Characterization of OfERF17 as a Key Regulator of Petal Senescence in Osmanthus fragrans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. RNA Extraction and Expression Analysis
2.3. Bioinformatics Analysis of OfERF17 Genes
2.4. Cloning and Characterization of the Gene Sequence of OfERF17
2.5. Subcellular Localization and Transcriptional Activation Activity of the OfERF17 Proteins
2.6. Agrobacterium-Mediated Transient Overexpression of O. fragrans
2.7. Agrobacterium-Mediated Overexpression of Nicotiana tabacum
2.8. Physiological Indicators and Quantitative Real-Time Analysis
2.9. Data Analysis
3. Results
3.1. Expression Analysis of OfERF17 Genes in Different Flowering Times
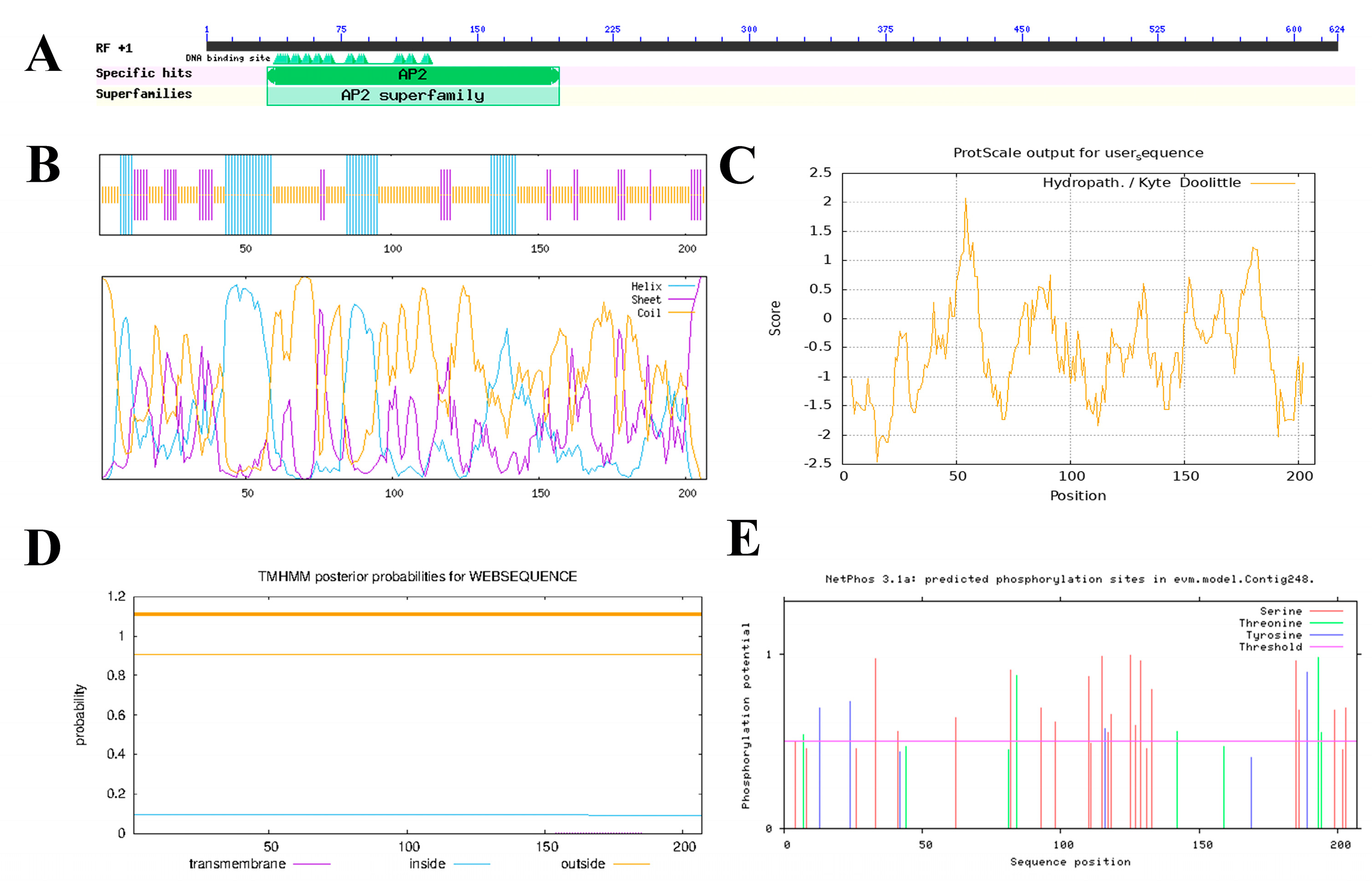
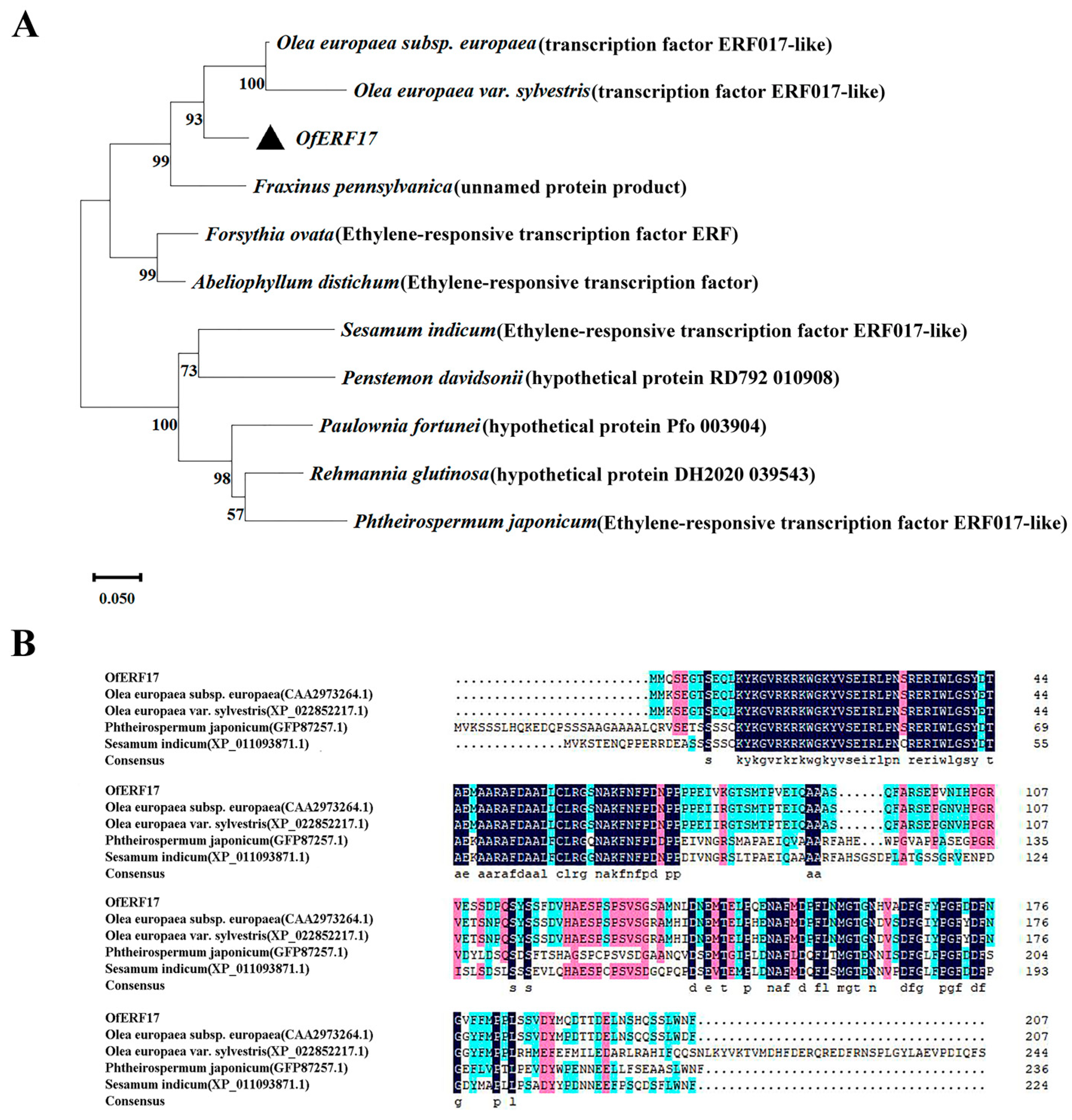
3.2. Bioinformatic Analysis of the OfERF17
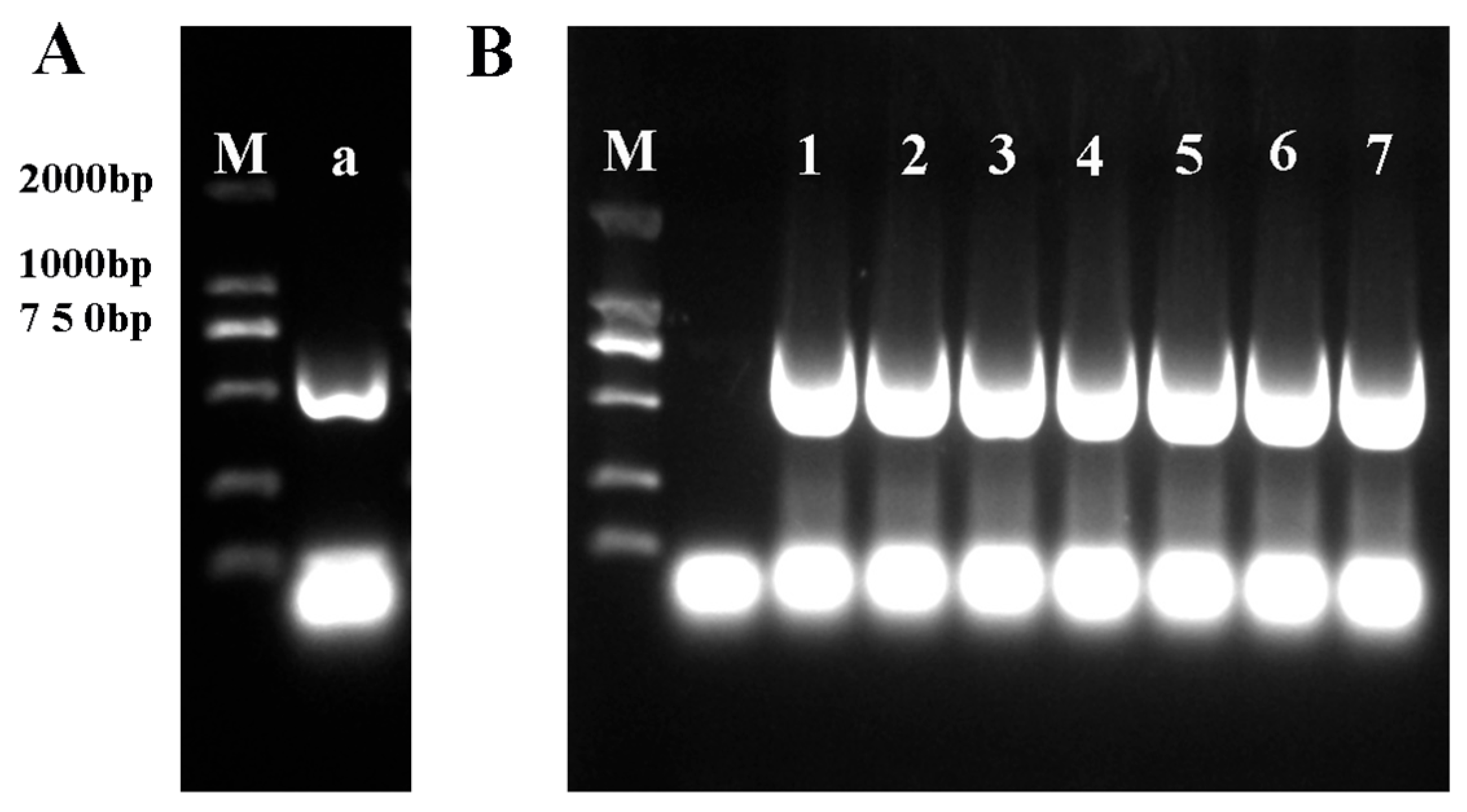
3.3. OfERF17 Construction of the Overexpression Vector
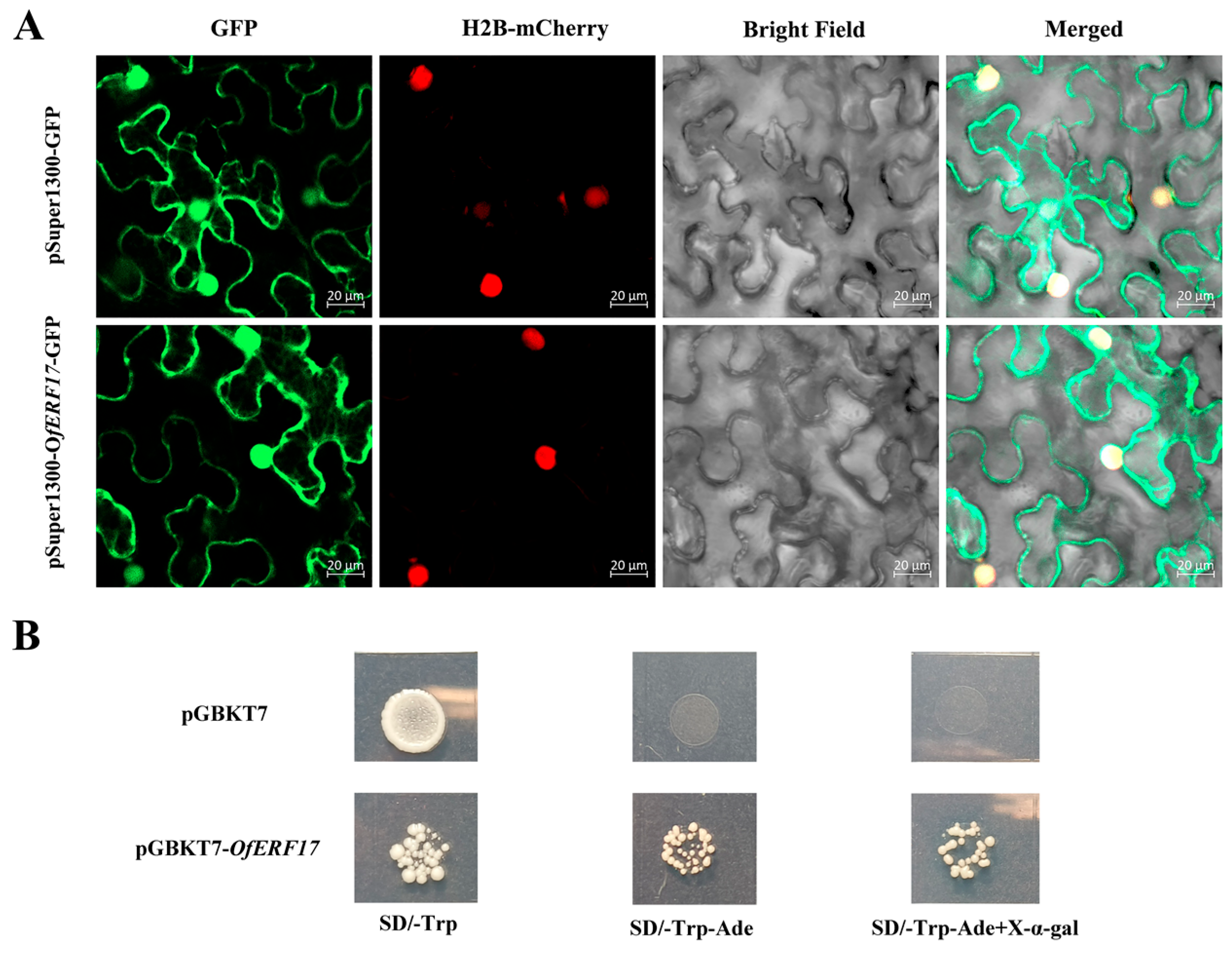
3.4. Subcellular Localization and Transcriptional Activation Analysis
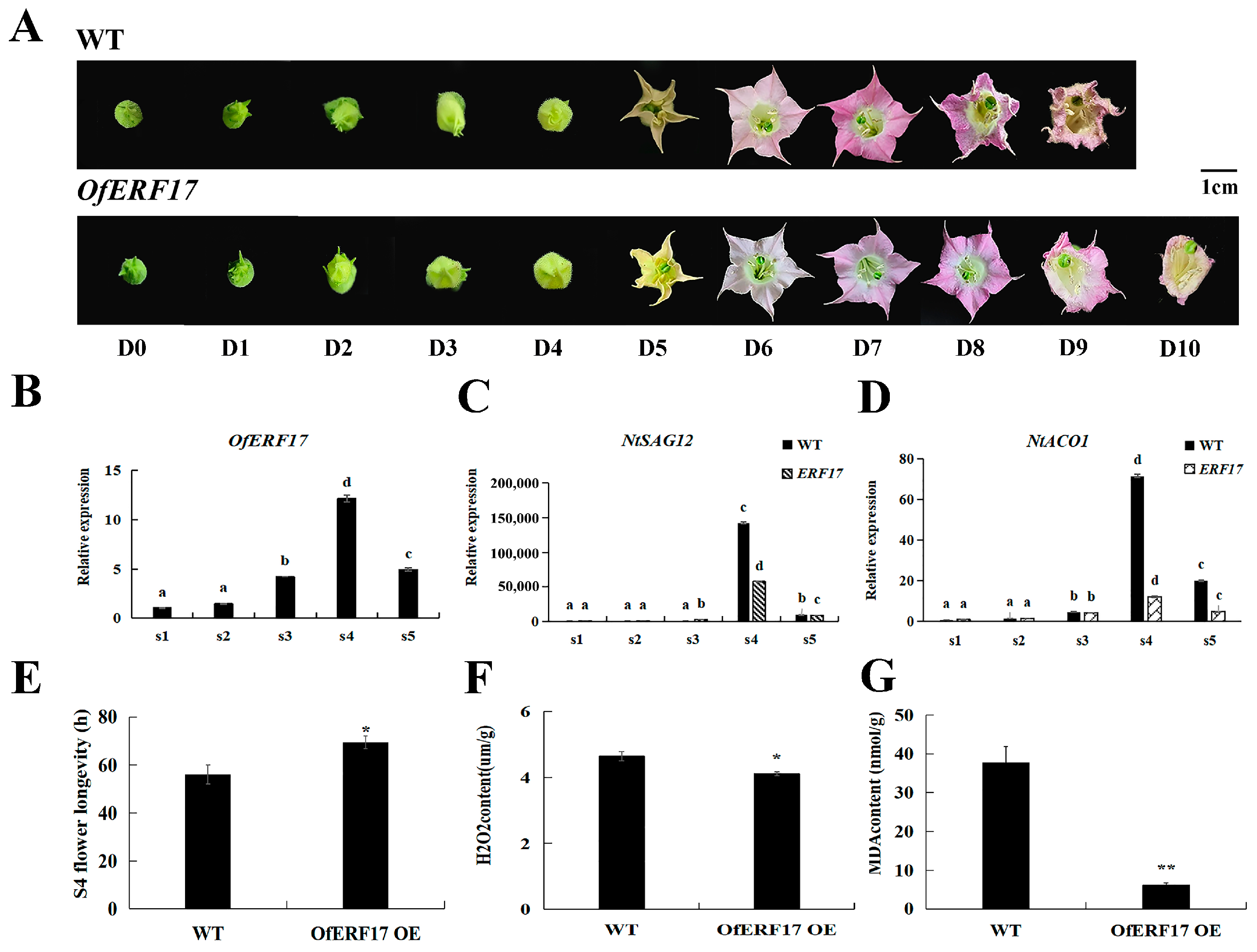
3.5. Overexpression of OfERF17 Delays Senescence of O. fragrans Petals
3.6. Overexpression of OfERF17 Delays the Senescence of Nicotiana tabacum Flower Petals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qian, Y.; Shan, L.; Zhao, R.; Tang, J.; Zhang, C.; Chen, M.; Duan, Y.; Zhu, F. Recent Advances in Flower Color and Fragrance of Osmanthus fragrans. Forests 2023, 14, 1403. [Google Scholar] [CrossRef]
- Wang, B.J.; Luan, F.; Bao, Y.W.; Peng, X.; Rao, Z.L.; Tang, Q.; Zeng, N. Traditional uses, phytochemical constituents and pharmacological properties of Osmanthus fragrans: A review. J. Ethnopharmacol. 2022, 293, 25. [Google Scholar] [CrossRef]
- Zou, J.J.; Zhou, Y.; Cai, X.; Wang, C.Y. Increase in DNA fragmentation and the role of ethylene and reactive oxygen species in petal senescence of Osmanthus fragrans. Postharvest Biol. Technol. 2014, 93, 97–105. [Google Scholar] [CrossRef]
- Rogers, H.J. From models to ornamentals: How is flower senescence regulated? Plant Mol. Biol. 2013, 82, 563–574. [Google Scholar] [CrossRef]
- Parveen, S.; Altaf, F.; Farooq, S.; Lone, M.L.; ul Haq, A.; Tahir, I. The swansong of petal cell death: Insights into the mechanism and regulation of ethylene-mediated flower senescence. J. Exp. Bot. 2023, 74, 3961–3974. [Google Scholar] [CrossRef] [PubMed]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Serek, M. Blue light postpones senescence of carnation flowers through regulation of ethylene and abscisic acid pathway-related genes. Plant Physiol. Biochem. 2020, 151, 103–112. [Google Scholar] [CrossRef]
- Dar, R.A.; Nisar, S.; Tahir, I. Ethylene: A key player in ethylene sensitive flower senescence: A review. Sci. Hortic. 2021, 290, 110491. [Google Scholar] [CrossRef]
- Haq, A.u.; Farooq, S.; Lone, M.L.; Parveen, S.; Altaf, F.; Tahir, I. Flower Senescence Coordinated by Ethylene: An Update and Future Scope on Postharvest Biology in the “Buttercup” Family. J. Plant Growth Regul. 2024, 43, 402–422. [Google Scholar] [CrossRef]
- Ma, N.; Ma, C.; Liu, Y.; Shahid, M.O.; Wang, C.; Gao, J. Petal senescence: A hormone view. J. Exp. Bot. 2018, 69, 719–732. [Google Scholar] [CrossRef]
- Qiu, H.; Chen, Y.; Fu, J.; Zhang, C. Expression of ethylene biosynthetic genes during flower senescence and in response to ethephon and silver nitrate treatments in Osmanthus fragrans. Genes Genom. 2024, 46, 399–408. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Zou, J. The spatial and temporal distribution of programmed cell death (PCD) during petal senescence of Osmanthus fragrans. Acta Hortic. 2017, 1185, 315–324. [Google Scholar] [CrossRef]
- Huang, T.H.; Hsu, W.H.; Mao, W.T.; Yang, C.H. The Oncidium Ethylene Synthesis Gene Oncidium 1-Aminocyclopropane-1 Carboxylic Acid Synthase 12 and Ethylene Receptor Gene Oncidium ETR1 Affect GA-DELLA and Jasmonic Acid Signaling in Regulating Flowering Time, Anther Dehiscence, and Flower Senescence in Arabidopsis. Front. Plant Sci. 2022, 13, 785441. [Google Scholar] [CrossRef]
- Thanomchit, K.; Imsabai, W.; Burns, P.; McAtee, P.A.; Schaffer, R.J.; Allan, A.C.; Ketsa, S. Differential expression of ethylene biosynthetic and receptor genes in pollination-induced senescence of Dendrobium flowers. Plant Physiol. Biochem. 2022, 188, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Hoppen, C.; Müller, L.; Albrecht, A.C.; Groth, G. The NOP-1 peptide derived from the central regulator of ethylene signaling EIN2 delays floral senescence in cut flowers. Sci. Rep. 2019, 9, 1287. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; He, L.; Sun, X.; Li, C.; Su, J.; Zhou, H.; Liu, X. Genome-Wide Analysis of the Rhododendron AP2/ERF Gene Family: Identification and Expression Profiles in Response to Cold, Salt and Drought Stress. Plants 2023, 12, 994. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Kesari, R.; Mila, I.; Regad, F. A dominant repressor version of the tomato Sl-ERF.B3 gene confers ethylene hypersensitivity via feedback regulation of ethylene signaling and response components. Plant J. 2013, 76, 406–419. [Google Scholar] [CrossRef]
- Yin, X.R.; Allan, A.C.; Chen, K.S.; Ferguson, I.B. Kiwifruit EIL and ERF Genes Involved in Regulating Fruit Ripening. Plant Physiol. 2010, 153, 1280–1292. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, H.; Han, Z.; Wang, S.; Wang, T.; Li, Q.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X. ERF4 affects fruit ripening by acting as a JAZ interactor between ethylene and jasmonic acid hormone signaling pathways. Hortic. Plant J. 2022, 8, 689–699. [Google Scholar] [CrossRef]
- Illgen, S.; Zintl, S.; Zuther, E.; Hincha, D.K.; Schmülling, T. Characterisation of the ERF102 to ERF105 genes of Arabidopsis thaliana and their role in the response to cold stress. Plant Mol. Biol. 2020, 103, 303–320. [Google Scholar] [CrossRef]
- Huan, X.; Wang, X.; Zou, S.; Zhao, K.; Han, Y.; Wang, S. Transcription Factor ERF194 Modulates the Stress-Related Physiology to Enhance Drought Tolerance of Poplar. Int. J. Mol. Sci. 2023, 24, 788. [Google Scholar] [CrossRef]
- Luo, J.; Chen, S.; Cao, S.; Zhang, T.; Li, R.; Chan, Z.L.; Wang, C. Rose (Rosa hybrida) Ethylene Responsive Factor 3 Promotes Rose Flower Senescence via Direct Activation of the Abscisic Acid Synthesis–Related 9-CIS-EPOXYCAROTENOID DIOXYGENASE Gene. Plant Cell Physiol. 2021, 62, 1030–1043. [Google Scholar] [CrossRef]
- Toshitsugu, N.; Masaki, F.; Yoko, S.; Yasuhiro, I. The AP2/ERF transcription factor SIERF52 functions in flower pedicel abscission in tomato. J. Exp. Bot. 2014, 65, 3111–3119. [Google Scholar] [CrossRef]
- Yang, X. Analysis of the Aging-Related AP2/ERF Transcription Factor Gene Family in Osmanthus fragrans. Int. J. Mol. Sci. 2024, 25, 8025. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, J.; Wang, Y.; Bao, Z.; Zhao, H. Identification of Suitable Reference Genes for Gene Expression Normalization in the Quantitative Real-Time PCR Analysis of Sweet Osmanthus (Osmanthus fragrans Lour.). PLoS ONE 2015, 10, e0136355. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, S.; Suo, J.; Chang, R.; Xu, X.; Xu, Z.; Yang, C.; Qu, C.; Liu, G. Bioinformatics analysis of PAE family in Populus trichocarpa and responsiveness to carbon and nitrogen treatment. 3 Biotech 2021, 11, 370. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Dong, S.; Yang, C.; Yang, S. Cloning and Expression Analysis of ATG8 (Autophagy-Related 8) Gene Family in Solanaceae. Plants 2024, 13, 2924. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Song, J.; Yang, W.; Zhang, X.; Zhu, Y.; Zhao, D.; Wang, H. Genome-wide characterization of AP2/ERF genes and their potential roles in bulb and bolt development in Allium sativum. Sci. Hortic. 2023, 317, 112359. [Google Scholar] [CrossRef]
- Sun, S.; Liang, X.; Chen, H.; Hu, L.; Yang, Z. Identification of AP2/ERF Transcription Factor Family Genes and Expression Patterns in Response to Drought Stress in Pinusmassoniana. Forests 2022, 13, 1430. [Google Scholar] [CrossRef]
- Wang, Y.; Du, X.; Liu, M.; Li, Y.; Shang, Z.; Zhao, L.; Yu, X.; Zhang, S.; Li, P.; Liu, J.; et al. Genome-Wide Exploration of the Ethylene-Responsive Element-Binding Factor Gene Family in Sweet Cherry (Prunus avium L.): Preliminarily Unveiling Insights into Normal Development and Fruit Cracking. Horticulturae 2024, 10, 247. [Google Scholar] [CrossRef]
- Dou, H.; Wang, T.; Zhou, X.; Feng, X.; Tang, W.; Quan, J.E.; Bi, H. Genome-Wide Identification and Expression of the AP2/ERF Gene Family in Morus notabilis. Forests 2024, 15, 697. [Google Scholar] [CrossRef]
- Cao, B.; Shu, L.; Li, A. Functional characterization of LkERF-B2 for improved salt tolerance ability in Arabidopsis thaliana. 3 Biotech 2019, 9, 263. [Google Scholar] [CrossRef]
- Lai, P.H.; Huang, L.M.; Pan, Z.J.; Jane, W.N.; Chen, H.H. PeERF1, a SHINE-Like Transcription Factor, Is Involved in Nanoridge Development on Lip Epidermis of Phalaenopsis Flowers. Front. Plant Sci. 2020, 10, 1709. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Yang, X.; Wassie, M.; Shi, H. Genome-wide identification and molecular characterization of the AP2/ERF superfamily members in sand pear (Pyrus pyrifolia). BMC Genom. 2023, 24, 32. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, X.; Xu, N.; Chen, G.; Qiao, X.; Gu, Q.; Wang, Y.; Sun, J. Fine mapping and identification of ERF transcription factor ERF017 as a candidate gene for cold tolerance in pumpkin. Theor. Appl. Genet. 2024, 137, 230. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhao, T.; Wu, Y.X.; Wang, H.; Wang, Y.X. Identification of AP2/ERF genes in apple (Malus × domestica) and demonstration that MdERF017 enhances iron deficiency tolerance. Plant Cell Tissue Organ Cult. 2020, 143, 465–482. [Google Scholar] [CrossRef]
- Wang, S.; Lv, S.; Zhao, T.; Jiang, M.; Liu, D.; Fu, S.; Hu, M.; Huang, S.; Pei, Y.; Wang, X. Modification of Threonine-825 of SlBRI1 Enlarges Cell Size to Enhance Fruit Yield by Regulating the Cooperation of BR-GA Signaling in Tomato. Int. J. Mol. Sci. 2021, 22, 7673. [Google Scholar] [CrossRef]
- Wang, S.; Hu, T.; Tian, A.; Luo, B.; Wang, X. Modification of Serine 1040 of SIBRI1 Increases Fruit Yield by Enhancing Tolerance to Heat Stress in Tomato. Int. J. Mol. Sci. 2020, 21, 7681. [Google Scholar] [CrossRef]
- Shi, T.; Shang, S.; Fan, L.; Zhou, S.; Gao, S.; Shaaban, M.; Fan, B.; Shi, G.; Wang, Z. The role of IpSAG12 in regulating florescence and senescence of cut Itoh peony flowers. Postharvest Biol. Technol. 2025, 222, 113352. [Google Scholar] [CrossRef]
- Grbić, V. Spatial expression pattern of SAG12:GUS transgene in tobacco (Nicotiana tabacum). Physiol. Plant. 2010, 116, 416–422. [Google Scholar] [CrossRef]
- Takahashi, S.; Yoshida, C.; Takahashi, H.; Nishihara, M. Isolation and Functional Analysis of EPHEMERAL1-LIKE (EPH1L) Genes Involved in Flower Senescence in Cultivated Japanese Gentians. Int. J. Mol. Sci. 2022, 23, 5608. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yang, H.; Yang, J.; Wang, Y.; Ye, T.; Xiang, L.; Chan, Z.; Wang, Y. Tulip transcription factor TgWRKY75 activates salicylic acid and abscisic acid biosynthesis to synergistically promote petal senescence. J. Exp. Bot. 2024, 75, 2435–2450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Yu, Z.C.; Peng, X.; Chen, M.; Liao, C.; Yang, Z.; Wang, C.; Li, C. Endogenous Ascorbic Acid Delays Ethylene-Induced Leaf Senescence in Arabidopsis thaliana. Photosynthetica 2020, 58, 720–731. [Google Scholar] [CrossRef]
- Houben, M.; Poel, B.V.D. 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef]
- Jia, M.; Li, X.; Wang, W.; Li, T.; Dai, Z.; Chen, Y.; Zhang, K.; Zhu, H.; Mao, W.; Feng, Q. SnRK2 subfamily I protein kinases regulate ethylene biosynthesis by phosphorylating HB transcription factors to induce ACO1 expression in apple. New Phytol. 2022, 235, 3429. [Google Scholar] [CrossRef]
- Tang, X.; Gomes, A.M.T.R.; Woodson, B.W.R. Pistil-Specific and Ethylene-Regulated Expression of 1-Aminocyclopropane- 1-Carboxylate Oxidase Genes in Petunia Flowers. Plant Cell 1994, 6, 1227–1239. [Google Scholar] [CrossRef][Green Version]
- Okamoto, M.; Niki, T.; Azuma, M.; Shibuya, K.; Ichimura, K. Expression of ethylene biosynthesis genes in the gynoecium and receptacle associated with sepal abscission during senescence in Delphinium grandiflorum. Plant Growth Regul. 2022, 97, 3429. [Google Scholar] [CrossRef]
- Naing, A.H.; Kyu, S.Y.; Pe, P.P.W.; Park, K.I.; Lee, J.M.; Lim, K.B.; Kim, C.K. Silencing of the phytoene desaturase (PDS) gene affects the expression of fruit-ripening genes in tomatoes. Plant Methods 2019, 15, 110. [Google Scholar] [CrossRef]
- Dek, M.S.P.; Padmanabhan, P.; Sherif, S.; Subramanian, J.; Paliyath, G. Upregulation of Phosphatidylinositol 3-Kinase (PI3K) Enhances Ethylene Biosynthesis and Accelerates Flower Senescence in Transgenic Nicotiana tabacum L. Int. J. Mol. Sci. 2017, 18, 1533. [Google Scholar] [CrossRef]
- Rogers, H.J. Is there an important role for reactive oxygen species and redox regulation during floral senescence? Plant Cell Environ. 2012, 35, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2013, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Sukpitak, C.; Munné-Bosch, S.; Seraypheap, K. Brassinosteroids alleviate postharvest water deficit stress in cut Dendrobium ’Khao Sanan’ orchid through modulation of protective mechanisms, osmotic regulation, and endogenous hormonal levels. Postharvest Biol. Technol. 2024, 211, 3429. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, S.-L.; Zhang, Z.-J.; Hu, L.-Y.; Jiang, C.-X.; Wei, Z.-J.; Liu, J.; Wang, H.-L.; Jiang, S.-T. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol. Technol. 2011, 59, 3429. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Ebrahimi, A.; Fard, J.R.; Sheikh-Assadi, M. Phytosulfokine α (PSKα) delays senescence in cut rose flowers by keeping intracellular ATP and ROS homeostasis. Sci. Hortic. 2024, 331, 3429. [Google Scholar] [CrossRef]
- Zhou, C.; Luo, L.; Miao, P.; Dong, Q.; Cheng, H.; Wang, Y.; Li, D.; Pan, C. A novel perspective to investigate how nanoselenium and melatonin lengthen the cut carnation vase shelf. Plant Physiol. Biochem. 2023, 196, 982–992. [Google Scholar] [CrossRef]
- Haddad, C.; Arkoun, M.; Jamois, F.; Schwarzenberg, A.; Yvin, J.-C.; Etienne, P.; Laîné, P. Silicon Promotes Growth of Brassica napus L. and Delays Leaf Senescence Induced by Nitrogen Starvation. Front. Plant Sci. 2018, 9, 516. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guo, Y.; Hao, X.; Li, Y.; He, W.; Zhao, X.; Cai, S.; Song, X. Functional Validation of Different Alternative Splicing Variants of the Chrysanthemum lavandulifolium ClNUM1 Gene in Tobacco. Curr. Issues Mol. Biol. 2024, 46, 5242–5256. [Google Scholar] [CrossRef]
- Qiu, Z.; Hou, Q.; Wen, Z.; Tian, T.; Hong, Y.; Yang, K.; Qiao, G.; Wen, X. Identification of PavHB16 gene in Prunus avium and validation of its function in Arabidopsis thaliana. Physiol. Mol. Biol. Plants 2024, 30, 559–570. [Google Scholar] [CrossRef]
- Chen, W.; Chen, L.; Cui, L.; Liu, Z.; Yuan, W. Genome-wide analysis of radish AHL gene family and functional verification of RsAHL14 in tomato. Front. Plant Sci. 2024, 15, 3429. [Google Scholar] [CrossRef]
- Krenek, P.; Samajova, O.; Luptovciak, I.; Doskocilova, A.; Komis, G.; Samaj, J. Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol. Adv. 2015, 33, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Rong, H.; Feng, Y.; Xin, Y.; Luan, X.; Zhou, Q.; Xu, M.; Xu, L.A. Protoplast isolation and transient transformation system for Ginkgo biloba L. Front. Plant Sci. 2023, 14, 3429. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, J.; Liu, H.; Guo, Y.; Niu, S. An efficient system for Agrobacterium-mediated transient transformation in Pinus tabuliformis. Plant Methods 2020, 16, 3429. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, R.; Yang, L.; Zuo, Z. Establishment of a Transient Transformation Protocol in Cinnamomum camphora. Forests 2023, 14, 1872. [Google Scholar] [CrossRef]
- Lei, Y.; Gao, J.; Li, Y.; Song, C.; Guo, Q.; Guo, L.; Hou, X. Functional Characterization of PoEP1 in Regulating the Flowering Stage of Tree Peony. Plants 2024, 13, 1642. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, H.; Shen, H.; Tian, Q.; Ding, W.; Yang, X.; Wang, L.; Yue, Y. Insights into the Cytochrome P450 Monooxygenase Superfamily in Osmanthus fragrans and the Role of OfCYP142 in Linalool Synthesis. Int. J. Mol. Sci. 2022, 23, 12150. [Google Scholar] [CrossRef]
- Zhu, M.; Bin, J.; Ding, H.; Pan, D.; Tian, Q.; Yang, X.; Wang, L.; Yue, Y. Insights into the trihelix transcription factor responses to salt and other stresses in Osmanthus fragrans. BMC Genom. 2022, 23, 3429. [Google Scholar] [CrossRef]
- Bin, J.; Zhu, M.; Ding, H.; Zai, Z.; Shi, T.; Wang, L.; Yang, X.; Yue, Y. New Insights into the Roles of Osmanthus Fragrans Heat-Shock Transcription Factors in Cold and Other Stress Responses. Horticulturae 2022, 8, 80. [Google Scholar] [CrossRef]
- Chen, G.; Chen, F.; Zhang, D.; Zhou, Y.; Gu, H.; Yue, Y.; Wang, L.; Yang, X. Osmanthus fragrans Ethylene Response Factor OfERF1-3 Delays Petal Senescence and Is Involved in the Regulation of ABA Signaling. Forests 2024, 15, 1619. [Google Scholar] [CrossRef]
- Wang, P.; Li, R.; Liu, X.; Zhao, X.; Hyden, B.; Han, Y.; Zhang, X.; Wang, J.; Chen, H.; Cao, H. Establishment of genetic transformation system of peach callus. Sci. Hortic. 2024, 323, 112501. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, R.; Tan, X.; Li, Z. Establishing an efficient callus genetic transformation system for the tung tree (Vernicia fordii). Ind. Crops Prod. 2024, 211, 118264. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, F.; Liu, J.; Liu, W.; Zhang, S.; Li, D.; Song, J.; Wang, R.; Yang, Y. Establishment of efficient callus genetic transformation system for Pyrus armeniacaefolia. Sci. Hortic. 2021, 289, 110429. [Google Scholar] [CrossRef]
- Gu, H.; Ding, W.; Shi, T.; Ouyang, Q.; Yang, X.; Yue, Y.; Wang, L. Integrated transcriptome and endogenous hormone analysis provides new insights into callus proliferation in Osmanthus fragrans. Sci. Rep. 2022, 12, 7609. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Zhang, D.; Chen, F.; Zhou, Y.; Gu, H.; Qin, X.; Yue, Y.; Wang, L.; Yang, X. Characterization of OfERF17 as a Key Regulator of Petal Senescence in Osmanthus fragrans. Forests 2025, 16, 615. https://doi.org/10.3390/f16040615
Chen G, Zhang D, Chen F, Zhou Y, Gu H, Qin X, Yue Y, Wang L, Yang X. Characterization of OfERF17 as a Key Regulator of Petal Senescence in Osmanthus fragrans. Forests. 2025; 16(4):615. https://doi.org/10.3390/f16040615
Chicago/Turabian StyleChen, Gongwei, Dandan Zhang, Fengyuan Chen, Yixiao Zhou, Heng Gu, Xuyang Qin, Yuanzheng Yue, Lianggui Wang, and Xiulian Yang. 2025. "Characterization of OfERF17 as a Key Regulator of Petal Senescence in Osmanthus fragrans" Forests 16, no. 4: 615. https://doi.org/10.3390/f16040615
APA StyleChen, G., Zhang, D., Chen, F., Zhou, Y., Gu, H., Qin, X., Yue, Y., Wang, L., & Yang, X. (2025). Characterization of OfERF17 as a Key Regulator of Petal Senescence in Osmanthus fragrans. Forests, 16(4), 615. https://doi.org/10.3390/f16040615





