The Effect of Hydrometeorological Factors on Tree Growth (Abies borisii-regis Mattf.) in Mountainous Watersheds (Central Greece)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling Process, and Statistical Analysis
2.2. Climatic Information and Trend Analysis
2.3. Tree Ring Width Correlation Analysis Under Different Climatic Conditions
3. Results
3.1. Tree Ring Time Series Statistical Analysis
3.2. Relationships Between Tree Ring Chronologies and Climatic Variables
4. Discussion
4.1. Tree Ring Chronologies’ (TRW, EW, and LW) Variability
4.2. Relations Between Climate Variability and Tree Growth
4.2.1. Precipitation
4.2.2. Temperature
4.2.3. Standardized Precipitation Evapotranspiration Index (SPEI)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novak, K.; de Luis, M.; Saz, M.A.; Longares, L.A.; Serrano-Notivoli, R.; Raventós, J.; Čufar, K.; Gričar, J.; Di Filippo, A.; Piovesan, G.; et al. Missing Rings in Pinus Halepensis—The Missing Link to Relate the Tree-Ring Record to Extreme Climatic Events. Front. Plant Sci. 2016, 7, 727. [Google Scholar] [CrossRef] [PubMed]
- Fritts Harold, C. Tree Rings and Climate; Academic Press: Cambridge, MA, USA, 1976; ISBN 9780122684500. [Google Scholar]
- Papadopoulos, A.; Raftoyannis, Y.; Pantera, A. Fir Decline in Greece: A Dendroclimatological Approach. In Proceedings of the 10th International Conference on Environmental Science and Technology, Kos Island, Greece, 5–7 September 2007; pp. 571–578. [Google Scholar]
- Roibu, C.-C.; Sfecla, V.; Mursa, A.; Ionita, M.; Nagavciuc, V.; Chiriloaei, F.; Lesan, I.; Popa, I. The Climatic Response of Tree Ring Width Components of Ash (Fraxinus excelsior L.) and Common Oak (Quercus robur L.) from Eastern Europe. Forests 2020, 11, 600. [Google Scholar] [CrossRef]
- Kastridis, A.; Margiorou, S.; Sapountzis, M. Post-Fire Soil Erosion: Two Years of Monitoring—First Time Detected Implications between Extremely Intense Consecutive Rainfall Events and Erosion Rates. Catena 2024, 243, 108194. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent Insects, Pathogens and Drought Shape Changing Patterns in Oak Decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Robson, J.R.M.; Conciatori, F.; Tardif, J.C.; Knowles, K. Tree-Ring Response of Jack Pine and Scots Pine to Budworm Defoliation in Central Canada. For. Ecol. Manag. 2015, 347, 83–95. [Google Scholar] [CrossRef]
- Barmpoutis, P.; Kamperidou, V.; Stathaki, T. Estimation of Extent of Trees and Biomass Infestation of the Suburban Forest of Thessaloniki (Seich Sou) Using UAV Imagery and Combining R-CNNs and Multichannel Texture Analysis. In Proceedings of the SPIE—The International Society for Optical Engineering, Amsterdam, The Netherlands, 31 January 2020; Volume 11433. [Google Scholar]
- Kamperidou, V. The Biological Durability of Thermally-and Chemically-Modified Black Pine and Poplarwood against Basidiomycetes and Mold Action. Forests 2019, 10, 1111. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To Die or Not to Die: Early Warnings of Tree Dieback in Response to a Severe Drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Lebourgeois, F. Climatic Signals in Earlywood, Latewood and Total Ring Width of Corsican Pine from Western France. Ann. For. Sci. 2000, 57, 155–164. [Google Scholar] [CrossRef]
- Panayotov, M.P.; Zafirov, N.; Cherubini, P. Fingerprints of Extreme Climate Events in Pinus Sylvestris Tree Rings from Bulgaria. Trees 2013, 27, 211–227. [Google Scholar] [CrossRef]
- Chavenetidou, M.; Kamperidou, V. Impact of Wood Structure Variability on the Surface Roughness of Chestnut Wood. Appl. Sci. 2024, 14, 6326. [Google Scholar] [CrossRef]
- De Luis, M.; Čufar, K.; Di Filippo, A.; Novak, K.; Papadopoulos, A.; Piovesan, G.; Rathgeber, C.B.K.; Raventós, J.; Saz, M.A.; Smith, K.T. Plasticity in Dendroclimatic Response across the Distribution Range of Aleppo Pine (Pinus halepensis). PLoS ONE 2013, 8, e83550. [Google Scholar] [CrossRef] [PubMed]
- Liphschitz, N.; Lev-Yadun, S. Cambial Activity of Evergreen and Seasonal Dimorphics around the Mediterranean. IAWA J. 1986, 7, 145–153. [Google Scholar] [CrossRef]
- Waszak, N.; Robertson, I.; Puchałka, R.; Przybylak, R.; Pospieszyńska, A.; Koprowski, M. Investigating the Climate-Growth Response of Scots Pine (Pinus sylvestris L.) in Northern Poland. Atmosphere 2021, 12, 1690. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Plastic Responses of Abies Pinsapo Xylogenesis to Drought and Competition. Tree Physiol. 2009, 29, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Carrer, M.; Nola, P.; Motta, R.; Urbinati, C. Contrasting Tree-Ring Growth to Climate Responses of Abies alba toward the Southern Limit of Its Distribution Area. Oikos 2010, 119, 1515–1525. [Google Scholar] [CrossRef]
- Camarero, J.J.; Rubio-Cuadrado, Á. Relating Climate, Drought and Radial Growth in Broadleaf Mediterranean Tree and Shrub Species: A New Approach to Quantify Climate-Growth Relationships. Forests 2020, 11, 1250. [Google Scholar] [CrossRef]
- Subotić, J.; Dukić, V.; Popov, T.; Trbić, G.; Maunaga, Z.; Petrović, D. Relationships Between Climatic Variables and Tree-Ring Width of Silver Fir (Abies alba Mill.) in Kozara National Park (Bosnia and Herzegovina). South-East. Eur. For. 2020, 11, 17–27. [Google Scholar] [CrossRef]
- Walder, D.; Krebs, P.; Bugmann, H.; Manetti, M.C.; Pollastrini, M.; Anzillotti, S.; Conedera, M. Silver Fir (Abies alba Mill.) Is Able to Thrive and Prosper under Meso-Mediterranean Conditions. For. Ecol. Manage 2021, 498, 119537. [Google Scholar] [CrossRef]
- Garfi, G. Climatic Signal in Tree-Rings of Quercus Pubescens s.l., and Celtis Australis L. in South-Eastern Sicily. Dendrochronologia 2000, 18, 41–51. [Google Scholar]
- Kastridis, A.; Kamperidou, V.; Stathis, D. Dendroclimatological Analysis of Fir (A. borisii-regis) in Greece in the Frame of Climate Change Investigation. Forests 2022, 13, 879. [Google Scholar] [CrossRef]
- Cherubini, P.; Gartner, B.L.; Tognetti, R.; Bräker, O.U.; Schoch, W.; Innes, J.L. Identification, Measurement and Interpretation of Tree Rings in Woody Species from Mediterranean Climates. Biol. Rev. Camb. Philos. Soc. 2003, 78, 119–148. [Google Scholar] [CrossRef] [PubMed]
- Stathi, E.; Kastridis, A.; Myronidis, D. Analysis of Hydrometeorological Trends and Drought Severity in Water-Demanding Mediterranean Islands under Climate Change Conditions. Climate 2023, 11, 106. [Google Scholar] [CrossRef]
- Stathi, E.; Kastridis, A.; Myronidis, D. Analysis of Hydrometeorological Characteristics and Water Demand in Semi-Arid Mediterranean Catchments under Water Deficit Conditions. Climate 2023, 11, 137. [Google Scholar] [CrossRef]
- Linares, J.C. Biogeography and Evolution of Abies (Pinaceae) in the Mediterranean Basin: The Roles of Long-Term Climatic Change and Glacial Refugia. J. Biogeogr. 2011, 38, 619–630. [Google Scholar] [CrossRef]
- Papadopoulos, A. Tree-Ring Patterns and Climate Response of Mediterranean Fir Populations in Central Greece. Dendrochronologia 2016, 40, 17–25. [Google Scholar] [CrossRef]
- Rathgeber, C.; Nicault, A.; Kaplan, J.O.; Guiot, J. Using a Biogeochemistry Model in Simulating Forests Productivity Responses to Climatic Change and [CO2] Increase: Example of Pinus Halepensis in Provence (South-East France). Ecol. Modell. 2003, 166, 239–255. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Serre-Bachet, F.; Tessier, L. Tree Ring to Climate Relationships of Aleppo Pine (Pinus halepensis Mill.) in Greece. Ecol. Mediterr. 2001, 27, 89–98. [Google Scholar] [CrossRef]
- Papadopoulos, A.M. Investigations Dendroclimatologiques Du Sapin de Céphalonie En Grèce Centrale. Geogr. Tech. 2009, 2, 34–38. [Google Scholar]
- Manetti, M.C.; Cutini, A. Tree-Ring Growth of Silver Fir (Abies alba Mill.) in Two Stands under Different Silvicultural Systems in Central Italy. Dendrochronologia 2006, 23, 145–150. [Google Scholar] [CrossRef]
- Akkemik, Ü.; D’Arrigo, R.; Cherubini, P.; Köse, N.; Jacoby, G.C. Tree-Ring Reconstructions of Precipitation and Streamflow for North-Western Turkey. Int. J. Climatol. 2008, 28, 173–183. [Google Scholar] [CrossRef]
- Touchan, R.; Anchukaitis, K.J.; Shishov, V.V.; Sivrikaya, F.; Attieh, J.; Ketmen, M.; Stephan, J.; Mitsopoulos, I.; Christou, A.; Meko, D.M. Spatial Patterns of Eastern Mediterranean Climate Influence on Tree Growth. Holocene 2014, 24, 381–392. [Google Scholar] [CrossRef]
- Rolland, C. Tree-Ring and Climate Relationships for Abies alba in the Internal Alps. Tree-Ring Bull. 1993, 53, 1–11. [Google Scholar]
- Pasho, E.; Toromani, E.; Alla, A.Q. Climatic Impact on Tree-Ring Widths in Abies Borisii-Regis Forests from South-East Albania. Dendrochronologia 2014, 32, 237–244. [Google Scholar] [CrossRef]
- Fund for the Administration and Management of University Forests. Pertouli University Forest Management Plan of the Decade 2009–2018; Fund for the Administration and Management of University Forests: Thessaloniki, Greece, 2018. [Google Scholar]
- Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; 192p. [Google Scholar]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating Crossdating Accuracy: A Manual and Tutorial for the Computer Program COFECHA. Tree Ring Res. 2001, 57, 205–221. [Google Scholar]
- Cook, E.R.; Holmes, R.L. Users Manual for Program ARSTAN. In Tree-Ring Chronologies of Western North America: California, Eastern Oregon and Northern Great Basin; Laboratory of TreeRing Research, University of Arizona: Tucson, AZ, USA, 1986; pp. 50–65. [Google Scholar]
- Cook, E.R.; Peters, K. The Smoothing Spline: A New Approach to Standardizing Forest Interior Tree-Ring Width Series for Dendroclimatic Studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree Ring Standardization (Dendrochronology, Forestry, Dendroclimatology, Autoregressive Process). Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series with Applications in Dendroclimatology and Hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Cook, E.R.; Palmer, J.G.; Cook, B.I.; Hogg, A.; D’Arrigo, R.D. A Multi-Millennial Palaeoclimatic Resource from Lagarostrobos Colensoi Tree-Rings at Oroko Swamp, New Zealand. Glob. Planet. Change 2002, 33, 209–220. [Google Scholar] [CrossRef]
- Buras, A. A Comment on the Expressed Population Signal. Dendrochronologia 2017, 44, 130–132. [Google Scholar] [CrossRef]
- Stefanidis, S.; Alexandridis, V. Precipitation and Potential Evapotranspiration Temporal Variability and Their Relationship in Two Forest Ecosystems in Greece. Hydrology 2021, 8, 160. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Tirivarombo, S.; Osupile, D.; Eliasson, P. Drought Monitoring and Analysis: Standardised Precipitation Evapotranspiration Index (SPEI) and Standardised Precipitation Index (SPI). Phys. Chem. Earth 2018, 106, 1–10. [Google Scholar] [CrossRef]
- Heikkinen, O. Relationships between Tree Growth and Climate in the Subalpine Cascade Range of Washington, USA. Ann. Bot. Fenn. 1985, 22, 1–14. [Google Scholar]
- Hauck, M.; Zimmermann, J.; Jacob, M.; Dulamsuren, C.; Bade, C.; Ahrends, B.; Leuschner, C. Rapid Recovery of Stem Increment in Norway Spruce at Reduced SO2 Levels in the Harz Mountains, Germany. Environ. Pollut. 2012, 164, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Koprowski, M.; Duncker, P. Tree Ring Width and Wood Density as the Indicators of Climatic Factors and Insect Outbreaks Affecting Spruce Growth. Ecol. Indic. 2012, 23, 332–337. [Google Scholar] [CrossRef]
- Serre-Bachet, F. La Dendrochronologie Dans Le Bassin Méditerranéen. Dendrochronologia 1985, 3, 77–92. [Google Scholar]
- Rigling, A.; Waldner, P.O.; Forster, T.; Bräker, O.U.; Pouttu, A. Ecological Interpretation of Tree-Ring Width and Intraannual Density Fluctuations in Pinus Sylvestris on Dry Sites in the Central Alps and Siberia. Can. J. For. Res. 2001, 31, 18–31. [Google Scholar] [CrossRef]
- Andreu, L.; Gutiérrez, E.; Macias, M.; Ribas, M.; Bosch, O.; Camarero, J.J. Climate Increases Regional Tree-growth Variability in Iberian Pine Forests. Glob. Chang. Biol. 2007, 13, 804–815. [Google Scholar] [CrossRef]
- Di Filippo, A.; Biondi, F.; Čufar, K.; De Luis, M.; Grabner, M.; Maugeri, M.; Presutti Saba, E.; Schirone, B.; Piovesan, G. Bioclimatology of Beech (Fagus sylvatica L.) in the Eastern Alps: Spatial and Altitudinal Climatic Signals Identified through a Tree-ring Network. J. Biogeogr. 2007, 34, 1873–1892. [Google Scholar] [CrossRef]
- Seim, A.; Büntgen, U.; Fonti, P.; Haska, H.; Herzig, F.; Tegel, W.; Trouet, V.; Treydte, K. Climate Sensitivity of a Millennium-Long Pine Chronology from Albania. Clim. Res. 2012, 51, 217–228. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Beeckman, H.; Cañellas, I. Influence of Drought on Tree Rings and Tracheid Features of Pinus Nigra and Pinus Sylvestris in a Mesic Mediterranean Forest. Eur. J. For. Res. 2013, 132, 33–45. [Google Scholar] [CrossRef]
- Koutavas, A. CO2 Fertilization and Enhanced Drought Resistance in Greek Firs from Cephalonia Island, Greece. Glob. Chang. Biol. 2013, 19, 529–539. [Google Scholar] [CrossRef]
- Hughes, M.K.; Kuniholm, P.; Eischeid, J.K.; Garfin, G.M.; Griggs, C.B.; Latini, C.E. Aegean Tree-Ring Signature Years Explained. Tree Ring Res. 2001, 57111, 67–73. [Google Scholar]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A. V Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Springer Science & Business Media: Berlin, Germany, 2006; Volume 183. [Google Scholar]
- von Felten, S.; Hättenschwiler, S.; Saurer, M.; Siegwolf, R. Carbon Allocation in Shoots of Alpine Treeline Conifers in a CO2 Enriched Environment. Trees 2007, 21, 283. [Google Scholar] [CrossRef]
- Żywiec, M.; Muter, E.; Zielonka, T.; Delibes, M.; Calvo, G.; Fedriani, J.M. Long-Term Effect of Temperature and Precipitation on Radial Growth in a Threatened Thermo-Mediterranean Tree Population. Trees 2017, 31, 491–501. [Google Scholar] [CrossRef]
- Izworska, K.; Muter, E.; Matulewski, P.; Zielonka, T. Tree Rings as an Ecological Indicator of the Reaction of Swiss Stone Pine (Pinus cembra L.) to Climate Change and Disturbance Regime in the Extreme Environment of Cliff Forests. Ecol. Indic. 2023, 148, 110102. [Google Scholar] [CrossRef]
- Stjepanović, S.; Matović, B.; Stojanović, D.; Lalić, B.; Levanič, T.; Orlović, S.; Gutalj, M. The Impact of Adverse Weather and Climate on the Width of European Beech (Fagus sylvatica L.) Tree Rings in Southeastern Europe. Atmos. 2018, 9, 451. [Google Scholar] [CrossRef]
- Bozkurt, A.E.; Şahan, E.A.; Köse, N. Growth Responses of Pinus Sylvestris L. to Climate from the Southeastern Limit of Its Natural Distribution Area, Turkey. Dendrochronologia 2021, 70, 125897. [Google Scholar] [CrossRef]
- Alkan, İ.; Irdem, C. The Effect of Climate on Tree-Ring of Fir, Spruce and Scotch Pine in Karçal Mountains. Artvin Çoruh Üniversitesi Orman. Fakültesi Derg. 2023, 24, 206–217. [Google Scholar] [CrossRef]
- Wilson, R.; Topham, J. Violins and Climate. Theor. Appl. Climatol. 2004, 77, 9–24. [Google Scholar] [CrossRef]
- van der Maaten-Theunissen, M.; Kahle, H.-P.; van der Maaten, E. Drought Sensitivity of Norway Spruce Is Higher than That of Silver Fir along an Altitudinal Gradient in Southwestern Germany. Ann. For. Sci. 2013, 70, 185–193. [Google Scholar] [CrossRef]
- Debel, A.; Meier, W.J.-H.; Bräuning, A. Climate Signals for Growth Variations of F. Sylvatica, P. Abies, and P. Sylvestris in Southeast Germany over the Past 50 Years. Forests 2021, 12, 1433. [Google Scholar] [CrossRef]
- Obojes, N.; Buscarini, S.; Meurer, A.K.; Tasser, E.; Oberhuber, W.; Mayr, S.; Tappeiner, U. Tree Growth at the Limits: The Response of Multiple Conifers to Opposing Climatic Constraints along an Elevational Gradient in the Alps. Front. For. Glob. Change 2024, 7, 1332941. [Google Scholar] [CrossRef]
- Rita, A.; Gentilesca, T.; Ripullone, F.; Todaro, L.; Borghetti, M. Differential Climate–Growth Relationships in Abies Alba Mill. and Fagus Sylvatica L. in Mediterranean Mountain Forests. Dendrochronologia 2014, 32, 220–229. [Google Scholar] [CrossRef]
- Bhuyan, U.; Zang, C.; Menzel, A. Different Responses of Multispecies Tree Ring Growth to Various Drought Indices across Europe. Dendrochronologia 2017, 44, 1–8. [Google Scholar] [CrossRef]
- Gadermaier, J.; Vospernik, S.; Grabner, M.; Wächter, E.; Keßler, D.; Kessler, M.; Lehner, F.; Klebinder, K.; Katzensteiner, K. Soil Water Storage Capacity and Soil Nutrients Drive Tree Ring Growth of Six European Tree Species across a Steep Environmental Gradient. For. Ecol. Manag. 2024, 554, 121599. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Graf Pannatier, E.; Rigling, A. Drought Alters Timing, Quantity, and Quality of Wood Formation in Scots Pine. J. Exp. Bot. 2011, 62, 2763–2771. [Google Scholar] [CrossRef]
- Candel-Pérez, D.; Lo, Y.-H.; Blanco, J.; Chiu, C.-M.; Camarero, J.; González de Andrés, E.; Imbert, J.; Castillo, F. Drought-Induced Changes in Wood Density Are Not Prevented by Thinning in Scots Pine Stands. Forests 2018, 9, 4. [Google Scholar] [CrossRef]
- Akhmetzyanov, L.; Sánchez-Salguero, R.; García-González, I.; Domínguez-Delmás, M.; Sass-Klaassen, U. Blue Is the Fashion in Mediterranean Pines: New Drought Signals from Tree-Ring Density in Southern Europe. Sci. Total Environ. 2023, 856, 159291. [Google Scholar] [CrossRef]
- Csilléry, K.; Buchmann, N.; Fady, B. Adaptation to Drought Is Coupled with Slow Growth, but Independent from Phenology in Marginal Silver Fir (Abies alba Mill.) Populations. Evol. Appl. 2020, 13, 2357–2376. [Google Scholar] [CrossRef]
- Mihai, G.; Alexandru, A.M.; Stoica, E.; Birsan, M.V. Intraspecific Growth Response to Drought of Abies alba in the Southeastern Carpathians. Forests 2021, 12, 387. [Google Scholar] [CrossRef]




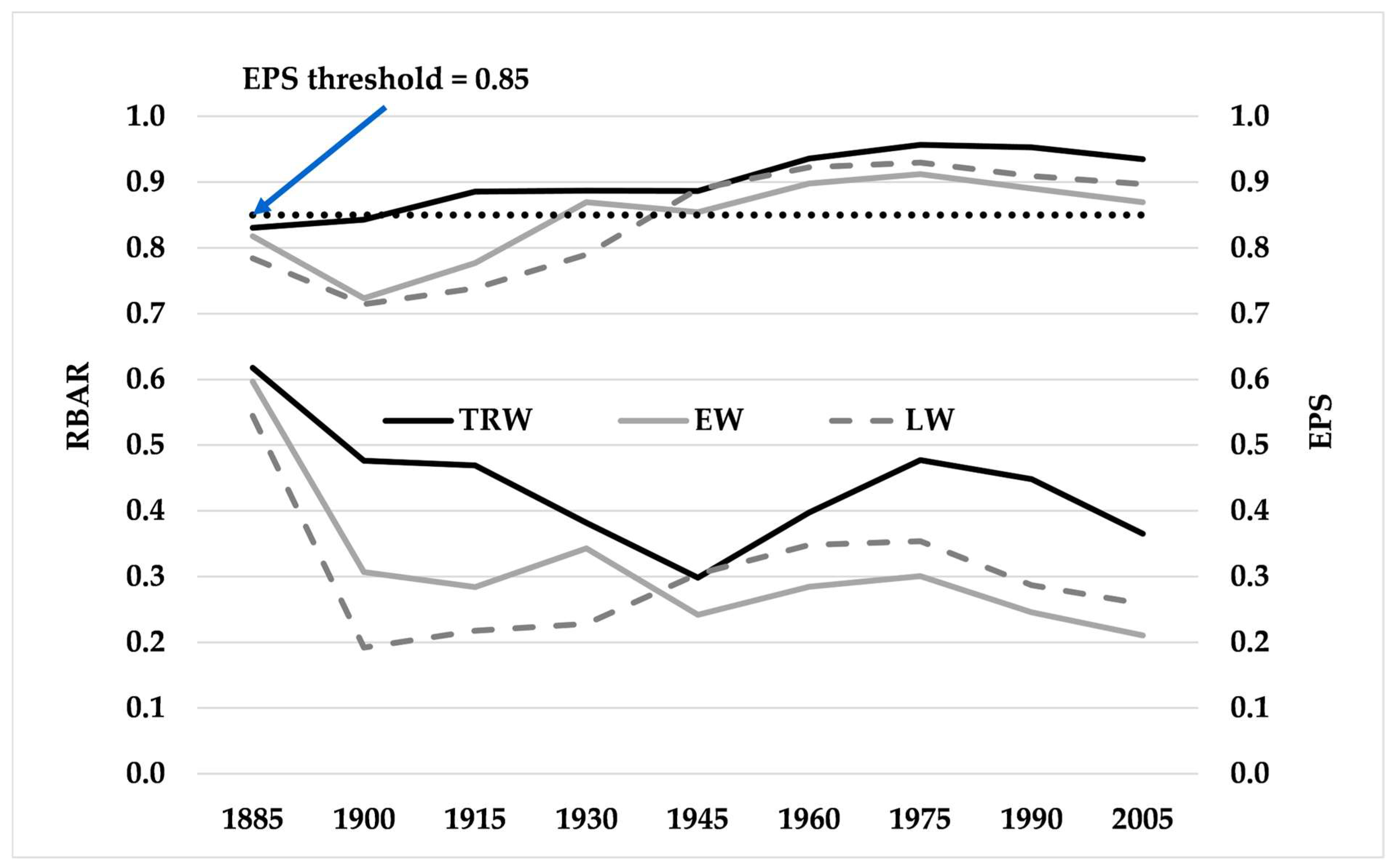

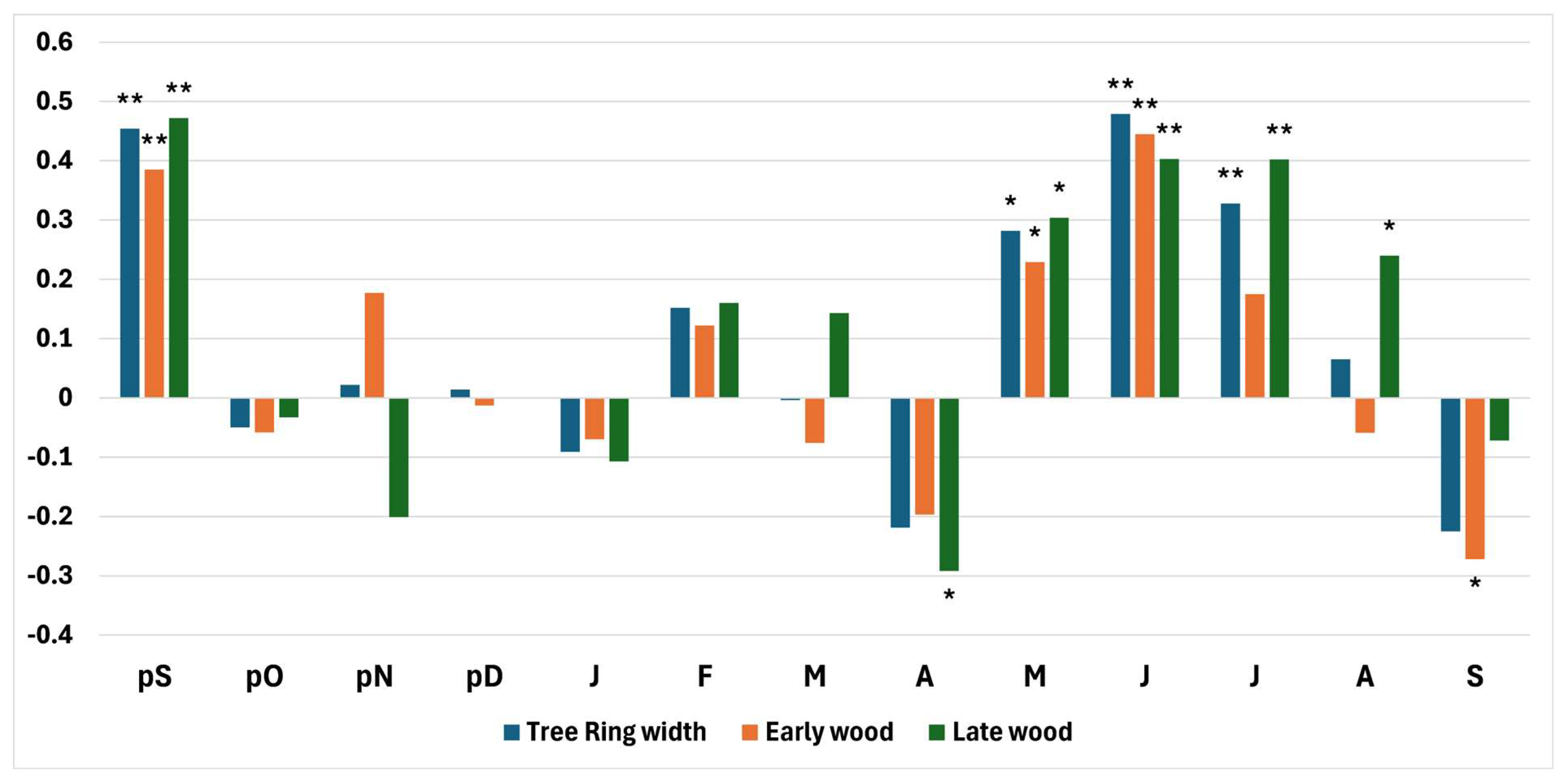
| Growth Chronologies | Trees | Period | MW (mm) | SD (mm) | AC1 | MS | RBAR | EPS | AC1st |
|---|---|---|---|---|---|---|---|---|---|
| TRW | 25 | 1833–2020 | 3.004 | 1.099 | 0.76 | 0.15 | 0.34 | 0.90 | 0.37 |
| EW | 25 | 1833–2020 | 2.109 | 0.739 | 0.71 | 0.15 | 0.24 | 0.85 | 0.28 |
| LW | 25 | 1833–2020 | 0.895 | 0.416 | 0.51 | 0.24 | 0.25 | 0.84 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastridis, A.; Koutsianitis, D.; Stathis, D. The Effect of Hydrometeorological Factors on Tree Growth (Abies borisii-regis Mattf.) in Mountainous Watersheds (Central Greece). Forests 2025, 16, 750. https://doi.org/10.3390/f16050750
Kastridis A, Koutsianitis D, Stathis D. The Effect of Hydrometeorological Factors on Tree Growth (Abies borisii-regis Mattf.) in Mountainous Watersheds (Central Greece). Forests. 2025; 16(5):750. https://doi.org/10.3390/f16050750
Chicago/Turabian StyleKastridis, Aristeidis, Dimitrios Koutsianitis, and Dimitrios Stathis. 2025. "The Effect of Hydrometeorological Factors on Tree Growth (Abies borisii-regis Mattf.) in Mountainous Watersheds (Central Greece)" Forests 16, no. 5: 750. https://doi.org/10.3390/f16050750
APA StyleKastridis, A., Koutsianitis, D., & Stathis, D. (2025). The Effect of Hydrometeorological Factors on Tree Growth (Abies borisii-regis Mattf.) in Mountainous Watersheds (Central Greece). Forests, 16(5), 750. https://doi.org/10.3390/f16050750






