]
- Wesołowski, T. Weather, food and predation shape the timing of Marsh Tit breeding in primaeval conditions: A long-term study. J. Ornithol. 2023, 164, 253–274. [Google Scholar] [CrossRef]
- Puzrina, N.; Psenichna, N.; Boiko, H.; Sendonin, S. Dominant pests and pathogens of urban plantings in Kyiv: Species composition and prevalence. Sci. J. Ukr. J. For. Wood Sci. 2023, 14, 64–77. [Google Scholar] [CrossRef]
- Paulin, M.; Hirka, A.; Eötvös, C.B.; Gáspár, C.; Fürjes-Mikó, Á.; Csóka, G. Known and predicted impacts of the invasive oak lace bug (Corythucha arcuata) in European oak ecosystems—A review. Folia Oecologica 2020, 47, 131–139. [Google Scholar] [CrossRef]



| Indicators | Kharkiv RS | Vovchansk FE | Chuguyevo-Babchansk FE | Zhovtneve FE |
|---|---|---|---|---|
| Total oak stands area, ha | 17,791.5 | 17,708.2 | 13,170.1 | 33,896.8 |
| Oak stands in D1, D2, ha | 17,602.2 | 16,931.3 | 12,657.5 | 32,981.3 |
| Oak stands of vegetative origin, ha | 14,141.8 | 14,498.5 | 9414.0 | 23,638.8 |
| Oak stands of 41–80 years, ha | 10,286.6 | 6327.2 | 3666.8 | 15,812.5 |
| Oak proportion ≥ 90%, ha | 9200.0 | 9116.6 | 6201.1 | 15,246.9 |
| Oak stands with relative stocking density < 65%, ha | 4802.9 | 2339.8 | 2071.7 | 9939.4 |
| Stands with the highest scores for all indicators, ha | 1121.6 | 167.7 | 9.0 | 533.5 |
| Indicators | Kharkiv RS | Vovchansk FE | Chuguyevo-Babchansk FE | Zhovtneve FE |
|---|---|---|---|---|
| Total oak stands area, ha | 17,746.6 | 17,306.9 | 13,310.1 | 33,965.9 |
| Oak stands in D1, D2, ha | 17,559.9 | 16,555.3 | 12,780.3 | 33,062.0 |
| Oak stands of vegetative origin, ha | 13,811.9 | 13,966.8 | 9845.3 | 23,085.3 |
| Oak stands of 41–80 years, ha | 5451.1 | 2787.3 | 2479.3 | 11,567.5 |
| Oak proportion ≥ 90%, ha | 7560.6 | 5696.2 | 4830.3 | 15,168.4 |
| Oak stands with relative stocking density < 65%, ha | 3350.8 | 2414.4 | 2734.3 | 8100.4 |
| Stands with the highest scores for all indicators, ha | 265.7 | 25.4 | 7.7 | 230.8 |
| Indicators | Kharkiv RS | Vovchansk FE | Chuguyevo-Babchansk FE | Zhovtneve FE |
|---|---|---|---|---|
| Total oak stands area, ha | −0.3 | −2.3 | 1.1 | 0.2 |
| Oak stands in D1, D2, ha | −0.2 | −2.2 | 1.0 | 0.2 |
| Oak stands of vegetative origin, ha | −2.3 | −3.7 | 4.6 | −2.3 |
| Oak stands of 41–80 years, ha | −47.0 | −55.9 | −32.4 | −26.8 |
| Oak proportion ≥ 90%, ha | −17.8 | −37.5 | −22.1 | −0.5 |
| Oak stands with relative stocking density < 65%, ha | −30.2 | 3.2 | 32.0 | −18.5 |
| Stands with the highest scores for all indicators, ha | −76.3 | −84.9 | −14.4 | −56.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshkova, V.; Stankevych, S.; Koshelyaeva, Y.; Korsovetskyi, V.; Borysenko, O. Climate-Induced Shift in the Population Dynamics of Tortrix viridana L. in Ukraine. Forests 2025, 16, 1005. https://doi.org/10.3390/f16061005
Meshkova V, Stankevych S, Koshelyaeva Y, Korsovetskyi V, Borysenko O. Climate-Induced Shift in the Population Dynamics of Tortrix viridana L. in Ukraine. Forests. 2025; 16(6):1005. https://doi.org/10.3390/f16061005
Chicago/Turabian StyleMeshkova, Valentyna, Serhij Stankevych, Yana Koshelyaeva, Volodymyr Korsovetskyi, and Oleksandr Borysenko. 2025. "Climate-Induced Shift in the Population Dynamics of Tortrix viridana L. in Ukraine" Forests 16, no. 6: 1005. https://doi.org/10.3390/f16061005
APA StyleMeshkova, V., Stankevych, S., Koshelyaeva, Y., Korsovetskyi, V., & Borysenko, O. (2025). Climate-Induced Shift in the Population Dynamics of Tortrix viridana L. in Ukraine. Forests, 16(6), 1005. https://doi.org/10.3390/f16061005







