Spatial Heterogeneity of Soil Respiration and Its Relationship with the Spatial Distribution of the Forest Ecosystem at the Fine Scale
Abstract
1. Introduction
2. Materials and Methods
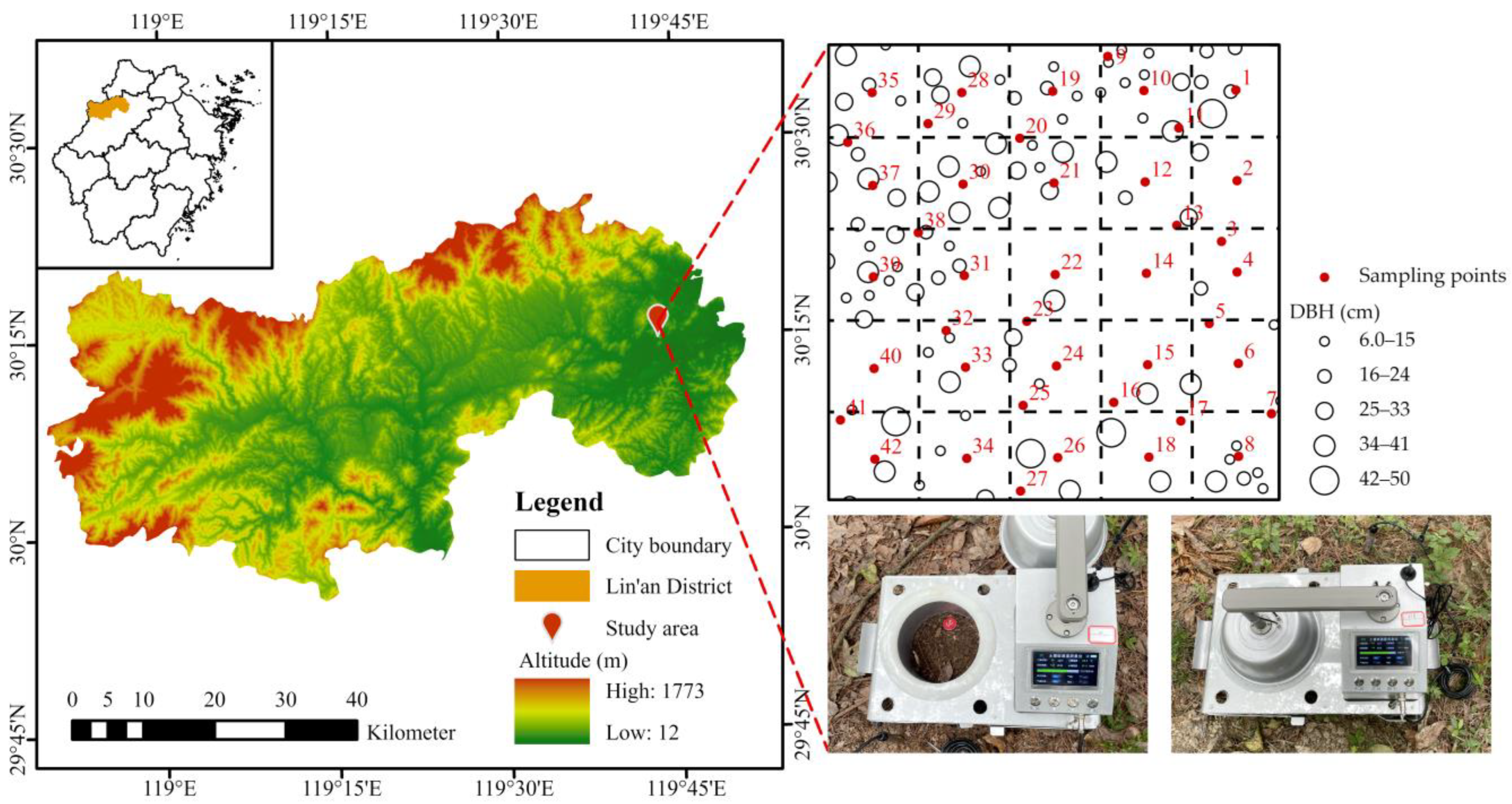
2.1. Study Area and Experimental Design
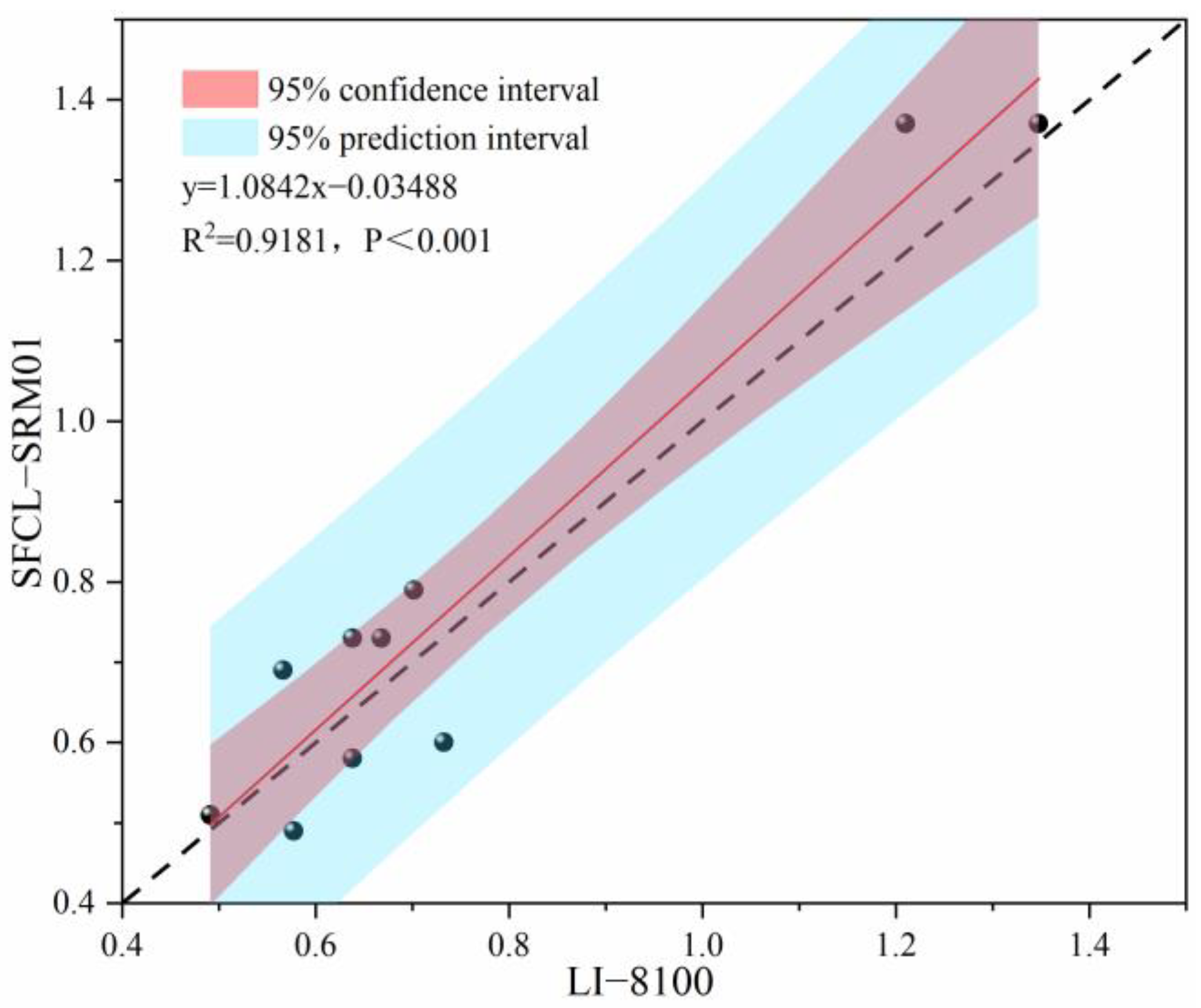
2.2. Measurements of Soil Respiration, Soil Temperature, and Water Content
2.3. Soil Sampling and Environmental Factor Investigation
2.4. Statistical Analyses
3. Results
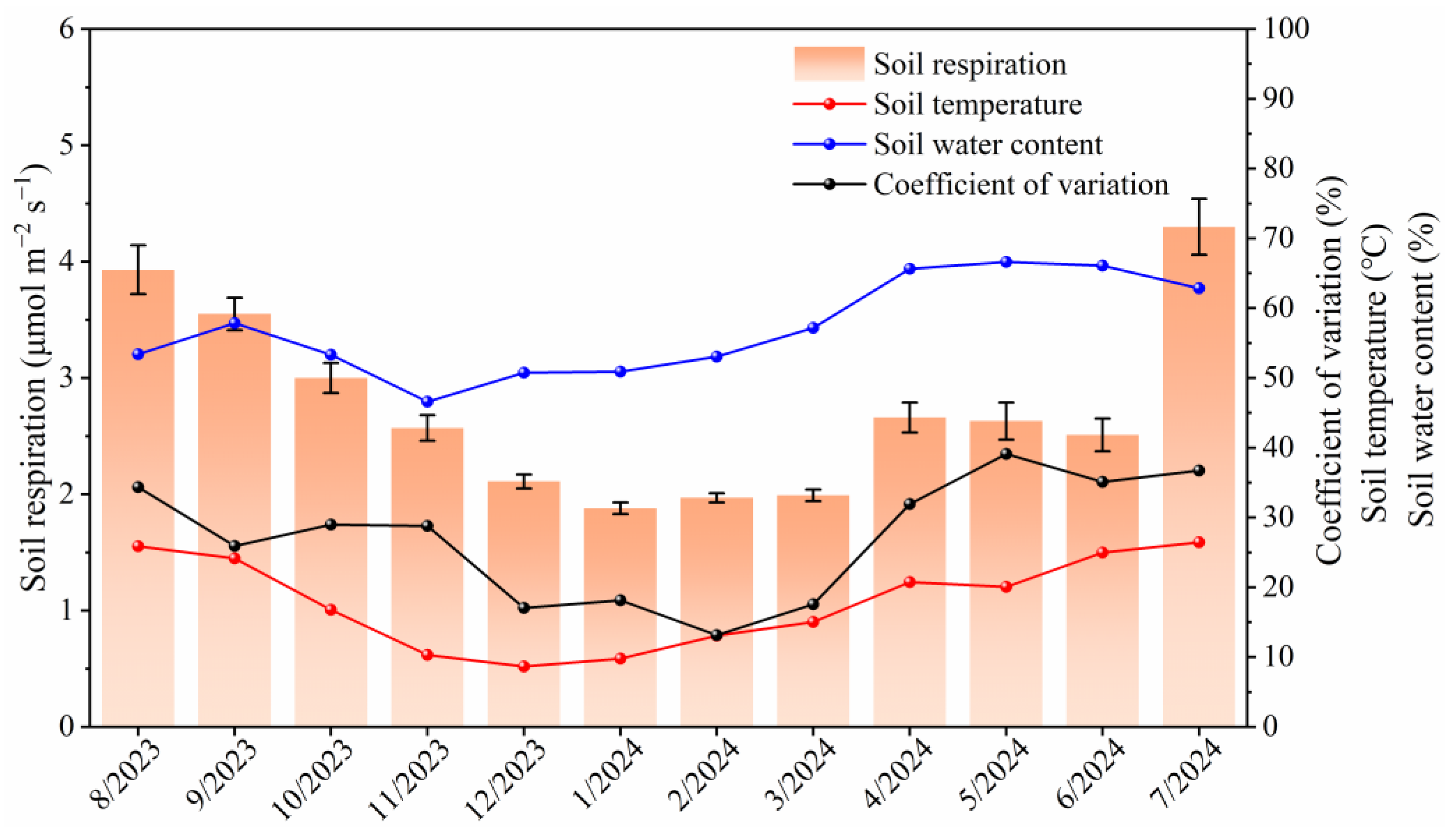
3.1. Temporal Variations in Soil Respiration and Soil Environmental Factors
3.2. Soil Respiration’s Spatial Distribution Characteristics and the Factors of Environment
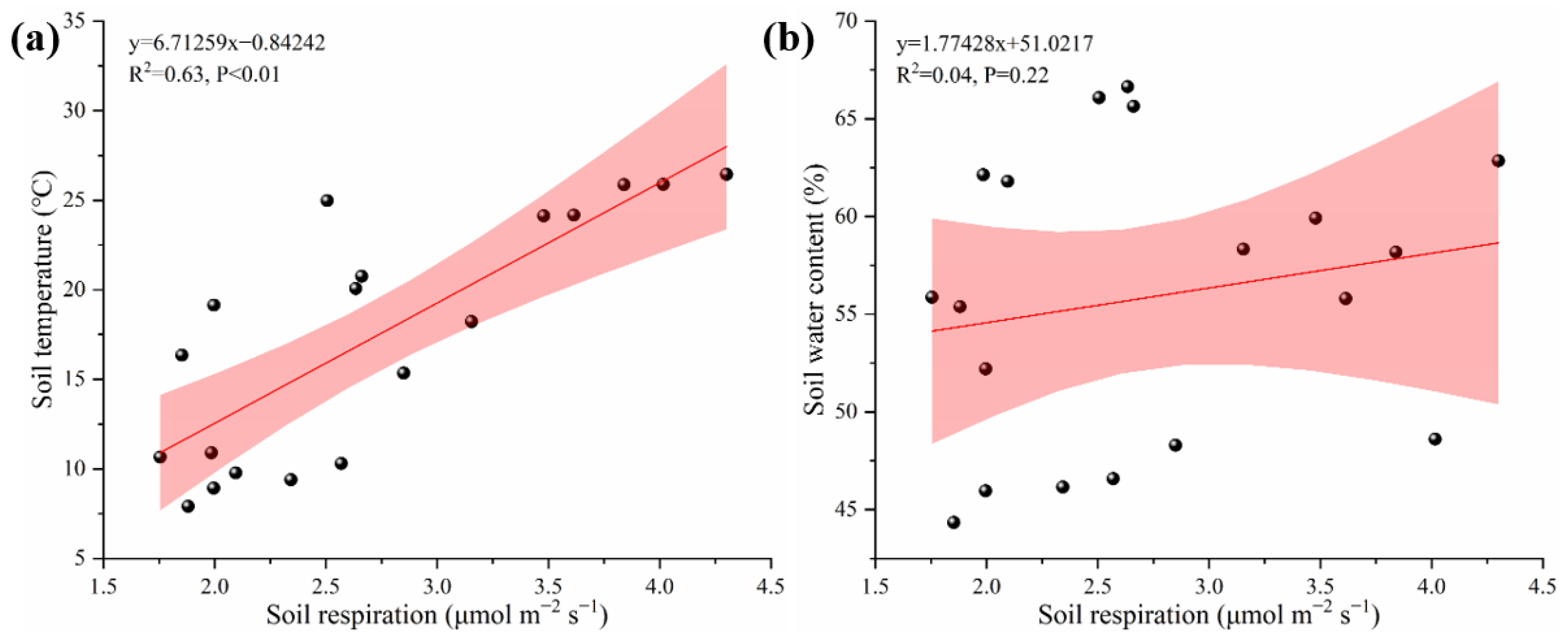
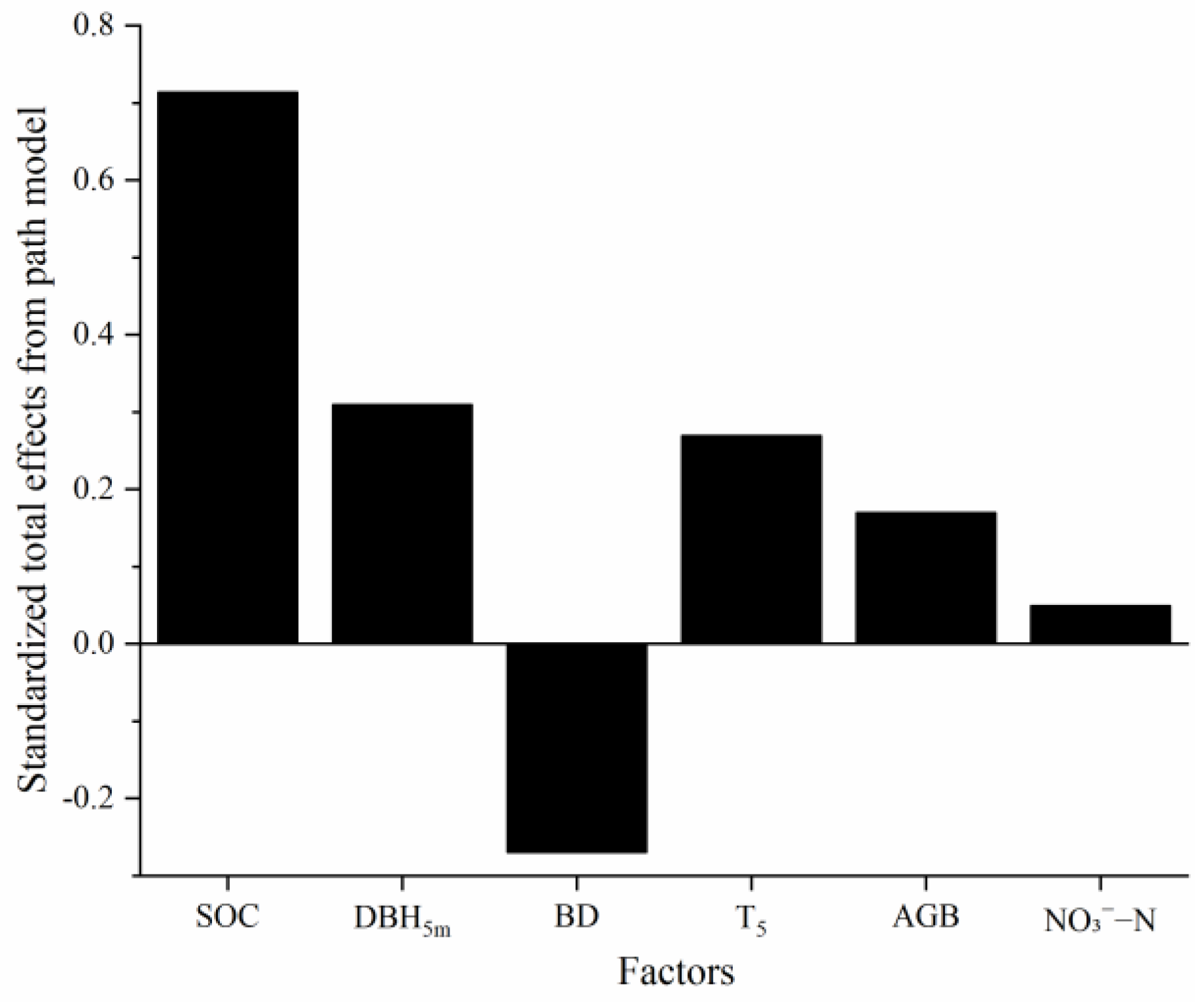
3.3. Main Drivers of Spatial Heterogeneity in Soil Respiration
4. Discussion
4.1. Spatial Variation of Soil Respiration
4.2. Drivers and Potential Pathways of Spatial Variability in Soil Respiration
4.3. Deficiencies and Prospects in the Study of Spatial Variability of Soil Respiration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Sampling Point | Number of Trees | Diameter at Breast Height (cm) | Vertical Canopy Coverage (%) | Sampling Point | Number of Trees | Diameter at Breast Height (cm) | Vertical Canopy Coverage (%) |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 152.4 | 88.23 | 22 | 1 | 33.5 | 78.06 |
| 2 | 1 | 32.2 | 75.34 | 23 | 2 | 59.6 | 45.15 |
| 3 | 1 | 25.2 | 23 | 24 | 3 | 9.3 | 33.6 |
| 4 | 1 | 18.8 | — | 25 | 3 | 73.4 | 71.88 |
| 5 | 1 | 18.8 | 27.07 | 26 | 2 | 80.8 | 72.06 |
| 6 | 1 | 33.9 | 38.5 | 27 | 2 | 72.7 | 68.24 |
| 7 | 2 | 26.4 | 41.28 | 28 | 5 | 95.6 | 72.01 |
| 8 | 6 | 113.7 | 63.29 | 29 | 4 | 36 | 66.35 |
| 9 | 5 | 70.7 | 55.02 | 30 | 4 | 110.1 | 68.59 |
| 10 | 6 | 58.8 | 39.02 | 31 | 4 | 41.7 | 54.78 |
| 11 | 5 | 172.9 | 52.07 | 32 | 2 | 15.8 | 39.3 |
| 12 | 2 | 54.5 | 80.37 | 33 | 3 | 73.5 | 33.35 |
| 13 | 1 | 25.2 | 95.15 | 34 | 2 | 17.6 | 64.93 |
| 14 | — | — | — | 35 | 4 | 97.9 | 56.62 |
| 15 | 2 | 70.9 | 73.12 | 36 | 5 | 122.9 | 76.62 |
| 16 | 2 | 79.2 | 55.86 | 37 | 4 | 99.7 | 76.68 |
| 17 | 2 | 70.9 | 68.11 | 38 | 5 | 104.5 | 67.12 |
| 18 | 2 | 80.3 | 79.41 | 39 | 7 | 98.6 | 68.3 |
| 19 | 5 | 76.3 | 76.2 | 40 | 1 | 6.5 | 38.57 |
| 20 | 4 | 81.6 | 69.92 | 41 | 2 | 6.5 | 36.28 |
| 21 | 5 | 113.1 | 67.52 | 42 | 3 | 108.4 | 64.69 |
| Factors | Model | C0 | C0 + C | C0/(C0 + C) | A (m) | R2 | RSS |
|---|---|---|---|---|---|---|---|
| RS | gaussian | 0.001 | 0.225 | 0.004 | 11.07 | 0.546 | 0.010 |
| T5 | gaussian | 0.008 | 0.311 | 0.026 | 21.17 | 0.847 | 0.011 |
| SWC | Spherical | 18.250 | 41.400 | 0.441 | 53.05 | 0.894 | 19.900 |

References
- Moyes, A.B.; Bowling, D.R. Plant community composition and phenological stage drive soil carbon cycling along a tree-meadow ecotone. Plant Soil 2016, 401, 231–242. [Google Scholar] [CrossRef]
- Fekadu, G.; Adgo, E.; Meshesha, D.T.; Tsunekawa, A.; Haregeweyn, N.; Peng, F.; Tsubo, M.; Masunaga, T.; Tassew, A.; Mulualem, T. Seasonal and diurnal soil respiration dynamics under different land management practices in the sub-tropical highland agroecology of Ethiopia. Environ. Monit. Assess. 2023, 195, 65. [Google Scholar] [CrossRef] [PubMed]
- Akande, O.J.; Ma, Z.; Huang, C.; He, F.; Chang, S.X. Meta-analysis shows forest soil CO2 effluxes are dependent on the disturbance regime and biome type. Ecol. Lett. 2023, 26, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bai, Z.; Jin, C.; Zhang, X.; Guan, D.; Wang, A.; Yuan, F.; Wu, J. The influence of tree species on small scale spatial heterogeneity of soil respiration in a temperate mixed forest. Sci. Total Environ. 2017, 590, 242–248. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, B.; Wang, W.; Li, B.; Wu, Z.; Chu, C. Topography and plant community structure contribute to spatial heterogeneity of soil respiration in a subtropical forest. Sci. Total Environ. 2020, 733, 139287. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, G.; Ma, J.; Zhang, K.; Wang, S.; Han, T.; Shang, S. Soil respiration in an irrigated oasis agroecosystem: Linking environmental controls with plant activities on hourly, daily and monthly timescales. Plant Soil 2020, 447, 347–364. [Google Scholar] [CrossRef]
- Wang, X.; Fan, K.; Yan, Y.; Chen, B.; Yan, R.; Xin, X.; Li, L. Controls of seasonal and interannual variations on soil respiration in a meadow steppe in Eastern Inner Mongolia. Agronomy 2022, 13, 20. [Google Scholar] [CrossRef]
- Berryman, E.M.; Barnard, H.; Adams, H.; Burns, M.; Gallo, E.; Brooks, P. Complex terrain alters temperature and moisture limitations of forest soil respiration across a semiarid to subalpine gradient. J. Geophys. Res. Biogeosci. 2015, 120, 707–723. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, Y.; Li, F.; Yang, G.; Wang, J.; Yu, J.; Zhou, G.; Yang, Y. Changes in above-/below-ground biodiversity and plant functional composition mediate soil respiration response to nitrogen input. Funct. Ecol. 2021, 35, 1171–1182. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Tuo, D.; Wang, C.; Fu, B.; Lv, Y.; Liu, G. Land use change and stand age regulate soil respiration by influencing soil substrate supply and microbial community. Geoderma 2020, 359, 113991. [Google Scholar] [CrossRef]
- Hereș, A.M.; Bragă, C.; Petritan, A.M.; Petritan, I.C.; Curiel Yuste, J. Spatial variability of soil respiration (Rs) and its controls are subjected to strong seasonality in an even-aged European beech (Fagus sylvatica L.) stand. Eur. J. Soil Sci. 2021, 72, 1988–2005. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Yan, J.; Li, J. Spatial variation characteristics of soil respiration in subalpine meadows at different sampling scales. Environ. Sci. 2014, 35, 4313–4320. [Google Scholar] [CrossRef]
- Warner, D.; Bond-Lamberty, B.; Jian, J.; Stell, E.; Vargas, R. Spatial predictions and associated uncertainty of annual soil respiration at the global scale. Glob. Biogeochem. Cycles 2019, 33, 1733–1745. [Google Scholar] [CrossRef]
- Cai, Y.; Sawada, K.; Hirota, M. Spatial variation in forest soil respiration: A systematic review of field observations at the global scale. Sci. Total Environ. 2023, 874, 162348. [Google Scholar] [CrossRef]
- Huang, N.; Wang, L.; Song, X.-P.; Black, T.A.; Jassal, R.S.; Myneni, R.B.; Wu, C.; Wang, L.; Song, W.; Ji, D. Spatial and temporal variations in global soil respiration and their relationships with climate and land cover. Sci. Adv. 2020, 6, eabb8508. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.; Warner, D.L.; Bond-Lamberty, B.P.; Stell, E.; Jian, J. Spatial predictions and associated uncertainty of annual soil respiration at the global scale. In Proceedings of the Chapman Conference on Understanding Carbon Climate Feedbacks, San Diego, CA, USA, 20–21 August 2019. [Google Scholar]
- Smith, I.A.; Hutyra, L.R.; Reinmann, A.B.; Thompson, J.R.; Allen, D.W. Evidence for edge enhancements of soil respiration in temperate forests. Geophys. Res. Lett. 2019, 46, 4278–4287. [Google Scholar] [CrossRef]
- Wang, J.; Teng, D.; He, X.; Qin, L.; Yang, X.; Lv, G. Spatial non-stationarity effects of driving factors on soil respiration in an arid desert region. Catena 2021, 207, 105617. [Google Scholar] [CrossRef]
- Hilt, S.; Grossart, H.P.; McGinnis, D.F.; Keppler, F. Potential role of submerged macrophytes for oxic methane production in aquatic ecosystems. Limnol. Oceanogr. 2022, 67, S76–S88. [Google Scholar] [CrossRef]
- Penne, C.; Ahrends, B.; Deurer, M.; Boettcher, J. The impact of the canopy structure on the spatial variability in forest floor carbon stocks. Geoderma 2010, 158, 282–297. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Zhao, T.; Yang, H.; Ali, W.; Chen, J. Different plant species exhibit contrasting root traits and penetration to variation in soil bulk density of clayey red soil. Agron. J. 2022, 114, 867–877. [Google Scholar] [CrossRef]
- Tang, X.; Fan, S.; Zhang, W.; Gao, S.; Chen, G.; Shi, L. Global variability in belowground autotrophic respiration in terrestrial ecosystems. Earth Syst. Sci. Data 2019, 11, 1839–1852. [Google Scholar] [CrossRef]
- Wang, R.; Guo, S.; Jiang, J.; Wu, D.; Li, N.; Zhang, Y.; Liu, Q.; Li, R.; Wang, Z.; Sun, Q. Tree-scale spatial variation of soil respiration and its influence factors in apple orchard in Loess Plateau. Nutr. Cycl. Agroecosyst. 2015, 102, 285–297. [Google Scholar] [CrossRef]
- Matsumoto, K.; Terasawa, K.; Taniguchi, S.; Ohashi, M.; Katayama, A.; Kume, T.; Takashima, A. Spatial and seasonal variations in soil respiration in a subtropical forest in Okinawa, Japan. Ecol. Res. 2023, 38, 479–490. [Google Scholar] [CrossRef]
- Cheng, Z.; Lu, D.; Li, G.; Huang, J.; Sinha, N.; Zhi, J.; Li, S. A random forest-based approach to map soil erosion risk distribution in Hickory Plantations in western Zhejiang Province, China. Remote Sens. 2018, 10, 1899. [Google Scholar] [CrossRef]
- Dong, Y.; Cai, Y.; Li, C.; Wang, H.; Zhou, L.; Sun, J.; Li, C.; Song, B.; Zhou, G. Vertical thermal environment of subtropical broad-leaved urban forests and the influence of canopy structure. Build. Environ. 2022, 224, 109521. [Google Scholar] [CrossRef]
- Davidson, E.; Savage, K.; Verchot, L.; Navarro, R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric. For. Meteorol. 2002, 113, 21–37. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 961–1010. [Google Scholar] [CrossRef]
- Khan, M.; Almazah, M.M.; EIlahi, A.; Niaz, R.; Al-Rezami, A.; Zaman, B. Spatial interpolation of water quality index based on Ordinary kriging and Universal kriging. Geomat. Nat. Hazards Risk 2023, 14, 2190853. [Google Scholar] [CrossRef]
- Klute, A. Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods; American Society of Agronomy, Inc.: Madison, WI, USA, 1986. [Google Scholar]
- Xu, W.; Li, X.; Liu, W.; Li, L.; Hou, L.; Shi, H.; Xia, J.; Liu, D.; Zhang, H.; Chen, Y. Spatial patterns of soil and ecosystem respiration regulated by biological and environmental variables along a precipitation gradient in semi-arid grasslands in China. Ecol. Res. 2016, 31, 505–513. [Google Scholar] [CrossRef]
- Stoyan, H.; De-Polli, H.; Böhm, S.; Robertson, G.P.; Paul, E.A. Spatial heterogeneity of soil respiration and related properties at the plant scale. Plant Soil 2000, 222, 203–214. [Google Scholar] [CrossRef]
- Yim, M.H.; Joo, S.J.; Shutou, K.; Nakane, K. Spatial variability of soil respiration in a larch plantation: Estimation of the number of sampling points required. For. Ecol. Manag. 2003, 175, 585–588. [Google Scholar] [CrossRef]
- Song, Q.-H.; Tan, Z.-H.; Zhang, Y.-P.; Cao, M.; Sha, L.-Q.; Tang, Y.; Liang, N.-S.; Schaefer, D.; Zhao, J.-F.; Zhao, J.-B. Spatial heterogeneity of soil respiration in a seasonal rainforest with complex terrain. Iforest-Biogeosci. For. 2013, 6, 65. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Zhu, H.; Li, Y. Spatial heterogeneity of soil moisture, microbial biomass carbon and soil respiration at stand scale of an arid scrubland. Environ. Earth Sci. 2013, 70, 3217–3224. [Google Scholar] [CrossRef]
- Herbst, M.; Prolingheuer, N.; Graf, A.; Huisman, J.; Weihermüller, L.; Vanderborght, J. Characterization and understanding of bare soil respiration spatial variability at plot scale. Vadose Zone J. 2009, 8, 762–771. [Google Scholar] [CrossRef]
- Knohl, A.; Søe, A.R.; Kutsch, W.L.; Göckede, M.; Buchmann, N. Representative estimates of soil and ecosystem respiration in an old beech forest. Plant Soil 2008, 302, 189–202. [Google Scholar] [CrossRef]
- Jordan, A.; Jurasinski, G.; Glatzel, S. Small scale spatial heterogeneity of soil respiration in an old growth temperate deciduous forest. Biogeosci. Discuss. 2009, 6, 9977–10005. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kwon, D.-J.; Park, C.-W.; Kim, S.-D. Appropriate sampling points and frequency of CO2 measurements for soil respiration analysis in a pine (Pinus densiflora) forest. Anim. Cells Syst. 2015, 19, 332–338. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, N.; Zeng, J.; Mohti, A. A simple model for partitioning forest soil respiration based on root allometry. Soil Biol. Biochem. 2021, 152, 108067. [Google Scholar] [CrossRef]
- Katayama, A.; Kume, T.; Komatsu, H.; Ohashi, M.; Nakagawa, M.; Yamashita, M.; Otsuki, K.; Suzuki, M.; Kumagai, T.O. Effect of forest structure on the spatial variation in soil respiration in a Bornean tropical rainforest. Agric. For. Meteorol. 2009, 149, 1666–1673. [Google Scholar] [CrossRef]
- Liang, Z.; Cao, B.; Jiao, Y.; Liu, C.; Li, X.; Meng, X.; Shi, J.; Tian, X. Effect of the combined addition of mineral nitrogen and crop residue on soil respiration, organic carbon sequestration, and exogenous nitrogen in stable organic matter. Appl. Soil Ecol. 2022, 171, 104324. [Google Scholar] [CrossRef]
- Cao, J.; He, X.; Chen, Y.; Chen, Y.; Zhang, Y.; Yu, S.; Zhou, L.; Liu, Z.; Zhang, C.; Fu, S. Leaf litter contributes more to soil organic carbon than fine roots in two 10-year-old subtropical plantations. Sci. Total Environ. 2020, 704, 135341. [Google Scholar] [CrossRef]
- Lajtha, K.; Peterson, F.; Nadelhoffer, K.; Bowden, R.D. Twenty Years of Litter and Root Manipulations: Insights into Multi-decadal SOM Dynamics; Soil Science Society of America: Madison, WI, USA, 2015. [Google Scholar]
- Fang, X.; Zhao, L.; Zhou, G.; Huang, W.; Liu, J. Increased litter input increases litter decomposition and soil respiration but has minor effects on soil organic carbon in subtropical forests. Plant Soil 2015, 392, 139–153. [Google Scholar] [CrossRef]
- Liu, L.; King, J.S.; Booker, F.L.; Giardina, C.P.; Lee Allen, H.; Hu, S. Enhanced litter input rather than changes in litter chemistry drive soil carbon and nitrogen cycles under elevated CO2: A microcosm study. Glob. Change Biol. 2009, 15, 441–453. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Change Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef] [PubMed]
- Guttières, R.; Nunan, N.; Raynaud, X.; Lacroix, G.; Barot, S.; Barré, P.; Girardin, C.; Guenet, B.; Lata, J.-C.; Abbadie, L. Temperature and soil management effects on carbon fluxes and priming effect intensity. Soil Biol. Biochem. 2021, 153, 108103. [Google Scholar] [CrossRef]
- Singh, S.; Mayes, M.A.; Shekoofa, A.; Kivlin, S.N.; Bansal, S.; Jagadamma, S. Soil organic carbon cycling in response to simulated soil moisture variation under field conditions. Sci. Rep. 2021, 11, 10841. [Google Scholar] [CrossRef]
- Schaeffer, S.M.; Homyak, P.M.; Boot, C.M.; Roux-Michollet, D.; Schimel, J.P. Soil carbon and nitrogen dynamics throughout the summer drought in a California annual grassland. Soil Biol. Biochem. 2017, 115, 54–62. [Google Scholar] [CrossRef]
- Manzoni, S.; Moyano, F.; Kätterer, T.; Schimel, J. Modeling coupled enzymatic and solute transport controls on decomposition in drying soils. Soil Biol. Biochem. 2016, 95, 275–287. [Google Scholar] [CrossRef]
- Rybar, J.; Bosela, M.; Marcis, P.; Ujházyová, M.; Polťák, D.; Hederová, L.; Ujházy, K. Effects of tree canopy on herbaceous understorey throughout the developmental cycle of a temperate mountain primary forest. For. Ecol. Manag. 2023, 546, 121353. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil bulk density as related to soil texture, organic matter content and available total nutrients of Coimbatore soil. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Fukumasu, J.; Jarvis, N.; Koestel, J.; Kätterer, T.; Larsbo, M. Relations between soil organic carbon content and the pore size distribution for an arable topsoil with large variations in soil properties. Eur. J. Soil Sci. 2022, 73, e13212. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Z.; Zhao, T.; Yang, H.; Long, Q.; He, Y. Rotation crop root performance and its effect on soil hydraulic properties in a clayey Utisol. Soil Tillage Res. 2021, 213, 105136. [Google Scholar] [CrossRef]
- Addo-Danso, S.D.; Prescott, C.E.; Smith, A.R. Methods for estimating root biomass and production in forest and woodland ecosystem carbon studies: A review. For. Ecol. Manag. 2016, 359, 332–351. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.; Bergeron, Y.; Paré, D. Coarse root biomass allometric equations for Abies balsamea, Picea mariana, Pinus banksiana, and Populus tremuloides in the boreal forest of Ontario, Canada. Biomass Bioenergy 2011, 35, 4189–4196. [Google Scholar] [CrossRef]
- Virgulino-Júnior, P.C.C.; Carneiro, D.N.; Nascimento, W.R., Jr.; Cougo, M.F.; Fernandes, M.E.B. Biomass and carbon estimation for scrub mangrove forests and examination of their allometric associated uncertainties. PLoS ONE 2020, 15, e0230008. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Frissell, M.; Hao, D.; Tang, X.; Berryman, E.; Bond-Lamberty, B. The global contribution of roots to total soil respiration. Glob. Ecol. Biogeogr. 2022, 31, 685–699. [Google Scholar] [CrossRef]
- Hicks Pries, C.; Angert, A.; Castanha, C.; Hilman, B.; Torn, M.S. Using respiration quotients to track changing sources of soil respiration seasonally and with experimental warming. Biogeosciences 2020, 17, 3045–3055. [Google Scholar] [CrossRef]
- Wang, R.; Bicharanloo, B.; Shirvan, M.B.; Cavagnaro, T.R.; Jiang, Y.; Keitel, C.; Dijkstra, F.A. A novel 13C pulse-labelling method to quantify the contribution of rhizodeposits to soil respiration in a grassland exposed to drought and nitrogen addition. New Phytol. 2021, 230, 857–866. [Google Scholar] [CrossRef]
- Hermans, R.; McKenzie, R.; Andersen, R.; Teh, Y.A.; Cowie, N.; Subke, J.-A. Net soil carbon balance in afforested peatlands and separating autotrophic and heterotrophic soil CO2 effluxes. Biogeosciences 2022, 19, 313–327. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Guan, D.; Chen, Y.; Motelica-Heino, M.; Peng, Y.; Lee, S.Y. The role of mangrove fine root production and decomposition on soil organic carbon component ratios. Ecol. Indic. 2021, 125, 107525. [Google Scholar] [CrossRef]
- Forsmark, B.; Nordin, A.; Maaroufi, N.I.; Lundmark, T.; Gundale, M.J. Low and high nitrogen deposition rates in northern coniferous forests have different impacts on aboveground litter production, soil respiration, and soil carbon stocks. Ecosystems 2020, 23, 1423–1436. [Google Scholar] [CrossRef]
- Xiao, H.; Shi, Z.; Li, Z.; Wang, L.; Chen, J.; Wang, J. Responses of soil respiration and its temperature sensitivity to nitrogen addition: A meta-analysis in China. Appl. Soil Ecol. 2020, 150, 103484. [Google Scholar] [CrossRef]
- Ma, J.; Liu, R.; Li, C.; Fan, L.; Xu, G.; Li, Y. Herbaceous layer determines the relationship between soil respiration and photosynthesis in a shrub-dominated desert plant community. Plant Soil 2020, 449, 193–207. [Google Scholar] [CrossRef]
- Jiang, L.; Wan, S.; Li, L. Species diversity and productivity: Why do results of diversity-manipulation experiments differ from natural patterns. J. Ecol. 2009, 97, 603–608. [Google Scholar] [CrossRef]
- Riaz, M.; Marschner, P.; Nutrition, P. Sandy soil amended with clay soil: Effect of clay soil properties on soil respiration, microbial biomass, and water extractable organic C. J. Soil Sci. 2020, 20, 2465–2470. [Google Scholar] [CrossRef]
- Sharmistha, P.; Marschner, P. Soil respiration, microbial biomass C and N availability in a sandy soil amended with clay and residue mixtures. Pedosphere 2016, 26, 643–651. [Google Scholar] [CrossRef]
- Fernandez, D.P.; Neff, J.C.; Belnap, J.; Reynolds, R.L. Soil respiration in the cold desert environment of the Colorado Plateau (USA): Abiotic regulators and thresholds. Biogeochemistry 2006, 78, 247–265. [Google Scholar] [CrossRef]
- Mavi, M.S.; Marschner, P.; Chittleborough, D.J.; Cox, J.W.; Sanderman, J. Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture. Soil Biol. Biochem. 2012, 45, 8–13. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, Z.; Fu, Z.; Zhang, Y.; Peng, X.; Zheng, J. Microscale heterogeneity controls macroscopic soil heterotrophic respiration by regulating resource availability and environmental stress. Biogeochemistry 2023, 164, 431–449. [Google Scholar] [CrossRef]


| Parameter | Range |
|---|---|
| Range | 0~5000 ppm |
| Accuracy | ±5% of the reading |
| Operating temperature | −10~50 °C |
| Operating humidity | 0~95% |
| Variables | Mean | Min. | Max. | SD. | CV(%) | S-W |
|---|---|---|---|---|---|---|
| SR (μmol m−2 s−1) | 2.71 | 1.89 | 4.83 | 0.51 | 18.82 | <0.001 |
| T5 (℃) | 17.33 | 16.48 | 18.84 | 0.50 | 2.89 | 0.016 |
| SWC (%) | 55.83 | 42.16 | 68.83 | 6.34 | 11.36 | 0.914 |
| AGB (g m−2) | 39.00 | 3.80 | 96.80 | 26.11 | 66.95 | 0.011 |
| DBH5m (cm) | 65.25 | 0 | 172.9 | 41.59 | 63.74 | 0.158 |
| VCC (%) | 60.54 | 0 | 95.15 | 17.76 | 29.33 | 0.046 |
| SOC (%) | 2.22 | 0.96 | 7.41 | 1.04 | 46.85 | <0.001 |
| MBC (mg kg−1) | 204.33 | 85.46 | 399.16 | 55.32 | 27.07 | 0.073 |
| NH4+-N (mg kg−1) | 9.31 | 3.34 | 23.03 | 4.05 | 43.50 | 0.003 |
| NO3−-N (mg kg−1) | 6.91 | 1.96 | 18.66 | 2.94 | 42.55 | <0.001 |
| pH | 6.21 | 5.68 | 6.62 | 0.28 | 4.51 | 0.001 |
| Sand (%) | 7.24 | 0.98 | 15.94 | 4.37 | 60.36 | 0.029 |
| Silt (%) | 76.56 | 63.72 | 85.57 | 5.35 | 6.99 | 0.006 |
| Clay (%) | 16.14 | 4.43 | 28.06 | 4.56 | 28.25 | 0.167 |
| BD (g cm−3) | 1.56 | 1.28 | 1.74 | 0.11 | 7.05 | 0.200 |
| SSA (m2 kg−1) | 531.29 | 318.30 | 764.90 | 98.50 | 18.54 | 0.440 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Cai, Y.; Pan, C.; Jiang, H.; Jia, Z.; Li, C.; Zhou, G. Spatial Heterogeneity of Soil Respiration and Its Relationship with the Spatial Distribution of the Forest Ecosystem at the Fine Scale. Forests 2025, 16, 678. https://doi.org/10.3390/f16040678
Chen Z, Cai Y, Pan C, Jiang H, Jia Z, Li C, Zhou G. Spatial Heterogeneity of Soil Respiration and Its Relationship with the Spatial Distribution of the Forest Ecosystem at the Fine Scale. Forests. 2025; 16(4):678. https://doi.org/10.3390/f16040678
Chicago/Turabian StyleChen, Zhihao, Yue Cai, Chunyu Pan, Hangjun Jiang, Zichen Jia, Chong Li, and Guomo Zhou. 2025. "Spatial Heterogeneity of Soil Respiration and Its Relationship with the Spatial Distribution of the Forest Ecosystem at the Fine Scale" Forests 16, no. 4: 678. https://doi.org/10.3390/f16040678
APA StyleChen, Z., Cai, Y., Pan, C., Jiang, H., Jia, Z., Li, C., & Zhou, G. (2025). Spatial Heterogeneity of Soil Respiration and Its Relationship with the Spatial Distribution of the Forest Ecosystem at the Fine Scale. Forests, 16(4), 678. https://doi.org/10.3390/f16040678







