Effect of Different Mixing Patterns on Carbon and Nitrogen Dynamics During the Decomposition of Deadwood in Subtropical Forest Ecosystems
Abstract
1. Introduction
2. Materials and Methods
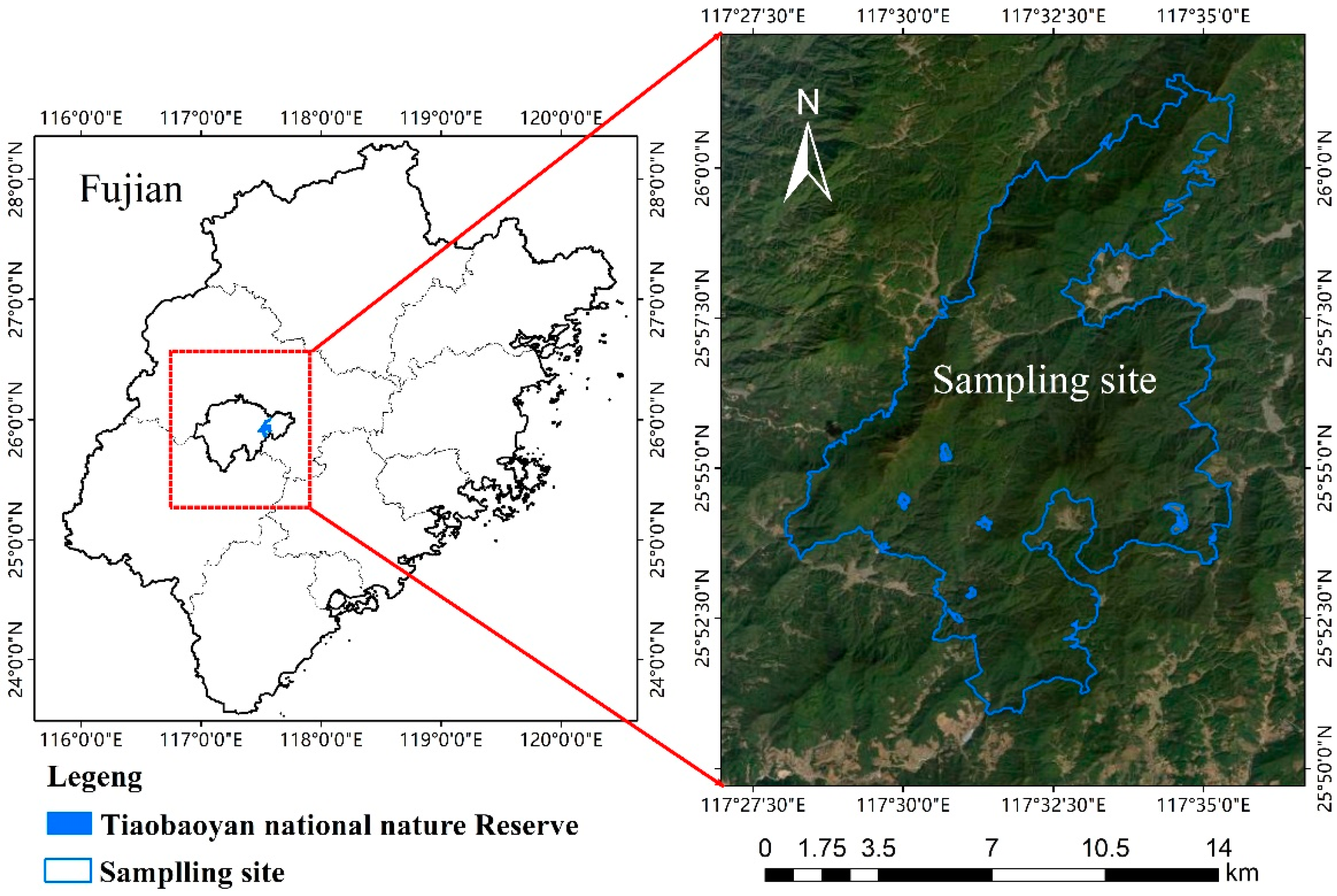
2.1. Study Area
2.2. Experimental Design
2.3. Classification of Coarse Woody Debris (CWD) Decay Levels
2.4. Sample Analysis
2.5. Statistical Analysis
3. Results
3.1. Comparison of Carbon and Nitrogen Content in the CWD of Different Tsuga longibracteata Forests
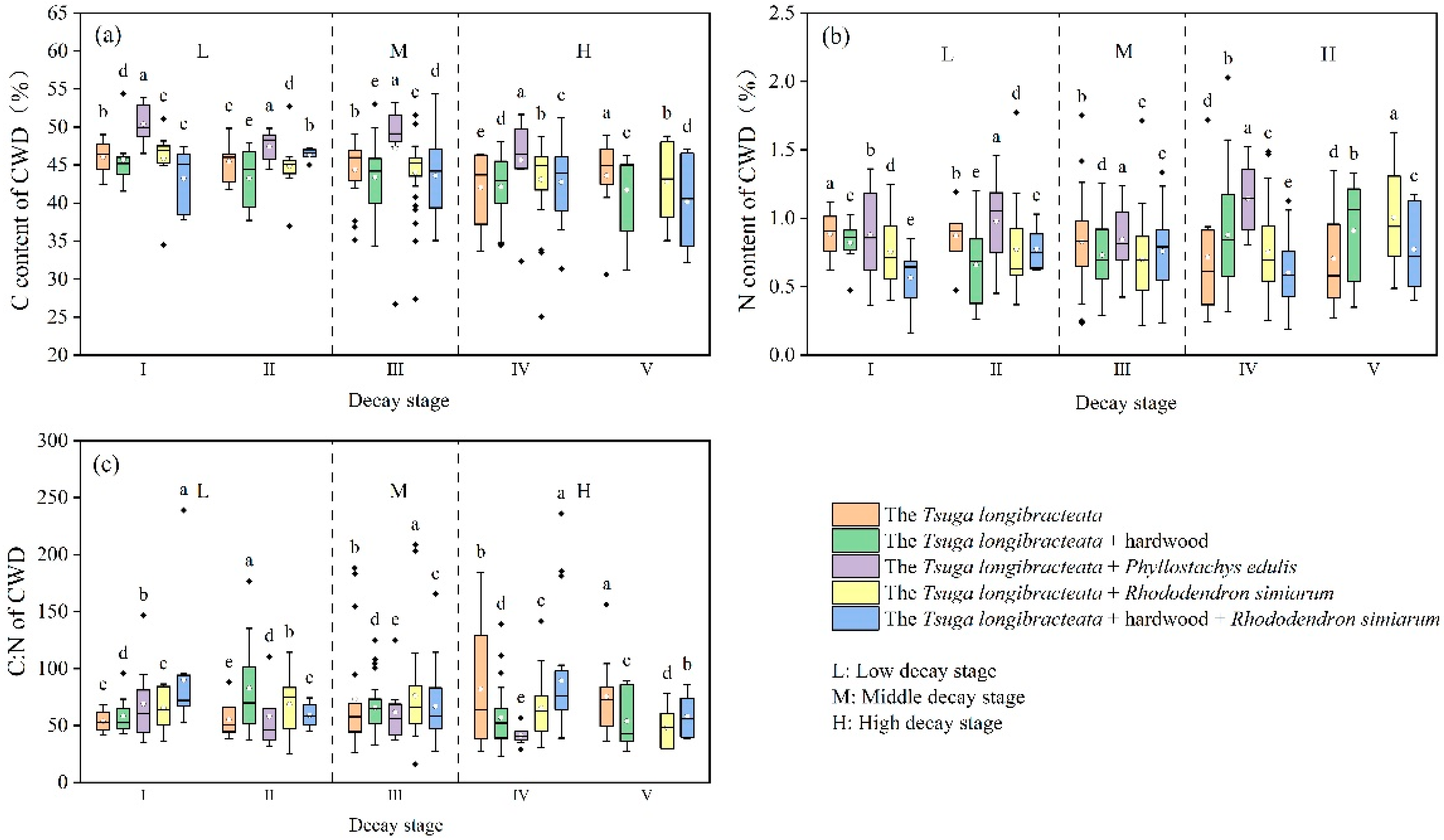
3.1.1. Comparison of Carbon and Nitrogen Content in CWD Across Different Decay Levels of Different Tsuga longibracteata Forests
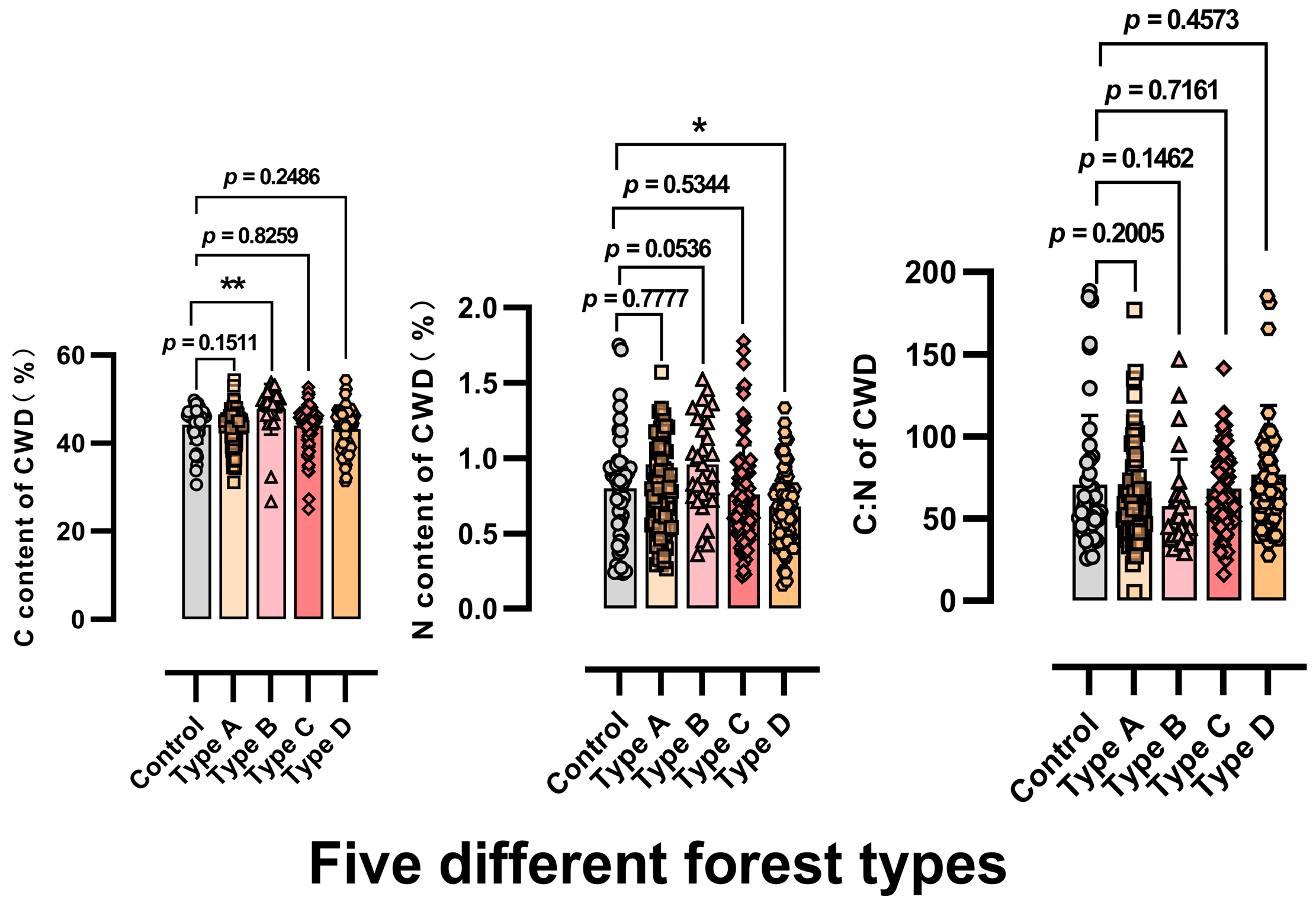
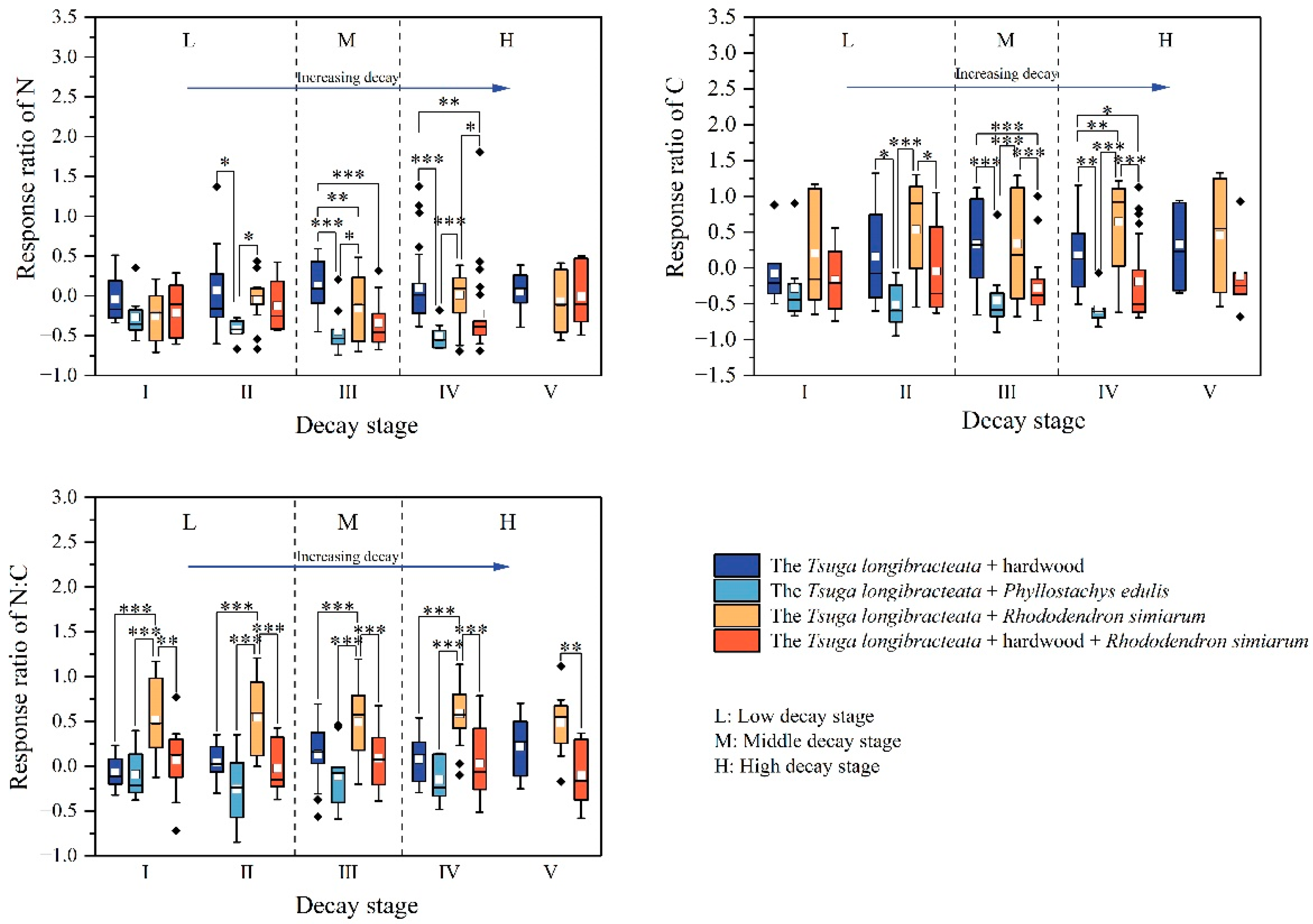
3.1.2. Effect of Tree Species Richness on the Carbon and Nitrogen Content of CWD in Tsuga longibracteata Forests
3.1.3. Effect of Vegetation Types on the Carbon and Nitrogen Content of CWD in Tsuga longibracteata Forests
3.2. Comparison of Carbon and Nitrogen Content in the Adjacent Soil Layers Beneath the CWD of Different Tsuga longibracteata Forests
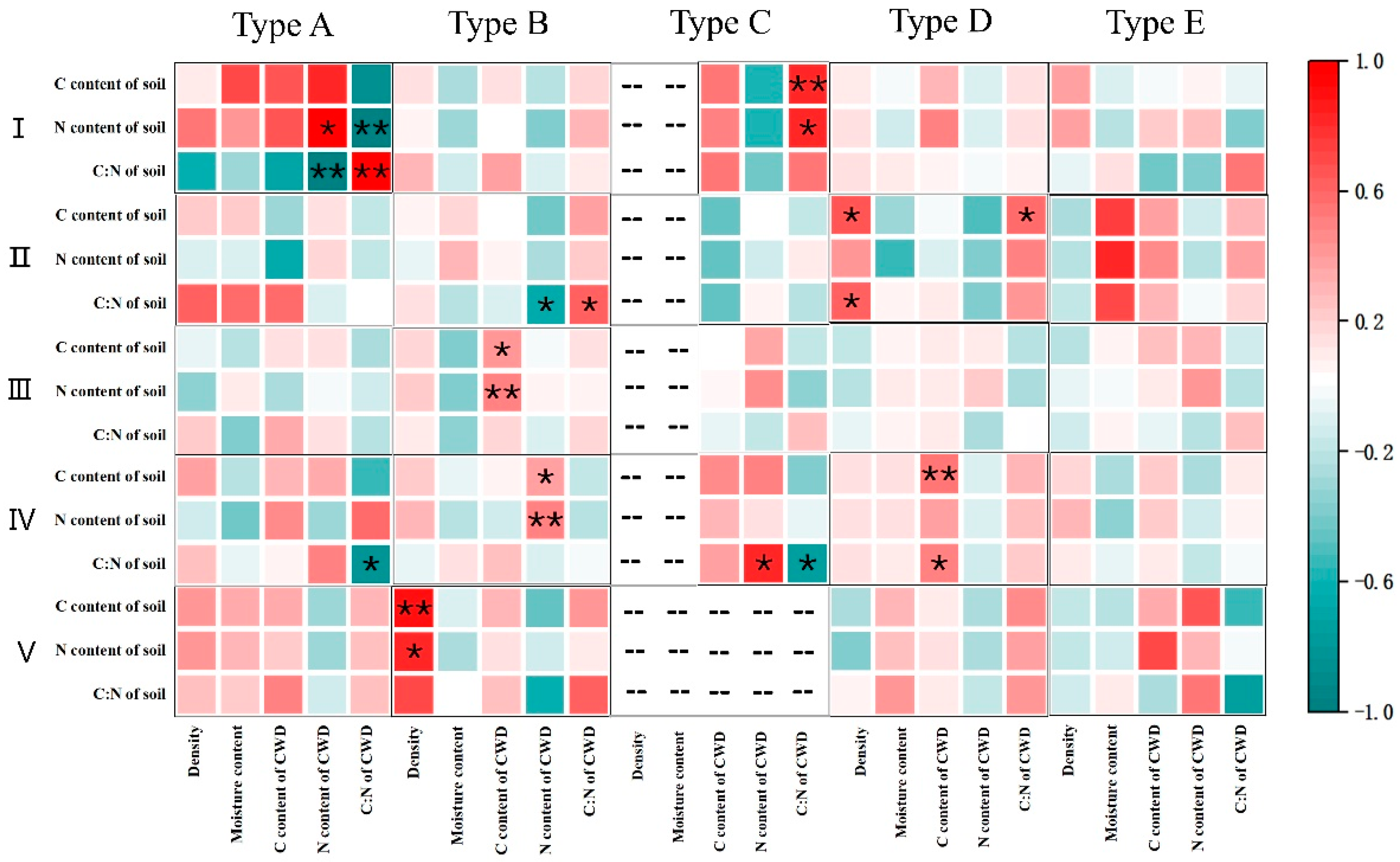
3.3. The Relationship Between C, N, and C:N Ratio in the Soil Layer Adjacent to CWD, and the Physicochemical Properties of CWD
4. Discussion
4.1. Analysis of the Variation in CWD Carbon and Nitrogen Content at Different Decomposition Stages in Different Forest Types
4.2. The Impact of Tree Species Richness on CWD Decomposition Rates and Soil Carbon and Nitrogen Content
4.3. The Impact of Vegetation Types on CWD Decomposition Rates and Soil Carbon and Nitrogen Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, L.; Lei, P.; Xiang, W.; Yan, W.; Liu, S. Soil Microbial Biomass Carbon and Nitrogen in Pure and Mixed Stands of Pinus massoniana and Cinnamomum camphora Differing in Stand Age. For. Ecol. Manag. 2014, 328, 150–158. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, S.; Lu, Y.; Froese, R.E.; Xu, X.; Zeng, J.; Ming, A.; Liu, X.; Xie, Y.; Li, Q. Thinning Effects on Forest Evolution in Masson Pine (Pinus massoniana Lamb.) Conversion from Pure Plantations into Mixed Forests. For. Ecol. Manag. 2020, 477, 118503. [Google Scholar] [CrossRef]
- Li, W.-Q.; Huang, Y.-X.; Chen, F.-S.; Liu, Y.-Q.; Lin, X.-F.; Zong, Y.-Y.; Wu, G.-Y.; Yu, Z.-R.; Fang, X.-M. Mixing with Broad-Leaved Trees Shapes the Rhizosphere Soil Fungal Communities of Coniferous Tree Species in Subtropical Forests. For. Ecol. Manag. 2021, 480, 118664. [Google Scholar] [CrossRef]
- Li, W.-Q.; Wu, Z.-J.; Zong, Y.-Y.; Wang, G.G.; Chen, F.-S.; Liu, Y.-Q.; Li, J.-J.; Fang, X.-M. Tree Species Mixing Enhances Rhizosphere Soil Organic Carbon Mineralization of Conifers in Subtropical Plantations. For. Ecol. Manag. 2022, 516, 120238. [Google Scholar] [CrossRef]
- Guo, J.; Feng, H.; McNie, P.; Liu, Q.; Xu, X.; Pan, C.; Yan, K.; Feng, L.; Adehanom Goitom, E.; Yu, Y. Species Mixing Improves Soil Properties and Enzymatic Activities in Chinese Fir Plantations: A Meta-Analysis. Catena 2023, 220, 106723. [Google Scholar] [CrossRef]
- Hulvey, K.B.; Hobbs, R.J.; Standish, R.J.; Lindenmayer, D.B.; Lach, L.; Perring, M.P. Benefits of Tree Mixes in Carbon Plantings. Nat. Clim. Change 2013, 3, 869–874. [Google Scholar] [CrossRef]
- González de Andrés, E. Interactions between Climate and Nutrient Cycles on Forest Response to Global Change: The Role of Mixed Forests. Forests 2019, 10, 609. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-Species Plantations of Eucalyptus with Nitrogen-Fixing Trees: A Review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-Species versus Monocultures in Plantation Forestry: Development, Benefits, Ecosystem Services and Perspectives for the Future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Kelty, M.J. The Role of Species Mixtures in Plantation Forestry. For. Ecol. Manag. 2006, 233, 195–204. [Google Scholar] [CrossRef]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant Species Diversity for Sustainable Management of Crop Pests and Diseases in Agroecosystems: A Review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S.; et al. Benefits of Increasing Plant Diversity in Sustainable Agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root Biomass and Exudates Link Plant Diversity with Soil Bacterial and Fungal Biomass. Sci. Rep. 2017, 7, 44641. [Google Scholar] [CrossRef]
- Haghverdi, K.; Kooch, Y. Effects of Diversity of Tree Species on Nutrient Cycling and Soil-Related Processes. Catena 2019, 178, 335–344. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Castro-Izaguirre, N.; Baruffol, M.; Brezzi, M.; Lang, A.; Li, Y.; Härdtle, W.; Von Oheimb, G.; Yang, X.; et al. Impacts of Species Richness on Productivity in a Large-Scale Subtropical Forest Experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Ferlian, O.; Huang, Y.; Luo, S.; Quosh, J.; Eisenhauer, N. Tree Diversity Effects on Productivity Depend on Mycorrhizae and Life Strategies in a Temperate Forest Experiment. Ecology 2023, 104, e3896. [Google Scholar] [CrossRef]
- Feng, Y.; Schmid, B.; Loreau, M.; Forrester, D.I.; Fei, S.; Zhu, J.; Tang, Z.; Zhu, J.; Hong, P.; Ji, C.; et al. Multispecies Forest Plantations Outyield Monocultures across a Broad Range of Conditions. Science 2022, 376, 865–868. [Google Scholar] [CrossRef]
- Dai, E.; Zhu, J.; Wang, X.; Xi, W. Multiple Ecosystem Services of Monoculture and Mixed Plantations: A Case Study of the Huitong Experimental Forest of Southern China. Land Use Pol. 2018, 79, 717–724. [Google Scholar] [CrossRef]
- Augusto, L.; Boča, A. Tree Functional Traits, Forest Biomass, and Tree Species Diversity Interact with Site Properties to Drive Forest Soil Carbon. Nat. Commun. 2022, 13, 1097. [Google Scholar] [CrossRef]
- Beugnon, R.; Bu, W.; Bruelheide, H.; Davrinche, A.; Du, J.; Haider, S.; Kunz, M.; von Oheimb, G.; Perles-Garcia, M.D.; Saadani, M.; et al. Abiotic and Biotic Drivers of Tree Trait Effects on Soil Microbial Biomass and Soil Carbon Concentration. Ecol. Monogr. 2023, 93, e1563. [Google Scholar] [CrossRef]
- Lieberman, M.; Lieberman, D. Nearest-neighbor Tree Species Combinations in Tropical Forest: The Role of Chance, and Some Consequences of High Diversity. Oikos 2007, 116, 377–386. [Google Scholar] [CrossRef]
- Forrester, D.I. The Spatial and Temporal Dynamics of Species Interactions in Mixed-Species Forests: From Pattern to Process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Wan, X.; Joly, F.; Jia, H.; Zhu, M.; Fu, Y.; Huang, Z. Functional Identity Drives Tree Species Richness-induced Increases in Litterfall Production and Forest Floor Mass in Young Tree Communities. New Phytol. 2023, 240, 1003–1014. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive Biodiversity-Productivity Relationship Predominant in Global Forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef]
- Schnabel, F.; Liu, X.; Kunz, M.; Barry, K.E.; Bongers, F.J.; Bruelheide, H.; Fichtner, A.; Härdtle, W.; Li, S.; Pfaff, C.-T.; et al. Species Richness Stabilizes Productivity via Asynchrony and Drought-Tolerance Diversity in a Large-Scale Tree Biodiversity Experiment. Sci. Adv. 2021, 7, eabk1643. [Google Scholar] [CrossRef]
- Urgoiti, J.; Messier, C.; Keeton, W.S.; Reich, P.B.; Gravel, D.; Paquette, A. No Complementarity No Gain—Net Diversity Effects on Tree Productivity Occur Once Complementarity Emerges during Early Stand Development. Ecol. Lett. 2022, 25, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Jucker, T.; Bouriaud, O.; Coomes, D.A. Crown Plasticity Enables Trees to Optimize Canopy Packing in Mixed-species Forests. Funct. Ecol. 2015, 29, 1078–1086. [Google Scholar] [CrossRef]
- Chiang, J.-M.; Spasojevic, M.J.; Muller-Landau, H.C.; Sun, I.-F.; Lin, Y.; Su, S.-H.; Chen, Z.-S.; Chen, C.-T.; Swenson, N.G.; McEwan, R.W. Functional Composition Drives Ecosystem Function through Multiple Mechanisms in a Broadleaved Subtropical Forest. Oecologia 2016, 182, 829–840. [Google Scholar] [CrossRef]
- Joly, F.; Milcu, A.; Scherer-Lorenzen, M.; Jean, L.; Bussotti, F.; Dawud, S.M.; Müller, S.; Pollastrini, M.; Raulund-Rasmussen, K.; Vesterdal, L.; et al. Tree Species Diversity Affects Decomposition through Modified Micro-environmental Conditions across European Forests. New Phytol. 2017, 214, 1281–1293. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, Y.; Zhao, K.; Niklaus, P.A.; Schmid, B.; He, J.-S. Positive Effects of Tree Species Diversity on Litterfall Quantity and Quality along a Secondary Successional Chronosequence in a Subtropical Forest. J. Plant Ecol. 2017, 10, 28–35. [Google Scholar] [CrossRef]
- Falconí-López, A.; Grella, N.; Donoso, D.A.; Feldhaar, H.; Tremlett, C.J.; Müller, J. Patterns of Deadwood Amount and Deadwood Diversity along a Natural Forest Recovery Gradient from Agriculture to Old-Growth Lowland Tropical Forests. Eur. J. For. Res. 2024, 143, 1321–1332. [Google Scholar] [CrossRef]
- Yuan, Y.; Cao, C.; Feng, Y.; Miao, Y.; Zhou, Z.; Zhang, S. Influence of Long-Term Ecological Reclamation on Carbon and Nitrogen Cycling in Soil Aggregates: The Role of Bacterial Community Structure and Function. Sci. Total Environ. 2024, 954, 176729. [Google Scholar] [CrossRef] [PubMed]
- Petritan, I.C.; Mihăilă, V.-V.; Yuste, J.C.; Bouriaud, O.; Petritan, A.M. Deadwood Density, C Stocks and Their Controlling Factors in a Beech-Silver Fir Mixed Virgin European Forest. For. Ecol. Manag. 2023, 539, 121007. [Google Scholar] [CrossRef]
- Magnússon, R.Í.; Tietema, A.; Cornelissen, J.H.C.; Hefting, M.M.; Kalbitz, K. Tamm Review: Sequestration of Carbon from Coarse Woody Debris in Forest Soils. For. Ecol. Manag. 2016, 377, 1–15. [Google Scholar] [CrossRef]
- Přívětivý, T.; Janík, D.; Unar, P.; Adam, D.; Král, K.; Vrška, T. How Do Environmental Conditions Affect the Deadwood Decomposition of European Beech (Fagus sylvatica L.)? For. Ecol. Manag. 2016, 381, 177–187. [Google Scholar] [CrossRef]
- Xiao, S.; You, H.; You, W.; Liu, J.; Cai, C.; Wu, J.; Ji, Z.; Zhan, S.; Hu, Z.; Zhang, Z.; et al. Rhizosphere and Bulk Soil Enzyme Activities in a Nothotsuga longibracteata Forest in the Tianbaoyan National Nature Reserve, Fujian Province, China. J. For. Res. 2017, 28, 521–528. [Google Scholar] [CrossRef]
- You, H.; He, D.; You, W.; Liu, J.; Cai, C. Effect of Environmental Gradients on the Quantity and Quality of Fallen Logs in Tsuga longibracteata Forest in Tianbaoyan National Nature Reserve, Fujian Province, China. J. Mt. Sci. 2013, 10, 1118–1124. [Google Scholar] [CrossRef]
- Cao, Y.; He, D.; Zhou, M.; Liu, J.; Cai, C.; You, W. Long-Term Dynamic Simulation of Forest Landscape Succession in Tianbaoyan National Nature Reserve under Climate Change Scenarios. Acta Ecol. Sin. 2023, 43, 3636–3649. (In Chinese) [Google Scholar] [CrossRef]
- Jaman, M.S.; Yu, Q.; Xu, C.; Jamil, M.; Ke, Y.; Yang, T.; Knapp, A.K.; Wilkins, K.; Collins, S.L.; Griffin-Nolan, R.J.; et al. Chronic Drought Decreased Organic Carbon Content in Topsoil Greater than Intense Drought across Grasslands in Northern China. Geoderma 2024, 443, 116832. [Google Scholar] [CrossRef]
- Yan, E.; Wang, X.; Huang, J. Concept and Classification of Coarse Woody Debris in Forest Ecosystems. Acta Ecol. Sin. 2005, 25, 158–167. (In Chinese) [Google Scholar] [CrossRef]
- Sollins, P. Input and Decay of Coarse Woody Debris in Coniferous Stands in Western Oregon and Washington. Can. J. For. Res. 1982, 12, 18–28. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, X.; Shu, X.; Liu, Y.; Zhang, Q. Linking Soil Bacterial and Fungal Communities to Vegetation Succession Following Agricultural Abandonment. Plant Soil 2018, 431, 19–36. [Google Scholar] [CrossRef]
- Li, S.; Lyu, M.; Deng, C.; Deng, W.; Wang, X.; Cao, A.; Jiang, Y.; Liu, J.; Lu, Y.; Xie, J. Input of High-Quality Litter Reduces Soil Carbon Losses Due to Priming in a Subtropical Pine Forest. Soil Biol. Biochem. 2024, 194, 109444. [Google Scholar] [CrossRef]
- Chen, W.; Hu, H.; Heal, K.; Sohi, S.; Tigabu, M.; Qiu, W.; Zhou, C. Linking Microbial Decomposition to Dissolved Organic Matter Composition in the Revegetation of the Red Soil Erosion Area. Forests 2023, 14, 270. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; pp. 154–196. [Google Scholar]
- Hsu, J.S.; Powell, J.; Adler, P.B. Sensitivity of Mean Annual Primary Production to Precipitation. Glob. Change Biol. 2012, 18, 2246–2255. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, X.; Wang, S.; Chen, M.; Chen, S.; Ren, T.; Xia, J.; Bai, Y.; Huang, J.; Han, X. Asymmetric Sensitivity of Ecosystem Carbon and Water Processes in Response to Precipitation Change in a Semi-arid Steppe. Funct. Ecol. 2017, 31, 1301–1311. [Google Scholar] [CrossRef]
- Thomas, S.C.; Martin, A.R. Carbon Content of Tree Tissues: A Synthesis. Forests 2012, 3, 332–352. [Google Scholar] [CrossRef]
- Herrmann, S.; Kahl, T.; Bauhus, J. Decomposition Dynamics of Coarse Woody Debris of Three Important Central European Tree Species. For. Ecosyst. 2015, 2, 27. [Google Scholar] [CrossRef]
- Köster, K.; Metslaid, M.; Engelhart, J.; Köster, E. Dead Wood Basic Density, and the Concentration of Carbon and Nitrogen for Main Tree Species in Managed Hemiboreal Forests. For. Ecol. Manag. 2015, 354, 35–42. [Google Scholar] [CrossRef]
- Mukhortova, L.; Pashenova, N.; Meteleva, M.; Krivobokov, L.; Guggenberger, G. Temperature Sensitivity of CO2 and CH4 Fluxes from Coarse Woody Debris in Northern Boreal Forests. Forests 2021, 12, 624. [Google Scholar] [CrossRef]
- Lecerf, A.; Risnoveanu, G.; Popescu, C.; Gessner, M.O.; Chauvet, E. Decomposition of Diverse Litter Mixtures in Streams. Ecology 2007, 88, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Arif, M.; Ding, D.; Li, J.; He, X.; Li, C. Invasive Plants and Species Richness Impact Litter Decomposition in Riparian Zones. Front. Plant Sci. 2022, 13, 955656. [Google Scholar] [CrossRef] [PubMed]


| Five Forest Types (Mean ± SD) | One-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | df | F | p | η2 | |
| C (%) | 44.22 ± 4.24 b | 43.05 ± 4.38 b | 47.72 ± 5.74 a | 44.04 ± 4.62 b | 43.19 ± 4.78 b | 4 | 5.974 | 0.000 | 0.162 |
| N (%) | 0.80 ± 0.36 | 0.90 ± 0.95 | 0.96 ± 0.32 | 0.76 ± 0.33 | 0.68 ± 0.26 | 4 | 2.169 | 0.072 | 0.065 |
| C:N | 70.66 ± 42.22 | 62.51 ± 28.40 | 57.53 ± 28.66 | 68.31 ± 31.32 | 76.77 ± 42.19 | 4 | 2.263 | 0.062 | 0.068 |
| Density | 0.36 ± 0.2 ab | 0.31 ± 0.14 b | - | 0.34 ± 0.16 b | 0.41 ± 0.16 a | 3 | 5.309 | 0.001 | 0.114 |
| moisture content (%) | 0.43 ± 0.19 | 0.45 ± 0.16 | - | 0.42 ± 0.17 | 0.39 ± 0.13 | 3 | 1.885 | 0.135 | 0.044 |
| Tree Species Richness | C (%) | N (%) | C:N | Density | Moisture Content (%) |
|---|---|---|---|---|---|
| 1 | 44.22 ± 4.24 | 0.80 ± 0.36 | 70.66 ± 44.22 | 0.36 ± 0.21 ab | 0.43 ± 0.19 |
| 2 | 44.18 ± 4.92 | 0.84 ± 0.65 | 64.41 ± 29.91 | 0.32 ± 0.15 b | 0.43 ± 0.17 |
| 3 | 43.19 ± 4.80 | 0.68 ± 0.26 | 67.92 ± 35.00 | 0.41 ± 0.16 a | 0.39 ± 0.13 |
| df | 2 | 2 | 2 | 2 | 2 |
| F | 1.102 | 2.270 | 2.609 | 7.270 | 1.888 |
| p | 0.334 | 0.105 | 0.079 | 0.001 | 0.156 |
| η2 | 0.018 | 0.035 | 0.040 | 0.105 | 0.030 |
| Vegetation Types | C (%) | N (%) | C:N | Density | Moisture Content (%) |
|---|---|---|---|---|---|
| Pure tree forests | 44.27 ± 4.9 ns | 0.88 ± 0.72 | 63.95 ± 33.19 | 0.32 ± 0.17 | 0.44 ± 0.17 ns |
| Tree–shrub mixed forests | 43.69 ± 4.69 | 0.73 ± 0.30 | 71.86 ± 36.39 | 0.37 ± 0.16 | 0.41 ± 0.16 |
| t-value | 1.073 | 2.471 | −1.994 | −2.155 | 1.887 |
| df | 309 | 312 | 307 | 286 | 286 |
| p | 0.284 | 0.014 | 0.047 | 0.032 | 0.060 |
| Five Forest Types (Mean ± SD) | One-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | df | F | p | η2 | |
| C (%) | 20.11 ± 8.42 b | 24.41 ± 11.02 ab | 11.02 ± 8.37 c | 29.21 ± 14.38 a | 16.94 ± 11.93 bc | 4 | 21.918 | 0.000 | 0.414 |
| N (%) | 1.58 ± 0.53 a | 1.72 ± 0.63 a | 0.93 ± 0.40 b | 1.44 ± 0.56 a | 1.44 ± 1.96 a | 4 | 3.421 | 0.009 | 0.099 |
| C:N | 12.81 ± 3.65 bc | 13.94 ± 3.50 b | 10.99 ± 4.33 c | 19.62 ± 4.51 a | 13.15 ± 4.68 bc | 4 | 38.681 | 0.000 | 0.555 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, Y.; Xu, Z.; You, W.; Cao, Y.; Xing, W.; He, D. Effect of Different Mixing Patterns on Carbon and Nitrogen Dynamics During the Decomposition of Deadwood in Subtropical Forest Ecosystems. Forests 2025, 16, 579. https://doi.org/10.3390/f16040579
Sang Y, Xu Z, You W, Cao Y, Xing W, He D. Effect of Different Mixing Patterns on Carbon and Nitrogen Dynamics During the Decomposition of Deadwood in Subtropical Forest Ecosystems. Forests. 2025; 16(4):579. https://doi.org/10.3390/f16040579
Chicago/Turabian StyleSang, Ying, Zhonglin Xu, Weibin You, Yan Cao, Wenli Xing, and Dongjin He. 2025. "Effect of Different Mixing Patterns on Carbon and Nitrogen Dynamics During the Decomposition of Deadwood in Subtropical Forest Ecosystems" Forests 16, no. 4: 579. https://doi.org/10.3390/f16040579
APA StyleSang, Y., Xu, Z., You, W., Cao, Y., Xing, W., & He, D. (2025). Effect of Different Mixing Patterns on Carbon and Nitrogen Dynamics During the Decomposition of Deadwood in Subtropical Forest Ecosystems. Forests, 16(4), 579. https://doi.org/10.3390/f16040579






