Abstract
Cattle grazing and selective logging alter the functioning of an ecosystem, but their impacts on forest regeneration, particularly in relation to forest successional stages, are yet poorly understood. This study examined how these activities affect the regeneration of Nothofagus antarctica (ñire or ñirre) and N. pumilio (lenga) pure forests in Patagonia and whether these effects vary between old-growth and secondary forests. We assessed seedlings by origin (sexual, asexual) and height classes (<0.3 m, 0.3–0.6 m, >0.6 m) across 88 plots (25 × 20 m). Selective logging intensity was measured via the basal area of tree stumps, and cattle grazing pressure via dung counts. Forest regeneration, as predicted by human disturbances, forest successional stage, and tree density (parent trees), was modeled using generalized linear models. For N. antarctica, regeneration was exclusively asexual and showed a positive influence for selective logging and cattle, but negative with both interacting. In contrast, the most recent regeneration (R1) was predominantly influenced by the density of parent trees and successional stage. Conversely, N. pumilio regeneration, entirely sexual, was unaffected by cattle grazing, relying instead on parent tree density, logging intensity, and successional stage. These findings highlight the species-specific dynamics of regeneration under anthropogenic pressures. Understanding the interactions between natural and human disturbances is critical for conserving Nothofagus forests. Our results provide a basis for targeted restoration efforts and policies to mitigate degradation and promote ecosystem resilience.
1. Introduction
The effects of anthropogenic disturbances on forest ecosystems have been thoroughly studied across the globe [1,2,3]. Research has highlighted forest degradation driven by both severe disturbances (e.g., land-use change, fires) and persistent, low-intensity pressures (e.g., grazing, selective logging, invasive species) in a wide range of forest systems [2,4,5,6]. Activities such as cattle grazing and selective logging modify species diversity and composition, potentially causing substantial impacts on community dynamics and ecosystem functioning [4]. In South America, previous published studies have focused on the impacts of introduced mammals on forest species such as Nothofagus dombeyi (Mirb.) Oerst. [7], Austrocedrus chilensis (D. Don) Pic-Serm. & Bizarri. [8,9,10], and Araucaria araucana (Mol.) K. Koch [11]. These human-induced disturbances and their impacts have been similarly reported in Patagonia, for example, in N. dombeyi-A. chilensis forest [12], and in N. antarctica and N. pumilio forests [13,14,15,16]. N. antarctica (G.Forst.) Oerst. and N. pumilio (Poepp. & Endl.) Krasser are two of the tree species with the widest geographic distribution in the temperate forests of Chile. Their distribution extends from the Maule Region to southern Patagonia in Magallanes (35° S–56° S; [17]). N. antarctica cover an area of 501,372 ha, with the largest concentration in the Aysén region, totaling 131,593 hectares (26%, [18]). This region also contains the largest area of forests dominated by N. pumilio, covering 1,409,260 ha (38%, [19]). N. antarctica has a high resprouting capacity from its roots, a strategy that allows it to persist in areas exposed to anthropogenic disturbances or natural disruptions [17]. On the other hand, N. pumilio is characterized by a strategy of sexual reproduction through seeds, without presenting the capacity for vegetative regrowth [17]. Along their distributions, these species form extensive pure and mixed forests adapted to extreme climatic conditions [20]. Both forests have been subjected to intensive and notable transformations, especially in the Aysén region [21,22].
Before European colonization in the 16th century, the transformations of the temperate rainforests in Chile, including Patagonia, were primarily driven by natural processes and the small-scale activities of local indigenous communities [23]. These ecosystems have since undergone significant degradation, largely due to fires and the expansion of two key human activities: selective logging and cattle ranching. This degradation has resulted in forests with lower-quality individuals, diminished productivity, and reduced vigor [24]. In Patagonia, selective logging remains one of the primary drivers of native forest degradation, which is characterized by continuous and low-intensity logging, typical of rural production systems [20]. Meanwhile, cattle impact forest ecosystems in multiple ways, affecting both regeneration [11,25] and soil conditions [13]. Notably, cattle lead to soil compaction, decreasing porosity and water infiltration capacity. Furthermore, intensive browsing reduces vegetation cover, leaving soil vulnerable to erosion [26]. Both selective logging and cattle represent serious threats to the conservation of the Nothofagus deciduous forests in Patagonia by negatively impacting these forest ecosystems, which are distributed throughout Chile and Argentina [27]. However, less attention has been paid to understanding how cattle and selective logging jointly impact forest regeneration in these ecosystems.
The evaluation of the effects of human-induced disturbances on the regeneration of forest species can be particularly informative as seedlings and saplings respond more rapidly to most of the low intensity and chronic human disturbances than long-lived adult trees do [28]. The process of forest regeneration is crucial for preserving the long-term ecological functions and benefits of forests [24] and maintaining the successive generations of trees [29]. Thus, studying how human disturbance affects tree regeneration can yield important insights into how these impacts may affect ecosystem functioning and forest community composition, as well as the long-term resilience and resistance of these ecosystems to environmental change [30]. The impacts of these activities may vary depending on the successional stage of the forests. In old-growth forests, which represent more advanced successional stages, selective logging and cattle may have more severe effects compared to in secondary forests due to the increased vulnerability of these ecosystems to disturbances [13]. Here, we investigated (1) the influences of cattle and selective logging on the regeneration of Nothofagus deciduous forests in Patagonia; (2) whether selective logging acts synergistically with cattle ranching; and (3) whether these impacts vary according to forest successional stage (old-growth and secondary forests).
2. Materials and Methods
2.1. Study Area
The study was carried out in Nothofagus antarctica (ñire or ñirre) and N. pumilio (lenga) forests, the main deciduous native species in western Patagonia, in the provinces of Coyhaique and General Carrera, Aysén region, Chilean Patagonia (southern Andes of South America, 45°–46° S, 72° W), in similar sites according to precipitation and temperature records, with a range of mean precipitation and temperature of 500–1000 mm and 7–9 °C, respectively. The area covers ca. 2500 km2 (Figure 1). Nothofagus deciduous forests are the dominant vegetation type and occupy 90% of the total forest cover in the study area [19].
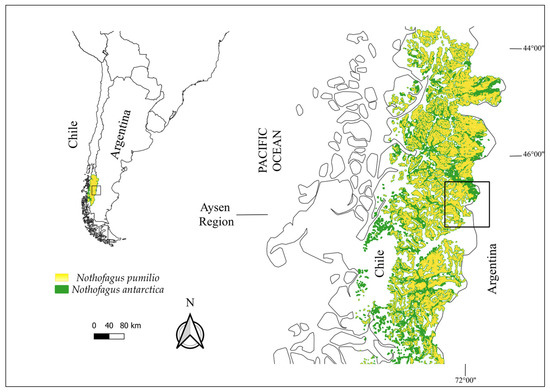
Figure 1.
Study area within Aysen region, western Patagonia, Chile.
Two successional forest stages were defined according to the native forests’ cadastre, one of the most comprehensive cartographic studies of natural vegetation developed in Chile [19]: old-growth and secondary forests. At least 50% canopy cover, considerable vertical heterogeneity, structural variety, and the presence of an emergent big canopy (>80 cm dbh, >25 m tall) are characteristics of old-growth forests, which are uneven-aged stands. Even-aged stands made up primarily of young trees that result from extensive disturbance, whether natural or man-made, are referred to as secondary forests. The study area is characterized by a mosaic of different land-use types and productive activities, mainly firewood production and cattle.
2.2. Sampling Methods
Plots were randomly distributed throughout the study area using a sample approach stratified by forest successional stage and were ensured to be at least one kilometer apart using a geographical information system (GIS). In the field, 84 25 × 20 m plots were set up in deciduous Nothofagus forests, at least 200 m from the forest boundaries. Plots were separated by an average of 1300 m. In the summer of 2023, 44 plots were located in N. antarctica (22 in old-growth forests, 22 in secondary forest) and 40 plots in N. pumilio forests (21 in old-growth forest, 19 in secondary forest). In each 25 × 20 m plot, two human disturbance related variables were measured, namely (1) the number and basal area of stumps of each tree species (a surrogate for selective logging) and (2) the number of cattle dung pats (a surrogate for current cattle density and trampling pressure). Henceforth, we will refer to these variables as ‘selective logging’ and ‘cattle intensity index’ (CAI, [11]), respectively. Basal area refers to the cross-sectional area of the tree stem measured at the upper level of the stump. In each plot, the number of parent trees of each species, defined as those individuals >5 cm in DBH, were recorded to account for density-dependent effects. In the central axis of each plot, 6 1 × 1 m subplots were established, and all seedlings were recorded according to height classes (R1: 0–0.3; R2: 0.3–0.6; and R3: >0.6 m tall and <5 cm in diameter at breast height [DBH]). Seedlings < 0.3 m represented recently established regeneration (<5 years old, [17]). Using a non-destructive technique, we dug around each sapling and seedling by hand using a hand shovel to differentiate between sexual (seeds) and asexual (resprouts, [31]) regeneration.
2.3. Data Analysis
The height classes of seedlings of each species were the response variable in regeneration models where the explanatory variables were human disturbances (CAI, selective logging), number of parent trees (tree density), forest type (N. antarctica, N. pumilio forest), and forest successional stage (old-growth, secondary forests). We used generalized linear models (GLM) with a log–log link function to linearize the observed exponential relationship between the response and the explanatory variables, and a negative binomial error distribution to account for overdispersion. In place of conventional hypothesis testing, we employed maximum likelihood techniques and model selection [32]. Given a range of different models, we estimated model parameters that maximized the likelihood of regeneration as measured in the field. We looked at 17 distinct nested models for each species, including the effects of tree density, successional stage, selective logging, CAI, and some specific interactions between the explanatory factors. Our most complex model for each response variable (Y) has the following form:
where Yi is the number of seedlings per hectare in plot i, CAIi is the cattle intensity index, SLi represents the selective logging pressure, and FSi is the forest successional stage. Interactions among covariates and between covariates and factors would indicate that the effect of a covariate in the response variable changes, depending on the value of the other covariate or factor level.
log(Yi) = log(CAIi) × log(SLi) + log(SLi) × FSi + log(CAIi) × FSi + Tree density
The Akaike information criterion (AIC, [33]) was used to compare alternative models. The AIC difference between each model and the best model in the set was determined as delta AIC (ΔAIC). Models are deemed to have similar empirical evidence if their AIC differences are less than two units [33]. Consequently, models with ΔAIC > 2 were not included in subsequent calculations. Akaike weights (wi) were calculated for the confidence set of models (ΔAIC < 2) to determine the weight of evidence in favor of each model and to estimate the relative importance (Wi) of each individual parameter in the set of candidate models. If no single model is clearly superior to the others in a set of models (model with wi < 0.9), a (weighted) model averaging approach should be used [33]. Therefore, we computed model-averaged estimates for variables in the confidence set of models and associated unconditional standard errors (SE) using the full set of plausible models (ΔAIC < 2). Regression coefficient estimates in all chosen subgroups are less affected by model selection bias through this method [33]. All analyses were performed using R (v4.4.3 2025) [34], including the ‘MASS’ [35], ‘vegan’ [36], and ‘MuMIn’ [37] packages.
3. Results
3.1. Cattle, Selective Logging and Forest Regeneration
N. antarctica forests exhibited, on average, higher cattle grazing intensity compared to N. pumilio forests (Table A1, Appendix A). For both forest types, this intensity was greater in old-growth forests than in secondary forests. On the other hand, in N. antarctica forests selective logging did not show significant variability between forest successional stages, while N. pumilio secondary forests exhibited the lowest basal area extraction values. In N. antarctica forests, a density of 780 to 1143 dung ha−1 was recorded (secondary and old-growth forests, respectively, Table A1, Appendix A), while in N. pumilio forests, it was from 282 to 589 dung ha−1. The extracted basal area for N. antarctica and N. pumilio forests ranged from 17 to 19 m2 ha−1 and 8 to 18 m2 ha−1, respectively. Tree density was higher in secondary than in old-growth forests, both in N. antarctica and N. pumilio-dominated forests. N. antarctica forests were generally characterized by higher tree densities compared to N. pumilio forests; however, N. pumilio showed a greater basal area. The sampled forests in the study area are monospecific, with each forest dominated by either N. antarctica or N. pumilio. Forest regeneration was species-specific: regeneration of N. antarctica occurred exclusively in forests dominated by this species, while N. pumilio regeneration occurred only in N. pumilio forests. Regeneration density was higher in N. pumilio forests compared to N. antarctica forests (Table 1). Regeneration was completely asexual (from resprouts) for N. antarctica and sexual (from seeds) for N. pumilio. Up to 90% of the total regeneration in both types of forests was concentrated in the first height class (<0.3 m), which was representative of recently established regeneration from seeds (ca. <5 years old).

Table 1.
Mean density (plants ha−1) and standard deviation (Sd) of N. antarctica and N. pumilio seedlings in sampled plots. All the regeneration of N. antarctica was of asexual origin (i.e., from resprouting), whereas that of N. pumilio was sexual (i.e., from seed). Height classes of forest regeneration are represented according to: R1, 0–0.3; R2, 0.3–0.6; and R3, >0.6 m tall and <5 cm in diameter at breast height [DBH].
3.2. Effects of Human Disturbances on the Dynamics of Forest Regeneration
Regeneration was differentially impacted by cattle grazing and selective logging (Table 2). For Nothofagus antarctica, the results suggest that selective logging and cattle have a positive influence on regeneration in higher height classes, but this effect becomes negative when both disturbances interact. In contrast, the most recent regeneration (R1) was predominantly influenced by parent tree density (positive effects) and successional stage (negative effects in secondary forests). These results indicate that a higher number of parent trees promotes regeneration via resprouting, except in secondary forests. Table 3 gives details of the best-fitting model parameters. For N. pumilio, a different dynamic was observed, with all regeneration originating from seeds (sexual). Our results suggest that cattle have no significant effects on the regeneration of this species. Regeneration was influenced exclusively by parent tree density, selective logging, and successional stage. The height classes R2 and R3 of N. pumilio were influenced by selective logging (positive effect), while smaller regeneration (R1) was associated with tree density and the forest successional stage (positive effect). The results indicated that the interaction between tree density and secondary forests had a negative effect, similar to its impact on total regeneration (R).

Table 2.
Ranking of the generalized linear models of individual species following an AIC-based model selection procedure. ΔAIC and wi correspond to AIC differences an Akaike weights, respectively. Abbreviations of the model parameters are as follows: FS, the forest successional stages; CAI, the cattle intensity index; SL, selective logging pressure. Height classes of forest regeneration are represented according to: R1, 0–0.3; R2, 0.3–0.6; and R3, >0.6 m tall and <5 cm in diameter at breast height [DBH]. R represents the sum of regeneration. Coefficients in bold indicate the best-fit models for each forest species.

Table 3.
Model-averaged estimates, standard errors, and relative variable importance (Wi) for selected variables in the best fitted models of the regeneration of individual tree species. Generalized linear models were used with a log–log link function and a negative binomial error distribution. Estimated coefficients therefore refer to the response variable on a log scale. Abbreviations of the model parameters are as follows: SF, secondary forest; CAI, cattle intensity index; SL, selective logging. Height classes of forest regeneration are represented according to: R1, 0–0.3; R2, 0.3–0.6; and R3, >0.6 m tall and < 5 cm in diameter at breast height [DBH]. R represents the sum of regeneration.
4. Discussion
Our results suggest that cattle grazing and selective logging have distinct impacts on the regeneration dynamics of pure deciduous Nothofagus forests in western Patagonia. These effects are primarily conditioned by tree density and the successional stages of the forests.
4.1. Influences of Human Disturbances on Forest Regeneration Dynamics
In our study, the response of N. antarctica regeneration to cattle grazing intensity varied across height classes. Early regeneration stages were positively associated with higher parent tree density, while taller seedlings were positively influenced by both selective logging and cattle grazing. However, this positive effect turned negative when both disturbances interacted, highlighting the complex relationship between human-induced disturbances and forest regeneration dynamics. The positive effect of tree density on regeneration diminishes at higher densities of parent trees, which is characteristic of secondary forests. The regeneration strategy of N. antarctica through resprouting (asexual reproduction) is related to parent tree density and appropriate light conditions for germination, according to its requirements as an intermediate shade-tolerant species [17]. N. antarctica forests are often semi-open rather than completely closed, providing frequent opportunities for regeneration to take advantage of constantly forming gaps [14]. Selective logging reduces tree density and facilitates cattle access and movement in the forests, but also modifies the microclimate (e.g., light and water availability, [38,39]), creating conditions that promote understory vegetation growth, often dominated by invasive exotic herbaceous species, which in turn serve as forage for cattle [40]. This alters the forest structure, as grazing inhibits seedling development due to the palatability of N. antarctica regeneration, thereby affecting forest dynamics and limiting the development of new individuals. Plant size is a critical factor influencing the extent of damage caused by herbivores [41]. Smaller plants, due to their greater accessibility, are more vulnerable to browsing in their meristematic regions [42]. In Patagonia, apical browsing of Nothofagus spp. saplings has been documented up to a height of 2.5 m [43]. Similarly, other studies have reported that saplings protected from cattle reach twice the maximum height compared to those left unprotected [12]. Previous studies have shown that damage to regeneration in these forests is correlated with forage availability and stocking rate [44]. Other authors have suggested that adjusting cattle stocking rates can mitigate damage to tree regeneration [45,46].
The lower densities of regeneration and the higher proportion of resprouts observed in our study, compared to previous research [13,15,47], suggest that N. antarctica forests have experienced degraded soil conditions due to compaction from prolonged and intensive cattle ranching, dating back to the early 1900s. These forests are used year-round for fodder and shelter, as well as for firewood and timber extraction. In fact, asexual reproduction through resprouting may have a higher likelihood of survival under intensive disturbances [25]. Resprouts benefit from established root systems with large surface areas for water and nutrient acquisition, as well as high energy reserves [48,49], allowing them to compete more effectively under disturbed conditions. Though N. antarctica can produce large quantities of lightweight seeds, it is considered the Nothofagus species that most heavily depends on resprouting for reproduction [50]. N. antarctica is capable of resprouting from its root system, branches, or basal structures (root collar or crown), either in relatively undisturbed forests or as a response to the loss of above-ground biomass. This species has demonstrated resilience to repeated and cumulative disturbances in Tierra del Fuego. Its ability to resprout and persist following silvicultural management and grazing has been well documented in the region [13,51,52]. The selective pressures that led to the resprouting ability of N. antarctica remain uncertain, but it is likely to have developed in response to grazing by the native herbivore Lama guanicoe or to wildfires further north within the species’ range [53]. As N. antarctica commonly occupies the ecotone between forest and grassland, it has historically been exposed to more consistent and intense grazing compared to other Nothofagus species, particularly from herbivores transitioning between grasslands and adjacent forests [54]. Although N. antarctica has adapted to selective pressures, prolonged trampling and browsing by livestock (cattle, sheep, and horses), as well as native herbivores, can still deform or hinder its regeneration over time [46,47], as suggested by the results of our study.
The contrasting ecological conditions of N. antarctica and N. pumilio forests further highlight their distinct regeneration dynamics. N. antarctica is typically found in flatter areas with shallow soils, strong winds, and low temperatures, resulting in forests with smaller trees in both height and diameter [17,27]. Conversely, N. pumilio generally grows in deeper soils at higher altitudes and on steeper slopes, where conditions are less restrictive, with lower exposure to cattle, leading to taller trees with larger diameters [55]. Thus, N. pumilio forests displayed a more favorable regeneration pattern, with the highest seedling densities in the first height class (≤0.3 m), and overall higher densities across all height classes compared to N. antarctica. These conditions contribute to more complex forest structures, greater plant diversity, and improved regeneration outcomes [17].
N. pumilio is classified as a mid-tolerant species due to its capacity to colonize new environments and persist under dense tree canopies [17,56]. These eco-physiological traits likely explain the species’ ability to thrive following canopy openings (e.g., clear-cuts). The species has demonstrated an adaptive capacity to grow under varying levels of soil moisture and light intensity, providing significant plasticity across a broad range of microenvironmental conditions [43,56]. Intermediate canopy cover (35%–45%) has been identified as the optimal condition for successful establishment and regeneration growth [57]. Nonetheless, the lower regeneration values recorded in this study, compared to previous research (e.g., [58]), may be associated with higher levels of logging. These intensified disturbances could have altered key environmental conditions, thereby limiting the species’ ability to regenerate effectively. Disturbances such as cattle grazing and wildfires, which can lead to large-scale loss of vegetative and reproductive structures, threaten the health and survival of N. pumilio regeneration [59]. In heavily grazed areas, cattle browsing can prevent tree regeneration from germinating or lead to stunted, shrub-like mature trees with reduced growth rates [46,59,60].
4.2. Prospects for the Restoration and Conservation
Regeneration is considered a crucial contributor to forest resilience [61] as it significantly impacts post-disturbance recovery processes [62] by influencing the development of different forest types or non-forest states [63]. Further investigation is needed to fully understand the multiple factors affecting the long-term maintenance of Nothofagus deciduous populations, as well as the interactions between natural and human-driven disturbances. While N. antarctica relies primarily on asexual reproduction through resprouting, N. pumilio depends on sexual reproduction via seedling establishment. These contrasting strategies likely explain the observed disparities in regeneration patterns. However, both species face significant challenges from human-induced disturbances, which disrupt regeneration processes and alter forest structure and composition. This underscores the need for improved management strategies to mitigate their detrimental effects on forest regeneration.
Productive activities such as cattle grazing and selective logging create distinct microenvironments that modify key factors, including the microclimate (solar radiation and water availability) and soil properties [64], playing a decisive role in the germination, growth, reproduction, and mortality of plant species. It is essential to maintain ongoing monitoring and evaluation of the successional dynamics in these forests subjected to cattle grazing and logging. Such efforts are crucial for identifying potential alterations in their influence on regeneration processes, particularly when considering the implications of climate change. This knowledge is essential for developing and promoting effective measures for the restoration and management of these forest ecosystems. Although many questions remain unanswered, our results provide valuable insights that can inform regulations and policies aimed at reversing degradation processes and promoting sustainable management practices for these forest ecosystems.
Author Contributions
Conceptualization, methodology, software, formal analysis, investigation; writing—original draft preparation; supervision; funding acquisition, C.Z.-E. Methodology, software, formal analysis, writing—review and editing, C.B.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID Fondecyt grant no. 11221074 and FONDAP/ANID 1523A0002 (Center for Climate and Resilience Research (CR2)).
Data Availability Statement
The data presented in this study are available upon request to the corresponding author.
Acknowledgments
We thank Diana Vera, Miguel Araneda, Manuel Vargas, Gonzalo Bravo, Pamela Almonacid, Jesse Palma, Franco Uribe, Jorge Sandoval, and Gustavo Pérez for their support during fieldwork. We also extend our gratitude to the rural landowners for facilitating access to the study areas, for their kindness and for sharing their experiences and knowledge.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Mean density and standard deviation of the main site variables (i.e., human disturbances and tree density) in sampled plots. CAI, cattle index (N ha−1); SL, selective logging (m2 ha−1); BA, basal area (m2 ha−1).
Table A1.
Mean density and standard deviation of the main site variables (i.e., human disturbances and tree density) in sampled plots. CAI, cattle index (N ha−1); SL, selective logging (m2 ha−1); BA, basal area (m2 ha−1).
| Forest Specie | Site Variables | Secondary Forests | Old-Growth Forests | ||
|---|---|---|---|---|---|
| Mean | Sd | Mean | Sd | ||
| Nothofagus antarctica | CAI (N ha−1) | 780 | 899 | 1143 | 1182 |
| SL (m2 ha−1) | 17 | 11 | 19 | 12 | |
| Tree density (N ha−1) | 1745 | 973 | 737 | 442 | |
| BA (m2 ha−1) | 33.4 | 9.4 | 36.4 | 11.3 | |
| N. pumilio | CAI (N ha−1) | 282 | 525 | 589 | 1365 |
| SL (m2 ha−1) | 8 | 14 | 18 | 28 | |
| Tree density (N ha−1) | 1226 | 873 | 333 | 252 | |
| BA (m2 ha−1) | 54.4 | 16.4 | 49 | 18 | |
References
- Belsky, A.J.; Blumenthal, D.M. Effects of livestock grazing on stand dynamics and soils in upland forests of the interior West. Conserv. Biol. 1997, 11, 315–327. [Google Scholar] [CrossRef]
- Clark, J.A.; Covey, K.R. Tree species richness and the logging of natural forests: A meta-analysis. For. Ecol. Manag. 2012, 276, 146–153. [Google Scholar] [CrossRef]
- Shuai, M.; Zhou, S.; Yu, B.; Song, J. Deforestation-induced runoff changes dominated by forest-climate feedbacks. Sci. Adv. 2024, 10, eadp3964. [Google Scholar] [CrossRef]
- Baraloto, C.; Hérault, B.; Paine, C.E.; Massot, H.; Blanc, L.; Bonal, D.; Molino, J.F.; Nicolini, E.; Sabatier, D. Contrasting taxonomic and functional responses of a tropical tree community to selective logging. J. Appl. Ecol. 2012, 49, 861–870. [Google Scholar] [CrossRef]
- Paudel, C.K.; Tiwari, A.; Baniya, C.B.; Shrestha, B.B.; Jha, P.K. High Impacts of Invasive Weed Lantana camara on Plant Community and Soil Physico-Chemical Properties across Habitat Types in Central Nepal. Forests 2024, 15, 1427. [Google Scholar] [CrossRef]
- Jones, M.W.; Veraverbeke, S.; Andela, N.; Doerr, S.H.; Kolden, C.; Mataveli, G.; Pettinari, M.L.; Le Quéré, C.; Rosan, T.M.; Van Der Werf, G.R.; et al. Global rise in forest fire emissions linked to climate change in the extratropics. Science 2024, 386, eadl5889. [Google Scholar] [CrossRef]
- Veblen, T.T.; Mermoz, M.; Martin, C.; Ramilo, E. Effects of exotic deer on forest regeneration and composition in northern Patagonia. J. Appl. Ecol. 1989, 26, 711–724. [Google Scholar] [CrossRef]
- Veblen, T.T.; Mermoz, M.; Martin, C.; Kitzberger, T. Ecological impacts of introduced animals in Nahuel Huapi National Park, Argentina. Conserv. Biol. 1992, 6, 71–83. [Google Scholar] [CrossRef]
- Relva, M.A.; Veblen, T.T. Impacts of introduced large herbivores on Austrocedrus chilensis forests in northern Patagonia, Argentina. For. Ecol. Manag. 1998, 108, 27–40. [Google Scholar] [CrossRef]
- Relva, M.A.; Sancholuz, L.A. Effects of simulated browsing on the growth of Austrocedrus chilensis. Plant Ecol. 2000, 151, 121–127. [Google Scholar] [CrossRef]
- Zamorano-Elgueta, C.; Cayuela, L.; González-Espinosa, M.; Lara, A.; Parra-Vázquez, M.R. Impacts of cattle on the South American temperate forests: Challenges for the conservation of the endangered monkey puzzle tree (Araucaria araucana) in Chile. Biol. Conserv. 2012, 152, 110–118. [Google Scholar] [CrossRef]
- Blackhall, M.; Raffaele, E.; Veblen, T.T. Cattle affect early post-fire regeneration in a Nothofagus dombeyi–Austrocedrus chilensis mixed forest in northern Patagonia, Argentina. Biol. Conserv. 2008, 141, 2251–2261. [Google Scholar] [CrossRef]
- Bahamonde, H.A.; Peri, P.L.; Monelos, L.H.; Martínez Pastur, G. Regeneración por semillas en bosques nativos de Nothofagus antarctica bajo uso silvopastoril en Patagonia Sur, Argentina. Bosque 2013, 34, 89–101. [Google Scholar] [CrossRef]
- Peri, P.L.; Bahamonde, H.A.; Lencinas, M.V.; Gargaglione, V.; Soler, R.; Ormaechea, S.; Pastur, G.M. A review of silvopastoral systems in native forests of Nothofagus antarctica in southern Patagonia, Argentina. Agroforest. Syst. 2016, 90, 933–960. [Google Scholar] [CrossRef]
- Bahamonde, H.A.; Peri, P.L.; Monelos, L.H.; Martínez Pastur, G. Aspectos ecológicos de la regeneración por semillas en bosques nativos de Nothofagus antarctica en Patagonia Sur, Argentina. Bosque 2011, 32, 20–29. [Google Scholar] [CrossRef]
- Peri, P.L.; Hansen, N.E.; Bahamonde, H.A.; Lencinas, M.V.; von Müller, A.R.; Ormaechea, S.; Gargaglione, V.; Soler, S.; Tejera, L.E.; Lloyd, C.E.; et al. Silvopastoral Systems Under Native Forest in Patagonia Argentina. In Silvopastoral Systems in Southern South America. Advances in Agroforestry, 1st ed.; Peri, P.L., Dube, F., Varella, A., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Donoso, C. Las Especies Arbóreas de los Bosques Templados de Chile y Argentina. Autoecología, 1st ed.; Marisa Cuneo Ediciones: Valdivia, Chile, 2006. [Google Scholar]
- Navarro-Cerrillo, R.; Hayas, A.; García-Ferrer, A.; Hernández-Clemente, R.; Duhalde, P.; González, L. Caracterización de la situación posincendio en el área afectada por el incendio de 2005 en el Parque Nacional de Torres del Paine (Chile) a partir de imágenes multiespectrales. Rev. Chil. Hist. Nat. 2008, 81, 95–110. [Google Scholar] [CrossRef]
- CONAF. Catastro de los Recursos Vegetacionales Nativos de Chile al Año 2020, 1st ed.; Departamento de Monitoreo de Ecosistemas Forestales: Santiago, Chile, 2021. [Google Scholar]
- Donoso, P.; Promis, A. Silvicultura en Bosques Nativos: Avances en la Investigación en Chile, Argentina y Nueva Zelandia, 1st ed.; Editorial Universitaria: Santiago, Chile, 2013. [Google Scholar]
- Otero, L. La Huella del Fuego: Historia de los Bosques Nativos; Poblamiento y Cambios en el Paisaje del sur de Chile, 1st ed.; Pehuén Ed.: Santiago, Chile, 2006. [Google Scholar]
- Quintanilla, V. Estado de recuperación del bosque nativo en una cuenca nordpatagónica de Chile, perturbada por grandes fuegos acaecidos 50 años atrás (44°–45° S). Rev. Geogr. Norte Gd. 2008, 39, 73–92. [Google Scholar] [CrossRef]
- Armesto, J.J.; Manuschevich, D.; Mora, A.; Smith-Ramírez, C.; Rozzi, R.; Abarzúa, A.; Marquet, P. From the Holocene to the Anthropocene: A historical framework for land cover change in southwestern South America in the past 15,000 years. Land Use Policy 2010, 27, 148–160. [Google Scholar] [CrossRef]
- Donoso, P.J.; Nyland, R.D. Seedling density according to structure, dominance, and understory cover in old-growth forest stands of the evergreen forest type in the coastal range of Chile. Rev. Chil. Hist. Nat. 2005, 78, 51–63. [Google Scholar] [CrossRef]
- Zamorano-Elgueta, C.; Cayuela, L.; Rey Benayas, J.M.; Donoso, P.J.; Geneletti, D.; Hobbs, R.J. The differential influences of human-induced disturbances on tree regeneration community: A landscape approach. Ecosphere 2014, 5, 90. [Google Scholar] [CrossRef]
- Blackhall, M.; Raffaele, E.; Veblen, T.T. Efectos combinados del fuego y el ganado en matorrales y bosques del noroeste patagónico. Ecol. Austral 2015, 25, 1–10. [Google Scholar] [CrossRef]
- Veblen, T.; Hill, R.; Read, J. Ecology and Biogeography of Nothofagus Forests, 1st ed.; Yale University Press: New Haven, CT, USA, 1996. [Google Scholar]
- Cayuela, L.; Golicher, D.J.; Rey Benayas, J.M.; González-Espinosa, M.; Ramírez-Marcial, N. Fragmentation, disturbance and tree diversity conservation in tropical montane forests. J. Appl. Ecol. 2006, 43, 1172–1181. [Google Scholar] [CrossRef]
- Barnes, B.V.; Zak, D.R.; Denton, S.R.; Spurr, S.H. Forest Ecology, 4th ed.; John Wiley and Sons: New York, NY, USA, 1998. [Google Scholar]
- Cadotte, M.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- González, M.; Cortés, M.; Izquierdo, F.; Gallo, L.; Echeverría, C.; Bekessy, S.; Montaldo, P. Araucaria araucana (Molina) K. Koch. Araucaria (o), Pehuén, Pino piñonero, Pino de Neuquén, Monkey Puzzle Tree. In Las Especies Arbóreas de los Bosques Templados de Chile y Argentina: Autoecología, 1st ed.; Donoso, C., Ed.; Marisa Cuneo Ediciones: Valdivia, Chile, 2006; pp. 36–53. [Google Scholar]
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Oksanen, J.; Blanchet, G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.B.; Simpson, G.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package, R package version 2.6-8; The Comprehensive R Archive Network: Vienna, Austria, 2024. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference, R package version 1.48.4; The Comprehensive R Archive Network: Vienna, Austria, 2024. [Google Scholar]
- Navarro-Cerrillo, R.; Rosenfeld, M.; Pérez-Aranda, J.; Padrón, E.; Guzmán, J.R.; Hernández Clemente, R.; González, L. Forest decline evaluation in Antarctic Beech Forests (Nothofagus antarctica) in Chilean Patagonia by using Landsat TM and ETM+. Bosque 2008, 29, 65–73. [Google Scholar]
- Martínez Pastur, G.; Rodríguez-Souilla, J.; Lencinas, M.V.; Cellini, J.M.; Chaves, J.E.; Aravena-Acuña, M.C.; Roig, F.A.; Peri, P.L. Microclimatic Conditions Restrict the Radial Growth of Nothofagus antarctica Regeneration Based on the Type of Forest Environment in Tierra del Fuego. Sustainability 2023, 15, 8687. [Google Scholar] [CrossRef]
- Bahamonde, H.A.; Peri, P.L.; Álvarez, R.; Barneix, A. Producción y calidad de gramíneas en un gradiente de calidades de sitio y coberturas en bosques de Nothofagus antarctica (G. Forster) Oerst. en Patagonia. Ecol. Austral 2012, 22, 62–73. [Google Scholar]
- Giorgis, M.; Cingolani, A.; Teich, I.; Renison, D.; Hensen, I. Do Polylepis australis trees tolerate herbivory? Seasonal patterns of shoot growth and its consumption by livestock. Plant Ecol. 2010, 207, 307–319. [Google Scholar] [CrossRef]
- Marquardt, S.; Marquez, A.; Bouillot, H.; Beck, S.G.; Mayer, A.C.; Kreuzer, M.; Alzérreca, H. Intensity of browsing on trees and shrubs under experimental variation of cattle stocking densities in southern Bolivia. For. Ecol. Manag. 2009, 258, 1422–1428. [Google Scholar] [CrossRef]
- Peri, P.; Ormaechea, S.; Huertas, L. Protección de renovales de ñire. Prod. For. 2009, 4, 15–16. [Google Scholar]
- Hansen, N.; Fertig, M.; Escalona, M.; Tejera, L.; Opazo, W. Ramoneo en regeneración de ñire y disponibilidad forrajera Eco-Nothofagus. In Proceedings of the Segunda Reunión sobre Nothofagus en la Patagonia—EcoNothofagus 2008, Esquel, Argentina, 22–24 April 2008; pp. 137–142. [Google Scholar]
- Martínez, N.; Cuerda, F.; Gomez, F.; Mondino, V.; Tejera, L.; Tarabini, M.; Bava, J.; von Müller, A.R. Direct and indirect estimations of aerial forage net primary productivity in Nothofagus antarctica forests under silvopastoral systems in Northwest of Chubut, Argentina. Agroforest. Syst. 2024, 98, 2027–2040. [Google Scholar] [CrossRef]
- Echevarría, D.; von Müller, A.; Hansen, N.; Bava, J. Efecto del ramoneo bovino en renovales de Nothofagus antarctica en Chubut, Argentina en relación con la carga ganadera y altura de las plantas. Bosque 2014, 35, 357–368. [Google Scholar] [CrossRef]
- Ruggirello, M.J.; Bustamante, G.; Fulé, P.Z.; Soler, R. Drivers of post-fire Nothofagus antarctica forest recovery in Tierra del Fuego, Argentina. Front. Ecol. Evol. 2023, 11, 1113970. [Google Scholar] [CrossRef]
- Simões, C.G.; Marques, M.C.M. The role of sprouts in the restoration of Atlantic Rainforest in southern Brazil. Rest. Ecol. 2007, 15, 53–59. [Google Scholar] [CrossRef]
- Miller, P.M.; Kauffman, J.B. Seedling and sprout response to slash-and-burn agriculture in a tropical deciduous forest. Biotropica 1998, 30, 538–546. [Google Scholar] [CrossRef]
- Premoli, A.C.; Steinke, L. Genetics of sprouting: Effects of long-term persistance in fireprone ecosystems. Mol. Ecol. 2008, 17, 3827. [Google Scholar] [CrossRef]
- Bahamonde, H.; Martínez Pastur, G.; Soler, R.; Monelos, L. Ten years of seed production and establishment of regeneration measurements in Nothofagus antarctica forests under different crown cover and quality sites, in Southern Patagonia. Agroforest. Syst. 2018, 92, 623–635. [Google Scholar] [CrossRef]
- Soler, R.; Lencinas, M.V.; Bustamante, G.; Martínez Pastur, G.M. Atributos de la regeneración natural de ñire (Nothofagus antarctica) en Tierra del Fuego: Beneficios y perjuicios que genera el uso silvopastoril. Ecosist 2018, 27, 41–47. [Google Scholar]
- Veblen, T.T.; Kitzberger, T.; Villalba, R.; Donnegan, J. Fire history in northern Patagonia: The roles of humans and climatic variation. Ecol. Monogr. 1999, 69, 47–67. [Google Scholar] [CrossRef]
- Iranzo, E.C.; Smith, C.; Moraga, C.A.; Radic-Schilling, S.; Corti, P. Patterns of guanaco distribution and microhabitat use in Tierra del Fuego: From protected to sheep ranching areas. Acta Oecol. 2022, 116, 103853. [Google Scholar] [CrossRef]
- Rivas, Y.; Godoy, R.; Valenzuela, E.; Leiva, J.; Oyarzún, C.; Alvear, M. Actividad biológica del suelo en dos bosques de Nothofagus del centro sur de Chile. Gayana Bot. 2007, 64, 81–92. [Google Scholar] [CrossRef]
- Martínez Pastur, G.; Lencinas, M.V.; Peri, P.; Arena, M. Photosynthetic plasticity of Nothofagus pumilio seedlings to light intensity and soil moisture. For. Ecol. Manag. 2007, 243, 274–282. [Google Scholar] [CrossRef]
- Cellini, J.M. Estructura y Regeneración Bajo Distintas Propuestas de Manejo de Bosques de Nothofagus pumilio (Poepp et. Endl) Krasser en Tierra del Fuego, Argentina. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, 2010. [Google Scholar] [CrossRef]
- Rodríguez-Souilla, J.; Cellini, J.M.; Lencinas, M.V.; Roig, F.A.; Chaves, J.E.; Aravena Acuña, M.C.; Peri, P.L.; Martínez Pastur, G. Variable retention harvesting and climate variations influence over natural regeneration dynamics in Nothofagus pumilio forests of Southern Patagonia. For. Ecol. Manag. 2023, 544, 121221. [Google Scholar] [CrossRef]
- Raffaele, E.; Veblen, T.T.; Blackhall, M.; Tercero-Bucardo, N. Synergistic influences of introduced herbivores and fire on vegetation change in northern Patagonia, Argentina. J. Veg. Sci. 2011, 22, 59–71. [Google Scholar] [CrossRef]
- Ruggirello, M.J.; Bustamante, G.N.; Soler, R.M. Nothofagus pumilio regeneration failure following wildfire in the sub-Antarctic forests of Tierra del Fuego, Argentina. For. Int. J. For. Res. 2025, 98, 40–49. [Google Scholar] [CrossRef]
- Rist, L.; Moen, J. Sustainability in forest management and a new role for resilience thinking. For. Ecol. Manag. 2013, 310, 416–427. [Google Scholar] [CrossRef]
- Albrich, K.; Rammer, W.; Turner, M.G.; Ratajczak, Z.; Braziunas, K.H.; Hansen, W.D.; Seidl, R. Simulating forest resilience: A review. Glob. Ecol. Biogeogr. 2020, 29, 2082–2096. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, G.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Martínez Pastur, G.; Soler, R.E.; Cellini, J.M.; Lencinas, M.V.; Peri, P.L.; Neyland, M.G. Survival and growth of Nothofagus pumilio seedlings under several microenvironments after variable retention harvesting in southern Patagonian forests. Ann. For. Sci. 2014, 71, 349–362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).