Epiphytes as Environmental Bioindicators in Forest Remnants of the Pisaca Reserve: Preserving the Unique Pre-Inca Artificial Wetland of Paltas, Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Desing and Data Sampling
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | ZB | ZM | ZA |
|---|---|---|---|
| Bryophytes | |||
| Bryopteris flicina (Sw.) Nees. | 0 | 6 | 4 |
| Frullania ericoides (Nees) Mont. | 30 | 2 | 16 |
| Lejeunea laetevirens Nees & Mont. | 9 | 9 | 21 |
| Diplasiolejeunea cavifolia Steph. | 1 | 7 | 5 |
| Metzgeria lechleri Steph. | 1 | 3 | 4 |
| Metzgeria rufula Spruce. | 1 | 3 | 7 |
| Plagiochila aff. simplex (Sw.) Lindenb. | 1 | 9 | 7 |
| Porella leiboldii (Lehm.) Trevis | 9 | 7 | 30 |
| Radula javanica Gottsche. | 0 | 11 | 0 |
| Radula quadrata Gottsche. | 0 | 1 | 0 |
| Thysananthus auriculatus (Wilson & Hook.) Sukkharak & Gradst. | 9 | 5 | 17 |
| Brachythecium plumosum (Hedw.) Schimp. | 5 | 0 | 2 |
| Campylopus richardii Brid. | 2 | 0 | 4 |
| Cryphaea patens Müll. Hal. | 4 | 0 | 3 |
| Cyrto-hypnum minutulum (Hedw.) W.R.Buck & H.A.Crum. | 0 | 1 | 0 |
| Fabronia ciliaris (Brid.) Brid. | 11 | 8 | 12 |
| Fissidens steerei Grout. | 5 | 15 | 6 |
| Leptodotium longicaule Mitt., J. Linn. Soc., Bot. var Longicaule. | 3 | 0 | 1 |
| Macromitrium podocarpi Müll. Hal. | 3 | 1 | 16 |
| Neckera chilensis Mont. | 0 | 25 | 22 |
| Porotrichum expansum (Taylor) Mitt. | 0 | 8 | 2 |
| Orthostichella pentasticha (Brid.) W.R. Buck. | 3 | 15 | 4 |
| Rhynchostegium scariosum (Taylor) A.Jaeger. | 0 | 3 | 1 |
| Squamidium macrocarpum (Mitt.) Broth. | 5 | 15 | 6 |
| Syntrichia amphidiacea (Müll. Hal.) R.H.Zander. | 1 | 0 | 1 |
| Lichens | |||
| Arthonia ilicina Taylor | 0 | 0 | 2 |
| Bacidia sp1 | 9 | 2 | 15 |
| Bacidia sp2 | 4 | 0 | 0 |
| Bulbothrix isidiza (Nyl.) Hale | 1 | 0 | 0 |
| Caloplaca sp1 | 2 | 0 | 1 |
| Caloplaca sp2 | 1 | 0 | 0 |
| Chrysothrix candelaris (L.) J. R. Laundon | 6 | 0 | 0 |
| Coccocarpia palmicola (Sprengel) Arv. y DJ Galloway | 0 | 0 | 1 |
| Coenogonium luteum (Dicks.) Kalb & Lücking | 0 | 0 | 2 |
| Coenogonium roumeguerianum (Müll. Arg.) Kalb | 0 | 1 | 6 |
| Coniocarpon cinnabarinum DC. | 1 | 0 | 0 |
| Dirinaria picta (Sw.) Clem. y esquivar | 4 | 0 | 3 |
| Fissurina columbina (Tuck.) Staiger | 0 | 1 | 2 |
| Flavoparmelia sp. | 3 | 0 | 2 |
| Flavoparmelia ecuadorensis T.H. Nash, Elix & J. Johnst. | 1 | 0 | 2 |
| Flavoplaca citrina (Hoffm.) Arup, Frödén & Søchting | 3 | 0 | 5 |
| Glyphis cicatricosa Ach. | 3 | 0 | 1 |
| Graphis elegans (Borrer ex Sm.) Ach. | 1 | 1 | 0 |
| Graphis leptoclada Müll. Arg. | 12 | 5 | 5 |
| Herpothallon granulare (Sipman) Aptroot & Lücking | 1 | 4 | 1 |
| Heterodermia sp1 | 1 | 0 | 1 |
| Heterodermia sp2 | 0 | 0 | 2 |
| Heterodermia granulifera (Ach.) Culb. | 11 | 1 | 16 |
| Hypotrachyna sp. | 1 | 0 | 0 |
| Hypotrachyna cirrhata (Fr.) Divakar, A. Crespo, Sipman, Elix & Lumbsch | 2 | 0 | 3 |
| Hypotrachyna lipidifera (Hale & M. Wirth) Divakar, A. Crespo, Sipman, Elix & Lumbsch | 0 | 2 | 0 |
| Lecanora chlarotera Nyl. | 2 | 1 | 1 |
| Lecanora leprosa Fée | 7 | 0 | 2 |
| Lecanora tropica Zahlbr. | 5 | 0 | 2 |
| Lepraria sp. | 2 | 0 | 3 |
| Leptogium chloromelum (Ach.) Nyl. | 3 | 2 | 16 |
| Leptogium milligranum Sierk | 8 | 2 | 20 |
| Leptogium phyllocarpum (Pers.) Mont. | 5 | 0 | 2 |
| Leptogium aff. pseudofurfuraceum P.M. Jørg. & Wallace | 0 | 0 | 2 |
| Lobariella exornata (Zahlbr.) Yoshim. | 0 | 0 | 2 |
| Lobariella subexornata (Yoshim.) Yoshim. | 0 | 7 | 18 |
| Leucodermia leucomelos (L.) Kalb | 10 | 0 | 0 |
| Parmotrema chinense (Osbeck) Hale & Ahti | 2 | 0 | 2 |
| Parmotrema fasciculatum (Van.) Hale | 1 | 0 | 1 |
| Parmotrema mellissii (C.W. Dodge) Hale | 4 | 0 | 3 |
| Parmotrema reticulatum (Taylor) M. Choisy | 5 | 0 | 7 |
| Parmotrema robustum (Degel.) Hale | 5 | 0 | 6 |
| Parmotrema subisidiosum (Müll. Arg.) Hale & Fletcher | 1 | 0 | 2 |
| Parmotrema subsumptum (Nyl.) Hale | 5 | 0 | 2 |
| Pertusaria sp. | 1 | 0 | 1 |
| Pertusaria texana Müll. Arg. | 20 | 12 | 13 |
| Phaeographis sp. | 5 | 0 | 0 |
| Phaeographis dendritica (Ach.) Müll. Arg. | 3 | 0 | 6 |
| Phaeographis scalpturata (Ach.) Staiger | 0 | 2 | 3 |
| Phyllopsora buettneri (Müll.Arg.) Zahlbr. | 0 | 3 | 1 |
| Phyllopsora parvifolia (Pers.) Müll. Arg. | 0 | 2 | 1 |
| Phyllopsora aff. parvifoliella (Nyl.) Müll. Arg. | 2 | 0 | 0 |
| Phyllopsora furfuracea (Pers.) Zahlbr. | 0 | 5 | 2 |
| Physcia lacinulata Müll. Arg. | 10 | 0 | 1 |
| Polyblastidium albicans (Pers.) SY Kondr., Lőkös & Hur | 0 | 0 | 2 |
| Porina aff. nucula Ach. | 5 | 3 | 3 |
| Pyrenula sp. | 2 | 1 | 7 |
| Ramalina celastri (Sprengel) Krog & Swinscow | 3 | 0 | 0 |
| Ramboldia aff. haematites (Fée) Kalb, Lumbsch & Elix | 2 | 0 | 0 |
| Sticta beauvoisii Delise | 1 | 0 | 5 |
| Sticta aff. damicornis (Sw.) Ach. | 1 | 1 | 2 |
| Syncesia farinacea (Fée) Tehler | 1 | 0 | 2 |
| Teloschistes flavicans (Sw.) Norman | 2 | 0 | 0 |
| Usnea cornuta Körb. | 7 | 0 | 0 |
| Usnea strigosa (Ach.) Eaton | 4 | 0 | 0 |
References
- Ministerio del Ambiente del Ecuador, MAE. Sistema de Clasificación de los Ecosistemas del Ecuador Continental; Subsecretaría de Patrimonio Natural: Quito, Ecuador, 2012. [Google Scholar]
- Werner, F.A.; Gradstein, S.R. Diversity of dry forest epiphytes along a gradient of human disturbance in the tropical andes. J. Veg. Sci. 2009, 20, 59–68. [Google Scholar] [CrossRef]
- Cueva, E.; Lozano, D.; Yaguana, C. Efecto de la gradiente altitudinal sobre la composición florística, estructura y biomasa arbórea del bosque seco andino, Loja, Ecuador. Bosque 2019, 40, 365–378. [Google Scholar]
- Cadena-Ortiz, H.; Varela, S.; Bahamonde-Vinueza, D.; Freile, J.F.; Bonaccorso, E. Birds of Bosque Protector Jerusalem, Guayllabamba Valley, Ecuador. Check List 2015, 11, 1770. [Google Scholar] [CrossRef][Green Version]
- de la Cadena-Mendoza, G.N.; Ramón-Cabrera, G.M. Diversity of Beetles (Coleoptera) in an Inter-Andean Dry Tropical Forest in Ecuador. Coleopt. Bull. 2023, 77, 561–580. [Google Scholar]
- Werner, F.A.; Gradstein, S.R. Spatial Distribution and Abundance of Epiphytes along a Gradient of Human Disturbance in an Interandean Dry Valley, Ecuador. Selbyana 2010, 30, 208–215. [Google Scholar]
- Benítez, Á.; Aragón, G.; Prieto, M. Lichen diversity on tree trunks in tropical dry forests is highly influenced by host tree traits. Biodivers. Conserv. 2019, 28, 2909–2929. [Google Scholar]
- Benítez, Á.; Ortiz, J.; Matamoros-Apolo, D.; Bustamante, A.; López, F.; Yangua-Solano, E.; Gusmán-Montalván, E. Forest disturbance determines diversity of epiphytic lichens and bryophytes on trunk bases in tropical dry forests. Forests 2024, 15, 1565. [Google Scholar] [CrossRef]
- Wolf, J.H.D. Diversity Patterns and biomass of epiphytic bryophytes and lichens along an altitudinal gradient in the Northern Andes. Ann. Missouri Bot. Gard. 1993, 80, 928–960. [Google Scholar] [CrossRef]
- Hauck, M.; Spribille, T. The significance of precipitation and substrate chemistry for epiphytic lichen diversity in spruce-fir forests of the Salish Mountains, northwestern Montana. Flora-Morphol. Distrib. Funct. Ecol. Plants 2005, 6, 547–562. [Google Scholar] [CrossRef]
- Werth, S.; Wagner, H.H.; Gugerli, F.; Holderegger, R.; Csencsics, D.; Kalwij, J.M.; Scheidegger, C. Quantifying dispersal and establishment limitation in a population of an epiphytic lichen. Ecology 2006, 87, 2037–2046. [Google Scholar]
- Aragón, G.; Martínez, I.; García, A. Loss of epiphytic diversity along a latitudinal gradient in southern Europe. Sci. Total Environ. 2012, 426, 188–195. [Google Scholar]
- Király, I.; Nascimbene, J.; Tinya, F.; Ódor, P. Factors influencing epiphytic bryophyte and lichen species richness at different spatial scales in managed temperate forests. Biodivers. Conserv. 2013, 22, 209–223. [Google Scholar]
- de Menezes, A.A.; da Silva Cáceres, M.E.; Bastos, C.J.P.; Lücking, R. The latitudinal diversity gradient of epiphytic lichens in the Brazilian Atlantic Forest: Does Rapoport’s rule apply? Bryologist 2018, 121, 480–497. [Google Scholar] [CrossRef]
- Zhang, Y.; He, N.; Liu, Y. Temperature factors are a primary driver of the forest bryophyte diversity and distribution in the southeast Qinghai-Tibet Plateau. For. Ecol. Manag. 2023, 527, 120610. [Google Scholar]
- Wolseley, P.A.; Aguirre-Hudson, B. The ecology and distribution of lichens in tropical deciduous and evergreen forests of Northern Thailand. J. Biogeogr. 1997, 24, 327–343. [Google Scholar] [CrossRef]
- Holz, I.; Gradstein, R.S. Cryptogamic epiphytes in primary and recovering upper montane oak forests of Costa Rica–species richness, community composition and ecology. Plant Ecol. 2005, 178, 89–109. [Google Scholar]
- Cáceres, M.E.; Lücking, R.; Rambold, G. Phorophyte specificity and environmental parameters versus stochasticity as determinants for species composition of corticolous crustose lichen communities in the Atlantic rain forest of northeastern Brazil. Mycol. Prog. 2007, 6, 117–136. [Google Scholar]
- Aragón, G.; Martínez, I.; de la Cruz, M.; Hurtado, P. High Host Preferences in Epiphytic Lichens Across Diverse Phorophyte Species in the Mediterranean Region. J. Fungi 2025, 11, 104. [Google Scholar] [CrossRef]
- Nöske, N.M.; Hilt, N.; Werner, F.A.; Brehm, G.; Fiedler, K.; Sipman, H.J.; Gradstein, S.R. Disturbance effects on diversity of epiphytes and moths in a montane forest in Ecuador. Basic Appl. Ecol. 2008, 9, 4–12. [Google Scholar] [CrossRef]
- Peñate-Pacheco, L.; Gil-Novoa, J.E.; Carillo-Fajardo, M.Y. Diversidad taxonómica y funcional de briófitos en diferentes coberturas de un bosque seco tropical, Córdoba (Colombia). Bol. Soc. Argent. Bot. 2022, 57, 687. [Google Scholar] [CrossRef]
- Kantvilas, G.; Jarman, S.J.; Minchin, P.R. Early impacts of disturbance on lichens, mosses and liverworts in Tasmania’s wet eucalypt production forests. Aust. For. 2015, 78, 92–107. [Google Scholar]
- Larsen, R.S.; Bell, J.N.B.; James, P.W.; Chimonides, P.J.; Rumsey, F.J.; Tremper, A.; Purvis, O.W. Lichen and bryophyte distribution on oak in London in relation to air pollution and bark acidity. Environ. Pollut. 2007, 146, 332–340. [Google Scholar]
- Mallen-Cooper, M.; Rodríguez-Caballero, E.; Eldridge, D.J.; Weber, B.; Büdel, B.; Höhne, H.; Cornwell, W.K. Towards an understanding of future range shifts in lichens and mosses under climate change. J. Biogeogr. 2023, 50, 406–417. [Google Scholar]
- Baquero, F.; Sierra, R.; Ordóñez, L.; Tipán, M.; Espinosa, L.; Belen Rivera, M.; Soria, P. La Vegetación de los Andes del Ecuador; EcoCiencia/CESLA/EcoPar/MAG SIGAGRO/CDC-JATUN SACHA/División Geográfica—IGM: Quito, Ecuador, 2004. [Google Scholar]
- Albarracín, M.; Ramón, G.; González, J.; Iñiguez-Armijos, C.; Zakaluk, T.; Martos-Rosillo, S. The ecohydrological approach in water sowing and harvesting systems: The case of the Paltas Catacocha ecohydrology demonstration site, Ecuador. Ecohydrol. Hydrobiol. 2021, 21, 454–466. [Google Scholar] [CrossRef]
- Díaz, L.; Campoverde, S.; Loarte, M.; Guaya, P. Importance of the landscape as a resource in tourist planning. Rev. Tur. Desenvolv. 2021, 37, 31–45. [Google Scholar] [CrossRef]
- Villacrés, D.; Flores, L.; Cartuche, H. Efecto del Acondicionador de Suelo Terracottem Sobre el Prendimiento y Desarrollo de Caesalpinia Spinosa Kuntze en la Reserva Pisaca, Cantón Paltas, Provincia de Loja. Bachelor’s Thesis, Universidad Nacional de Loja, Loja, Ecuador, 2013; pp. 1–123. [Google Scholar]
- Encalada, D.; Castro, L.M.; Cabrera, O.; Ramón, P.; Reyes-Bueno, F.; Paul, C. Factors influencing the expressed willingness to transition from collection to cultivation of non-timber forest products: The case of Caesalpinia spinosa in southern Ecuador. For. Policy Econ. 2025, 170, 103366. [Google Scholar] [CrossRef]
- Paladines, J.E. Ruta Arqueológica Vivencial del Cantón Paltas. Bachelor’s Thesis, Universidad del Azuay, Cuenca, Ecuador, 2019; pp. 1–177. [Google Scholar]
- Benítez, A.; Aragón, G.; González, Y.; Prieto, M. Functional traits of epiphytic lichens in response to forest disturbance and as predictors of total richnes and diversity. Ecol. Indic. 2018, 86, 18–26. [Google Scholar] [CrossRef]
- Guerra, G.; Arrocha, C.; Rodríguez, G.; Déleg, J.; Benítez, Á. Briófitos en los troncos de árboles como indicadores de la alteración en bosques montanos de Panamá. Rev. Biol. Trop. 2020, 68, 492–502. [Google Scholar] [CrossRef]
- Brodo, I.M. Lichens of North America; Yale University Press: New Haven, CT, USA, 2001; Volume 828. [Google Scholar]
- Nash, T.H., III; Ryan, B.D.; Gries, C.; Bungartz, F. Lichen Flora of the Greater Sonoran Desert Region; Lichens Unlimited: Tempe, AZ, USA, 2002; Volume 1. [Google Scholar]
- Nash, T.H., III; Ryan, B.D.; Diederich, P.; Gries, C.; Bungartz, F. Lichen Flora of the Greater Sonoran Desert Region; Lichen Unlimited: Tempe, AZ, USA, 2004; Volume 2. [Google Scholar]
- Nash, T.H., III; Gries, C.; Bungartz, F. Lichen Flora of the Greater Sonoran Desert Region; Lichen Unlimited: Tempe, AZ, USA, 2007; Volume 3. [Google Scholar]
- Churchill, S.P.; Linares, C.E. Prodromus Bryologiae Novo-Granatensis: Introduccion a la Flora de Musgos de Colombia; Instituto de Ciencias Naturales: Bogota, Colombia, 1995. [Google Scholar]
- Gradstein, S.R.; da Costa, D.P. The Hepaticae and Anthocerotae of Brazil; New York Botanical Garden Press: New York, NY, USA, 2003; Volume 87, pp. 1–318. [Google Scholar]
- Gradstein, S.R. Checklist of the liverworts and hornworts of Ecuador. Frahmia 2020, 17, 1–40. [Google Scholar]
- Gradstein, S.R. The Liverworts and Hornworts of Colombia and Ecuador; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Tehler, A. Syncesia (Arthoniales, Euascomycetidae). Flora Neotrop. 1997, 74, 1–48. [Google Scholar]
- Rivas-Plata, E.R.; Lücking, R.; Aptroot, A.; Sipman, H.J.M.; Chaves, J.L.; Umaña, L.; Lizano, D. A first assessment of the Ticolichen biodiversity inventory in Costa Rica: The genus Coenogonium (Ostropales: Coenogoniaceae), with a world-wide key and checklist and a phenotype-based cladistic analysis. Fungal Divers. 2006, 23, 255–321. [Google Scholar]
- Aptroot, A. A world key to the species of Anthracothecium and Pyrenula. Lichenologist 2012, 44, 5–53. [Google Scholar] [CrossRef]
- Lücking, R.; Plata, E.R. Clave y guía ilustrada para géneros de Graphidaceae. Glalia 2008, 1, 1–39. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.A. Analyzing Ecological Data; Springer: New York, NY, USA, 2007. [Google Scholar]
- Oksanen, J.; Ovaskainen, O.; de Jonge, M.M.J.; Lehikoinen, A.; Ábrego, N.; Opedal, Ø.H.; Tikhonov, G. Modelado conjunto de distribución de especies con el paquete r Hmsc. Métodos Ecol. Evol. 2019, 11, 442–447. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Roberts, D.W. Package “Labdsv”. 2012. Available online: http://cran.rproject.org/web/packages/labdsv/labdsv.pdf (accessed on 1 September 2021).
- Benítez, Á.; Prieto, M.; Aragón, G. Large trees and dense canopies: Key factors for maintaining high epiphytic diversity on trunk bases (bryophytes and lichens) in tropical montane forests. For. J. For. Res 2015, 88, 521–527. [Google Scholar] [CrossRef]
- Benítez, Á.; Nagua, R.; Medina, J.; Lapo, G.; Yangua-Solano, E.; Andrade-Hidalgo, R. Bryophytes as indicators of disturbance in one of the last Remnants of the mountain forests of el Oro province, Ecuador. Plants 2025, 14, 184. [Google Scholar] [CrossRef]
- Benítez, Á.; Prieto, M.; González, Y.; Aragón, G. Effects of tropical montane forest disturbance on epiphytic macrolichens. Sci. Total Environ. 2012, 441, 169–175. [Google Scholar] [CrossRef]
- España-Puccini, P.; Gómez, J.P.; Muñoz-Acevedo, A.; PosadaEcheverría, D.; Martínez-Habibe, M.C. Analysis of the Diversity of Corticolous Lichens Associated with Tree Trunks in the Understories of Four Tropical Dry Forests of the Atlántico Department in Colombia. Forests 2024, 15, 2000. [Google Scholar] [CrossRef]
- Zárate-Arias, N.; Moreno-Palacios, M.; Torres-Benitez, A. Diversidad, especificidad de forófito y preferencias microambientales de líquenes cortícolas de un bosque subandino en la región centro de Colombia. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2019, 43, 737–745. [Google Scholar]



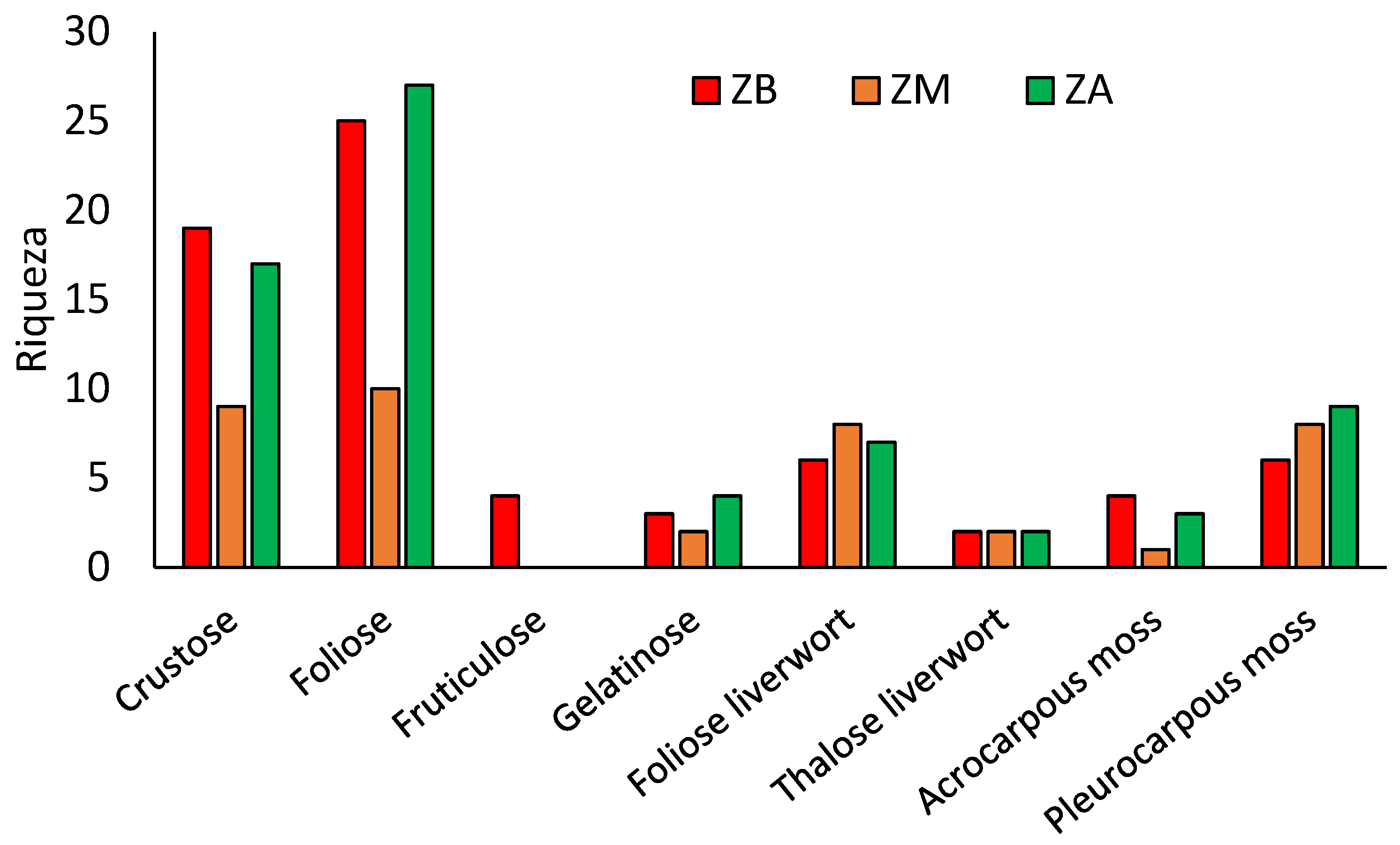
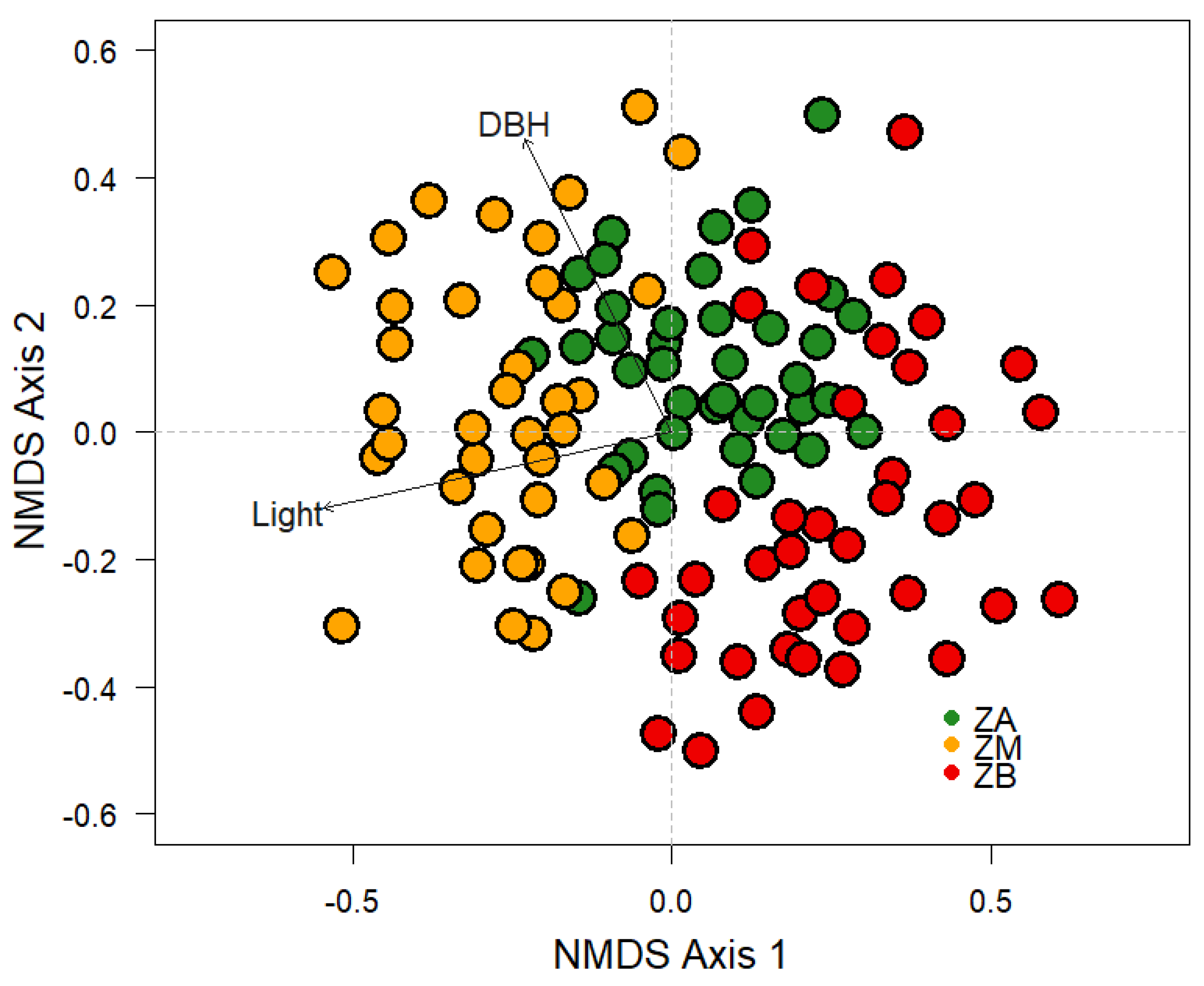


| Richness | Stimator | St | t | p-Value |
|---|---|---|---|---|
| Zone ZA | 8.60051 | 1.27718 | 6.734 | <0.0001 |
| Zone ZB | −2.65262 | 0.68202 | −3.889 | 0.00016 |
| Zone ZM | −5.25705 | 0.81233 | −6.472 | <0.0001 |
| Light | 0.06294 | 0.07862 | 0.801 | 0.42500 |
| DAP | 0.02876 | 0.02493 | 1.154 | 0.25104 |
| Coverage | ||||
| Zone ZA | 94.6510 | 16.5333 | 5.725 | <0.0001 |
| Zone ZB | −23.6311 | 8.8288 | −2.677 | 0.0085 |
| Zone ZM | −23.1332 | 10.5158 | −2.200 | 0.0298 |
| Light | 0.4593 | 0.3228 | 1.423 | 0.1573 |
| DAP | −0.1243 | 1.0177 | −0.122 | 0.9030 |
| Shannon-Weaver | ||||
| Zone ZA | 1.891972 | 0.189714 | 9.973 | <0.0001 |
| Zone ZB | −0.364373 | 0.101308 | −3.597 | 0.000476 |
| Zone ZM | −0.805877 | 0.120665 | −6.679 | <0.0001 |
| Light | 0.008208 | 0.011678 | 0.703 | 0.483580 |
| DAP | 0.001573 | 0.003704 | 0.425 | 0.671860 |
| Simpson | ||||
| Zone ZA | 0.8148280 | 0.0634774 | 12.837 | <0.0001 |
| Zone ZB | −0.0838579 | 0.0338972 | −2.474 | 0.0148 |
| Zone ZM | −0.2083502 | 0.0403738 | −5.161 | <0.0001 |
| Light | 0.0013782 | 0.0039074 | 0.353 | 0.7249 |
| DAP | 0.0002617 | 0.0012392 | 0.211 | 0.8331 |
| Factor | Df | SS | R2 | F | p-Value |
|---|---|---|---|---|---|
| Zone | 2 | 5.721 | 0.12049 | 8.1466 | 0.001 |
| Light | 1 | 0.751 | 0.01581 | 2.1381 | 0.005 |
| DAP | 1 | 0.630 | 0.01327 | 1.7951 | 0.015 |
| Residual | 115 | 40.380 | 0.85043 | ||
| Total | 119 | 48.482 | 1.00000 |
| Species | Zone | Indicator Value | p-Value | Growth Forms |
|---|---|---|---|---|
| Bryophytes | ||||
| Frullania ericoides (Nees) Mont. | ZB | 56.03 | 0.0001 | Foliose liverwort |
| Porella leiboldii (Lehm.) Trevis | ZA | 53.28 | 0.0001 | Foliose liverwort |
| Neckera chilensis Mont. | ZM | 34.93 | 0.0003 | Pleurocarpous moss |
| Macromitrium podocarpi Müll. Hal. | ZA | 31.49 | 0.0001 | Acrocarpous moss |
| Radula javanica Gottsche | ZM | 27.5 | 0.0001 | Foliose liverwort |
| Fissidens steerei Grout. | ZM | 27.08 | 0.0012 | Acrocarpous moss |
| Squamidium macrocarpum (Mitt.) Broth. | ZM | 27.08 | 0.0012 | Pleurocarpous moss |
| Thysananthus auriculatus (Wilson & Hook.) Sukkharak & Gradst. | ZA | 26.89 | 0.0007 | Foliose liverwort |
| Orthostichella pentasticha (Brid.) W.R. Buck. | ZM | 26.72 | 0.0001 | Pleurocarpous moss |
| Lejeunea laetevirens Nees & Mont | ZA | 18.44 | 0.0407 | Foliose liverwort |
| Porotrichum expansum (Taylor) Mitt. | ZM | 17.07 | 0.0012 | Pleurocarpous moss |
| Metzgeria rufula Spruce | ZA | 11.25 | 0.0228 | Thalose liverwort |
| Diplasiolejeunea cavifolia Steph | ZM | 10.94 | 0.0399 | Foliose liverwort |
| Brachythecium plumosum (Hedw.) Schimp | ZB | 10.34 | 0.0281 | Pleurocarpous moss |
| Bryopteris flicina (Sw.) Nees. | ZM | 9.057 | 0.0495 | Foliose liverwort |
| Lichens | ||||
| Lobariella subexornata (Yoshim.) Yoshim. | ZA | 37.5 | 0.0001 | Foliose |
| Leptogium chloromelum (Ach.) Nyl. | ZA | 32.86 | 0.0001 | Gelatinose |
| Leptogium milligranum Sierk | ZA | 28.53 | 0.0004 | Gelatinose |
| Leucodermia leucomelos (L.) Kalb | ZB | 25 | 0.0001 | Foliose |
| Pertusaria texana Müll. Arg. | ZB | 22.94 | 0.0247 | Crustose |
| Physcia lacinulata Müll. Arg. | ZB | 21.55 | 0.0001 | Foliose |
| Graphis leptoclada Müll. Arg. | ZB | 17.56 | 0.0079 | Crustose |
| Usnea cornuta Körb | ZB | 17.5 | 0.0006 | Fruticulose |
| Chrysothrix candelaris (L.) J. R. Laundon | ZB | 15 | 0.001 | Crustose |
| Lecanora leprosa Fée | ZB | 13.75 | 0.0051 | Crustose |
| Coenogonium roumeguerianum (Müll. Arg.) Kalb | ZA | 13.7 | 0.0032 | Crustose |
| Phaeographis dendritica (Ach.) Müll. Arg. | ZA | 11.25 | 0.0193 | Crustose |
| Phyllopsora furfuracea (Pers.) Zahlbr. | ZM | 11.18 | 0.02 | Squmulose |
| Sticta beauvoisii Delise | ZA | 10.94 | 0.0136 | Foliose |
| Usnea strigosa (Ach.) Eaton | ZB | 10 | 0.0118 | Fruticulose |
| Lecanora tropica Zahlbr. | ZB | 9.375 | 0.033 | Crustose |
| Leptogium phyllocarpum (Pers.) Mont. | ZB | 9.375 | 0.0331 | Gelatinose |
| Ramalina celastri (Sprengel) Krog & Swinscow | ZB | 7.5 | 0.0338 | Fruticulose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganazhapa-Plasencia, M.; Yangua-Solano, E.; Ruiz, L.; Andrade-Hidalgo, R.; Benítez, Á. Epiphytes as Environmental Bioindicators in Forest Remnants of the Pisaca Reserve: Preserving the Unique Pre-Inca Artificial Wetland of Paltas, Ecuador. Forests 2025, 16, 628. https://doi.org/10.3390/f16040628
Ganazhapa-Plasencia M, Yangua-Solano E, Ruiz L, Andrade-Hidalgo R, Benítez Á. Epiphytes as Environmental Bioindicators in Forest Remnants of the Pisaca Reserve: Preserving the Unique Pre-Inca Artificial Wetland of Paltas, Ecuador. Forests. 2025; 16(4):628. https://doi.org/10.3390/f16040628
Chicago/Turabian StyleGanazhapa-Plasencia, María, Erika Yangua-Solano, Leslye Ruiz, Rolando Andrade-Hidalgo, and Ángel Benítez. 2025. "Epiphytes as Environmental Bioindicators in Forest Remnants of the Pisaca Reserve: Preserving the Unique Pre-Inca Artificial Wetland of Paltas, Ecuador" Forests 16, no. 4: 628. https://doi.org/10.3390/f16040628
APA StyleGanazhapa-Plasencia, M., Yangua-Solano, E., Ruiz, L., Andrade-Hidalgo, R., & Benítez, Á. (2025). Epiphytes as Environmental Bioindicators in Forest Remnants of the Pisaca Reserve: Preserving the Unique Pre-Inca Artificial Wetland of Paltas, Ecuador. Forests, 16(4), 628. https://doi.org/10.3390/f16040628






