Abstract
Soil salinization significantly exacerbates the deficiency in plant-available phosphorus in the soil, thereby adversely affecting plant growth and development. Through various processes, phosphate-solubilizing bacteria in the rhizosphere significantly increase soil-soluble phosphorus content, boosting plant development and stress resistance. This study focused on annual R. soongorica seedlings to examine how rhizosphere phosphate-solubilizing bacteria enhance growth under NaCl-induced stress conditions. This study isolated and characterized rhizosphere phosphate-solubilizing bacteria, evaluating their phosphate solubilization capacity and effects on R. soongorica seedling growth and physiology under NaCl stress through pot experiments, with potential applications in saline soil improvement and desert ecosystem restoration. This study used four treatment groups (control group, NaCl treatment group, bacterial inoculation treatment group, and bacterial and NaCl mixed-treatment group) with twelve treatments and four replicates per treatment. The experimental results demonstrated that five phosphate-solubilizing bacterial strains exhibited a significant phosphate solubilization capacity, accompanied by a notable reduction in pH within the inorganic phosphorus medium. Compared to the NaCl treatment, the net growth of the plant height of R. soongorica seedlings inoculated with strains J23, J24, and M1 under NaCl stress increased significantly (p < 0.05), and all of them more than doubled, and the net growth of the stem diameter of R. soongorica seedlings inoculated with strain J24 increased significantly by 144.17%. The physiological characteristics of R. soongorica seedlings demonstrated significant alterations following inoculation with the five phosphate-solubilizing bacterial strains. The inoculation of R. soongorica seedlings with the five phosphate-solubilizing bacterial resulted in a statistically significant increase in both foliar total phosphorus content and available phosphorus levels within the rhizosphere soil (p < 0.05). Additionally, under NaCl stress conditions, R. soongorica seedlings inoculated with the five phosphate-solubilizing bacterial strains exhibited varying degrees of salt tolerance, with the following descending order of effectiveness: J24 > P2 > J23 > P3 > M1. In conclusion, the rhizosphere phosphate-solubilizing bacteria J24 represents a potentially valuable microbial resource for saline soil amelioration, demonstrating the most pronounced enhancement in both the growth parameters and salt tolerance of R. soongorica seedlings under 300 mmol·L−1 NaCl stress.
1. Introduction
Salinized soil is found throughout China with a total area of 3.69 × 107 hm2 and comes in various forms. It is particularly prevalent in arid and semi-arid regions, where it significantly negatively influences the long-term viability of forestry and agriculture [1]. Soil salinization occurs when soluble salts accumulate excessively in the soil, driven by human activities and climatic factors. Key contributors include inappropriate tillage techniques, the misuse of chemical fertilizers, and climatic conditions such as high evaporation and precipitation. NaCl is the most prevalent of them [2]. Similar to other forms of abiotic stress, the excessive accumulation of soluble salts adversely affects plant growth, development, physiology, and biochemical processes. These effects include osmotic stress, ion homeostasis disruption, and reduced plant nutrient uptake [3]. Therefore, soil salinization remediation is crucial to enhancing soil usage and maintaining ecosystem stability.
Over the long-term evolution process, numerous halophytes have emerged in nature, capable of thriving in high-salinity environments and mitigating salt stress damage through diverse defence mechanisms [4]. On the one hand, plants can alleviate salt stress by maintaining cellular ion homeostasis. On the other hand, plants employ both non-enzymatic antioxidants and antioxidant enzymes to synergistically reduce the accumulation of reactive oxygen species (ROS). Additionally, the accumulation of osmoregulatory substances supports plant cell expansion and maintains adequate water uptake [5]. Furthermore, various growth-promoting bacteria in the rhizosphere may also relate to salt tolerance. Phosphate-solubilizing bacteria (PSB), a group within plant growth-promoting rhizobacteria (PGPR) in halophytes, can solubilize insoluble phosphate or mineralize organic phosphorus [6]. They enhance plants’ absorption and utilization of soluble phosphorus while preventing the re-fixation of the released soluble phosphorus [7]. Additionally, they promote plant growth and improve stress tolerance through various pathways, including the production of organic acids, iron carriers, auxin, and other beneficial substances [8]. The phosphate-solubilizing bacterium Curtobacterium luteum has been isolated from a saline–alkali seagrass meadow. In quantitative analysis, the phosphate-solubilizing amount of the strain was 208.41 μg/mL. By inoculating three crops under saline–alkali stress, the results showed that it promoted the growth of the three crops, and the soil available phosphorus content increased significantly by 67.23%, 37.61%, and 7.47% [9]. Additionally, phosphate-solubilizing bacteria enhance the decomposition of soil insoluble or fixed phosphorus by actively participating in soil phosphorus cycling. A study inoculated MWP-1 (Pseudomonas poae), a highly efficient phosphorus-solubilizing bacterium, into the high-altitude Muli mining area of Qinghai Province to investigate its effects on soil properties and nutrient cycling. The results showed that the strain was able to significantly increase soil quick-acting phosphorus content by over 50%. Additionally, it altered the relative abundance of genes regulating phosphorus uptake, transport, and inorganic phosphorus solubilization within the bacterial community [10]. Therefore, rhizosphere phosphate-solubilizing bacteria in halophytes hold significant potential for mitigating soil salinization.
Phosphorus, an essential mineral nutrient for plant growth and development, plays a critical role in metabolic processes such as photosynthesis, respiration, signal transduction, and energy transfer. It is also vital for improving plant resistance to abiotic stress [11]. Under conditions of excessive salt accumulation, phosphorus is essential for maintaining the functional diversity of soil ecosystems and supporting normal plant growth. Wu Yang demonstrated a strong correlation between soil multifunctionality under salt stress and the functional diversity of soil phosphorus [12]. At the same salt concentration, reductions in treatment levels led to declines in the phosphorus content, chlorophyll content, plant height, and dry weight of Sorghum bicolor ‘Dochna’ leaves [13]. Although phosphorus is abundant in natural soil, plants can only absorb and utilize a small amount. Simultaneously, excessive soil salt accumulation causes osmotic stress and ion toxicity, impairing plant phosphorus absorption and transport. This leads to phosphorus fixation and mineralization in the soil due to cation interactions and competition with phosphate, disrupting plant phosphorus transport, absorption, and utilization and exacerbating the deficiency in plant-available phosphorus [14]. Studies show that treating Medicago sativa with a certain quantity of NaCl considerably decreases its phosphorus content [15]. Consequently, improving plants’ phosphorus absorption and utilization is crucial to increase their salt tolerance.
R. soongorica is a Tamaricaceae shrub that is highly xerophytic and stress-resistant. It serves as a key constructive and dominant species in the arid and semi-arid regions of Northwest China, playing a vital role in ecological protection and vegetation restoration in desert and grassland areas [16]. R. soongorica possesses a unique salt gland structure, enabling it to transport internal salts to large vesicles under salt stress, which are then excreted through the secretory cells’ external membranes [17]. Some studies have found that R. soongorica, which belongs to salt-secreting plants, can secrete salt through its leaves to alleviate salt stress [16]. Despite its strong salt tolerance, R. soongorica has weak seedling resistance and slow growth, limiting its widespread use in large-scale desert vegetation restoration. The research on R. soongorica is predominantly focused on three main aspects: physiological characteristics [18], the influence of exogenous substances on stress resistance [19], and the screening of resistance genes. There are relatively few studies on the characteristics of bacterial community composition in the rhizosphere of R. soongorica and the effect of rhizosphere phosphate-solubilizing bacteria on the salt tolerance of R. soongorica. How does the inoculation of rhizosphere phosphate-solubilizing bacteria affect R. soongorica’s growth and physiological characteristics when exposed to NaCl stress? How has the phosphorus content of its rhizosphere soil and leaves changed? A series of problems, such as salt tolerance, can be improved by inoculating phosphate-solubilizing bacteria. Therefore, this paper took R. soongorica seedlings as the research object, analysed the effects of phosphate-solubilizing bacteria on the growth and physiological characteristics of the seedlings by applying NaCl and phosphate-solubilizing bacteria, and explored the effects of inoculating rhizosphere phosphate-solubilizing bacteria on the phosphorus content of R. soongorica. This study aimed to establish a scientific foundation for enhancing R. soongorica’s resistance to salt and to serve as a guide for improving saline soil.
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Overview of the Study Area and Soil Sample Collection
Soil samples were collected in the Qingtu Lake area of Minqin County, Gansu Province, with an average annual temperature of about 7.6 °C and an average annual precipitation of 113.2 mm. The latitude and longitude of the sampling site is 39°8′10.65″ N, 103°30′19.60″ E, and the altitude is 1030.2 m. The area experiences low rainfall, extensive evaporation, and a significant temperature difference between day and night, characteristic of a typical temperate continental desert climate [20]. The dominant vegetation is mainly herbaceous plants and drought-tolerant shrubs, including herbaceous plants such as Limonium aureum and Agriophyllum pungens and shrubs such as R. soongorica and Haloxylon ammodendron [21].
In April 2023, in this area, three sample plots with R. soongorica populations as the dominant population or a single population were set up as standard sample plots with a size of 100 m × 100 m. Within the standard sample plots set, five small sample plots with a size of 10 m × 10 m were selected using the five-point method, and then five plants of R. soongorica with the same growth and no pests or diseases were randomly selected in the small sample plots. The debris on the soil’s surface was scooped away, the soil was dug up around the roots, and R. soongorica roots in the 0–20 cm soil layer were collected. After shaking off the loose soil block from the root surface, a small brush was used to lightly brush the soil attached to the root surface of about 2 mm as rhizosphere soil. The researchers sterilized all experimental tools to avoid contamination during sample collection. After completing the sampling, the rhizosphere soil obtained from the small sample was evenly put into sterile bags and then transported back to the laboratory at a low temperature and stored in a refrigerator at −80 °C for subsequent separation and screening of phosphate-solubilizing bacterial strains.
2.1.2. Testing Material
The rhizosphere soil obtained from the small sample was sampled evenly. The seeds of R. soongorica used in the experiment were collected from the Qingtu Lake area of Minqin County, Wuwei City, Gansu Province, in November 2022 and preserved by low-temperature drying, and the annual R. soongorica seedlings were cultivated artificially for the test.
The phosphate-solubilizing bacteria used in the experiment were isolated from the rhizosphere soil of R. soongorica in the Qingtu Lake area. After 16S rRNA sequencing, combined with Gram staining and phylogenetic analysis, five strains of phosphate-solubilizing bacteria were finally identified as P2 (Bacillus pumilus), P3 (Enterobacter mori), J23 (Enterobacter cloacae), J24 (Bacillus safensis), and M1 (Bacillus megaterium).
The medium used in the experiment was LB liquid medium: tryptone 10 g·L−1, yeast extract 5 g·L−1, NaCl 10 g·L−1, and pH = 7.2 (solid medium with agar 10–15 g·L−1); inorganic phosphorus liquid medium: glucose 10 g·L−1, (NH4)2SO4 0.5 g·L−1, yeast extract 0.5 g·L−1, NaCl 0.3 g·L−1, KCl 0.3 g·L−1, MgSO4·7H2O 0.3 g·L−1, FeSO4·7H2O 0.03 g·L−1, MnSO4·H2O 0.03 g·L−1, Ca3(PO4)2 5 g·L−1, and pH = 7.2 (solid medium with agar 10–15 g·L−1).(The above reagents are from Haibo Biology in Qingdao, Shandong Province, China)
2.2. Determination Indexes and Methods
2.2.1. Isolation and Identification of Phosphate-Solubilizing Bacteria
The 16S rRNA sequencing technology was used to determine the taxonomic status of the selected dephosphorylated strains, and the genes of the strains were amplified by PCR using 27F (5′-AGAGAGTTTGATCCTGGGCTCAG-3′) and 1492R (5′-TACGGGYTACCTTGTTAYGACTT-3′) as the primers. The reaction conditions were as follows: pre-denaturation at 95 °C for 2 min; denaturation at 95 °C for 15 s, annealing at 53 °C for 15 s, extension at 72 °C for 15 s, and the repetition of this cycle 31 times; and total extension at 72 °C for 5 min. The PCR products were detected by agarose gel electrophoresis with 1% agarose to ensure that the concentration was up to the standard, and then, the resulting amplified genes were sent to Guangdong Meige Genetic Science and Technology Co. The obtained sequences were compared and analysed with the NCBI database using the Blast program, and the gene sequences of standard strains with high similarity were selected. A phylogenetic tree was constructed using the MEGA 11 neighbour-joining method. The above methods were used to determine the classification status of the strains.
The dilution-coated plate method and plate scribing method were used to isolate and purify the phosphate-solubilizing bacteria from R. soongorica rhizosphere soil. In total, 1 g of R. soongorica rhizosphere soil was weighed into a triangular vial containing 100 mL of sterile water, placed in a 150 rpm·min−1 shaker for 30 min, and then subjected to gradient dilution (10−2–10−6), with three replicates set for each gradient. Then, 0.1 mL soil dilutions of different gradients were spread on LB solid medium and inverted in an incubator at a constant temperature of 28 °C for 24 h–72 h. Different morphological colonies growing well on the medium were labelled and numbered, and single colonies were picked with inoculation rings. Subsequently, the LB solid medium was delineated by the trichotomous delineation method and inverted in a constant-temperature incubator at 28 °C for 24 h. After three rounds of purified delineation, the resulting purified strains were placed in 25%–30% glycerol tubes and stored in a refrigerator at −80 °C for spare use [22].
2.2.2. Functional Characteristics of Phosphate-Solubilizing Bacteria
The isolation and purification of phosphate-solubilizing bacteria from the rhizosphere soil of R. soongorica were conducted by the dilution-coated plate and plate streaking methods. The selected phosphate-solubilizing bacteria were inoculated in an LB liquid medium and placed on a shaker at 28 °C and 180 rpm·min−1 for 24 h to produce a bacterial suspension with an OD600 = 0.8–1. The fermentation broth was inoculated into the inorganic phosphorus-dissolving liquid medium with 1% inoculation amount, and the inorganic phosphorus liquid medium without inoculation was used as the control. It was placed on a shaker at 28 °C and 180 rpm·min−1 for 7 days. The fermentation broth was taken on the 1st, 3rd, 5th, and 7th days. The researchers determined the available phosphorus content using molybdenum–antimony resistance colorimetry and measured the pH with a pH meter (German Sartorius PB10, Gottingen, Germany) [22].
2.2.3. Pot Experiments
The experiment was conducted at the science and technology base on the Gansu Agricultural University campus, with good ventilation, sufficient light, and an artificial rain shelter. Before seedling cultivation, the full, uniform, and pest-free R. soongorica seeds were soaked in 75% alcohol for 1 min and then rinsed with sterile distilled water 3–5 times to complete seed disinfection. The substrate used in the experiment was composed of peat soil, vermiculite, and perlite mixed according to the volume ratio of 3:1:1. Seedlings began to grow in the tray on 17 April 2023 and were transplanted on 17 June 2023 into uniformly sized pots filled with about 5.2 kg of substrate per pot. When the plant height reached 10–12 cm, the researchers selected R. soongorica seedlings with uniform growth for the experiment and placed two plants in each pot.
The experiment was set up with four treatment groups, which included a total of twelve treatments, with each treatment replicated four times for a total of 48 pots, as follows: (1) control group (CK); (2) NaCl treatment group (concentration 300 mmol·L−1); (3) phosphate-solubilizing bacteria treatment group (P2, P3, J23, J24, M1); and (4) phosphate-solubilizing bacteria and salt mixed-treatment group (P2 + NaCl, P3 + NaCl, J23 + NaCl, J24 + NaCl, M1 + NaCl). The previous research results of the research group have shown that when NaCl reaches 300 mmol·L−1, it can slightly inhibit the growth of R. soongorica seedlings, so the authors selected to use this concentration of NaCl to treat R. soongorica. A progressive salt application method was used to treat the R. soongorica seedlings with NaCl to prevent acute salt damage to seedlings. The experiment started with 50 mmol·L−1 NaCl solution per day and gradually increased the target concentration, reaching 50 mmol·L−1 NaCl solution daily [23]. We irrigated each pot with 1500 mL of water each time and added the same amount of sterile water without NaCl treatment. The inoculation of plants with phosphate-solubilizing bacteria was conducted after 7 days of NaCl treatment. Five strains of phosphate-solubilizing bacteria were inoculated in LB liquid medium at 1% and placed on a shaker at 28 °C and 180 rpm·min−1 for 24 h. After centrifugation at 6000 rpm·min−1 for 6 min to collect phosphate-solubilizing bacteria, the OD600 was adjusted to 0.8–1 with sterile saline (0.9% NaCl) to obtain a phosphate-solubilizing bacterial suspension. R. soongorica was inoculated with 100 mL phosphate-solubilizing bacterial suspension per pot by the root-drenching method. After 14 days, the same process was used for secondary inoculation, and the same amount of sterile normal saline was added to the non-inoculated treatment. Seven days after the last treatment, the plant seedling heights and stem thicknesses were measured, and plant and rhizosphere soil samples were collected.
After collecting plant samples, the physiological characteristics and phosphorus content were determined. Superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) were determined by the nitrogen blue tetrazolium (NBT) method, ultraviolet absorption method, and guaiacol method, respectively. The contents of soluble sugar, soluble protein, and free proline were determined by the anthrone colorimetry method, Coomassie brilliant blue method, and acid ninhydrin colorimetry, respectively [24]. The content of available phosphorus in rhizosphere soil and total phosphorus in plant leaves was determined by the 0.5 mol·L−1 NaHCO3 method and molybdenum–vanadium yellow colorimetric method, respectively [25]. (The above reagents are from Shanghai Sinopharm Group, Shanghai, China)
2.3. Data Statistics and Analysis
We used Microsoft Excel 2010 and SPSS 26.0 software to complete the statistical data analysis. We also used Pearson correlation analysis of soluble phosphorus content and pH of phosphate-solubilizing bacteria in the rhizosphere soil of R. soongorica. One-way ANOVA was used to compare the growth characteristics, physiological characteristics, and phosphorus content of R. soongorica seedlings under different treatments. The ability of phosphorus-solubilizing bacteria to improve the salt tolerance of R. soongorica was compared by membership function analysis. Origin 2021 software was used for mapping.
3. Results
3.1. Determination of Phosphorus-Solubilizing Capacity of Phosphate-Solubilizing Bacteria
3.1.1. Phosphate-Solubilizing Ability of Different Strains
Figure 1 and Figure 2 shows the phylogenetic evolutionary tree of five strains of phosphate-solubilizing bacteria, combined with 16S rRNA to determine the taxonomic status of the strains. Table 1 shows the phosphate-solubilizing effects of five strains of phosphate-solubilizing bacteria, P2, P3, J23, J24, and M1, in inorganic phosphorus-solubilizing solid medium. The colony diameters ranged from 0.39 to 0.74 cm, the diameters of solubilized phosphorus circles ranged from 0.42 to 1.44 cm, and the solubilizing index (SI) ranged from 2.08 to 3.55. Among them, the phosphorus solubilization index of the P2 strain was the highest, and the phosphorus solubilization index of the J24 strain was the lowest. The preliminary conclusion is that these five strains can dissolve inorganic phosphorus.
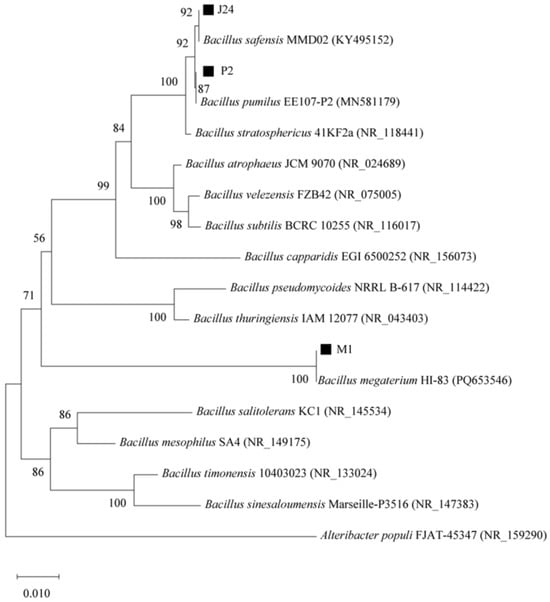
Figure 1.
The phylogenetic tree of phosphate-solubilizing bacteria (strains P2, J24, M1) in the rhizosphere of Bacillus was constructed based on 16S rRNA gene sequence.
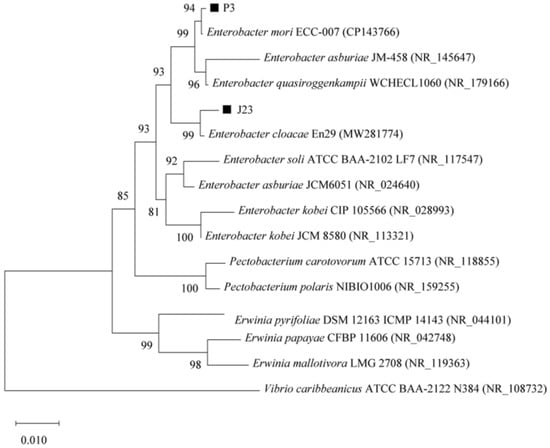
Figure 2.
The phylogenetic tree of phosphate-solubilizing bacteria (strains P3 and J23) in the rhizosphere of Enterobacter was constructed based on 16S rRNA gene sequence.

Table 1.
The effect of different phosphorus-solubilizing bacteria in solid inorganic phosphorus-solubilizing medium.
3.1.2. Growth Dynamics of Phosphate-Solubilizing Bacteria During Phosphate-Solubilizing Process
Five strains of phosphate-solubilizing bacteria were inoculated in an inorganic phosphorus medium containing Ca3(PO4)2 as the sole inorganic phosphorus source, without bacteria as the control (CK). The dissolved phosphorus content and pH were measured during the incubation period, as illustrated in Figure 3. The five strains could solubilize inorganic phosphorus compared to the CK. As the culture time increased, the phosphorus solubilization capacities of strains P2, P3, and J23 reached the maximum on the first day of culture, which were 126.53 mg·L−1, 58.74 mg·L−1, and 26.47 mg·L−1, respectively, before declining with further incubation. The soluble phosphorus content of the M1 strain increased with the rise in culture time, reached the maximum value of 44.15 mg·L−1 on the fifth day, and then decreased with the increase in culture time. The soluble phosphorus content of the J24 strain increased with the rise in culture time, and the maximum soluble phosphorus content reached 1.71 mg·L−1 on the seventh day. During the culture time, the pH value of the inorganic phosphorus-solubilizing medium inoculated with five strains of phosphorus-solubilizing bacteria was lower than that of the CK, and the changing trend of different strains was different.
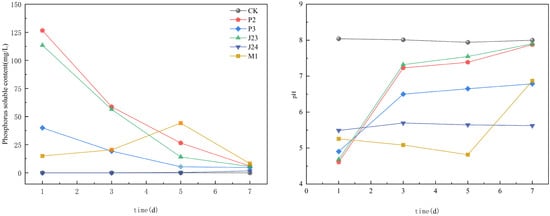
Figure 3.
Changes in soluble phosphorus content and pH in inorganic phosphorus medium under different treatments of phosphate-solubilizing bacteria.
In contrast, the pH value of the CK did not significantly change and stabilized at about 8.0. The pH value of the inorganic phosphorus-solubilizing medium of the P2, P3, and J23 strains was the smallest on the first day of inoculation, which was 4.61, 4.91, and 4.69, respectively, and then increased rapidly. After three days of inoculation, the rise rate slowed until it was close to the CK on the seventh day. The pH value of the J24 strain minimized to 5.49 on the first day of inoculation, then increased until it decreased slowly after three days. The pH of the M1 strain decreased after one day of inoculation until the third day when the pH reached a minimum value of 5.09 and then increased rapidly.
Table 2 shows the results of the correlation analysis between the available phosphorus content and pH in the inorganic phosphorus medium under different phosphate-solubilizing bacteria treatments. There was a significant negative correlation between the available phosphorus content and pH in the inoculated inorganic phosphorus medium of strains P2, P3, and J23 (p < 0.01) and a significant negative correlation between the available phosphorus content and pH of strain M1 (p < 0.05).

Table 2.
Correlation analysis of dissolved phosphorus content and pH in inorganic phosphorus medium treated with different phosphate-solubilizing bacteria.
3.2. Effect of Phosphate-Solubilizing Bacteria on Growth of R. soongorica
Figure 4 shows that inoculation with different phosphate-solubilizing bacteria significantly affected the growth characteristics of R. soongorica seedlings (p < 0.05). Under the NaCl stress treatment, there were substantial differences in the plant height net growth and root biomass compared with the CK (p < 0.05), which decreased by 42.65% and 43.68%, respectively. In contrast, inoculation with different phosphate-solubilizing bacteria had various positive effects on the growth of R. soongorica seedlings under NaCl stress. The net growth in the plant height of R. soongorica seedlings inoculated with the J23, J24, and M1 strains under NaCl stress increased significantly (p < 0.05) compared to the NaCl treatment, which was more than doubled. The net growth of the base diameter of R. soongorica seedlings inoculated with the J24 strain increased significantly by 144.17%. The total biomass of R. soongorica seedlings inoculated with the P2 and J23 strains significantly increased by 7.35% and 21.01%, respectively. The root biomass of R. soongorica inoculated with five strains increased significantly, by 124.37%, 33.04%, 106.88%, 63.35%, and 40.34%. The biomass of R. soongorica seedling leaves inoculated with the J23 strain increased significantly by 11.03%.
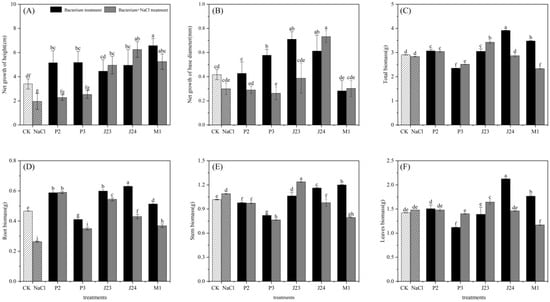
Figure 4.
Effects of different treatments on growth indexes of R. soongorica seedlings. Different letters show significant difference in R. soongorica seedling growth between treatments (p < 0.05). (A) is net growth height, (B) is net growth of diameter, (C) is total biomass, (D) is root biomass, (E) is stem biomass, (F) is leaves biomass.
3.3. Effect of Phosphate-Solubilizing Bacteria on Antioxidant Enzyme Activity of R. soongorica Seedlings
As shown in Figure 5, the POD activity of R. soongorica seedlings increased significantly by 42.53% compared with the CK under NaCl stress, indicating that the 300 mmol·L−1 NaCl treatment inhibited the growth of R. soongorica seedlings. Under NaCl stress, the SOD activity of R. soongorica seedlings inoculated with the P3 and M1 strains was significantly different from that of the NaCl treatment (p < 0.05), in which the SOD activity of R. soongorica seedlings inoculated with the M1 strain increased by 30.13%. The difference in the POD activity of R. soongorica seedlings inoculated with the P3, J24, and M1 strains under NaCl stress was significant (p < 0.05) compared with the NaCl treatment, and the POD activity of R. soongorica seedlings inoculated with the J24 strain increased by 87.55%. There was no significant difference in the CAT activity between R. soongorica inoculated with P3 and seedlings inoculated with the J24 strain (p > 0.05).
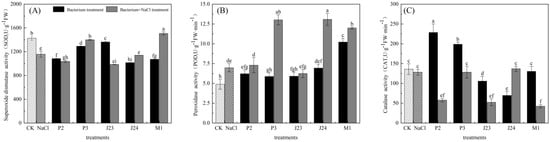
Figure 5.
Effect of different treatments on antioxidant enzyme activities of R. soongorica seedlings. Different letters show significant difference in R. soongorica seedling growth between treatments (p < 0.05). (A) is superoxide dismutase activity(SOD), (B) is peroxidase cetivity(POD), (C) is catalase activity(CAT).
3.4. Effect of Phosphate-Solubilizing Bacteria on Osmotic Adjustment Substance of R. soongorica Seedlings
As shown in Figure 6, the NaCl stress treatment significantly reduced the soluble sugar content of R. soongorica seedlings by 35.69%, and the soluble protein and proline contents were different from the CK (p < 0.05), increasing by 70.98% and 9.46%, respectively. The soluble sugar content of R. soongorica seedlings inoculated with the P2, J23, and M1 strains under NaCl stress was significantly reduced by 24.91%, 45.55%, and 22.91%, respectively, compared with the NaCl stress treatment. The soluble protein of R. soongorica seedlings inoculated with the P2, P3, J23, and M1 strains under NaCl stress was significantly different from that under NaCl stress (p < 0.05) and more than three times higher. The proline content of R. soongorica seedlings inoculated with the P3 and J23 strains under NaCl stress significantly differed from that under NaCl stress (p < 0.05), increasing by 27.06% and 19.67%, respectively.
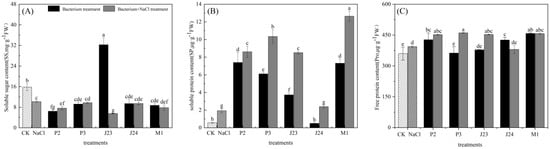
Figure 6.
Effects of different treatments on osmoregulatory substances in R. soongorica seedlings. Different letters show significant difference in R. soongorica seedling growth between treatments (p < 0.05). (A) is soluble sugar content(ss), (B) is soluble protein(SP), (C) is free protein(SP).
3.5. Effects of Phosphate-Solubilizing Bacteria on Phosphorus Content in Leaves and Rhizosphere Soil of R. soongorica Seedlings
As shown in Figure 7, the contents of total phosphorus in the leaves and available phosphorus in the rhizosphere soil of R. soongorica seedlings under NaCl stress were significantly different from those of the CK (p < 0.05), decreasing by 60.65% and 45.86%, respectively. The total phosphorus content of R. soongorica seedling leaves inoculated with five strains under NaCl stress was significantly increased compared with the NaCl stress treatment (p < 0.05), and the total phosphorus content of R. soongorica seedling leaves inoculated with the J24 strain increased by more than four times. The content of available phosphorus in the rhizosphere soil of R. soongorica seedlings inoculated with the P2, J23, J24, and M1 strains was significantly increased compared with that under NaCl stress (p < 0.05), and the content of available phosphorus in the rhizosphere soil of R. soongorica seedlings increased by more than five times following inoculation with P2.
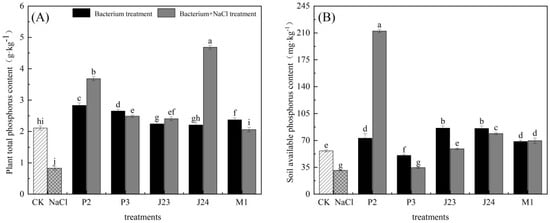
Figure 7.
Effects of different treatments on total phosphorus content and rhizosphere soil available phosphorus content of R. soongorica seedlings. Different letters show significant difference in R. soongorica seedling growth between treatments (p < 0.05). (A) is plant total phosphorus content (TP), (B) is soil available phosphrus content (AP).
3.6. Comprehensive Evaluation of Effects of Different Phosphate-Solubilizing Bacteria on Salt Tolerance of R. soongorica Seedlings Under NaCl Stress
As shown in Figure 8, a correlation analysis of 14 indexes of R. soongorica seedlings inoculated with phosphate-solubilizing bacteria under NaCl stress was carried out. The total biomass had a negative correlation with SOD; TP had a negative correlation with SP; the net stem gross growth had a negative correlation with proline and SP. SS had a positive correlation with POD, and SP had a positive correlation with proline. Many of the indicators are significantly associated, and the evaluation information overlaps. Therefore, it is necessary to use principal component analysis to further screen the key factors affecting the growth and physiological characteristics of R. soongorica seedlings under NaCl stress.
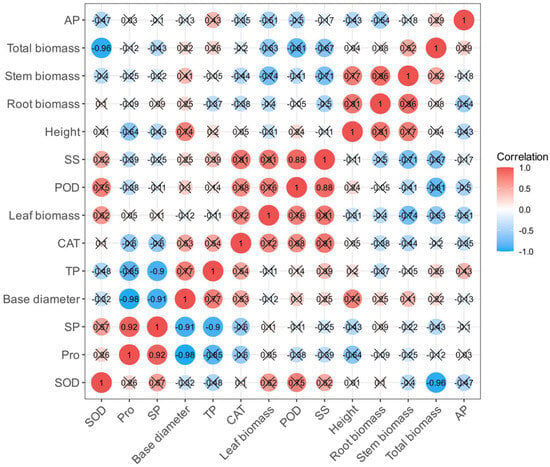
Figure 8.
The correlation heatmap of every single index.
As shown in Table 3, we performed principal component analysis on 14 individual indicators of R. soongorica seedlings inoculated with phosphate-solubilizing bacteria under NaCl stress. The contribution rates of the first four comprehensive indicators are 44.515%, 34.894%, 12.778%, and 7.812%. The cumulative contribution rate was greater than 85%, and the eigenvalue was more significant than 1. The data show that the eigenvalue of the first principal component was 6.232, and the coefficients of total biomass, root biomass, and stem biomass were more significant in the first principal component. The eigenvalue of the second principal component was 4.885, and the coefficients of net stem thickness growth, TP, and CAT were more prominent in the second principal component. The eigenvalue of the third principal component was 1.789, and AP, TP, and soluble sugar had significant coefficients in the third principal component. The characteristic value of the fourth principal component was 1.094, and the coefficients of CAT, leaf biomass, and total biomass were more extensive in the fourth principal component. This exhaustive evaluation selected the first four comprehensive indicators as independent indicators. We calculated the relevant, comprehensive index values based on the standardized values of the four individual indexes under NaCl stress and the eigenvector coefficients of each comprehensive index.

Table 3.
Principal component analysis of growth and physiological indexes of R. soongorica seedlings inoculated with different phosphate-solubilizing bacteria under NaCl stress.
The effects of different phosphate-solubilizing bacteria on the salt tolerance of R. soongorica seedlings under NaCl stress were evaluated by the membership function method. As shown in Table 4, according to the comprehensive evaluation D value of each index, the effect of five strains of phosphate-solubilizing bacteria on the salt tolerance of R. soongorica seedlings under 300 mmol·L−1 NaCl stress was J24 > P2 > J23 > P3 > M1.

Table 4.
Comprehensive Evaluation of the Effects of Inoculating Different Phosphate-Solubilizing Bacteria on the Salt Tolerance of R. soongorica Seedlings under NaCl Stress.
4. Discussion
4.1. Functional Characteristics of Rhizosphere Phosphorus-Solubilizing Bacteria
Phosphate-solubilizing bacteria drive the soil phosphorus cycle and can increase the soil’s phosphorus concentration. Plants can utilize this enhanced phosphorus in multiple ways to improve the soil’s ecological environment [26,27]. However, the distribution of phosphate-solubilizing bacteria is influenced by various factors, including soil environmental conditions, plant species, and diversity. Researchers have not thoroughly studied the resources of phosphate-solubilizing bacteria in the rhizosphere of desert plants. In this study, the five strains of phosphate-solubilizing bacteria isolated from the rhizosphere soil of the desert plant R. soongorica belong to Bacillus and Enterobacter. Their maximum inorganic phosphorus-solubilizing content was 126.53 mg·L−1, 58.74 mg·L−1, 26.47 mg·L−1, 44.15 mg·L−1, and 1.71 mg·L−1. Some researchers have isolated phosphate-solubilizing bacteria from the rhizosphere soil of Phragmites australis in saline and alkaline areas, mainly Bacillus, Enterobacter, and Pseudomona, and the phosphorus-solubilizing amount ranged from 55.8 to 722.3 mg·L−1 [28], which is similar to the phosphate-solubilizing bacteria groups screened from the rhizosphere saline–alkali soil of plants in different regions, and the amount of inorganic phosphorus-solubilizing content is different between them. Therefore, we speculated that Bacillus and Enterobacter are necessary phosphorus-solubilizing bacterial resources widely distributed in desert saline–alkali soil environments, which can better adapt to the extreme climate of the desert. However, due to the different types of phosphate-solubilizing bacterial strains and culture environments, the amount of inorganic phosphorus-solubilizing content will also be different.
Furthermore, phosphate-solubilizing bacteria dissolve insoluble phosphorus through processes such as acidification, proton secretion, and enzymatic hydrolysis. The acidification principle is mainly based on producing organic acid-chelating cations or releasing hydrogen ions [29]. This study found that inoculating the inorganic phosphorus-solubilizing medium with the P2, P3, J23, and M1 strains resulted in a lower pH value compared to the CK during the culture time, and the soluble phosphorus content showed a significant negative correlation with pH. Studies have shown that Burkholderia cepacia and Bacillus pumilus contain different kinds of organic acids in the supernatant [30]. The combined application of phosphate-solubilizing bacteria and phosphate fertilizer significantly enhances rhizosphere phosphatase activity and organic acid content in wheat [31]. We therefore hypothesize that different phosphate-solubilizing bacteria secrete varying types and concentrations of organic acids or H+ during respiration and fermentation, et cetera, leading to differing degrees of pH reduction in the medium [32]. The pH of strain J24 was lower than that of the blank control. However, the correlation between its soluble phosphorus content and pH was weak, likely because this strain dissolves phosphorus not only through acid production but also via extracellular phosphatase secretion and other mechanisms.
4.2. Effects of Phosphate-Solubilizing Bacteria on Growth and Physiological Characteristics of R. soongorica Seedlings Under NaCl Stress
Increased soil salinity leads to the accumulation of soluble ions such as Na+ and Cl− in plants roots, adversely affecting plant growth and development, ion toxicity, reactive oxygen species accumulation, osmotic stress, and other mechanisms [33]. Phosphate-solubilizing bacteria in the rhizosphere play a crucial role in the root environment, producing beneficial compounds such as IAA, hydrolases, and siderophores, which positively influence plant growth. They enhance plant salt tolerance by promoting plant growth, increasing antioxidant enzyme activity, accumulating osmoregulatory substances, and boosting plant available phosphorus content, with phosphate solubilization being the most beneficial function [34]. When researchers subject plants to salt stress, growth indicators such as plant height and stem diameter can intuitively reflect the effects on their appearance. Bacillus amyloliquefaciens and Bacillus pumilus can improve the salt tolerance of Cicer Argentum by increasing plant height, root length, and leaf phosphorus content [35]. This study found that inoculating R. soongorica seedlings with five different strains of phosphate-solubilizing bacteria enhanced growth under NaCl stress, increasing the net growth of the plant height, the net growth of the stem diameter, and biomass. However, the effects varied among strains, likely due to differences in their phosphate-solubilizing capacity and environmental adaptability.
Under salt stress, changes in antioxidant enzymes and osmoregulatory substances help scavenge accumulated reactive oxygen species (ROS) and regulate osmotic balance, mitigating salt-induced damage. These changes are key indicators of plant salt tolerance. Inoculating phosphorus-solubilizing bacteria with a growth-promoting ability could improve plant salt tolerance [36]. This study found that SOD and POD in R. soongorica seedlings inoculated with phosphate-solubilizing bacteria under NaCl stress increased to varying degrees compared with the NaCl treatment. We speculated that phosphate-solubilizing bacteria alleviate salt stress in R. soongorica by enhancing SOD and POD activity, maintaining ion homeostasis, and reducing reactive oxygen species (ROS) accumulation. Additionally, inoculation with five phosphate-solubilizing bacterial strains increased the soluble protein levels in R. soongorica seedlings under NaCl stress to varying degrees. In comparison, the soluble sugar content of R. soongorica seedlings inoculated with five strains of phosphate-solubilizing bacteria decreased to varying degrees, which was inconsistent with the results for Oryza sativa inoculated with Bacillus subtilis under salt stress [37]. We suggest that under 300 mmol·L−1 NaCl stress, soluble sugars in R. soongorica leaves may act as energy sources or secondary metabolites in plant metabolism, while soluble proteins and proline function as osmoregulatory substances to enhance seedling salt tolerance.
4.3. Effects of Rhizosphere Phosphate-Solubilizing Bacteria on Phosphorus Content of R. soongorica Seedlings Under NaCl Stress
In arid and semi-arid regions, soil salinization exacerbates the fixation of soluble phosphorus, impairing root nutrient absorption and worsening phosphorus deficiency. In [38], it was shown that adding an appropriate amount of phosphate fertilizer could enhance the salt tolerance of two varieties of Medicago sativa. However, excess phosphorus fertilizer added to saline soils is adsorbed and fixed, forming insoluble phosphates that plants cannot absorb or utilize. Studies have shown that rhizosphere phosphate-solubilizing bacteria exhibit functional traits such as phosphorus solubilization and IAA secretion. Generally, they lower soil pH and chelate metal ions by producing small-molecule organic acids like gluconic and acetic acid while also secreting phosphatases to decompose insoluble phosphorus [39]. Meanwhile, it was found that Bacillus anthracis, B. licheniformis, and B. cereus, which have a phosphate-solubilizing function, were all able to produce IAA [40]. Additionally, they stimulate plant roots, induce root secretions, and indirectly enhance plant growth. Thus, applying phosphate-solubilizing bacteria to increase soil-soluble phosphorus content significantly improves saline soil. This study found that inoculating R. soongorica seedlings with five phosphate-solubilizing bacterial strains under NaCl stress significantly increased leaf total phosphorus and rhizosphere soil available phosphorus content compared to NaCl stress alone (p < 0.05). In conclusion, phosphate-solubilizing bacteria enhance soluble phosphorus content in saline soils and improve plant phosphorus absorption and utilization efficiency.
4.4. Comprehensive Evaluation of Effect of Rhizosphere Phosphate-Solubilizing Bacteria on Salt Tolerance of R. soongorica Seedlings Under NaCl Stress
Plants respond to environmental stress through changes in multiple growth and physiological indicators; thus, resistance evaluation should not rely solely on all indicators or a single indicator [41]. This study employed correlation analysis, principal component analysis, and membership function analysis to comprehensively evaluate the impact of phosphate-solubilizing bacteria on R. soongorica seedling salt tolerance, using total biomass, organ biomass, stem net growth, CAT activity, soluble sugars, proline, leaf total phosphorus, and rhizosphere soil available phosphorus as indicators. This experimentation result is consistent with the evaluation index of the salt tolerance of Melia azedarach, Ulmus pumila, and Robinia pseudoacacia [42]. Finally, the membership function values indicated that the effectiveness of the five phosphate-solubilizing bacterial strains in enhancing R. soongorica seedling salt tolerance under NaCl stress followed the order J24 > P2 > J23 > P3 > M1. J24 phosphate-solubilizing bacteria enhance R. soongorica seedling growth and salt tolerance under stress by regulating antioxidant enzyme activity, accumulating osmoregulatory substances, and increasing available phosphorus.
5. Conclusions
The five rhizosphere phosphate-solubilizing bacteria lower medium pH and solubilize phosphate, enhancing R. soongorica seedling salt tolerance through improvements in growth, physiology, phosphorus content, and rhizosphere soil enzyme activity. The comprehensive evaluation revealed that seedlings inoculated with J24 exhibited the strongest salt tolerance, while those inoculated with M1 showed the weakest. These findings offer valuable insights for desert ecological restoration and saline soil improvement.
Author Contributions
Conceptualization, data curation, visualization, review, and editing, X.W.; funding acquisition, investigation, supervision, review, and editing, P.C.; investigation, review, and editing, X.B.; data curation, investigation, and methodology, F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Foundation of China, grant number 32160407, the Major Special Project of Gansu Provincial Science and Technology Plan—International Cooperation Field—grant number 25ZDWA007, and the Gansu Provincial Key Research and Development Program, grant number 23YFFA0065.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are thankful to the National Natural Foundation of China (32160407) for providing financial support. The authors are also thankful to the Gansu Provincial Key Research and Development Program (23YFFA0065) for providing financial support. The authors are thankful to the Major Special Project of Gansu Provincial Science and Technology Plan—International Cooperation Field (25ZDWA0070).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Su, T.; Wang, X.; Ning, S.; Sheng, J.; Jiang, P.; Gao, S.; Yang, Q.; Zhou, Z.; Cui, H.; Li, Z. Enhancing Soil Salinity Evaluation Accuracy in Arid Regions: An Integrated Spatiotemporal Data Fusion and AI Model Approach for Arable Lands. Land 2024, 13, 1837. [Google Scholar] [CrossRef]
- Potestio, S.; Giannelli, G.; Degola, F.; Vamerali, T.; Fragni, R.; Cocconi, E.; Sandei, L.; Visioli, G. Salt stress mitigation and improvement in fruit nutritional characteristics of tomato plants: New opportunities from the exploitation of a halotorelant Agrobacterium strain. Plant Stress 2024, 13, 100558. [Google Scholar]
- Chang, W.; Zhang, Y.; Ping, Y.; Li, K.; Qi, D.-D.; Song, F.-Q. Label-free quantitative proteomics of arbuscular mycorrhizal Elaeagnus angustifolia seedlings provides insights into salt-stress tolerance mechanisms. Front. Plant Sci. 2023, 13, 1098260. [Google Scholar]
- Al-Huqail, A.A.; Aref, N.M.A.; Khan, F.; Sobhy, S.E.; Hafez, E.E.; Khalifa, A.M.; Saad-Allah, K.M. Azolla filiculoides extract improved salt tolerance in wheat (Triticum aestivum L.) is associated with prompting osmostasis, antioxidant potential and stress-interrelated genes. Sci. Rep. 2024, 14, 11100. [Google Scholar]
- Banzouzi Bouzika, V.J.; Chong, P.-f.; Jia, X.-y.; Lu, W.-t.; Tin, Y.-l. Effects of foliar-spraying nitric oxide on the carbon metabolism enzymes activities and nutrients in leaves and roots of Reaumuria soongorica (pall.) maxim seedlings under NaCl stress. Plant Stress 2022, 5, 100096. [Google Scholar]
- Teles, E.A.P.; Xavier, J.F.; Arcênio, F.S.; Amaya, R.L.; Gonçalves, J.V.S.; Rouws, L.F.M.; Zonta, E.; Coelho, I.S. Characterization and evaluation of potential halotolerant phosphate solubilizing bacteria from Salicornia fruticosa rhizosphere. Front. Plant Sci. 2024, 14, 1324056. [Google Scholar]
- Bakki, M.; Banane, B.; Marhane, O.; Esmaeel, Q.; Hatimi, A.; Barka, E.A.; Azim, K.; Bouizgarne, B. Phosphate solubilizing Pseudomonas and Bacillus combined with rock phosphates promoting tomato growth and reducing bacterial canker disease. Front. Microbiol. 2024, 15, 1289466. [Google Scholar]
- Cheng, Y.; Yuan, J.; Wang, G.; Hu, Z.; Luo, W.; Zhao, X.; Guo, Y.; Ji, X.; Hu, W.; Li, M. Phosphate-solubilizing bacteria improve the antioxidant enzyme activity of Potamogeton crispus L. and enhance the remediation effect on Cd-contaminated sediment. J. Hazard. Mater. 2024, 470, 134305. [Google Scholar]
- Saranya, K.; Sundaramanickam, A.; Manupoori, S.; Kanth, S.V. Screening of multi-faceted phosphate-solubilising bacterium from seagrass meadow and their plant growth promotion under saline stress condition. Microbiol. Res. 2022, 261, 127080. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Kou, J.; Li, Q.; Liu, J.; Chi, L.; Zhang, Y.; Liu, Q.; Yu, Y. Impacts of phosphate-solubilizing bacterium strain MWP-1 on vegetation growth, soil characteristics, and microbial communities in the Muli coal mining area, China. Front. Microbiol. 2024, 15, 1500070. [Google Scholar]
- Wang, J.; Qin, Q.; Pan, J.; Sun, L.; Sun, Y.; Xue, Y.; Song, K. Transcriptome analysis in roots and leaves of wheat seedlings in response to low-phosphorus stress. Sci. Rep. 2019, 9, 19802. [Google Scholar]
- Wu, Y. Effects of High Salt Stress on Soil Carbon, Nitrogen, and Phosphorus Metabolism and Its Microbiological Mechanism; Northwest Agriculture and Forestry University: Xi’an, China, 2023. [Google Scholar]
- Zhang, X.J. Effect of Low Phosphorus on Light Energy Utilization of Sorghum Bicolor ‘Dochna’ Under Salt Stress; Shandong Normal University: Jinan, China, 2021. [Google Scholar]
- Ibeas, M.A.; Salinas-Grenet, H.; Johnson, N.R.; Pérez-Díaz, J.; Vidal, E.A.; Alvarez, J.M.; Estevez, J.M. Filling the gaps on root hair development under salt stress and phosphate starvation using current evidence coupled with a meta-analysis approach. Plant Physiol. 2024, 196, 2140–2149. [Google Scholar] [PubMed]
- Zhao, L.; Su, R.; He, H. Effects of soil salt stress on phosphorus utilization of Medicago sativa. J. Soil Water Conserv. 2019, 38, 414–422. [Google Scholar]
- He, F.-L.; Bao, A.-K.; Wang, S.-M.; Jin, H.-X. NaCl stimulates growth and alleviates drought stress in the salt-secreting xerophyte Reaumuria soongorica. Environ. Exp. Bot. 2019, 162, 433–443. [Google Scholar]
- Liu, H.; Chong, P.; Yan, S.; Liu, Z.; Bao, X.; Tan, B. Transcriptome and Proteome Association Analysis to Screen Candidate Genes Related to Salt Tolerance in Reaumuria soongorica Leaves under Salt Stress. Plants 2023, 12, 3542. [Google Scholar] [CrossRef]
- Yan, S.; Chong, P.; Zhao, M.; Liu, H. Physiological response and proteomics analysis of Reaumuria soongorica under salt stress. Sci. Rep. 2022, 12, 2539. [Google Scholar]
- Shan, L.; Yang, C.; Li, Y.; Duan, Y.; Geng, D.; Li, Z.; Zhang, R.; Duan, G.; Васильевич, Ж.А. Effects of drought stress on root physiological traits and root biomass allocation of Reaumuria soongorica. Acta Ecol. Sin. 2015, 35, 155–159. [Google Scholar]
- Zhang, S.; Zhao, J.; Yang, J.; Xie, J.; Sun, Z. Feature Selection and Regression Models for Multisource Data-Based Soil Salinity Prediction: A Case Study of Minqin Oasis in Arid China. Land 2024, 13, 877. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Zhang, S.; Shao, M.; Ding, Z.; Zhou, Y.; Su, C. The Process of Soil Carbon Sequestration in Different Ecological Zones of Qingtu Lake in the Arid–Semi-Arid Region of Western China. Microorganisms 2024, 12, 2122. [Google Scholar] [CrossRef]
- Zeng, Q.; Tang, L.; Zhang, Y.; Shao, Y.; Wu, W.; Wang, J.; Ding, X.; Han, X.; Bilal, M. Isolation and characterization of phosphate-solubilizing bacteria from rhizosphere of poplar on road verge and their antagonistic potential against various phytopathogens. BMC Microbiol. 2023, 23, 221. [Google Scholar]
- Liu, H.; Chong, P.; Liu, Z.; Bao, X.; Tan, B. Exogenous hydrogen sulfide improves salt stress tolerance of Reaumuria soongorica seedlings by regulating active oxygen metabolism. PeerJ 2023, 11, e15881. [Google Scholar]
- Gao, J.F. Plant Physiology Experiment Instruction; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Janati, W.; Mikou, K.; El Ghadraoui, L.; Errachidi, F. Growth stimulation of two legumes (Vicia faba and Pisum sativum) using phosphate-solubilizing bacteria inoculation. Front. Microbiol. 2023, 14, 1212702. [Google Scholar]
- Pan, L.; Cai, B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, D.L.; Zhou, Q.; Zhang, T.; Li, M.Y. Screening and growth-promoting characteristics of phosphate-solubilizing strains from the rhizosphere of Phragmites australis in saline-alkali soil. Jiangsu J. Agric. 2024, 40, 64–74. [Google Scholar]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Fan, Y.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar] [PubMed]
- Luo, Y.H.; Ke, Z.B.; Zhong, C.; Chen, Y.J. Isolation, identification, and characteristics of phosphate-solubilizing bacteria from mangrove soil. China Environ. Sci. 2020, 40, 2664–2673. [Google Scholar]
- Khourchi, S.; Elhaissoufi, W.; Ibnyasser, A.; Haddine, M.; Ghani, R.; Zeroual, Y.; Delaplace, P.; Bargaz, A. Integrated use of polyphosphate and P-solubilizing bacteria enhanced P use efficiency and growth performance of durum wheat. Front. Microbiol. 2023, 14, 1211397. [Google Scholar]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef]
- Nurrahma, A.H.I.; Harsonowati, W.; Putri, H.H.; Iqbal, R. Current Research Trends in Endophytic Fungi Modulating Plant Adaptation to Climate Change-associated Soil Salinity Stress. J. Soil Sci. Plant Nutr. 2024, 24, 6446–6466. [Google Scholar] [CrossRef]
- Wael, D.; El-Amier, Y.; Saber, W.I.A.; Elsayed, A. Plant-associated halotolerant bacteria improving growth of Vicia faba L. Mariout-2 under salinity conditions. Sci. Rep. 2024, 14, 16737. [Google Scholar] [CrossRef]
- Rahimi Chegeni, A.; Fatehi, F.; Ebrahimi, A.; Maleki, M. Phosphate-Solubilizing Bacteria Modulated Salinity Stress in the Presence of Phosphorous through Improving Growth, Biochemical Properties, and Gene Expression of Chickpea (Cicer arientnum L.). J. Soil Sci. Plant Nutr. 2023, 23, 4450–4462. [Google Scholar]
- AbuQamar, S.F.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; Elrys, A.S.; El-Mageed, T.A.A.; Semida, W.M.; Abdelkhalik, A.; Mosa, W.F.A.; Al Kafaas, S.S.; et al. Halotolerant plant growth-promoting rhizobacteria improve soil fertility and plant salinity tolerance for sustainable agriculture—A review. Plant Stress 2024, 12, 100482. [Google Scholar]
- Siddika, A.; Rashid, A.A.; Khan, S.N.; Khatun, A.; Karim, M.M.; Prasad, P.V.V.; Hasanuzzaman, M. Harnessing plant growth-promoting rhizobacteria, Bacillus subtilis and B. aryabhattai to combat salt stress in rice: A study on the regulation of antioxidant defense, ion homeostasis, and photosynthetic parameters. Front. Plant Sci. 2024, 15, 1419764. [Google Scholar]
- Su, R.; Zhang, Z.; Chang, C.; Peng, Q.; Cheng, X.; Pang, J.; He, H.; Lambers, H. Interactive effects of phosphorus fertilization and salinity on plant growth, phosphorus and sodium status, and tartrate exudation by roots of two alfalfa cultivars. Ann. Bot. 2022, 129, 53–64. [Google Scholar]
- Koczorski, P.; Furtado, B.U.; Baum, C.; Weih, M.; Ingvarsson, P.; Hulisz, P.; Hrynkiewicz, K. Large effect of phosphate-solubilizing bacteria on the growth and gene expression of Salix spp. at low phosphorus levels. Front. Plant Sci. 2023, 14, 1218617. [Google Scholar]
- Khosravi, M.; Heydari, M.; Alikhani, A.H. Mitigating negative impacts of drought on oak seedlings performances through plant growth-promoting rhizobacteria. J. Environ. Manag. 2025, 375, 124163. [Google Scholar]
- Yan, S.; Chong, P.F.; Zhao, M. Effect of salt stress on the photosynthetic characteristics and endogenous hormones, and: A comprehensive evaluation of salt tolerance in Reaumuria soongorica seedlings. Plant Signal. Behav. 2022, 17, 2031782. [Google Scholar]
- Gan, H.H.; Gong, S.; Liu, H.; Chu, J.M. Evaluation of salt tolerance of seedlings of three afforestation tree species in China and screening of indicators. For. Sci. Res. 2024, 37, 156–168. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).