Assessing the Role of Asymptomatic Infected Trees in Pine Wilt Disease Spread in Japan—Insights from Tree Health Monitoring
Abstract
1. Introduction
2. Materials and Methods
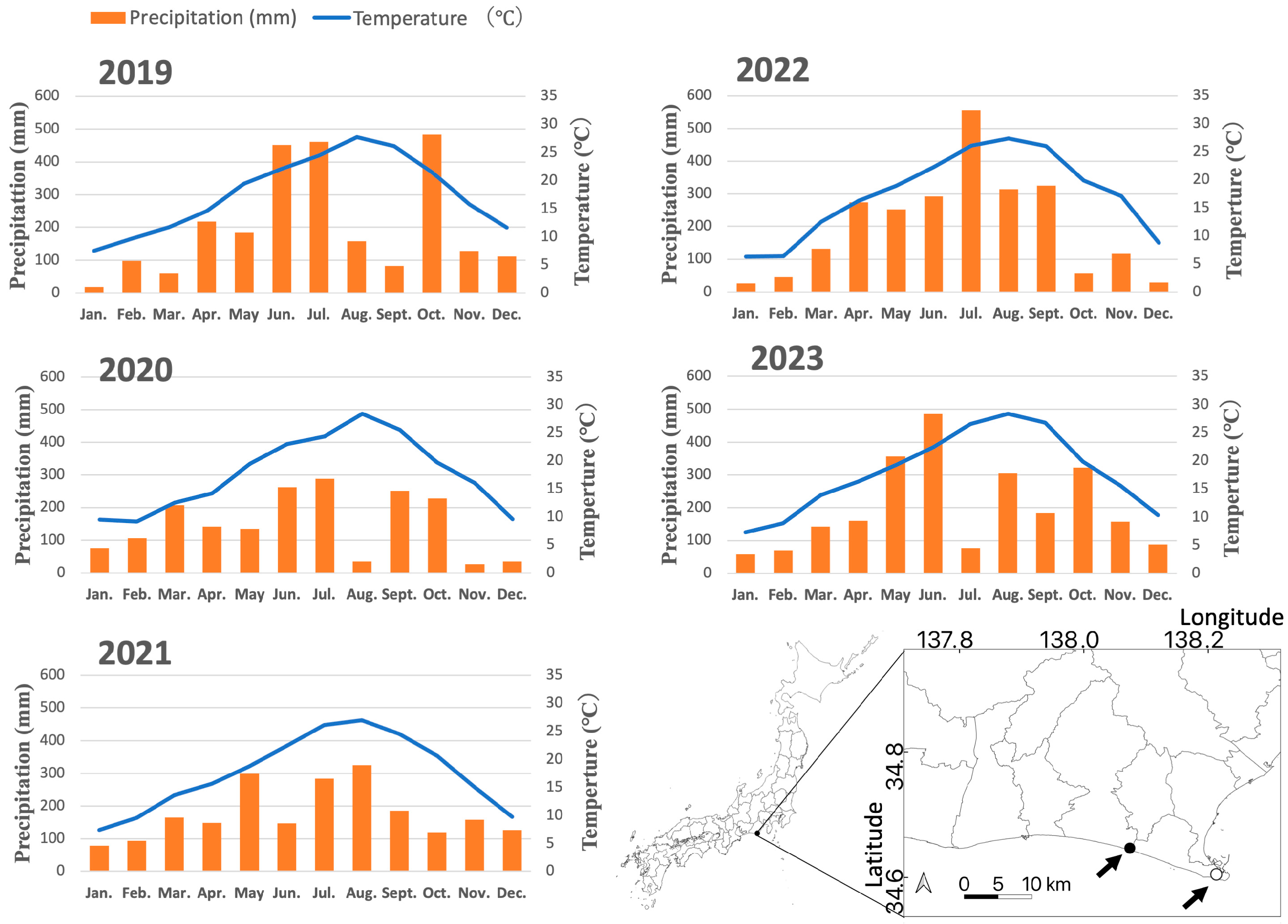
2.1. Study Site
2.2. Tree Health Monitoring
2.3. Statistical Analysis
3. Results
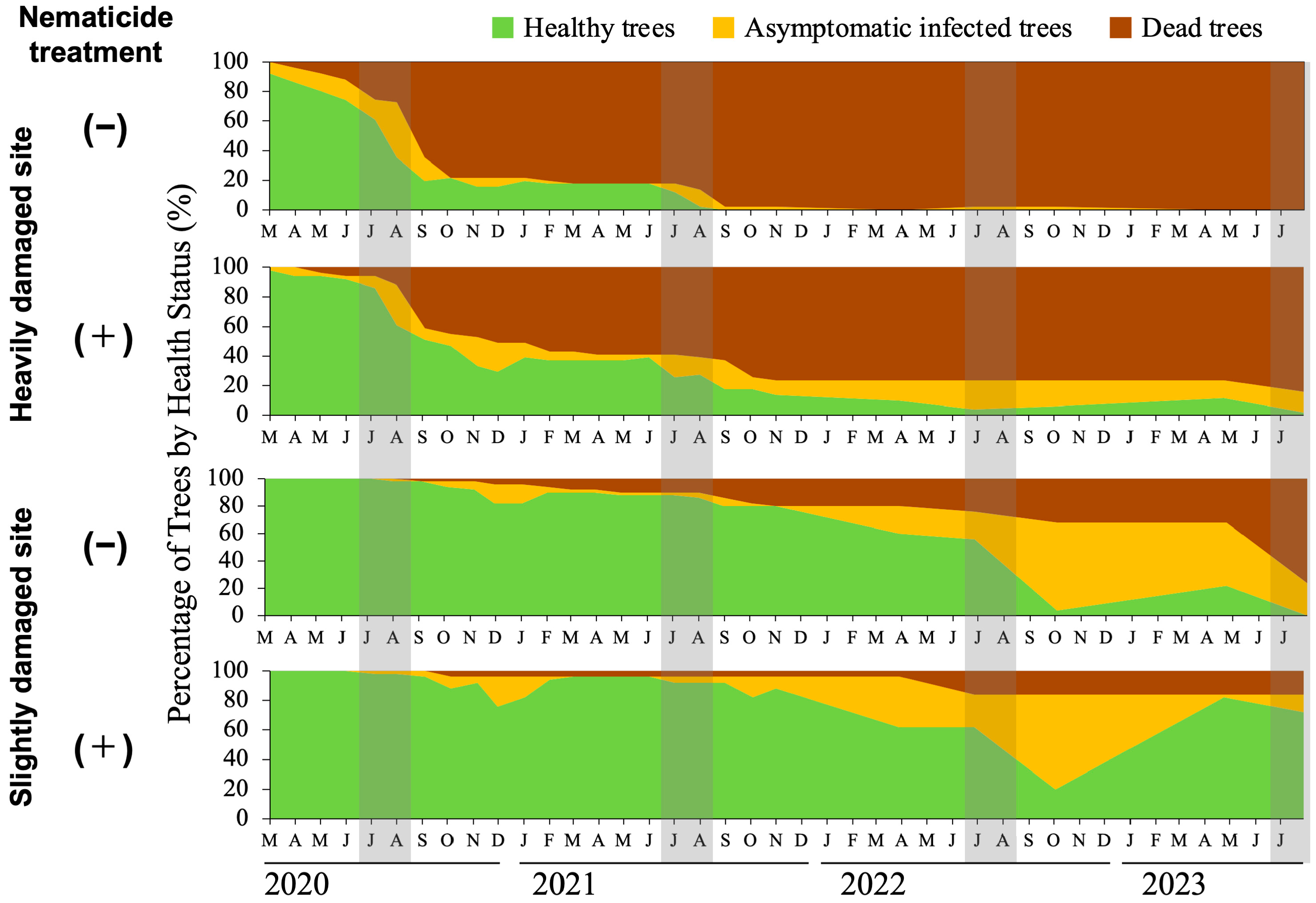
3.1. Relationship Between Initial PWD Damage, Nematicide Treatment, and Damage Progression
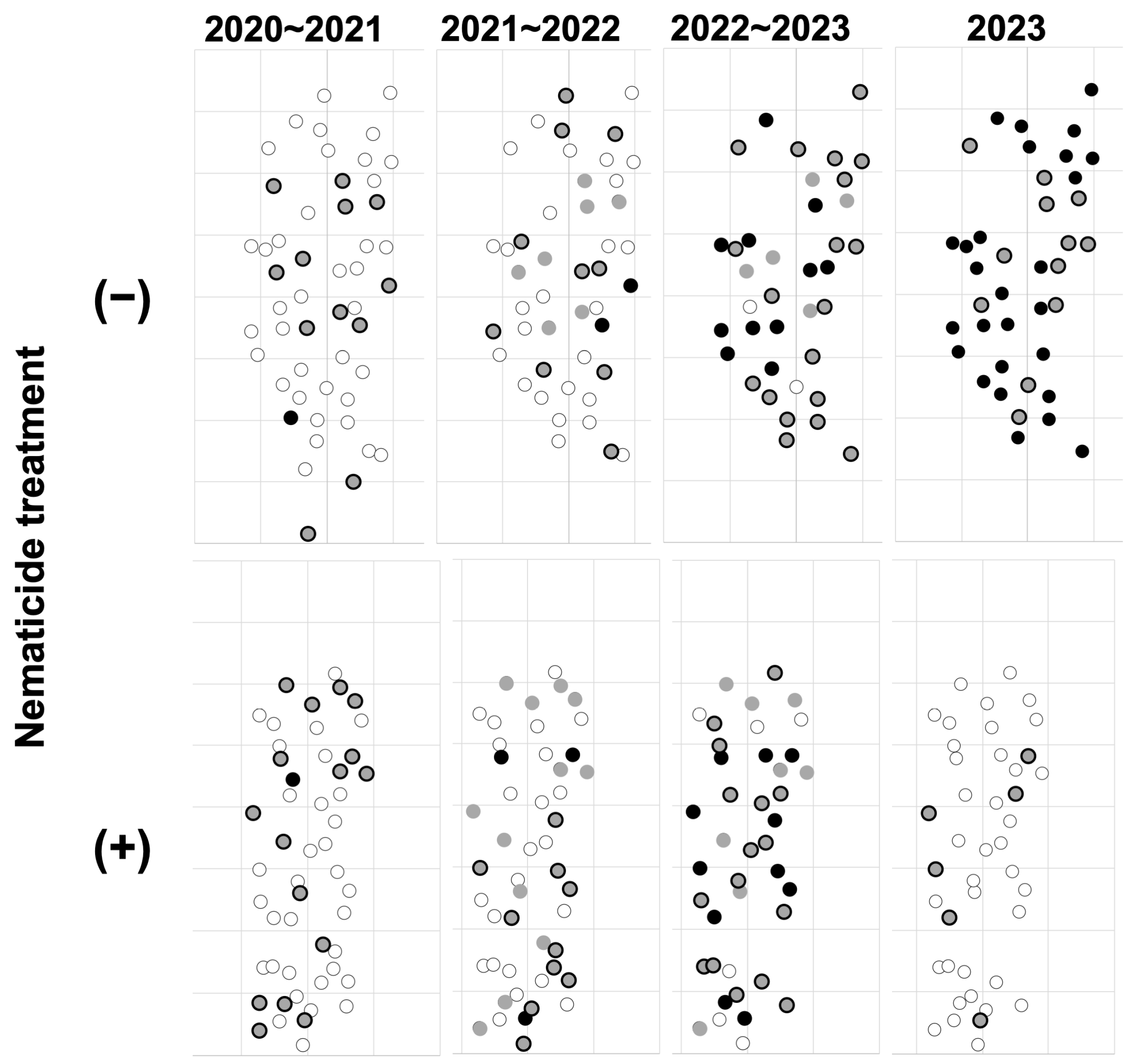
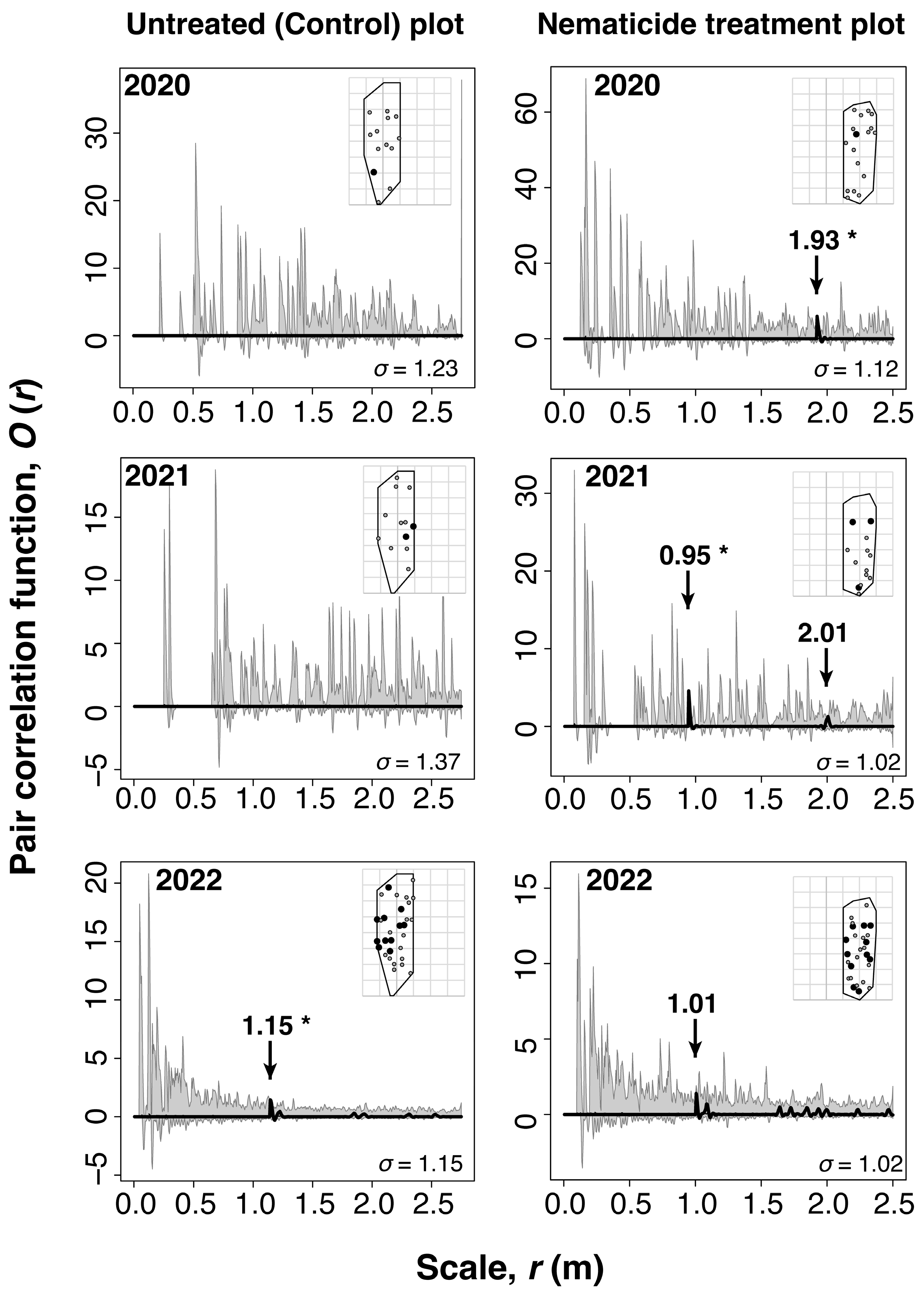
3.2. Spatial Overlap Between Oviposition Target Trees and Asymptomatic Infected Trees in the Slightly Damaged Site
4. Discussion
4.1. Suggestions for PWD Control
4.2. Suggestions for PWD Control Considering Asymptomatic Infected Trees
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamiya, Y. History of pine wilt disease in Japan. J. Nematol. 1988, 20, 219–226. [Google Scholar] [PubMed]
- Zhao, B.G. Pine wilt disease in China. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 18–25. ISBN 978-4-431-75655-2. [Google Scholar] [CrossRef]
- Hao, Z.; Huang, J.; Li, X.; Sun, H.; Fang, G. A multi-point aggregation trend of the outbreak of pine wilt disease in China over the past 20 years. For. Ecol. Manag. 2022, 505, 119890. [Google Scholar] [CrossRef]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Back, M.A.; Bonifácio, L.; Inácio, M.L.; Mota, M.; Boa, E. Pine wilt disease: A global threat to forestry. Plant Pathol. 2024, 73, 1026–1041. [Google Scholar] [CrossRef]
- Akbulut, S.; Stamps, W.T. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE 2017, 12, e0182837. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine wilt disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Annual Report on Forest and Forestry in Japan: Fiscal Year 2023. Available online: https://www.rinya.maff.go.jp/j/kikaku/hakusyo/r5hakusyo/index.html (accessed on 14 February 2025). (In Japanese with English Summary).
- Kiyohara, T.; Tokushige, Y. Inoculation experiments of a nematode, Bursaphelenchus sp., onto pine trees. J. Jpn. For. Soc. 1971, 53, 210–218, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Mamiya, Y.; Enda, N. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Coleoptera: Cerambycidae). Nematologica 1972, 18, 159–162. [Google Scholar] [CrossRef]
- Kishi, Y. Invasion of pine trees by Bursaphelenchus lignicolus M. & K. (Nematoda: Aphelenchoidae) from Monochamus alternatus Hope (Coleoptera: Cerambycidae). J. Jpn. For. Soc. 1978, 60, 179–182. (In Japanese) [Google Scholar] [CrossRef]
- Togashi, K. Transmission curves of Bursaphelenchus xylophilus (Nematoda:Aphelenchoididae) from its vector, Monochamus alternatus (Coleoptera:Cerambycidae), to pine trees with reference to population performance. Appl. Entomol. Zool. 1985, 20, 246–251. [Google Scholar] [CrossRef]
- Linit, M.J. Transmission of pinewood nematode through feeding wounds of Monochamus carolinensis (Coleoptera: Cerambycidae). J. Nematol. 1990, 22, 231–236. [Google Scholar] [PubMed]
- Togashi, K. Vector-nematode relationships and epidemiology in pine wilt disease. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 162–183. ISBNs 978-4-431-75654-5/978-4-431-75655-2. [Google Scholar] [CrossRef]
- Togashi, K. Variation in external symptom development of pine wilt disease in field grown Pinus thunbergii. J. Jpn. For. Soc. 1989, 71, 442–448. [Google Scholar] [CrossRef]
- Kuroda, K. Mechanism of cavitation development in the pine wilt disease. Eur. J. For. Pathol. 1991, 21, 82–89. [Google Scholar] [CrossRef]
- Fukuda, K. Physiological process of the symptom development and resistance mechanism in pine wilt disease. J. For. Res. 1997, 2, 171–181. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y.; Wei, Y. Two terpene synthases in resistant contribute to defence against Bursaphelenchus xylophilus. Plant Cell Environ. 2021, 44, 257–274. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Li, D.; Liu, Z.; Wang, X.; Zhang, W.; Wen, X.; Zhang, X. Bursaphelenchus xylophilus venom allergen protein BxVAP2 responds to terpene stress, triggers plant defense in Nicotiana benthamiana. Forests 2024, 15, 1929. [Google Scholar] [CrossRef]
- Linit, M.J. Nematode-vector relationships in the pine wilt disease system. J. Nematol. 1988, 20, 227–235. [Google Scholar]
- Zhao, L.; Zhang, S.; Wei, W.; Hao, H.; Zhang, B.; Butcher, R.A.; Sun, J. Chemical signals synchronize the life cycles of a plant-parasitic nematode and its vector beetle. Curr. Biol. 2013, 23, 2038–2043. [Google Scholar] [CrossRef]
- Kirino, H.; Maehara, N.; Shinya, R. How did Bursaphelenchus nematodes acquire a specific relationship with their beetle vectors, Monochamus? Front. Physiol. 2023, 14, 1209695. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K. Inhibitory activity of fenitrothion against maturation feeding of Japanese pinesawyer, Monochamus alternatus. Jpn. J. Appl. Entomol. Zool. 1988, 32, 245–251, (In Japanese with English Summary). [Google Scholar] [CrossRef][Green Version]
- Kwon, T.-S.; Song, M.-Y.; Shin, S.-C.; Park, Y.-S. Effects of aerial insecticide sprays on ant communities to control pine wilt disease in Korean pine forests. Appl. Entomol. Zool. 2005, 40, 563–574. [Google Scholar] [CrossRef]
- Suh, D.Y.; Jung, J.-K.; Lee, S.K.; Seo, S.-T. Effect of aerial spraying of thiacloprid on pine sawyer beetles (Monochamus alternatus) and honey bees (Apis mellifera) in pine forests. Entomol. Res. 2021, 51, 83–89. [Google Scholar] [CrossRef]
- Fujishita, A. Trunk injection of chemicals for the control of the pine wilt disease caused by Bursaphelenchus xylophilus. Bull. Shizuoka Prefect. For. Exp. Station. 1985, 13, 23–34, (In Japanese with English Summary). [Google Scholar]
- Sousa, E.; Naves, P.; Vieira, M. Prevention of pine wilt disease induced by Bursaphelenchus xylophilus and Monochamus galloprovincialis by trunk injection of emamectin benzoate. Phytoparasitica 2013, 41, 143–148. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Yang, T.; Xu, M.; Li, Y.; Pei, Y.; Tang, J.; Zheng, Z.; Sun, Z.; Cheng, G.; et al. Optimized emamectin benzoate trunk injection: Addressing temperature limitations for pine wilt disease control. Pest Manag. Sci. 2025, 81, 892–902. [Google Scholar] [CrossRef]
- Yoshimura, A.; Kawasaki, K.; Takasu, F.; Togashi, K.; Futai, K.; Shigesada, N. Modeling the spread of pine wilt disease caused by nematodes with pine sawyers as vector. Ecology 1999, 80, 1691–1702. [Google Scholar] [CrossRef]
- Togashi, K.; Shigesada, N. Spread of the pinewood nematode vectored by the Japanese pine sawyer: Modeling and analytical approaches. Popul. Ecol. 2006, 48, 271–283. [Google Scholar] [CrossRef]
- Xia, C.; Chon, T.-S.; Takasu, F.; Choi, W.I.; Park, Y.-S. Simulating pine wilt disease dispersal with an individual-based model incorporating individual movement patterns of vector beetles. Front. Plant Sci. 2022, 13, 886867. [Google Scholar] [CrossRef]
- Yu, R.; Luo, Y.; Zhou, Q.; Zhang, X.; Wu, D.; Ren, L. Early detection of pine wilt disease using deep learning algorithms and UAV-based multispectral imagery. For. Ecol. Manag. 2021, 497, 119493. [Google Scholar] [CrossRef]
- Tahir, S.; Hassan, S.S.; Yang, L.; Ma, M.; Li, C. Detection methods for pine wilt disease: A comprehensive review. Plants 2024, 13, 2876. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, J.; Wang, T.; Zhao, J.; Gadekallu, T.R.; Fang, K. Detection of pine wilt disease using AAV remote sensing with an improved YOLO model. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2024, 17, 19230–19242. [Google Scholar] [CrossRef]
- Futai, K. Role of asymptomatic carrier trees in epidemic spread of pine wilt disease. J. For. Res. 2003, 8, 253–260. [Google Scholar] [CrossRef]
- Futai, K. Two infectious diseases: “COVID-19” and “pine wilt disease”. Forests 2024, 15, 1724. [Google Scholar] [CrossRef]
- Halik, S.; Bergdahl, D.R. Long-term survival of Bursaphelenchus xylophilus in living Pinus sylvestris in an established plantation. Eur. J. For. Pathol. 1994, 24, 357–363. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Futai, K. Asymptomatic carrier trees in pine stands naturally infected with Bursaphelenchus xylophilus. Nematology 2007, 9, 243–250. [Google Scholar] [CrossRef]
- Bergdahl, D.R.; Halik, S. Persistence of the pine wood nematode in asymptomatic Scots pines. In The Pinewood Nematode, Bursaphelenchus xylophilus; Mota, M., Vieira, P., Eds.; Brill: Berlin, Germany, 2004; pp. 177–185. ISBN 978-90-474-1309-7. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Kanzaki, N.; Futai, K. Volatile compounds in pine stands suffering from pine wilt disease: Qualitative and quantitative evaluation. Nematology 2006, 8, 869–879. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Yi, S.; Wei, Y.; Wang, M. Odorant-binding protein 19 in Monochamus alternatus involved in the recognition of a volatile strongly emitted from ovipositing host pines. Insect Sci. 2024, 31, 134–146. [Google Scholar] [CrossRef]
- Kato, T.; Kenmochi, A.; Yamada, Y.; Futai, K. Confirmation of the presence of asymptomatic carrier trees of pine wilt disease after thorough control procedure, and the effectiveness of trunk injection to suppress disease development. J. Jpn. For. Soc. 2019, 101, 46–51, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Koiwa, T. Survey of asymptomatic carrier trees of pine wilt disease in Iwate Prefecture, northeastern Japan. J. Jpn. For. Soc. 2019, 101, 35–45, (In Japanese with English Summary). [Google Scholar] [CrossRef][Green Version]
- Cho, Y.; Jung, K. Countermeasures against asymptomatic carriers in pine wilt disease in Korea. J. Jpn. For. Soc. 2019, 101, 26–29, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Ishiguro, H.; Futai, K. When coming to a wilting pine tree, Monochamus alternatus causes pinewood nematode infection on the surrounding healthy trees. Tree For. Health 2022, 26, 59–64, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Oda, K. Target trees of pine wilt disease and its diagnostic method. For. Pests 1967, 16, 263–266. (In Japanese) [Google Scholar]
- Wiegand, T.; Moloney, K.A. Rings, circles, and null-models for point pattern analysis in ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Baddeley, A.; Turner, R. Spatstat: An R package for analyzing spatial point patterns. J. Stat. Softw. 2005, 12, 1–42. [Google Scholar] [CrossRef]
- Berman, M.; Diggle, P. Estimating weighted integrals of the second-order intensity of a spatial point process. J. R. Stat. Soc. Ser. B (Methodol.) 1989, 51, 81–92. [Google Scholar] [CrossRef]
- Togashi, K. Spatial pattern of pine wilt disease caused by Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) within a Pinus thunbergii stand. Res. Popul. Ecol. 1991, 33, 245–256. [Google Scholar] [CrossRef]
- Yuyan, W.; Chaoran, S.; Haiyan, L.; Zhihong, G. A study on the techniques of the rapid quarantine detection for diseased wood caused by pine wood nematode. Sci. Silvae Sin. 2000, 36, 59–62, (In Chinese with English Summary). [Google Scholar]
- Naves, P.M.; Camacho, S.; De Sousa, E.M.; Quartau, J.A. Transmission of the pine wood nematode Bursaphelenchus xylophilus through feeding activity of Monochamus galloprovincialis (Col., Cerambycidae). J. Appl. Entomol. 2007, 131, 21–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchiyama, Y.; Futai, K. Assessing the Role of Asymptomatic Infected Trees in Pine Wilt Disease Spread in Japan—Insights from Tree Health Monitoring. Forests 2025, 16, 583. https://doi.org/10.3390/f16040583
Uchiyama Y, Futai K. Assessing the Role of Asymptomatic Infected Trees in Pine Wilt Disease Spread in Japan—Insights from Tree Health Monitoring. Forests. 2025; 16(4):583. https://doi.org/10.3390/f16040583
Chicago/Turabian StyleUchiyama, Yoshimasa, and Kazuyoshi Futai. 2025. "Assessing the Role of Asymptomatic Infected Trees in Pine Wilt Disease Spread in Japan—Insights from Tree Health Monitoring" Forests 16, no. 4: 583. https://doi.org/10.3390/f16040583
APA StyleUchiyama, Y., & Futai, K. (2025). Assessing the Role of Asymptomatic Infected Trees in Pine Wilt Disease Spread in Japan—Insights from Tree Health Monitoring. Forests, 16(4), 583. https://doi.org/10.3390/f16040583




