Post-Fire Recovery of Soil Multiple Properties, Plant Diversity, and Community Structure of Boreal Forests in China
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling Sites, Experimental Design, and Soil Sampling
2.2. Soil Nutrients and GRSP Determination
2.3. Calculation of Plant Diversity and Community Traits
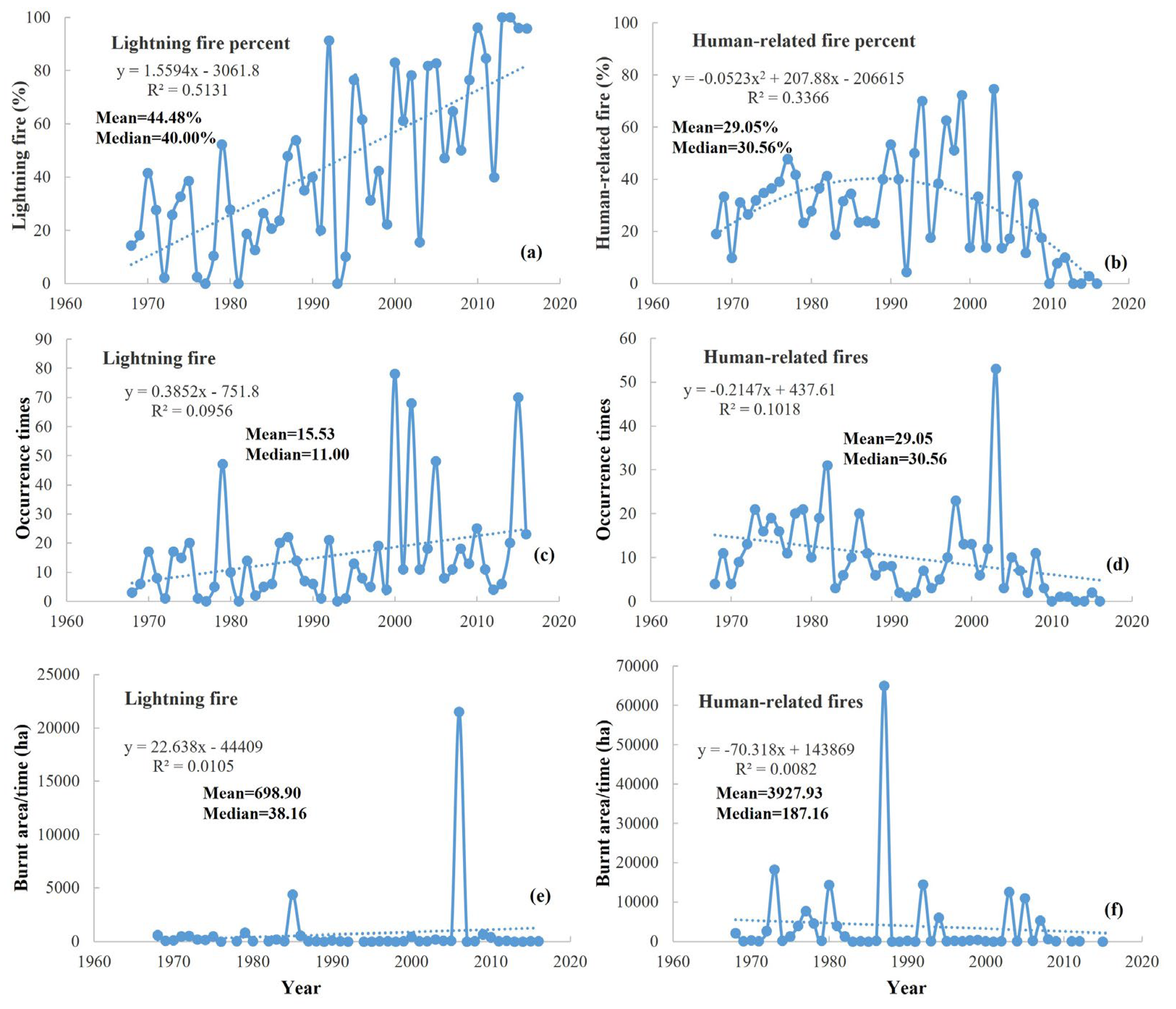
2.4. Fire Characteristics, Temporal Changes, and Comparison with Other Countries
2.5. Statistical Analysis
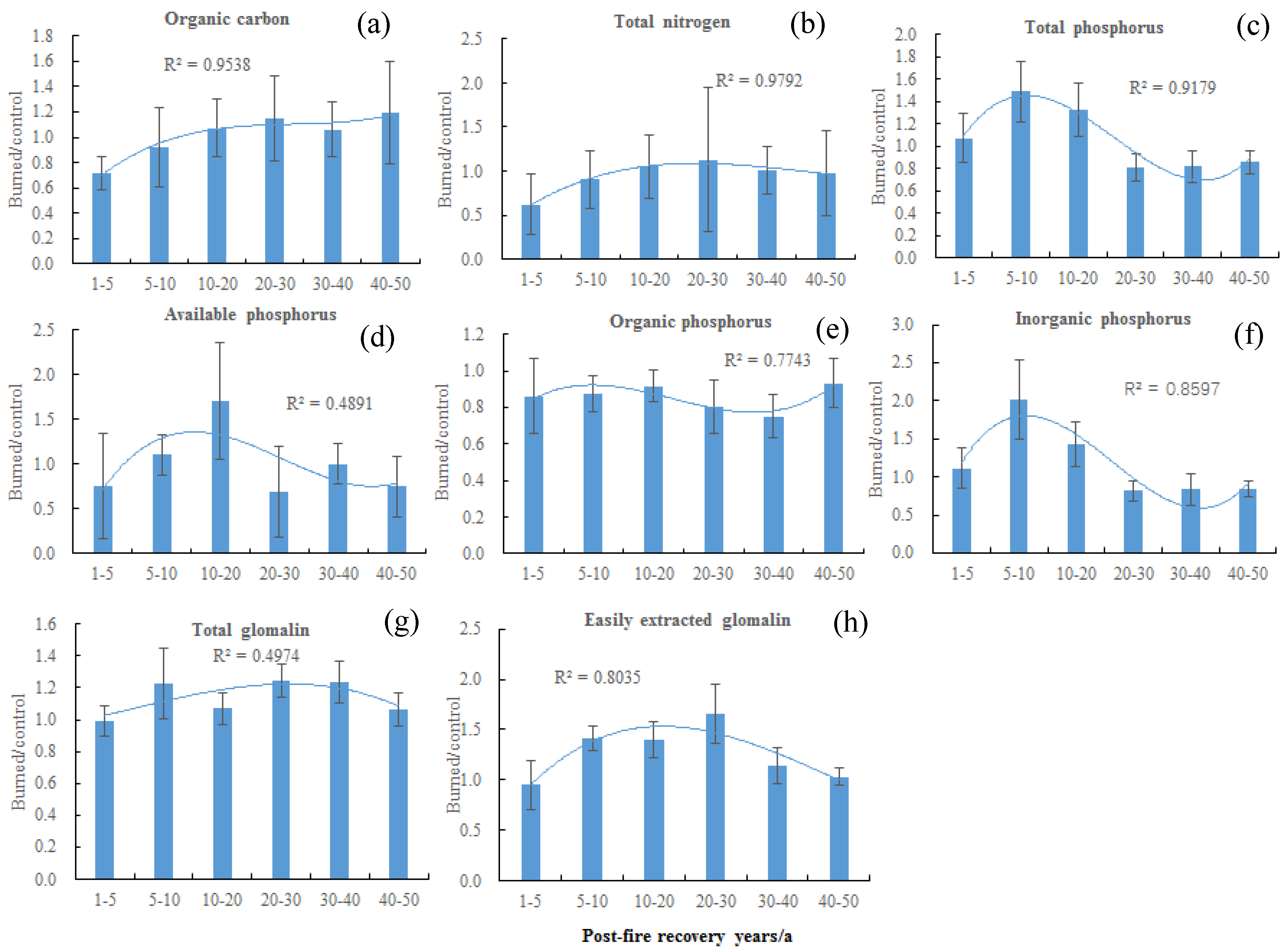
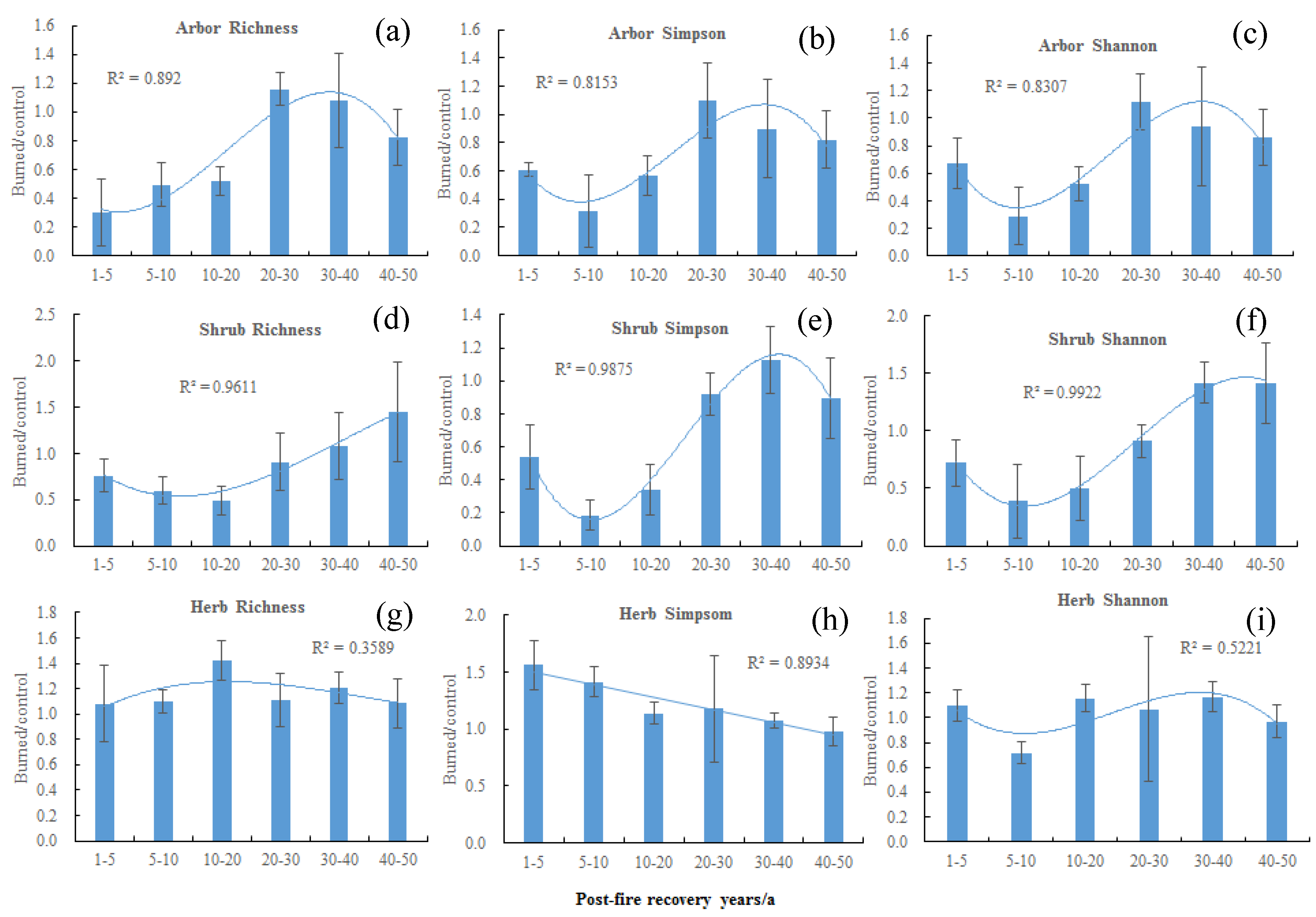
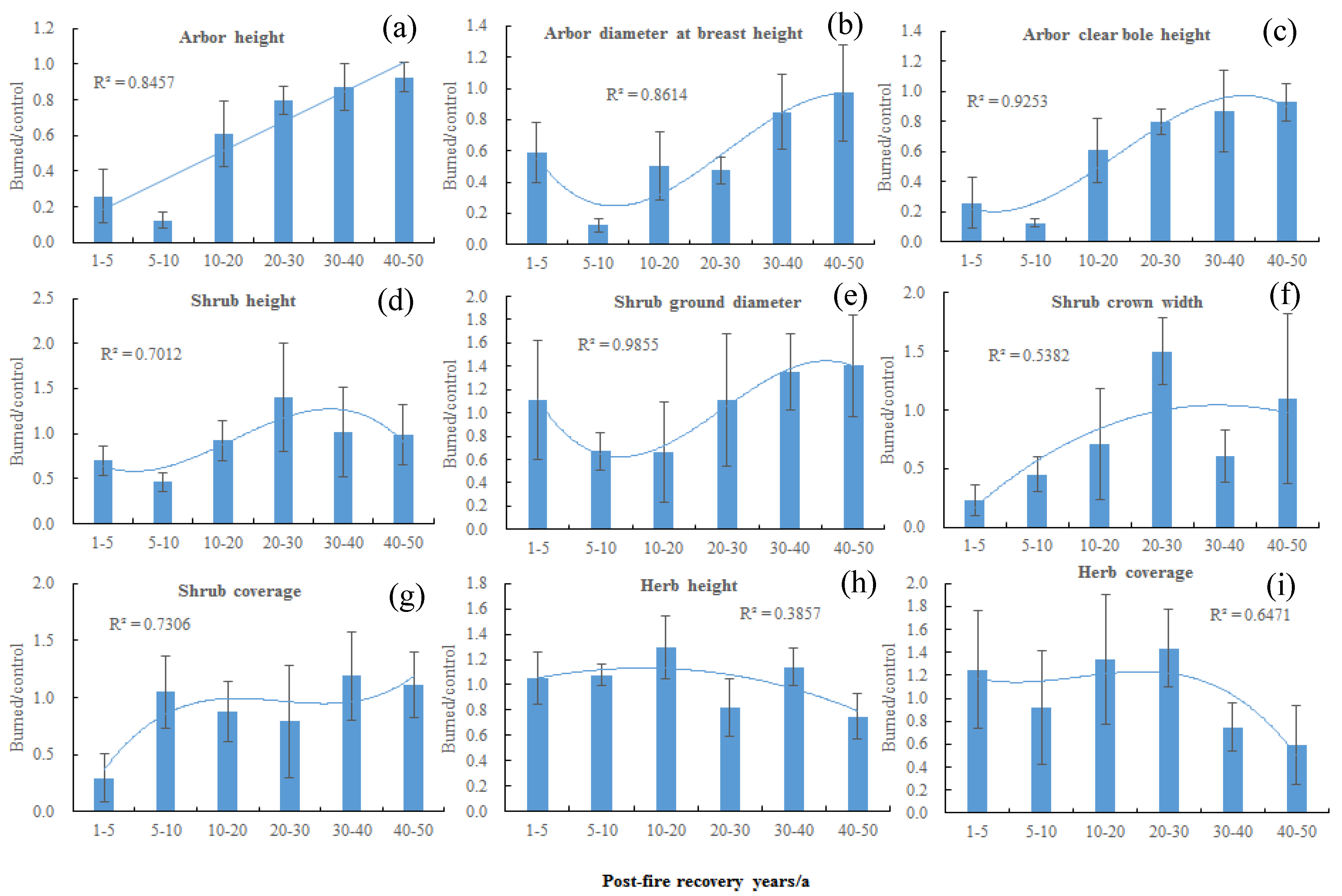
3. Results
3.1. Paired T-Test in Burnt and Control Sites
3.2. Effects of Post-Fire Recovery Years on Soil Nutrients, Plant Diversity, and Community Structure
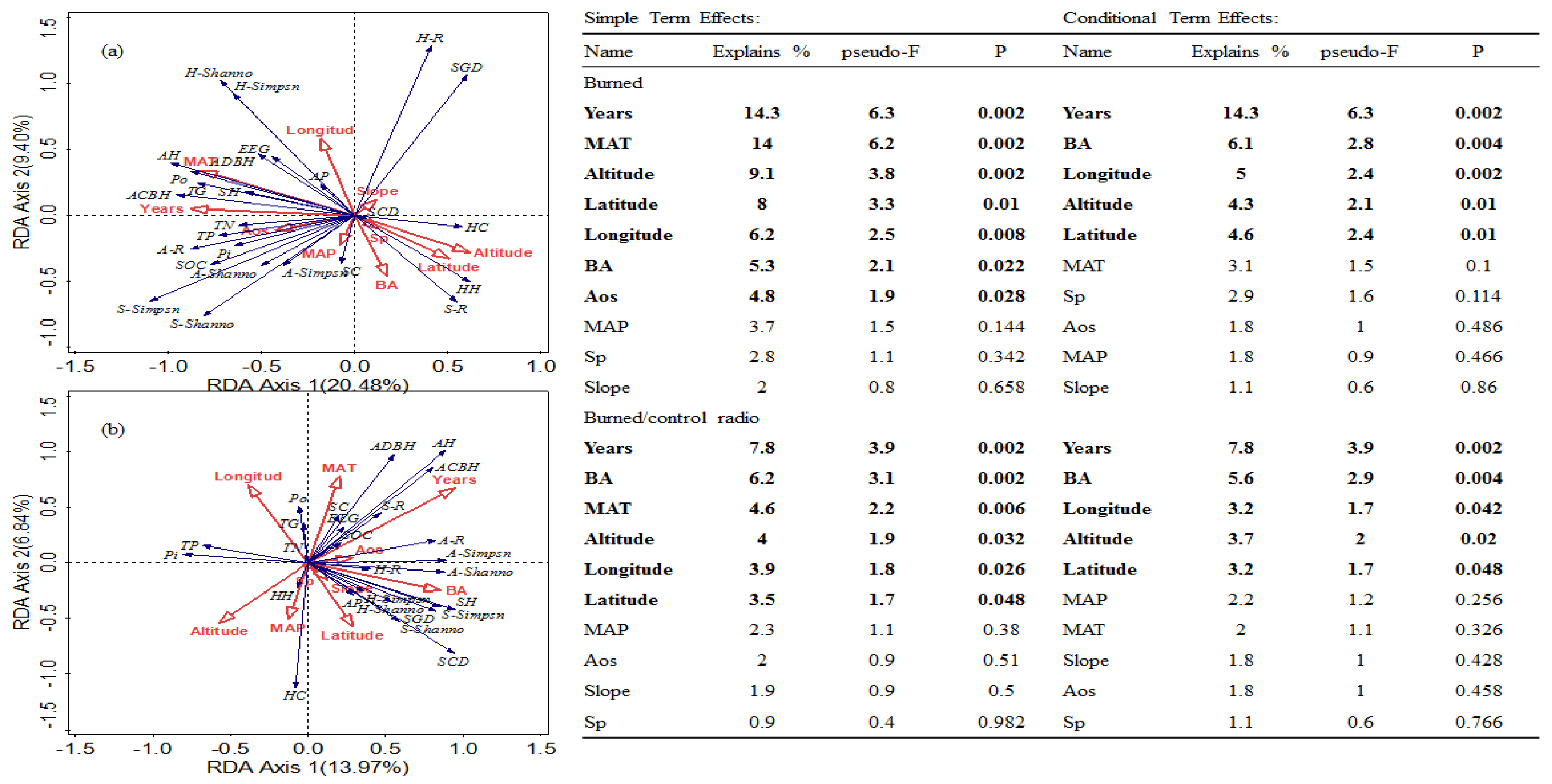
3.3. Redundancy Analysis for Identifying Significant Factors Responsible for the Variations
3.4. PLS-PM Analysis for Finding the Driving Paths for the Variations
3.5. Fire Changes in the Studied Region and China Forest Fires Compared with Other Countries
4. Discussion
4.1. Comparison with Previous Publications
4.2. Recovery Time by Effect Size Analysis: Scientific Evaluation and Stand Management
4.3. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Duran, J.; Fernandez-Palacios, J.M.; Gallardo, A. Short-term wildfire effects on the spatial pattern and scale of labile organic-N and inorganic-N and P pools. For. Ecol. Manag. 2009, 257, 739–746. [Google Scholar] [CrossRef]
- Sun, L.; Dou, X.; Hu, T. Research progress on the effects of forest fire on forest ecosystem C-N-P ecological stoichiometry characteristics. J. Nanjing For. University. Nat. Sci. Ed. 2021, 45, 1–9. [Google Scholar]
- Ling, L.; Fu, Y.Y.; Jeewani, P.H.; Tang, C.X.; Pan, S.T.; Reid, B.J.; Gunina, A.; Li, Y.F.; Li, Y.C.; Cai, Y.J.; et al. Organic matter chemistry and bacterial community structure regulate decomposition processes in post-fire forest soils. Soil Biol. Biochem. 2021, 160, 108311. [Google Scholar] [CrossRef]
- Liu, X.J.; Pan, C.D. Effects of recovery time after fire and fire severity on stand structure and soil of larch forest in the Kanas National Nature Reserve, Northwest China. J. Arid Land 2019, 11, 811–823. [Google Scholar] [CrossRef]
- Running, S.W. Is global warming causing more, larger wildfires? Science 2006, 313, 927–928. [Google Scholar] [CrossRef]
- Dieleman, C.M.; Rogers, B.M.; Potter, S.; Veraverbeke, S.; Johnstone, J.F.; Laflamme, J.; Solvik, K.; Walker, X.J.; Mack, M.C.; Turetsky, M.R. Wildfire combustion and carbon stocks in the southern Canadian boreal forest: Implications for a warming world. Glob. Change Biol. 2020, 26, 6062–6079. [Google Scholar] [CrossRef]
- Rumpel, C. Soils linked to climate change. Nature 2019, 572, 442–443. [Google Scholar] [CrossRef]
- Hu, T.X.; Zhao, B.Q.; Li, F.; Dou, X.; Hu, H.Q.; Sun, L. Effects of fire on soil respiration and its components in a Dahurian larch (Larix gmelinii) forest in northeast China: Implications for forest ecosystem carbon cycling. Geoderma 2021, 402, 115273. [Google Scholar] [CrossRef]
- Huang, C.; He, H.S.; Liang, Y.; Wu, Z.W.; Hawbaker, T.J.; Gong, P.; Zhu, Z.L. Long-term effects of fire and harvest on carbon stocks of boreal forests in northeastern China. Ann. For. Sci. 2018, 75, 42. [Google Scholar] [CrossRef]
- Perez-Valera, E.; Verdu, M.; Navarro-Cano, J.A.; Goberna, M. Soil microbiome drives the recovery of ecosystem functions after fire. Soil Biol. Biochem. 2020, 149, 107948. [Google Scholar] [CrossRef]
- Dai, Z.M.; Lv, X.F.; Ma, B.; Chen, N.; Chang, S.X.; Lin, J.H.; Wang, X.H.; Su, W.Q.; Liu, H.T.; Huang, Y.L.; et al. Concurrent and rapid recovery of bacteria and protist communities in Canadian boreal forest ecosystems following wildfire. Soil Biol. Biochem. 2021, 163, 108452. [Google Scholar] [CrossRef]
- Liu, B.; Biswas, S.R.; Yang, J.; Lite, Z.H.; He, H.S.; Liang, Y.; Lau, M.K.; Fang, Y.T.; Han, S.J. Strong influences of stand age and topography on post-fire understory recovery in a Chinese boreal forest. For. Ecol. Manag. 2020, 473, 118307. [Google Scholar] [CrossRef]
- Yang, G.; Di, X.-Y.; Zeng, T.; Shu, Z.; Wang, C.; Yu, H.-Z. Prediction of area burnt under climatic change scenarios: A case study in the Great Xing’an Mountains boreal forest. J. For. Res. 2010, 21, 213–218. [Google Scholar] [CrossRef]
- Hu, H.; Wei, S.; Sun, L. Estimation of carbon emissions due to forest fire in Daxingan Mountains from 1965 to 2010. Acta Phytoecol. Sin. 2012, 36, 629–644. [Google Scholar]
- Jayen, K.; Leduc, A.; Bergeron, Y. Effect of fire severity on regeneration success in the boreal forest of northwest Quebec, Canada. Ecoscience 2006, 13, 143–151. [Google Scholar] [CrossRef]
- Kelly, J.; Ibanez, T.S.; Santin, C.; Doerr, S.H.; Nilsson, M.C.; Holst, T.; Lindroth, A.; Kljun, N. Boreal forest soil carbon fluxes one year after a wildfire: Effects of burn severity and management. Glob. Change Biol. 2021, 27, 4181–4195. [Google Scholar] [CrossRef]
- Feng, X.; Merow, C.; Liu, Z.H.; Park, D.S.; Roehrdanz, P.R.; Maitner, B.; Newman, E.A.; Boyle, B.L.; Lien, A.; Burger, J.R.; et al. How deregulation, drought and increasing fire impact Amazonian biodiversity. Nature 2021, 597, 516–521. [Google Scholar] [CrossRef]
- Han, J.; Ying, L.; Li, G.; Shen, Z. Spatial patterns of species diversity in the herb layer of early post-fire regeneration in mixed Pinus yunnanensis forests. Chin. J. Plant Ecol. 2016, 40, 200–211. [Google Scholar]
- Zhao, H.M.; Tong, D.Q.; Lin, Q.X.; Lu, X.G.; Wang, G.P. Effect of fires on soil organic carbon pool and mineralization in a Northeastern China wetland. Geoderma 2012, 189, 532–539. [Google Scholar] [CrossRef]
- Weng, Y.T.; Yang, G.; Wang, L.X.; Quan, X.K.; Di, X.Y.; Yu, H.Z.; Han, D.D. Changes in water-soluble nitrogen and organic carbon in the post-fire litter layer of Dahurian larch forests. Plant Soil 2021, 464, 131–148. [Google Scholar] [CrossRef]
- Dove, N.C.; Taş, N.; Hart, S.C. Ecological and genomic responses of soil microbiomes to high-severity wildfire: Linking community assembly to functional potential. ISME J. 2022, 16, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Ma, B.; Tang, C.X.; Yu, H.D.; Lv, X.F.; Rodrigues, J.L.M.; Dahlgren, R.A.; Xu, J.M. Habitat heterogeneity induced by pyrogenic organic matter in wildfire-perturbed soils mediates bacterial community assembly processes. ISME J. 2021, 15, 1943–1955. [Google Scholar] [CrossRef]
- Gao, C.Y.; He, J.B.; Zhang, Y.; Cong, J.X.; Han, D.X.; Wang, G.P. Fire history and climate characteristics during the last millennium of the Great Hinggan Mountains at the monsoon margin in northeastern China. Glob. Planet. Change 2018, 162, 313–320. [Google Scholar] [CrossRef]
- Song, Z.P.; Tian, H.X.; Li, Z.L.; Luo, Y.Q.; Liu, Y.H. Changes in plant nutrient utilization during ecosystem recovery after wildfire. J. Environ. Manag. 2021, 295, 112994. [Google Scholar] [CrossRef]
- Kong, F.; Li, X.; Wang, X.; Shi, B.; Gao, Z. Advance in study on the forest restoration in the burnt blank. Chin. J. Ecol. 2003, 22, 60–64. [Google Scholar]
- Chen, J.; Di, X.-Y. Forest fire prevention management legal regime between China and the United States. J. For. Res. 2015, 26, 447–455. [Google Scholar] [CrossRef]
- Xiong, Q.; Luo, X.; Liang, P.; Xiao, Y.; Xiao, Q.; Sun, H.; Pan, K.; Wang, L.; Li, L.; Pang, X. Fire from policy, human interventions, or biophysical factors? Temporal–spatial patterns of forest fire in southwestern China. For. Ecol. Manag. 2020, 474, 118381. [Google Scholar] [CrossRef]
- Li, W.-k.; Yin, J.-y.; Guo, Z.-q.; Guo, Y.-j.; Hu, T.-x. Analysis of forest fires in representative countries and regions in the world in 2019. Fire Sci. Technol. 2020, 39, 1280–1284. [Google Scholar]
- Wang, Y.Y.; Wen, H.; Wang, K.; Sun, J.X.; Yu, J.H.; Wang, Q.G.; Wang, W.J. Forest plant and macrofungal differences in the Greater and Lesser Khingan Mountains in Northeast China: A regional-historical comparison and its implications. J. For. Res. 2022, 33, 623–641. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; He, X.; Zhou, W.; Zhai, C.; Wang, P.; Tang, Z.; Wei, C.; Zhang, B.; Xiao, L.; et al. Urbanization-induced glomalin changes and their associations with land-use configuration, forest characteristics, and soil properties in Changchun, Northeast China. J. Soils Sediments 2019, 19, 2433–2444. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.J.; Zhong, Z.L.; Wang, H.M.; Fu, Y.J. Variation in glomalin in soil profiles and its association with climatic conditions, shelterbelt characteristics, and soil properties in poplar shelterbelts of Northeast China. J. For. Res. 2020, 31, 279–290. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Yu, N.; Zou, H. Effect of control lower limit of subsurface irrigation on phosphorus distribution in soil profiles of protected land. Trans. Chin. Soc. Agric. Eng. 2009, 25, 66–70. [Google Scholar]
- Bei, Z.; Zhang, Q.; Zheng, W.; Yang, L.; Chen, Y.; Yang, Y. Effects of simulated warming on soil phosphorus availability in subtropical Chinese fir plantation. Acta Ecol. Sin. 2018, 38, 1106–1113. [Google Scholar]
- Zhao, S.; Yu, W.; Zhang, L.; Shen, S. Latitudinal differentiation of phosphorus in black soil in Northeast of China. Trans. Chin. Soc. Agric. Eng. 2005, 21, 34–37. [Google Scholar]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Laxton, R.R. The measure of diversity. J. Theor. Biol. 1978, 70, 51–67. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Xiao, L.; Chen, S.; Zhong, Z.; Gao, W.; Wang, W. Characteristics of plant diversity and community structure in Duobukuer Nature Reserve in Daxing′anling area. Acta Ecol. Sin. 2022, 42, 176–185. [Google Scholar]
- Yao, M.; Rui, J.; Niu, H.; Heděnec, P.; Li, J.; He, Z.; Wang, J.; Cao, W.; Li, X. The differentiation of soil bacterial communities along a precipitation and temperature gradient in the eastern Inner Mongolia steppe. CATENA 2017, 152, 47–56. [Google Scholar] [CrossRef]
- Li, J.; Pei, J.; Liu, J.; Wu, J.; Li, B.; Fang, C.; Nie, M. Spatiotemporal variability of fire effects on soil carbon and nitrogen: A global meta-analysis. Glob. Change Biol. 2021, 27, 4196–4206. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, H.; Jing, S. Dynamic characteristics of forest soil phosphorus in the early succession after fire in Great Xing’an Mountains. Chin. J. Ecol. 2017, 36, 1515–1523. [Google Scholar]
- Chen, S.; Zhou, Z.; Tsang, D.C.W.; Wang, J.; Gao, Y. Glomalin-related soil protein reduces the sorption of polycyclic aromatic hydrocarbons by soils. Chemosphere 2020, 260, 127603. [Google Scholar] [CrossRef] [PubMed]
- Dove, N.C.; Klingeman, D.M.; Carrell, A.A.; Cregger, M.A.; Schadt, C.W. Fire alters plant microbiome assembly patterns: Integrating the plant and soil microbial response to disturbance. New Phytol. 2021, 230, 2433–2446. [Google Scholar] [CrossRef]
- Meng, M.; Wang, B.; Zhang, Q.; Tian, Y. Driving force of soil microbial community structure in a burnt area of Daxing’anling, China. J. For. Res. 2021, 32, 1723–1738. [Google Scholar] [CrossRef]
- Su, W.Q.; Tang, C.X.; Lin, J.H.; Yu, M.J.; Dai, Z.M.; Luo, Y.; Li, Y.; Xu, J.M. Recovery patterns of soil bacterial and fungal communities in Chinese boreal forests along a fire chronosequence. Sci. Total Environ. 2022, 805, 150372. [Google Scholar] [CrossRef]
- Ammitzboll, H.; Jordan, G.J.; Baker, S.C.; Freeman, J.; Bissett, A. Contrasting successional responses of soil bacteria and fungi to post-logging burn severity. For. Ecol. Manag. 2022, 508, 120059. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Cui, X. Temporal and spatial dynamics of soil available potassium in a post-fire Larix gmelinii forest. J. Nanjing For. Univ. Nat. Sci. Ed. 2020, 44, 141–147. [Google Scholar]
- Wang, L.; Xin, Y.; Zou, M.; Zhao, Y.; Liu, S.; Ren, Q. Plants diversity and biomass distribution of vegetation restoration in burnt area of Great Xing’ an Mountains. J. Beijing For. Univ. 2015, 37, 41–47. [Google Scholar]
- Wang, D.; Wang, Z.; Feng, Q.; Shu, Y.; Zhou, M.; Zhao, P. Species Composition and Diversity of Severe Burnt Phytocoenosium After Different Regenerate Years. Bull. Soil Water Conserv. 2017, 37, 56–61. [Google Scholar]
- Nilsson, M.C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Bian, B.; Guo, Q.; Ci, B.; Luo, D. Effects of gap in primitive subalpine fir forest on diversity of herd and shrub in tibet. J. Appl. Ecol. 2004, 15, 191–194. [Google Scholar]
- Tepley, A.J.; Thomann, E.; Veblen, T.T.; Perry, G.L.W.; Holz, A.; Paritsis, J.; Kitzberger, T.; Anderson-Teixeira, K.J. Influences of fire-vegetation feedbacks and post-fire recovery rates on forest landscape vulnerability to altered fire regimes. J. Ecol. 2018, 106, 1925–1940. [Google Scholar] [CrossRef]
- Boucher, Y.; Perrault-Hebert, M.; Fournier, R.; Drapeau, P.; Auger, I. Cumulative patterns of logging and fire (1940-2009): Consequences on the structure of the eastern Canadian boreal forest. Landsc. Ecol. 2017, 32, 361–375. [Google Scholar] [CrossRef]
- Fischer, R. The Long-Term Consequences of Forest Fires on the Carbon Fluxes of a Tropical Forest in Africa. Appl. Sci. 2021, 11, 4696. [Google Scholar] [CrossRef]
- Chu, T.; Guo, X.L.; Takeda, K. Effects of Burn Severity and Environmental Conditions on Post-Fire Regeneration in Siberian Larch Forest. Forests 2017, 8, 76. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wu, Z.W.; Gu, X.L.; Fu, J.J.; Yan, S.J. Effects of fire severity and recovery time on organic carbon content of forest soil in Great Xing’an Mountains, China. Chin. J. Appl. Ecol. 2018, 29, 2455–2462. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Li, S.; Zhong, Z.; Hu, X.; Wang, W. Characteristics of plant diversity and community structure in Shuanghe Nature Reserve in Daxing’anling area of northeastern China. J. Beijing For. Univ. 2021, 43, 79–87. [Google Scholar] [CrossRef]
- Wang, w.; Wang, K.; Wang, Y.; Wen, H.; Yu, J.; Wang, Q.; Han, S. Comparative study on tree, shrub and herb composition and carbon sink function between coniferous and broadleaved forests in Northeast China. J. Beijing For. Univ. 2022, 44, 52–67. [Google Scholar] [CrossRef]
- Gong, D.; Yan, C.; Guo, Z. Vegetation Regeneration After Grassland Fire Based on Remote Sensing Index. J. Northwest For. Univ. 2021, 36, 204–210. [Google Scholar]
- Ramo, R.; Roteta, E.; Bistinas, I.; van Wees, D.; Bastarrika, A.; Chuvieco, E.; van der Werf, G.R. African burnt area and fire carbon emissions are strongly impacted by small fires undetected by coarse resolution satellite data. Proc. Natl. Acad. Sci. USA 2021, 118, e2011160118. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Zhang, X.; Yang, Y.; Wang, H.; Wang, W. Effects of after-burning rehabilitation times on soil phosphorus and its availability in the Daxing’anling forests. Acta Ecol. Sin. 2019, 39, 7977–7986. [Google Scholar]
- Jin, W.; He, H.S.; Shifley, S.R.; Wang, W.J.; Kabrick, J.M.; Davidson, B.K. How can prescribed burning and harvesting restore shortleaf pine-oak woodland at the landscape scale in central United States? Modeling joint effects of harvest and fire regimes. For. Ecol. Manag. 2018, 410, 201–210. [Google Scholar] [CrossRef]
- Barker, J.W.; Price, O.F.; Jenkins, M.E. High severity fire promotes a more flammable eucalypt forest structure. Austral Ecol. 2022, 47, 519–529. [Google Scholar] [CrossRef]
- Buisson, E.; Archibald, S.; Fidelis, A.; Suding, K.N. Ancient grasslands guide ambitious goals in grassland restoration. Science 2022, 377, 594–598. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, H.Y.H.; Chang, S.X. Meta-analysis shows that plant mixtures increase soil phosphorus availability and plant productivity in diverse ecosystems. Nat. Ecol. Evol. 2022, 6, 1112–1121. [Google Scholar] [CrossRef]
- Ning, J.; Yang, G.; Liu, X.; Geng, D.; Wang, L.; Li, Z.; Zhang, Y.; Di, X.; Sun, L.; Yu, H. Effect of fire spread, flame characteristic, fire intensity on particulate matter 2.5 released from surface fuel combustion of Pinus koraiensis plantation—A laboratory simulation study. Environ. Int. 2022, 166, 107352. [Google Scholar] [CrossRef]
- Cui, Y.; Di, H.; Xing, Y.; Chang, X.; Shan, W. Spatial and temporal distributions of forest fires in Heilongjiang Province from 2001 to 2018 based on MODIS data. J. Nanjing For. University. Nat. Sci. Ed. 2021, 45, 205–211. [Google Scholar]


| Year Elapsed | Fire Features from 1967–2016 | Selected Plot | |||
|---|---|---|---|---|---|
| Time Period | Fire Frequency | Total Burnt Area (ha) | Plot Number in This Paper | Total Burnt Area (ha) | |
| 1–5 | 2012–2016 | 133 | 1615.01 | 5 | 296.73 |
| 5–10 | 2007–2011 | 109 | 45,539.51 | 5 | 1246.92 |
| 10–20 | 1997–2006 | 446 | 1,110,409.33 | 8 | 4826.92 |
| 20–30 | 1987–1996 | 179 | 1,162,317.37 | 14 | 448,359.4 |
| 30–40 | 1977–1986 | 473 | 1,672,661.27 | 8 | 60,008.4 |
| 40–50 | 1967–1976 | 406 | 1,835,516.66 | 8 | 11,806.76 |
| Burnt | Control | p-Level | ||
|---|---|---|---|---|
| Soil nutrients | SOC (g·kg−1) | 89.93 ± 8.38 | 81.92 ± 7.22 | 0.21 |
| TN (g·kg−1) | 2.76 ± 0.32 | 2.81 ± 0.28 | 0.48 | |
| TP (g·kg−1) | 0.88 ± 0.07 | 0.97 ± 0.09 | 0.19 | |
| AP (mg·kg−1) | 25.85 ± 2.90 | 30.11 ± 3.22 | 0.27 | |
| Po (g·kg−1) | 0.15 ± 0.01 | 0.18 ± 0.02 | 0.01 | |
| Pi (g·kg−1) | 0.73 ± 0.07 | 0.78 ± 0.08 | 0.36 | |
| TG (g·kg−1) | 19.23 ± 1.09 | 16.25 ± 0.96 | 0.007 | |
| EEG (g·kg−1) | 1.33 ± 0.08 | 1.02 ± 0.07 | 0.004 | |
| Plant diversity | A-Simpson | 0.32 ± 0.04 | 0.37 ± 0.03 | 0.16 |
| A-Shannon | 0.56 ± 0.06 | 0.64 ± 0.06 | 0.23 | |
| A-R | 2.54 ± 0.23 | 3.12 ± 0.24 | 0.02 | |
| S-Simpson | 0.48 ± 0.06 | 0.58 ± 0.04 | 0.12 | |
| S-Shannon | 0.72 ± 0.09 | 0.77 ± 0.07 | 0.59 | |
| S-R | 1.91 ± 0.20 | 2.52 ± 0.27 | 0.03 | |
| H-Simpson | 0.73 ± 0.03 | 0.64 ± 0.03 | 0.01 | |
| H-Shannon | 1.80 ± 0.10 | 1.75 ± 0.11 | 0.64 | |
| H-R | 6.93 ± 0.61 | 6.14 ± 0.45 | 0.10 | |
| Community structure | AH (m) | 10.01 ± 1.03 | 13.58 ± 0.46 | 0.009 |
| ACBH (m) | 9.00 ± 0.91 | 11.94 ± 0.58 | 0.003 | |
| ADBH (cm) | 4.47 ± 0.52 | 8.52 ± 0.55 | 0.008 | |
| SH (m) | 0.64 ± 0.06 | 0.57 ± 0.05 | 0.28 | |
| SC (%) | 20.08 ± 2.27 | 23.26 ± 2.45 | 0.30 | |
| SCD (m) | 0.16 ± 0.03 | 0.18 ± 0.03 | 0.53 | |
| SGD (cm) | 0.68 ± 0.09 | 0.67 ± 0.14 | 0.94 | |
| HH (cm) | 21.45 ± 1.85 | 22.62 ± 1.41 | 0.47 | |
| HC (%) | 10.00 ± 1.12 | 8.42 ± 0.91 | 0.28 |
| Incidence/Year | Burnt Area/Year (hm2) | Burnt Area/Time (hm2) | |||
|---|---|---|---|---|---|
| Range | Mean | Range | Mean | ||
| USA | 50,500–71,000 | 60,750 | 1.49 × 106–4.1 × 106 | 2,795,000 | 46 |
| Russia | 11,700–22,000 | 16,850 | 2.8 × 106–1.0 × 107 | 6,400,000 | 380 |
| Canada | 4090–7200 | 5645 | 1.4 × 106–4.55 × 106 | 2,975,000 | 527 |
| China | 2000–4750 | 3375 | 6 × 103–2.5 × 104 | 15,500 | 5 |
| Mediterranean region of France | 1050–2350 | 1700 | 3 × 103–2 × 104 | 11,500 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; She, D.; Wang, K.; Yang, Y.; Hu, X.; Feng, P.; Yan, X.; Gavrikov, V.; Wang, H.; Han, S.; et al. Post-Fire Recovery of Soil Multiple Properties, Plant Diversity, and Community Structure of Boreal Forests in China. Forests 2025, 16, 806. https://doi.org/10.3390/f16050806
Zhang X, She D, Wang K, Yang Y, Hu X, Feng P, Yan X, Gavrikov V, Wang H, Han S, et al. Post-Fire Recovery of Soil Multiple Properties, Plant Diversity, and Community Structure of Boreal Forests in China. Forests. 2025; 16(5):806. https://doi.org/10.3390/f16050806
Chicago/Turabian StyleZhang, Xiting, Danqi She, Kai Wang, Yang Yang, Xia Hu, Peng Feng, Xiufeng Yan, Vladimir Gavrikov, Huimei Wang, Shijie Han, and et al. 2025. "Post-Fire Recovery of Soil Multiple Properties, Plant Diversity, and Community Structure of Boreal Forests in China" Forests 16, no. 5: 806. https://doi.org/10.3390/f16050806
APA StyleZhang, X., She, D., Wang, K., Yang, Y., Hu, X., Feng, P., Yan, X., Gavrikov, V., Wang, H., Han, S., & Wang, W. (2025). Post-Fire Recovery of Soil Multiple Properties, Plant Diversity, and Community Structure of Boreal Forests in China. Forests, 16(5), 806. https://doi.org/10.3390/f16050806








