Two of the Most Promising Potential Agents from Kazakhstan for the Biocontrol of Russian Olive (Elaeagnus angustifolia) in the USA with an Annotated List of Its Pest Insects from Central Asia
Abstract
1. Introduction
2. Materials and Methods
3. Results
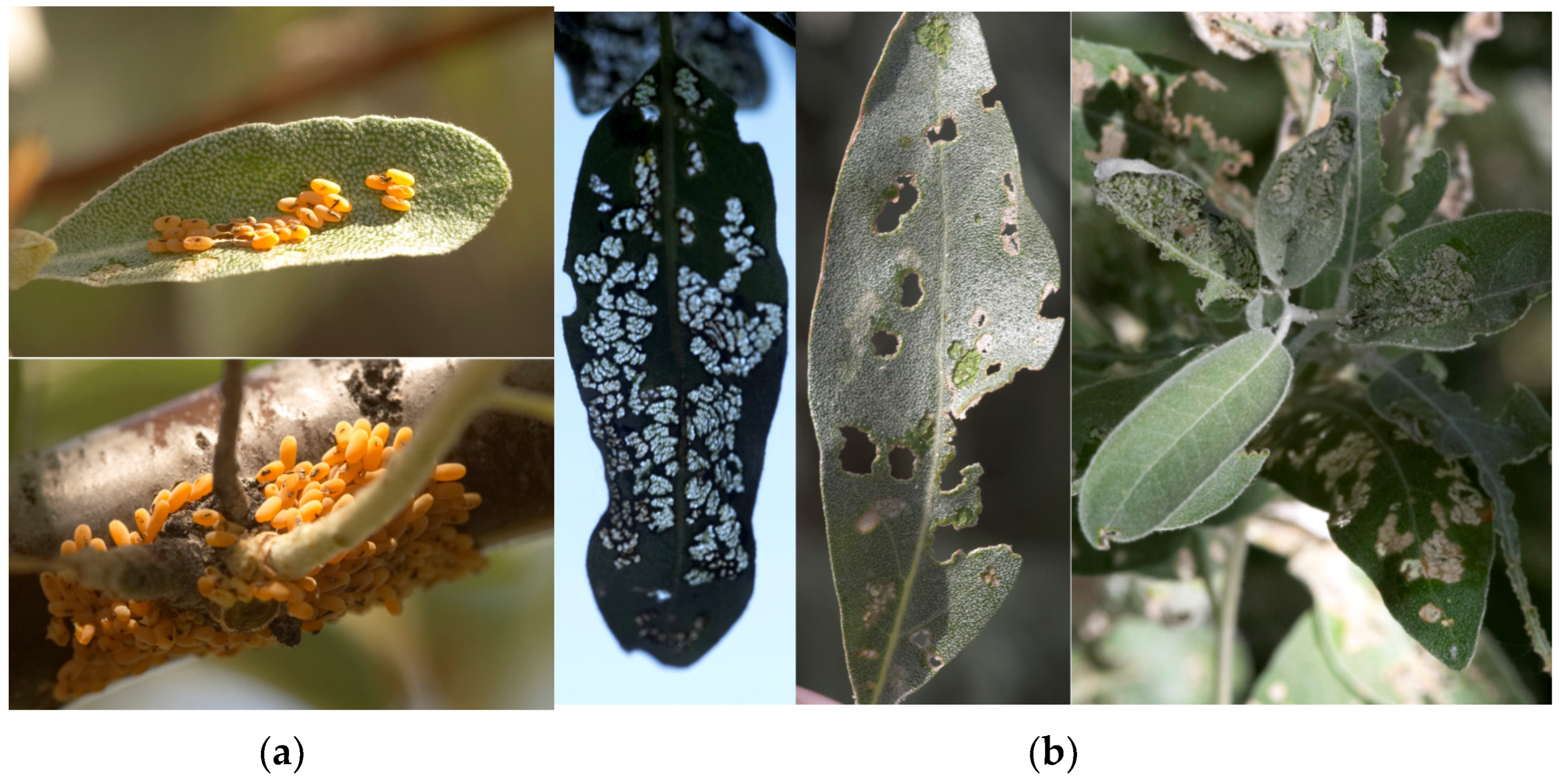
3.1. Trioza Magnisetosa Loginova, 1964
3.1.1. Distribution
3.1.2. Habitats
3.1.3. Host Plants
3.1.4. Biological and Phenological Features
3.1.5. Parasites
3.1.6. Efficiency for Biological Control
3.1.7. Testing in Nature
3.2. Altica Balassogloi (Jakobson, 1892) Coleoptera, Chrysomelidae (=Haltica Suvorovi Ogl., H. Lopatini Pal.)
3.2.1. Distribution
3.2.2. Habitats
3.2.3. Host Plants
3.2.4. Biological and Phenological Features
3.2.5. Monitoring of Modern Populations
- (1)
- The Ili population (40 km north of Kapchagai, Russian olive thickets in the floodplain of the Ili River) was found on 13 June 2006. The population size for all the time of observations was very low; every year in the second decade of June, there were only about 15–20 beetles and 25–30 larvae of 1–2 ages. Near the place where the leaf beetle was found, a large young grove grows on the site of a previous fire, but it was impossible to find a single individual in it.
- (2)
- The Tasmurun population (70 km north of Kapchagai, near Miyaly village, Russian olive trees grew between the beginning of the Tasmurun canal and the Ili River) was found on 14 June 2006. Unburned bushes were still preserved in this part of the riparian forest, where a small number (27 individuals) of beetles, 2 oviposits, and several larvae of 1–3 ages were found. The number of insects in this population also remained very low in 2006–2018.
- (3)
- The Topar population (10–12 km south-west of the village of Topar, in the lower reaches of the Ili River, a small oleaster forest along a moist roadside depression on the edge of dunes), was discovered on 16 June 2006. In mid-June, 2nd–3rd instar larvae were already predominant here, and egg-laying and larvae of the 1st instar were rare. Despite the relatively high number of insects (an average of about 100 individuals per tree), this population did not cause much damage to its host plants in 2006. However, observations in 2007 showed that beetles and larvae of the overwintering generation had severely damaged individual trees growing along roadside ditches, and on some young bushes, all the leaves were eaten entirely. Compared to 2006, the number of leaf beetles in 2007 increased to an average of 120 individuals per tree. In 2008, the number of oleaster leaf beetles became even higher and reached an average of 160 individuals per 1 tree, although the number of leaf beetles in the adjacent barkhan (sand dune) area did not change and remained at the level of 2006—100 individuals per tree. The damage to host plants in 2008 was noticeably more remarkable than the previous year: devoid of leaves, which caused the drying and death of individual branches.
- (4)
- The Karatal population 1 (15 km north of Ushtobe City, a grove of sparse old trees) was found for the first time on 15 May 2007. At this time, in the lower part of the crown of one of the Russian olive trees, on the upper side of the leaf, only five larvae of the 2nd age were found, one larva that had just transformed to the 3rd age, one ovipositor, and one female. This is all that could be found in this reasonably extensive grove. The appearance of beetles, the laying of eggs, and the development of larvae occurred in the early spring cycle.
- (5)
- The Karatal population 2 (22 km north of Ushtobe City, a vast stretch of floodplain forest consisting of old and medium-aged Russian olive trees mixed with willows) was discovered on 15 May 2007. This leaf beetle population was represented by small scattered spots, but the number of leaf beetles in individual spots was very high. In mid-May 2007, egg-laying larvae of 1–3 instars (mostly 1–2), males, and egg-laying females were recorded here. The leaves were usually damaged, mainly in the lower half of the crown. Most of the eggs were laid on the leaves, and in one ovipositor, the number of eggs located on the upper side of the leaf was usually less (5–15 eggs) than in the ovipositor on the lower side of the leaves (30–43 eggs). Large oviposits were also found on young branches, in the forks of branches, and on the petioles of leaves. Such egg-laying was sometimes observed in the 1950s in powerful centers of mass reproduction of the species on overpopulated bushes. An average of 5–10 adults and 15–20 oviposits were usually observed on the same branch length of 1.5 m. In this population, emerging larvae of the 1st age of the wintering generation were generally observed in the third decade of May. In general, this leaf beetle population developed almost a month earlier than the Topar population over the entire period of observations in 2007–2018. This is due to the peculiarities of the microclimate in this place.
- (6)
- The Karatal population 3 (55 km north of Ushtobe City, a moistened depression in the floodplain on the border with dunes along the drainage channel) was found for the first time on 15 May 2007, when this population lived only on a few low young bushes; the number of leaf beetles here was relatively high, about 430 beetles per plant. At this time, only copulating beetles were observed, and no egg-laying was found. The appearance of beetles from wintering sites on the oleaster crown here occurred 10–15 days later than in the Karatal population 2, even though they are located relatively close to each other (23 km). Here, local features of the microclimate and wintering places of this population are also affected. Unfortunately, observations in 2008 showed that this population has completely disappeared—no damage or beetles were found on the bushes. The leading causes of population death are drying up of the coastal lowlands, fire, and livestock that completely destroyed the litter under the Russian olive bushes.
3.2.6. Efficiency for Biological Control
3.2.7. Testing in Nature Control
3.3. Preliminary Annotated List of Insect Species Damaging to Russian Olive in Central Asia
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baitenov, M.S. Flora Kazakhstana, Rodovoy Kompleks Flory (=Flora of Kazakhstan, Generic Complex of Flora); Gylym: Almaty, Kazakhstan, 2001; Volume 2, pp. 1–280. [Google Scholar]
- Abdulina, S.A. Spisok Sosydistykh Rasteniy Kazakhstana (=List of Vascular Plants in Kazakhstan); Kamelin, R.V., Ed.; Gylym: Almaty, Kazakhstan, 1999; pp. 1–187. [Google Scholar]
- Flora of Kazakhstan; Academy of Sciences of the KazSSR: Almaty, Kazakhstan, 1963; Volume 6, pp. 223–224.
- Pavlovskiy, E.N. (Ed.) Vrednye Zhivotnye Sredney Azii; USSR Academy of Sciences: Leningrad, Moscow, 1949; pp. 1–404. [Google Scholar]
- Vorontsov, A.I. Vrediteli Lesomeliorativnykh Posadok Zapadnogo Kazakhstana i Zavolzhiya. Itogi Raboty VIZR za 1936 g (=Pests of Forest Landings in Western Kazakhstan and the Volga Region. Results of the VIZR’s Work for 1936); Part 1; VIZR: Leningrad, Russia; pp. 202–205.
- Grechkin, V.P. Ocherki po Biologii Vrediteley Lesa (=Essays on the Biology of Forest Pests); VIZR: Leningrad, Russia, 1951; pp. 75–90, 128–135. [Google Scholar]
- Vorontsov, A.I.; Zakharchenko, I.S. Lokhoviy izmenchiviy usach i mery boriby s nim (=The grape wood borer (Chlorophorus varius) and measures to combat it). In Sbornik Rabot po Lesozaschite; Moscow Forestry Institute: Moscow, Russia, 1957; pp. 46–54. [Google Scholar]
- Makhnovskiy, I.K. Vrediteli Zaschitnykh Lesonasazhdeniy Sredney Azii i Boriba s Nimi (=Pests of Protective Forest Plantations in Central Asia and Their Control); SIL UASN: Tashkent, Uzbekistan, 1955; pp. 43–166. [Google Scholar]
- Makhnovskiy, I.K. Vrediteli Drevesno-Kustarnikovoy Rastitelnosti Chirchik-Angrenskogo Gorno-Lesnogo Massiva i Boriba s Nimi (=Pests of Wood and Shrub Vegetation of the Chirchik-Angren Mountain Forest Massif and Their Control). In Trudy Sredneaziatskogo Instituta Lesovodstva Uzbekskoy Akademii Selskokhozyastvennykh Nauk; SIL UASN: Tashkent, Uzbekistan, 1959; pp. 13–56. [Google Scholar]
- Rafes, M.P. Vrednye nasekomye lokha, dzhuzguna i tamariska, proizrastayuschikh na Narynskikh peskakh polupustynnogo Zavolzhiya (=Pest insects of oleaster, Calligonum and tamarisk, that grow on the Naryn Sands of the semi-desert Zavolzhye). Entomol. Obozr. 1956, 35, 805–817. [Google Scholar]
- Parfentiev, V.Y. Lokhoviy listed Haltica suvorovi Ogl. (Coleoptera, Chrysomelidae) v tugainykh lesakh Kazakhstana (=Oleaster leaf beetle Haltica suvorovi Ogl. (Coleoptera, Chrysomelidae) in the tugai forests of Kazakhstan). Entomol. Obozr. 1957, 36, 96–97. [Google Scholar]
- Kuznetsov, A.V. Lokhovoya moli Anarsia eleagnella W. Kuzn. sp. n. (Lepidoptera, Gelechiidae)—Novyi vreditel lokha v SSSR (=Oleaster moth Anarsia eleagnella W. Kuzn. sp. n. (Lepidoptera, Gelechiidae)—New pest of oleaster in the USSR). Zool. Zhurnal 1957, 36, 1096–1098. [Google Scholar]
- Matesova, G.Y. Notes on the Biology of Mealybugs and Scale Insects (Homoptera, Coccoidea) of South-Eastern Kazakhstan. In Trudy of Instituta Zoologii Akademii Nauk Kazakhskoy SSR; Institute of Zoology: Alma-Ata, Kazakhstan, 1958; pp. 130–137. [Google Scholar]
- Kostin, I.A. Materialy po Faune Koroedov Kazakhstana (Coleoptera, Ipidae) (=Materials on Bark Beetle Fauna in Kazakhstan (Coleoptera, Ipidae). In Trudy of Instituta Zoologii Akademii Nauk Kazakhskoy SSR; Institute of Zoology: Alma-Ata, Kazakhstan, 1960; pp. 129–136. [Google Scholar]
- Mityaev, I.D. K Faune Nasekomykh—Vrediteley Lokha v Kazakhstane (=To the Fauna of Oleaster Insect Pests in Kazakhstan). In Trudy of Instituta Zoologii Akademii Nauk Kazakhskoy SSR; Institute of Zoology: Alma-Ata, Kazakhstan, 1960; pp. 108–128. [Google Scholar]
- Mityaev, I.D. Nasekomye, Vredyaschie Lokhu (Eleagnus angustifolia L.) v Kulturnoy Zone Yuzhnykh Oblastey Kazakhstana (=Insects that Harm Russian Olive (Elaeagnus angustifolia L.) in the Cultural Zone of the Southern Regions of Kazakhstan). In Trudy of Instituta Zoologii Akademii Nauk Kazakhskoy SSR; Institute of Zoology: Alma-Ata, Kazakhstan, 1962; pp. 61–68. [Google Scholar]
- Sinadskiy, Y.V. Vrediteli Tugainykh Lesov Sredney Azii i Mery Boriby s Nimi (=Pests of Central Asian tugai Forests and Measures to Control Them); Nauka USSR: Leningrad, Russia, 1963; pp. 1–150. [Google Scholar]
- Skopin, N.G. Nasekomye—Vrediteli lesoposadok v peskakh Bolishie Barsuki i puti boriby s nimi (=Insects—Pests of forest plantations in the sand Big Barsuki and ways to fight them). Uchenye Zap. KazGU 1955, 27, 84–101. [Google Scholar]
- Sinadskiy, Y.V. Siniy Listoed—Haltica Deserticola Parf., Vreditel Lokha Uzkolistnogo v Tugayakh Reki Syrdariya (=Blue Leaf Beetle—Haltica Deserticola Parf., Russian Olive Pest in the Riparian Forests of the Syr Darya River); Nauchnye Doklady Vyshey Shkoly. Lesoinzhenernoye Delo: Leningrad, Russia, 1958; pp. 47–50. [Google Scholar]
- Sinadskiy, Y.V. Vrednaya entomofauna lokha (dzhidy) v tugainykh lesakh Sredney Azii i Kazakhstana (=Harmful entomofauna of oleaster (dzhida) in the tugai forests of Central Asia and Kazakhstan). Zoologicheskiy Zhurnal 1961, 40, 1019–1029. [Google Scholar]
- Sinadskiy, Y.V. Dendrofilinye Nasekomye Pustyn’ Sredney Azii i Kazakhstana I Mery Boriby s Nimi (=Dendrophilous Insects of the Deserts of Central Asia and Kazakhstan and Measures to Combat Them); Nauka: Moscow, Russia, 1968; pp. 1–126. [Google Scholar]
- Emeljanov, A.F. Noviy vid roda Macropsis Low. (Homoptera, Cicadellidae) s lokha (=New species of the genus Macropsis Low. (Homoptera, Cicadellidae) from the Elaeagnus). Doklady Acad. Sci. Tajik SSR 1964, 7, 47–48. [Google Scholar]
- Pripisnova, M.G. Vrednaya Entomofauna Tugaynoi Drevesnoo-Kustarnikovoy Rastitelnosti Yuzhnogo Tadzhikistana (=Harmful Entomofauna of Tugai Tree and Shrub Vegetation of Southern Tajikistan); Tajik University: Dushanbe, Tajikistan, 1965; pp. 1–118. [Google Scholar]
- Kuznetsov, A.V. Nasaekomye i Kleschi, Povrzhdayuschie Plodovye i Dekorativnye Nasazhdeniya Severnogo Pribalkhashiya, i Biologocheskie Osnovy Meropriyatiy po Boribe s Nimi (=Insects and Mites That Damage Fruit and Ornamental Plantings of the Northern Balkhash Region, and the Biological Basis of Measures to Combat Them); Abstract of the dissertation on competition of a scientific degree of candidate of agricultural sciences; Kazakh State University: Alma-Ata, Kazakhstan, 1971; pp. 1–22. [Google Scholar]
- Kostin, I.A. Zhiki-Dendrofagi Kazakhstana (Koroedy, Drovoseki, Zlatki) (=Dendrophagous Beetles of Kazakhstan (Bark Beetles, Longhorn, Buprestids); Nauka: Alma-Ata, Kazakhstan, 1974; pp. 1–286. [Google Scholar]
- Yagdyev, A. Stvolovye vrediteli turangi v Turkmenii (=Trunk pests of Turanga in Turkmenia). Izv. Akad. Nauk. Turkm. SSR Ser. Biol. Nauk. 1975, 6, 60–64. [Google Scholar]
- Yagdyev, A. Obzor nasekomykh-ksilofagov lesov Centralnogo Kopetdaga (=A review of the xylophagous insects of the forests of the Central Kopetdag). Entomol. Obozr. 1979, 58, 776–781. [Google Scholar]
- Yagdyev, A. Vrediteli dekorativnykh rasteniy v gorodakh Turkmenistana (=Pests of ornamental plants in towns of Turkmenistan). Izv. Akad. Nauk. Turkm. SSR Ser. Biol. Nauk. 1987, 1, 47–50. (In Russian) [Google Scholar]
- Asanova, R.B.; Iskakov, B.V. Vrednye i Poleznye Poluzhestkokrylye (Heteroptera) Kazakhstann (Opredelitel) (=Harmful and Useful Hemipterans (Heteroptera) of Kazakhstan (Key Table); Kainar: Alma-Ata, Kazakhstan, 1977; pp. 1–204. [Google Scholar]
- Lopatin, I.K.; Kulenova, K.Z. Zhuki-Listoedy (Coleoptera, Chrysomelidae) Kazakhstana: Opredelitel (=Leaf Beetles (Coleoptera, Chrysomelidae) of Kazakhstan: Key Table); Nauka: Alma-Ata, Kazakhstan, 1986; pp. 1–199. [Google Scholar]
- Danzig, E.M. Fauna of Russia and Adjoining States, Suborder Coccids (Coccinea), Families Phoenicoccidae and Diaspididae; Nauka: Sanct-Petersburg, Russia, 1993; pp. 1–453. [Google Scholar]
- Ponomarenko, M.G. Trophic relationships of caterpillars of subfamily Dichomeridinae (Lepidoptera, Gelechiidae) of the fauna of Russia and adjacent countries. In Lecturing for the Memory of A.I. Kurenzov; Nauka: Leningrad, Russia, 1993; pp. 41–48. [Google Scholar]
- Ponomarenko, M.G. Catalogue of the subfamily Dichomeridinae (Lepidoptera, Gelechiidae) of the Asia. Far East. Entomol. 1997, 50, 1–67. [Google Scholar]
- Fedotova, Z.A. Gallitsy-Fitofagi (Diptera, Cecidomyiidae) Pustyn i gor Kazakhstana: Morphologiya, Biologiya, Rasprostranenie, Filogeniya i Sistematika; Samara State Agricultiral Academy: Samara, Russia, 2000; pp. 1–803. [Google Scholar]
- Mityaev, I.D. Fauna, ecology, and zoogeography of cicadas (Homoptera, Cicadinea) in Kazakhstan. Tethys Entomol. Res. 2002, 5, 3–168. [Google Scholar]
- Aitzhanova, M.O.; Kadyrbekov, R.K. To the fauna of aphids (Homoptera, Aphididae) of the tugai forests of the Karatal river basin. Vestn. KazNU Ser. Biol. 2006, 2, 82–87. [Google Scholar]
- Kadyrbekov, R.K.; Aitzhanova, M.O. To the fauna of aphids (Homoptera, Aphididae) of the tugai forests between the rivers Aksu and Lepsy. Izv. NAS RK Ser. Biol. Med. 2006, 3, 7–13. [Google Scholar]
- Jashenko, R.; Mityaev, I.; DeLoach, C.J. Potential agents for Russian biocontrol in USA. In Proceedings of the XII International Symposium on Biological Control of Weeds, Le Grand Motte, France, 22–27 April 2007; p. 249. [Google Scholar]
- Jashenko, R.V. Annotated list of species of the family Margarodidae (Homoptera, Coccinea) of Central Asia and Kazakhstan. Izv. NAS RK Ser. Biol. Med. 2007, 2, 3–10. [Google Scholar]
- Schaffner, U. Update on CABI’s Weed Biocontrol Program; CABI: Delémont, Switzerland, 2009; pp. 1–27. [Google Scholar]
- Schaffner, U.; Hinz, H.L.; Cristofaro, M. Biological Control of Russian Olive, Elaeagnus Angustifolia; Annual report 2007; CABI: Delémont, Switzerland, 2007; pp. 1–20. [Google Scholar]
- Schaffner, U.; Grözinger, F.; Cristofaro, M. Biological Control of Russian Olive, Elaeagnus Angustifolia; Annual report 2007; CABI: Delémont, Switzerland, 2008; pp. 1–20. [Google Scholar]
- Schaffner, U.; Dingle, K.; Swart, C.; Cristofaro, M. Biological Control of Russian Olive, Elaeagnus Angustifolia; Annual report 2011; CABI: Delémont, Switzerland, 2012; pp. 1–20. [Google Scholar]
- Schaffner, U.; Asadi, G.; Chetverikov, P.; Ghorbani, R.; Khamraev, A.; Petanović, R.; Rajabov, T.; Scott, T.; Vidović, B.; Cristofaro, M. Biological Control of Russian Olive, Elaeagnus Angustifolia; Annual report 2013; CABI: Delémont, Switzerland, 2014; pp. 1–20. [Google Scholar]
- Bean, D.; Norton, A.; Jashenko, R.; Cristofaro, M.; Shaffner, U. Status of Russian olive biological control in North America. Ecol. Restor. 2008, 26, 105–107. [Google Scholar] [CrossRef]
- Weyl, P.; Schaffner, U.; Asadi, G.; Klötzli, J.; Vidović, B.; Petanović, R.; Cristofaro, M. Biological Control of Russian Olive, Elaeagnus Angustifolia; Annual Report 2016; CABI: Delémont, Switzerland, 2017; pp. 1–20. [Google Scholar]
- Weyl, P.; Closça, C.; Asad, G.; Vidović, B.; Petanović, R.; Marini, F.; Cristofaro, M. Biological Control of Russian Olive, Elaeagnus Angustifolia; Annual Report 2018; CABI: Delémont, Switzerland, 2019; pp. 1–20. [Google Scholar]
- Fasulati, K.K. Polevoe Izuchenie Nazemnykh Bespozvonochnykh; (=Field study of terrestrial invertebrates), Vyshaya Shkola: Moscow, Russia, 1961; pp. 1–304. [Google Scholar]
- Loginova, M.M. Podotrjad Psyllinea. In Opredelitel Nasekomykh Evropeiskoi Tchasti SSSR, Part 1; Bei-Bienko, G.Y., Ed.; Nauka: Moscow, Russia, 1964; pp. 437–482. [Google Scholar]
- Loginova, M.M. New species of psyllids (Homoptera, Psylloidea). In Trudy Zoologicheskogo Instituta AN SSSR; Nauka: Leningrad, Russia, 1978; pp. 30–123. [Google Scholar]
- Baeva, V.G. Fauna of the Tajik SSR, Psyllids or Jumping Plant-Lice (Homoptera, Psylloidea); Donish: Dushanbe, Tajikistan, 1985; pp. 1–329. [Google Scholar]
- Loginova, M.M. New data on the fauna and biology of the Caucasian Psylloidea (Homoptera). In Trudy Vsesoyuznogo Entomologicheskogo Obshchestva; Akademiya Nauk SSSR: Moscow, Russia, 1968; pp. 275–328. [Google Scholar]
- Gegechkori, F.M. Psyllids (Homoptera, Psylloidea) of Caucasia; Metsniereba: Tbilisi, Georgia, 1984; pp. 1–294. [Google Scholar]
- Klimaszewski, S.M.; Lodos, N. New informations about Jumping Plant Lice of Turkey (Homoptera: Psylloidea). Ege Üniversitesi Ziraat Fakültesi Derg. 1977, 14, 1–9. [Google Scholar]
- Burckhardt, D.; Önuçar, A. A review of Turkish jumping plant-lice (Homoptera, Psylloidea). Rev. Suisse Zool. 1993, 100, 547–574. [Google Scholar]
- Drohojowska, J.; Burckhardt, D. The jumping plant-lice (Hemiptera: Psylloidea) of Turkey: A checklist and new records. Turk. J. Zool. 2014, 38, 559–568. [Google Scholar]
- Burckhardt, D.; Lauterer, P. The jumping plant-lice of Iran (Homoptera, Psylloidea). Rev. Suisse Zool. 1993, 100, 829–898. [Google Scholar]
- Ahmadi, Z.; Mehrvar, A.; Lotfalizadeh, H.; Gharekhani, G. Comparative study of the superfamily Psylloidea in Iran and East-Azarbaijan. Iran. J. Entomol. Res. 2013, 5, 183–193. [Google Scholar]
- Li, F. Psyllidomorpha of China (Insecta: Hemiptera); part 1–2; Huayu Nature Book Trade Co., Ltd.: Beijing, China, 2011; pp. 1–1976. [Google Scholar]
- Kuznetsov, A.V. Lokhovoya listobloshka Trioza magnisetosa Log. (Homoptera, Triozidae) v Severnom Pribalkhashiye (=Oleaster jumping plant-lice Trioza magnisetosa Log. (Homoptera, Triozidae) in North Balkhash Lake area). Tr. Kazakh State Agric. Inst. 1970, 13, 62–70. [Google Scholar]
- Kuznetsov, A.V. Morfologiya lokhovoy listobloshki (Trioza magnisetosa Log.), povrezhdayuschey lokh uzkolistniy v Severnom Pribalkhashie (=Morphology of the oleaster jumping plant-lice (Trioza magnisetosa Log.), damaged Russian Olive in North Balkhash region). In Aktualnye Voprosy Ozeleneniya i Ustoichivosti Derevesnykh i Kustarnikovykh Porod v Centralnom Kazakhstane; Kainar: Alma-Ata, Kazakhstan, 1975; pp. 148–157. [Google Scholar]
- Khlebutina, L.G. Insects of the South and East of Kazakhstan, part 2, section 1, Psyllids of East Kazakhstan. In Report of the Entomological Laboratory of the Institute of Zoology of the Academy of Sciences of the Kazakh SSR; Institute of Zoology: Alma-Ata, Kazakhstan, 1982; pp. 4–45. [Google Scholar]
- Hodkinson, I.D. Life cycle variation and adaptation in jumping plant lice (Insecta: Hemiptera: Psylloidea): A global synthesis. J. Nat. Hist. 2009, 43, 65–179. [Google Scholar]
- Parfentiev, V.Y. Vredityeli Urdinskikh lesnykh nasazhdeniy (=Pests of Urda forest plantations). In Trudy of Republican Station of Plant Protection (Kazakh Branch of All-Union Lenin Academy of Agricultural Sciences); Academy of Sciences of Kazakh SSR: Alma-Ata, Kazakhstan, 1953; pp. 59–61. [Google Scholar]
- Lopatin, I.K. Leaf Beetles (Insecta, Coleoptera, Chrysomelidae) of Central Asia; Belarus University Publ.: Minsk, Belarus, 2010; pp. 1–511. [Google Scholar]
- Kulenova, K.Z. Fauna i ekologicheskie osobennosti Zhukov-listoedov (Coleoptera, Chrysomelidae) yugo-vostoka Kazakhstana (=Fauna and ecological features of leaf beetles (Coleoptera, Chrysomelidae) South-East of Kazakhstan). In Trudy of the Institute of Zoology of the Academy of Sciences of the Kazakh SSR; Nauka: Alma-Ata, Kazakhstan, 1968; pp. 158–183. [Google Scholar]
- Putshkov, V.G. Species of the genus Glaucopterum Wagner 1963 (Heteroptera, Miridae) of the Soviet Union fauna. Dokl. Akad. Nauk. Ukr. SSR Ser. B 1975, 11, 1037–1042. [Google Scholar]
- Kerzhner, I.M. Novye i maloizvestnye vidy Heteroptera iz Mongolii i. Sopredelnikh rayonov SSSR. IV. Miridae, 1 (=New and little-known species of Heteroptera from Mongolia and adjacent regions of the USSR. IV. Miridae, 1). In Nasekomye Mongolii; Nauka: Leningrad, Russia, 1984; pp. 35–72. [Google Scholar]
- Vinokurov, N.N.; Dubatolov, V.V. Desert shield bug Brachynema germarii (Heteroptera: Pentatomidae) is found in the south of Eastern Siberia, Russia. Zoosystematica Ross. 2018, 27, 146–149. [Google Scholar]
- Yagdyev, A.; Tashlieva, A.O. Zhuki-vrediteli orekha i lokha v Turkmenii (=Beetle pests of walnut and oleaster in Turkmenia). In Ekologicheskoe i Khozyaistvennoe Znachenie Nasekomykh Turkmenii; Ilim: Ashghabad, Turkmenistan, 1976; pp. 83–92. [Google Scholar]
- Aeolesthes sarta. EPPO Bull. 2005, 35, 387–389. [CrossRef]
- Legalov, A.A.; Korotyaev, B.A. A new species of the genus Temnocerus Thunb. (Coleoptera: Rhynchitidae) from Kazakhstan. Balt. J. Coleopterol. 2006, 6, 125–127. [Google Scholar]
- Popescu-Gorj, A. Apotomis lutosana Kennel (Lepidoptera, Tortricidae) en Roumanie, espèce nouvelle pour la faune d’Europe. Trav. Du Muséum D’histoire Nat. Grigore Antipa 1984, 25, 237–238. [Google Scholar]
- Rikhter, V.A.; Durdyev, S.K. Tachinids (Diptera, Tachinidae)—parasites of Lepidoptera—orchard pests in Turkmenia. Vestn. Zool. 1988, 1, 62. [Google Scholar]
- Kuznetsov, V.I. Tortricidae. In Keys to the Insects of the European Part of the USSR, IV Lepidoptera Part 1; Nauka: Leningrad, Russia, 1987; pp. 279–967. [Google Scholar]
- Bulyginskaya, M.A.; Akhmedov, A.M.; Dusmanov, I.E. Biological characteristics of the green tortricid Pandemis chondrillana H.-S. (Lepidoptera, Tortricidae) in the Penjikent district of Tajikistan. Entomol. Obozr. 1994, 73, 234–237. [Google Scholar]
- Skou, P. The Geometroid Moths of North Europe (Lepidoptera, Drepanidae and Geometridae); Scandinavian Science Press: Copenhagen, Denmark, 1986; pp. 1–348. [Google Scholar]
- Forest Pests on the Territories of the Former USSR; European and Mediterranean Plant Protection Organization: Paris, France, 2000; pp. 1–117.

| Months | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV | V | VI | VII | VIII | IX | X-XII-I-III | ||||||||||||
| Decades | Decades | Decades | Decades | Decades | Decades | |||||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Normal Spring | ||||||||||||||||||
| 3 generation (overwintered adults) | 1generation (adults) | 2 generation (adults) | 3 generation (overwintered adults) | |||||||||||||||
| W | W | W | W | |||||||||||||||
| ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ||
| ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ||
| ◎ | ◎ | ◎ | ◎ | ◎ | ◎ | ◎ | ◎ | ◎ | ◎ | ◎ | ◎ | |||||||
| oo | oo | oo | oo | oo | oo | oo | oo | oo | ||||||||||
| L1 | L1 | L1 | L1 | L1 | L1 | |||||||||||||
| L2 | L2 | L2 | L2 | L2 | L2 | |||||||||||||
| L3 | L3 | L3 | L3 | L3 | L3 | |||||||||||||
| L4 | L4 | L4 | L4 | L4 | L4 | |||||||||||||
| L5 | L5 | L5 | L5 | L5 | L5 | |||||||||||||
| Months | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV | V | VI | VII | VIII | IX | X-XII-I-III | ||||||||||||
| Decades | Decades | Decades | Decades | Decades | Decades | |||||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Normal Spring | ||||||||||||||||||
| W | W | W | W | W | W | W | ||||||||||||
| ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ||||||||||||
| ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ||||||||||||
| ◎ | ◎ | ◎ | ◎ | |||||||||||||||
| oo | oo | oo | oo | |||||||||||||||
| L1 | L1 | L1 | L1 | |||||||||||||||
| L2 | L2 | L2 | L2 | |||||||||||||||
| L3 | L3 | L3 | L3 | |||||||||||||||
| P | P | P | P | |||||||||||||||
| Earlier Warm Spring | ||||||||||||||||||
| W | W | W | W | W | ||||||||||||||
| ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ||||||||
| ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ♀ | ||||||||
| ◎ | ◎ | ◎ | ◎ | |||||||||||||||
| oo | oo | oo | oo | oo | ||||||||||||||
| L1 | L1 | L1 | L1 | L1 | L1 | |||||||||||||
| L2 | L2 | L2 | L2 | L2 | L2 | |||||||||||||
| L3 | L3 | L3 | L3 | L3 | L3 | |||||||||||||
| P | P | P | P | P | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jashenko, R.; DeLoach, C.J.; Ilina, V. Two of the Most Promising Potential Agents from Kazakhstan for the Biocontrol of Russian Olive (Elaeagnus angustifolia) in the USA with an Annotated List of Its Pest Insects from Central Asia. Forests 2025, 16, 614. https://doi.org/10.3390/f16040614
Jashenko R, DeLoach CJ, Ilina V. Two of the Most Promising Potential Agents from Kazakhstan for the Biocontrol of Russian Olive (Elaeagnus angustifolia) in the USA with an Annotated List of Its Pest Insects from Central Asia. Forests. 2025; 16(4):614. https://doi.org/10.3390/f16040614
Chicago/Turabian StyleJashenko, Roman, C. Jack DeLoach, and Viktoriya Ilina. 2025. "Two of the Most Promising Potential Agents from Kazakhstan for the Biocontrol of Russian Olive (Elaeagnus angustifolia) in the USA with an Annotated List of Its Pest Insects from Central Asia" Forests 16, no. 4: 614. https://doi.org/10.3390/f16040614
APA StyleJashenko, R., DeLoach, C. J., & Ilina, V. (2025). Two of the Most Promising Potential Agents from Kazakhstan for the Biocontrol of Russian Olive (Elaeagnus angustifolia) in the USA with an Annotated List of Its Pest Insects from Central Asia. Forests, 16(4), 614. https://doi.org/10.3390/f16040614






